Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Immediate Effects of Mobile Phone App for Depressed Mood in Young Adults with Subthreshold Depression: A Pilot Randomized Controlled Trial
Authors Ejiri H, Uchida H, Tsuchiya K, Fujiwara K, Kikuchi S, Hirao K
Received 5 April 2023
Accepted for publication 25 July 2023
Published 31 July 2023 Volume 2023:19 Pages 1695—1707
DOI https://doi.org/10.2147/NDT.S415937
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Taro Kishi
Hitomi Ejiri,1 Hiroyuki Uchida,2 Kenji Tsuchiya,3 Kazuhiko Fujiwara,4 Senichiro Kikuchi,1,5 Kazuki Hirao1,5
1Department of Occupational Therapy, Faculty of Medicine, Gunma University, Maebashi, Japan; 2Department of Rehabilitation, Kurashiki Heisei Hospital, Kurashiki, Japan; 3Department of Rehabilitation, Faculty of Health Sciences, Nagano University of Health and Medicine, Nagano, Japan; 4Department of Rehabilitation Sciences, Faculty of Rehabilitation Sciences, Nishikyushu University, Kanzaki, Japan; 5Graduate School of Health Sciences, Gunma University, Maebashi, Japan
Correspondence: Kazuki Hirao, Graduate School of Health Sciences, Gunma University, 3-39-22 Showa, Maebashi, Gunma, 371-8514, Japan, Tel +81-27-220-8952, Fax +81-27-220-8952, Email [email protected]
Background: Preventive interventions for subthreshold depression (StD) are essential to reduce the incidence of major depressive disorder. Our smartphone application presenting positive word stimulation in video (ie, Subliminal Priming with Supraliminal Reward Stimulation, SPSRS) was suggested to improve depressive symptoms in people with StD, although it is unclear whether it can immediately improve depressed mood. This pilot randomized controlled trial (RCT) aimed to investigate the preliminary efficacy of SPSRS application intervention on depressive mood in people with StD.
Methods: Thirty-two participants with StD were randomly assigned to the experimental (n = 16) or control group (n = 16). The experimental group received SPSRS application intervention (10-minute video with positive word stimulation) and the control group received YouTube application intervention (10-minute video without positive word stimulation). Both groups used identical iPhones managed by the research team. The primary outcome was the change from baseline in depression-dejection on the Profile of Mood States 2nd Edition-Adult Short (POMS 2-A Short) after the intervention.
Results: No participants dropped out of the study. The experimental group showed a small improvement in depression-dejection on the POMS 2-A Short score (adjusted Hedges’s g = − 0.32) compared to the control group. Post-hoc power analyses estimated a sample size of 56 per group (112 total) to evaluate depression-dejection on the POMS 2-A Short in a future full-scale RCT.
Conclusion: SPSRS application intervention may be effective in immediately improving depressive mood in people with StD. A future full-scale RCT based on a formally calculated sample size should be conducted to replicate these findings.
Keywords: depressed mood, subthreshold depression, mobile application
Introduction
In recent years, interventions for subthreshold depression (StD) have become increasingly important.1,2 StD does not meet the diagnostic criteria for Major Depressive Disorder (MDD) but is characterized by clinically significant depressive symptoms.3 Not only is the prevalence of StD (4.0%–53.2%) higher than the prevalence of MDD,4–7 but StD causes a lower quality of life,3,8,9 increased mortality,10 poorer health,11 reduced daily activities,5,11 and increased economic costs,12 even though it does not meet the criteria for MDD. Therefore, StD itself is an important entity requiring clinical intervention. In addition, StD is a precursor to MDD and a risk factor for the development of MDD.9,13–15 Given that once established, MDD tends to follow a recurrent and often treatment-resistant course, the development of preventive interventions for StD (ie, adaptive prevention) is essential to reduce its incidence, which is a significant public health problem.16
Many studies have focused on the improvement of depressive symptoms in people with StD.1,2 However, improving depressed mood itself in people with StD can be an important step in improving depressive symptoms and preventing the development of MDD. Mood is the temporary emotional and physiological state of a person.17 Empty, irritable, and sad moods are considered characteristics of depressed mood.18 Previous study have suggested that depressed mood causes an increase in depressive symptoms.19 This finding demonstrates the clinical importance of depressed mood in StD and its potential as a target for reducing the increase in depressive symptoms and ultimately preventing the development of MDD.
There is substantial evidence that psychotherapy can reduce depressive symptoms in people with StD.1,2,20,21 However, these interventions may not be useful in improving depressed mood in StD. In many cases, psychotherapy has focused on the cumulative effects of repeated interventions.2,21 Such a cumulative intervention effect may be useful in addressing chronic depressive symptoms. However, psychotherapy may not be able achieve a reduction of depressive moods resulting from unpredictable stimuli that frequently occur in daily life. In addition, given the need for qualified professional therapists in the delivery of psychotherapy, the stigma attached to the intervention, and the long waiting times, it is essential to develop effective interventions that can help people with StD improve their depressive mood promptly.1,22–24
An application presenting positive word stimulation in video (SPSRS) we developed may be an innovative approach to immediately improve the depressed mood of people with StD.25 This application was designed based on evidence from word stimulation studies and the positive words identified by qualitative studies for people with StD.4,26–29 Its goal is to improve depressive symptoms in people with StD and thereby prevent progression to MDD.25
Subliminal Priming with Supraliminal Reward Stimulation (SPSRS) applications show several advantages towards improving depressed mood in people with StD. First, the SPSRS application is a free video viewing application that uses smartphones. In recent years, mobile devices have been increasingly used to provide mental health services.30 In addition, many people always carry their smartphones and even use them instead of computers to access the internet.30,31 These smartphone features could be used in ambulatory care and rural populations if mental health expertise and equipment are not available. In addition, the SPSRS application is free of charge and enables smartphones to process interventions at any time without restrictions. Thus, users can not only overcome the stigma of face-to-face treatment but also continue their normal daily routines (eg, commuting) because they can receive mental health services when professional interventions are unavailable. The SPSRS application can address the three availability issues in mental health interventions: location-based availability (direct physical access to treatment), time-based availability (inability to receive needed treatment due to time of day), and cost-based availability (inability to access services beyond the recommended minimum weekly hours).31–35 Secondly, the SPSRS application uses the YouTube Application Programming Interface. This makes it possible to watch videos uploaded on YouTube with the SPSRS application. Given the rapid increase in video viewing worldwide and the variety of videos uploaded to YouTube worldwide,36 people with StD can choose the videos that suit their preferences and work towards reducing their depressive mood. Third, the SPSRS application is a StD-only application. Currently, many applications for MDD are being developed.37 However, no smart phone application specific to people with StD has been developed,38 despite suggestions that people with StD need disease-specific interventions.37 Therefore, our SPSRS application may be more effective in improving the symptoms of people with StD than existing applications for MDD. In fact, a previous pilot randomized controlled trial (RCT) showed the preliminary efficacy of SPSRS application intervention in improving depressive symptoms, psychological distress, and anxiety symptoms in people with StD.39 Considering these advantages, the SPSRS application may be an effective intervention strategy to immediately improve depressed mood in people with StD.
However, there are no published RCT data on the immediate effects of SPSRS application intervention on depressed mood in people with StD. Therefore, it was difficult to perform a high-quality RCT because no information was available for formal sample size calculation and preliminary validation by a pilot study was particularly desirable.40 Here, we conducted a pilot RCT to test the preliminary efficacy of the SPSRS application on depressed mood in people with StD, and to obtain information for calculating the sample size needed for a full-scale RCT.
Methods
Trial Design
This study, designed as a single-center, open-label, two-arm, parallel-group, pilot RCT, administered SPSRS application intervention to people with StD and assessed depressed mood at baseline and immediately after the 10-minute intervention. More details on the study design can be found in the protocol paper.41 This study was conducted under the CONSORT 2010 extension to randomized pilot and feasibility trials.42
Participants
We recruited people with StD at Gunma University in Japan. The eligibility criteria for participants are as follows.
Inclusion Criteria
1) Male and female
2) 20 to 39 years old
3) Beck Depression Inventory-II (BDI-II) score of 10 points or more43–45
4) Written informed consent provided prior to participation.
Exclusion Criteria
1) Being diagnosed with a psychiatric disorder at least once in their lifetime, regardless of its type.
2) Currently receiving treatment from a professional for mental health issues.
3) Vision or hearing problems that interfere with daily life.
4) Having had a major depressive episode in the last two weeks according to the Mini-International Neuropsychiatric Interview (MINI).46
Generally, the cutoff value for BDI-II is 14 points or higher.47,48 However, several previous studies on StD have used BDI-II with a cutoff value of 10 points or higher.43,44 Therefore, in this study, the cutoff value for BDI-II was set at 10 points or higher.
The mean (SD) age of participants who met the eligibility criteria was 20.88 (0.72) years for the experimental group and 21.19 (1.68) years for the control group. The percentage of women was 100% in the experimental group and 87.5% in the control group. The percentage of alcohol consumption was 25% in the experimental group and 6.3% in the control group. The mean (SD) duration of sleep was 6.22 (1.28) hours for the experimental group and 6.97 (1.06) hours for the control group. The percentage of smokers was 0% in both the experimental and control groups. The mean (SD) score of the BDI-II was 17.00 (7.03) for the experimental group and 15.25 (3.28) for the control group (Table 1).
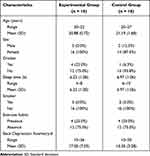 |
Table 1 Baseline Characteristics of the Two Groups |
Settings and Recruitment
Participants were recruited from Gunma University in the Gunma Prefecture, Japan, from March to June 2021. Advertising, e-mail, and social networking services were used to recruit participants. Potential participants who wished to participate in the study were given a detailed explanation of the purpose and procedures of the study using handouts. The URL of the online questionnaire was then sent to potential participants interested in the study to obtain electronic informed consent. Consent to participate in the study was obtained by the participant selecting the consent option. Participants who gave informed consent continued to complete the online questionnaire to assess their eligibility. The researcher then interviewed potential participants using the MINI. After completing a baseline assessment, participants were randomly assigned to the experimental or control groups. The baseline assessment was conducted by the investigator within one week after the eligibility assessment was conducted.
Interventions
The experimental group received a video viewing intervention using the SPSRS application, while the control group received a video viewing intervention via YouTube.
Experimental Group
The experimental group received a video viewing intervention using the SPSRS smartphone application. Two types of word stimuli are displayed in the SPSRS application. First, “able”, “let us try”, “good luck”, “can”, and “do not worry” were randomly displayed in the four corners of the screen as word stimuli to boost confidence.4 These words were displayed for 17 milliseconds. Immediately after that, positive word stimuli, “great”, “fantastic”, “nice”, “satisfactory”, and “enjoyable”, are displayed in the center of the screen for 150 milliseconds.29 These words appeared on the screen repeatedly every five seconds. The SPSRS application was installed on iPhones managed by the research team, and participants watched a predetermined 10-minute video (of a basketball game). Originally, the SPSRS application was designed for participants to independently select and watch videos. However, we decided to select the videos to be viewed during the design phase of the study because the influences of different videos on depressive mood may vary across participants. It is unclear whether the videos we selected are optimal for this study. However, some studies suggest that watching sports may improve viewers’ moods.49,50 In addition, the SPSRS application has a filtering function that prevents participants from viewing videos that may be inappropriate for them (eg, violent videos).39 Therefore, the videos of basketball players used in this study were unlikely to disturb participants and were considered appropriate. Intervention implementers used the SPSRS application following the user manual developed by the research team to ensure fidelity of the intervention.
Control Group
The control group watched the same video as the experimental group for 10 minutes using the YouTube application. Therefore, there was no confidence-boosting or positive words in the video. The control group used the same iPhones as the experimental group and also received the intervention by the same provider as that of the experimental group. As in the experimental group, the intervention implementers used the YouTube application following the user manual developed by the research team to ensure the fidelity of the intervention.
Assessment Measures
The study collected demographic data such as age and gender as well as lifestyle characteristics such as sleep duration, smoking habits, exercise habits, and drinking habits as part of the baseline assessment. Sleep time was defined as the average sleep time per week.51 Drinking habits were defined as drinking at least one bottle (approximately 500 mL) of beer per day, ≥3 days a week.52 Smoking habits were defined as “daily smoker” or “have occasional smoking days.53” Exercise habits were defined as exercise periods “once 30 min or more” performed at least twice a week and continued for at least 1 year.54
Primary Outcome
The primary outcome of the study was the change from baseline in depression-dejection on the Profile of Mood States 2nd Edition-Adult Short (POMS 2-A Short) after the intervention.55,56 The POMS 2-A Short is a 35-item self-administered questionnaire for measuring mood state. It consists of seven subscale scores (anger-hostility, confusion-bewilderment, depression-dejection, fatigue-inertia, tension-anxiety, vigor-activity, and friendliness). Each subscale is rated on a 5-point Likert scale (0 = not at all, 1 = a little, 2 = moderately, 3 = quite a lot, and 4 = extremely). High scores indicate better vigor-activity and friendliness are better but more severe symptoms in the five other domains. In addition, the Total Mood Disturbance score (TMD) is calculated from anger-hostility, confusion-bewilderment, depression-dejection, fatigue-inertia, tension-anxiety, and vigor-activity. TMD is calculated based on a standard value (mean of 50 and standard deviation of 10). POMS 2-A Short showed high internal consistency (Cronbach’s α = 0.80–0.95).57
Secondary Outcomes
Anger-hostility, confusion-bewilderment, fatigue-inertia, tension-anxiety, vigor-activity, friendliness, and TMD as measured by the POMS 2-A Short.55,56
State-Trait Anxiety Inventory State (STAI-S)
The STAI-S is a 20-item self-administered questionnaire used to measure anxiety state.58–60 Each item on the STAI-S is rated on a scale of 1 to 4, with a total score of 20 to 80. The higher the score, the stronger the anxiety state. STAI-S showed high internal consistency (Cronbach’s α = 0.87–0.91).61,62
Blinding
This was an open-label study in which participants, interventionists, and the assessor were not blinded. However, we tried to minimize the bias caused by a lack of blinding. First, a baseline assessment was performed prior to the random assignment. Second, we also used standardized methods to measure outcomes for auditing and maintaining the quality of the data. In addition, the interventions for the experimental and control groups were conducted according to the manual developed by the research team.
Sample Size
Sample size calculation requires four factors: significance level (α = 0.05), power (80%), and group difference and standard deviation in primary outcome.63 However, group difference and standard deviation are determined based on past research data, and there is no research to date examining the immediate effects of the SPSRS application intervention on depressed mood in people with StD. Therefore, it was difficult to calculate the sample size based on actual data. In the absence of data from previous studies, a pilot RCT is a reliable way to obtain information prerequisite to sample size calculations (mean and standard deviation of differences between groups for the primary outcome) for later full-scale RCTs.40 In fact, one of the main objectives of this pilot study is to inform sample size calculations for subsequent full-scale RCT.40,64 For these reasons, pilot RCTs do not require formal sample size calculations.40 However, a sufficient sample size is necessary to ensure the scientific validity of the pilot study. In general, the sample size of a pilot study intended for subsequent full-scale studies requires 15 to 20 participants per group.65 Therefore, the goal of this study was to gather 16 participants per group, for a total of 32 participants.
Randomization
Participants who met eligibility criteria were randomly assigned to either the experimental or control group in a 1:1 ratio after baseline assessment. The randomization list was created by a third party not involved in the study, using Excel software, and based on a permuted block method (block size 4). The prepared randomized list was sent to the central registration center established at the Kurashiki Heisei Hospital in Japan. Each time a participant was enrolled in the study, the central registration center was contacted and a random assignment was made. Thus, the process of randomization was concealed.
Statistical Analysis
All randomly assigned participants were analyzed based on intention-to-treat (ITT). To investigate the effect of the SPSRS application on primary and secondary outcomes, a linear mixed model (LMM) with a restricted maximum likelihood estimation method was used.66 LMMs can use all available data to produce estimates.66 Therefore, LMMs can address the deficiencies that occur in clinical trials. The fixed-effect factors considered the group, time, and group and time interactions, while the random-effect factors considered the participants. The model used a type III test of fixed effects. SPSS v.27.0 (IBM Japan, Tokyo, Japan) was used for these analyses, with statistical significance set at P < 0.05 for the two-tailed test. We also reported the Hedges’ g between groups to report the standardized mean difference.67,68 Hedges’s g is capable of correcting the upward bias of Cohen’s d caused by the size of the sample size.67,68 Hedges’s g between groups was calculated pre and post, and Hedges’s g adjusted for baseline differences was reported by subtracting the pre-Hedges’s g from the post-Hedges’s g.69 For the standardized mean difference between groups, 0.2 was considered a small difference, 0.5 a medium difference, and 0.8 a large difference.70
Ethical Issues
It was approved by the Ethical Review Board for Medical Research Involving Human Subjects of Gunma University (approval number: HS2020-157) and was registered with ClinicalTrials.gov (NCT04707495). Each participant’s data was recorded and processed as a participant number to protect the participant’s privacy and maintain confidentiality. In addition, no information regarding the identity of the individual was entered into the electronic database. Names and other identifying information, such as informed consent, were stored separately from the survey materials identified by participant number.
Results
Enrollment and Baseline Characteristics
This study was conducted between March 2021 and June 2021, and the final evaluation was performed. Figure 1 shows the flow of this trial. Among 222 participants, 32 (aged 20–27 years) met the eligibility criteria for the study and were randomized to the experimental (n = 16) or control group (n = 16) after baseline assessment. All participants completed the assigned intervention and received a final assessment immediately following the intervention. Therefore, no participants discontinued the study after the start of intervention. Table 1 shows the demographic and lifestyle characteristics of participants at baseline. The mean age (SD) was 20.88 (0.72) in the experimental group and 21.19 (1.68) in the control group. The percentage of females was 100% in the experimental group and 87.5% in the control group.
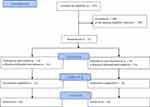 |
Figure 1 Flowchart of trial. |
Effects of SPSRS Application Intervention on Outcomes
Table 2 shows the estimated effects of the SPSRS application on outcomes based on LMM analysis for the experimental and control groups. In addition, Table 3 shows the mean and SD of the baseline and post-intervention outcome measures, as well as the Hedges’s g between the two groups. The effects of time (P < 0.001) and group (P = 0.03) on the primary outcome, the depression-dejection score, were significant, but the group and time interaction (P = 0.14) was not significant. Adjusted Hedges’s g between the two groups was −0.32. The effect of time on the secondary outcome, the anger-hostility score, was significant (P < 0.001), but those of group (P = 0.36) and the group and time interaction (P = 1.00) were not significant. The Adjusted Hedges’s g between the two groups was 0.28. The effect of time on the confusion-bewilderment score was significant (P < 0.001), but those of group (P = 0.14) and the group and time interaction (P = 0.97) were not significant. Adjusted Hedges’s g between the two groups was 0.27. The effect of time on the fatigue-inertia score was significant (P < 0.001), but those of group (P = 0.23) and the group and time interaction (P = 0.40) were not significant. The adjusted Hedges’s g between the two groups was 0.23. The effect of time on the tension-anxiety score was significant (P < 0.001), but those of group (P = 0.15) and the group and time interaction (P = 0.85) were not significant. The adjusted Hedges’s g between the two groups was 0.01. The effects of time (P < 0.79), group (P = 0.94), and group and time interaction (P = 0.76) on vigor-activity scores were not significant. The adjusted Hedges’s g between the two groups was 0.10. The effects of time (P < 0.25), group (P = 0.90), and group and time interaction (P = 0.83) on the friendliness score were not significant. The adjusted Hedges’s g between the two groups was −0.04. The effect of time on the TMD was significant (P < 0.001), but those of group (P = 0.19) and the group and time interaction (P = 0.49) were not significant. Adjusted Hedges’s g between the two groups was −0.26. The effect of time on the STAI-S score was significant (P = 0.01), but those of the group (P = 0.53) and the group and time interaction (P=0.62) were not significant. The adjusted Hedges’s g between the two groups was −0.15.
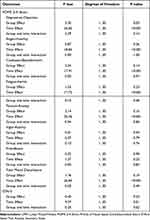 |
Table 2 Results of the LMM Analysis of Experimental and Control Groups |
 |
Table 3 Results of Effect Size Analysis Between Groups |
Post-Hoc Power Analyses
Data from this trial was used to calculate the sample size required to achieve a two-sided α = 0.05, 80% power. The difference in the mean change in the depression-dejection score between the experimental and control groups was 2.37 points. The mean SD of the two groups was 4.41 points. Therefore, a sample size of 56 per group (112 total) was estimated to assess depression-dejection.
Discussion
This study used an RCT to determine whether the SPSRS application could immediately improve depressed mood in people with StD. This pilot RCT showed that a video viewing intervention using the SPSRS application may lead to a small improvement in depressed mood in people with StD immediately after the intervention compared to that of a video viewing intervention using the YouTube application (adjusted Hedges’s g = −0.32). The results of this study agree with trends observed in other RCTs that suggest the effectiveness of SPSRS application-based interventions for treating depressive symptoms in people with StD.39 The immediate intervention effect of the SPSRS application on depressed mood in people with StD may be small. However, the SPSRS application could improve depressed mood in people with StD with a short intervention time, regardless of time and place. In addition, SPSRS applications can be offered at a low cost, which addresses many of the availability issues (location-based availability, time-based availability, cost-based availability) in mental health interventions.31–35 Therefore, SPSRS applications can be incorporated into routine practice as a cost-effective adjunctive intervention to improve depressive mood in people with StD, potentially reducing the burden on the health care system and its practitioners as well as accessibility barriers.
A previous study showed that an intervention of 30 minutes of aerobic exercise for patients with MDD led to small improvements in depressed mood (standardized mean difference = 0.26–0.48) compared to no intervention.71 Therefore, the intervention effect of SPSRS application on depressed mood is comparable to that of aerobic exercise. Considering the non-clinical sample of participants in this study as well as the short intervention time (10 minutes) of the SPSRS application, the SPSRS application is considered as effective as or more effective than existing interventions for an immediate improvement of depressive mood. Therefore, the SPSRS application can be a new approach for people who have difficulty participating in training with aerobic and other exercises.
Subliminal Priming with Supraliminal Reward Stimulation (SPSRS) application intervention was suggested to not be superior to a regular video viewing intervention using YouTube in improving anger-hostility (adjusted Hedges’s g = 0.28), confusion-bewilderment (adjusted Hedges’s g = 0.27), fatigue-inertia (adjusted Hedges’s g = 0.23), tension-anxiety (adjusted Hedges’s g = 0.01), vigor-activity (adjusted Hedges’s g = 0.10), friendliness (adjusted Hedges’s g = −0.04), and STAI-S (adjusted Hedges’s g = −0.15) in people with StD. There are two possible explanations for these results. Firstly, word stimuli used in the SPSRS application focus on reducing depressive symptoms in people with StD and did not focus on mood (anger-hostility, confusion-bewilderment, fatigue-inertia, tension-anxiety, vigor-activity, and friendliness) and anxiety state,4,25 which may have made it difficult to improve these symptoms. Secondly, regarding the intervention time for SPSRS applications, a previous RCT suggested that SPSRS application intervention (70+ minutes of video viewing per week for 5 weeks) for people with StD had a medium effectiveness in improving depressive symptoms and a small effectiveness in improving psychological distress and anxiety symptoms.39 This finding indicates that the SPSRS application may be more effective for depressive symptoms than for psychological distress and anxiety symptoms, given a comparable intervention time. Based on this previous study, it is possible that the 10-minute SPSRS application intervention was not a sufficient intervention time to produce immediate changes in mood scores other than depressed mood. On the other hand, interestingly, TMD was suggested to be slightly improved by using the SPSRS application (adjusted Hedges’s g = −0.26). This result supports the overall mood-improving effect of SPSRS application intervention. Since TMD is a broad concept that reflects overall mood, it may indicate a wide range of effects for SPSRS applications beyond the domain of each subscale.
Based on the results of this study, we were able to calculate a reasonable sample size (56 patients per group, 112 patients in total) that would allow for appropriate evaluation in a full-scale RCT in the future. Therefore, considering strategies to recruit larger samples is essential for the success of a future full-scale RCT. For example, advertising on online portals and social media, online recruitment using relevant websites of academic societies and recruitment agencies, and multi-site implementation may improve recruitment rates.72
Strengths and Limitations
The strength of this study is that it examined the immediate effects of SPSRS application intervention on depressed mood in people with StD. Considering psychological factors such as conditioning and expectations, an immediate improvement in depressed mood may motivate people with StD to continue treatment and facilitate their participation in longer-term interventions that produce further treatment effects. Additionally, spontaneous remission of disease, regression to the mean in clinical studies, and other factors that disrupt the internal validity of results are expected to be smaller in studies examining the immediate effects of interventions than in study designs examining cumulative intervention effects. This RCT may also guide future digital mental health interventions. Although previous studies have highlighted the importance of digital mental health interventions, they suggest that the field of research and practice has not yet been fully explored.34,73 They concluded that these interventions cannot be reliably evaluated by a single best-practice approach.73 Other studies have also described the following potential study design issues when conducting RCTs on medical devices: randomization, acceptability, blinding, and determining appropriate outcomes.74 Therefore, trials that evaluate the effectiveness of digital mental health interventions must be carefully designed to minimize the risk of bias. This RCT was rigorously designed based on standardized and robust procedures to ensure scientific validity.75 As such, this study can guide future RCTs on various digital mental health interventions.
However, there are some limitations to this study. First, no formal sample size calculation was performed, and the sample size was the minimum necessary to ensure the scientific validity of the results. Therefore, the comparative analysis may not have functioned properly. Post-hoc power analyses showed that a sample of 56 participants per group was needed to detect differences between the two groups. Future trials should replicate the intervention effects of the SPSRS application on depressed mood in people with StD, based on formally calculated sample sizes. Second, this was an open-label study with no blinding. Therefore, bias may have occurred among intervention implementers, participants, and evaluators. Due to the nature of the intervention, it is difficult to blind intervention implementers and participants. Future full-scale trials will need to blind evaluators and address the possibility of bias. Third, the study sample was mainly young people (20–27 years old) and the generalizability of its findings to other age groups is unknown. Future trials should examine the impact of the SPSRS application intervention on the depressed mood of people with StD in various age groups.
Conclusions
This is the first RCT on the immediate effects of SPSRS application intervention on depressed mood in people with StD. The current findings extend the literature on application-based interventions for depressed mood in people with StD. It provides evidence that SPSRS application intervention has a small effect on depressive mood in people with StD compared to regular video viewing, requiring further study as an intervention to improve depressed mood in people with StD. Future full-scale RCTs to test the effect of the intervention based on formal sample sizes may help determine whether it reduces depressive mood in StD and prevents the development of MDD.
Data Sharing Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by JSPS KAKENHI Grant Number 19K19724 and 22K11111.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhou T, Li X, Pei Y, Gao J, Kong J. Internet-based cognitive behavioural therapy for subthreshold depression: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):356.
2. Cuijpers P, Koole SL, van Dijke A, Roca M, Li J, Reynolds CF. Psychotherapy for subclinical depression: meta-analysis. Br J Psychiatry. 2014;205(4):268–274.
3. Rodríguez MR, Nuevo R, Chatterji S, Ayuso-Mateos JL. Definitions and factors associated with subthreshold depressive conditions: a systematic review. BMC Psychiatry. 2012;12:181.
4. Takahashi K, Takada K, Inoue A, et al. Identification of common words to improve self-confidence in Japanese students with subthreshold depression. Int J Adolesc Med Health. 2019;31(3):1–7.
5. Xiang X, Leggett A, Himle JA, Kales HC. Major Depression and Subthreshold Depression among Older Adults Receiving Home Care. Am J Geriatr Psychiatry. 2018;26(9):939–949.
6. Vaccaro R, Borrelli P, Abbondanza S, et al. Subthreshold Depression and Clinically Significant Depression in an Italian Population of 70-74-Year-Olds: prevalence and Association with Perceptions of Self. Biomed Res Int. 2017;2017:3592359.
7. Meeks TW, Vahia IV, Lavretsky H, Kulkarni G, Jeste DV. A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord. 2011;129(1–3):126–142.
8. Goldney RD, Fisher LJ, Dal Grande E, Taylor AW. Subsyndromal depression: prevalence, use of health services and quality of life in an Australian population. Soc Psychiatry Psychiatr Epidemiol. 2004;39(4):293–298.
9. Cuijpers P, Smit F, van Straten A. Psychological treatments of subthreshold depression: a meta-analytic review. Acta Psychiatr Scand. 2007;115(6):434–441.
10. Ho C, Jin A, Nyunt MS, Feng L, Ng TP. Mortality rates in major and subthreshold depression: 10-year follow-up of a Singaporean population cohort of older adults. Postgrad Med. 2016;128(7):642–647.
11. Ayuso-Mateos JL, Nuevo R, Verdes E, Naidoo N, Chatterji S. From depressive symptoms to depressive disorders: the relevance of thresholds. Br J Psychiatry. 2010;196(5):365–371.
12. Cuijpers P, Smit F, Oostenbrink J, de Graaf R, Ten Have M, Beekman A. Economic costs of minor depression: a population-based study. Acta Psychiatr Scand. 2007;115(3):229–236.
13. Wesselhoeft R, Sørensen MJ, Heiervang ER, Bilenberg N. Subthreshold depression in children and adolescents - a systematic review. J Affect Disord. 2013;151(1):7–22.
14. Naber D, Bullinger M. Should antidepressants be used in minor depression? Dialogues Clin Neurosci. 2018;20(3):223–228.
15. Lee YY, Stockings EA, Harris MG, et al. The risk of developing major depression among individuals with subthreshold depression: a systematic review and meta-analysis of longitudinal cohort studies. Psychol Med. 2019;49(1):92–102.
16. Narang P, Retzlaff A, Brar K, Lippmann S. Deep Brain Stimulation for Treatment-Refractory Depression. South Med J. 2016;109(11):700–703.
17. Nettle D. An evolutionary model of low mood states. J Theor Biol. 2009;257(1):100–103.
18. Pemberton R, Fuller Tyszkiewicz MD. Factors contributing to depressive mood states in everyday life: a systematic review. J Affect Disord. 2016;200:103–110.
19. Fu JX, Luo Y, Chen MZ, et al. Associations among menopausal status, menopausal symptoms, and depressive symptoms in midlife women in Hunan Province, China. Climacteric. 2020;23(3):259–266.
20. Reins JA, Buntrock C, Zimmermann J, et al. Efficacy and Moderators of Internet-Based Interventions in Adults with Subthreshold Depression: an Individual Participant Data Meta-Analysis of Randomized Controlled Trials. Psychother Psychosom. 2021;90(2):94–106.
21. Cuijpers P, Pineda BS, Ng MY, et al. A Meta-analytic Review: psychological Treatment of Subthreshold Depression in Children and Adolescents. J Am Acad Child Adolesc Psychiatry. 2021;60(9):1072–1084.
22. Apolinário-Hagen J, Vehreschild V, Alkoudmani RM. Current Views and Perspectives on E-Mental Health: an Exploratory Survey Study for Understanding Public Attitudes Toward Internet-Based Psychotherapy in Germany. JMIR Mental Health. 2017;4(1):e8.
23. Pedersen ER, Paves AP. Comparing perceived public stigma and personal stigma of mental health treatment seeking in a young adult sample. Psychiatry Res. 2014;219(1):143–150.
24. Cuijpers P, van Straten A, Andersson G. Internet-administered cognitive behavior therapy for health problems: a systematic review. J Behav Med. 2008;31(2):169–177.
25. Takahashi K, Takada K, Hirao K. Feasibility and preliminary efficacy of a smartphone application intervention for subthreshold depression. Early Interv Psychiatry. 2019;13(1):133–136.
26. Azukizawa K, Hirose K, Morigami Y, Higashi N, Uchida H, Hirao K. Positive-word stimuli via a smartphone application have no immediate-term effects on multi-directional reach ability in standing position: a randomized controlled trial. Ann Med. 2021;53(1):1402–1409.
27. Aarts H, Custers R, Marien H. Preparing and motivating behavior outside of awareness. Science. 2008;319(5870):1639.
28. Aoyama Y, Uchida H, Sugi Y, et al. Immediate effect of subliminal priming with positive reward stimuli on standing balance in healthy individuals: a randomized controlled trial. Medicine. 2017;96(28):e7494.
29. Takarada Y, Nozaki D. Maximal voluntary force strengthened by the enhancement of motor system state through barely visible priming words with reward. PLoS One. 2014;9(10):e109422.
30. Firth J, Torous J, Nicholas J, et al. The efficacy of smartphone-based mental health interventions for depressive symptoms: a meta-analysis of randomized controlled trials. World Psychiatry. 2017;16(3):287–298.
31. Kolenik T. Methods in Digital Mental Health: smartphone-Based Assessment and Intervention for Stress, Anxiety, and Depression. In: Comito C, Forestiero A, Zumpano E, editors. Integrating Artificial Intelligence and IoT for Advanced Health Informatics: AI in the Healthcare Sector. Cham: Springer International Publishing; 2022:105–128.
32. Freedman N, Hoffenberg JD, Vorus N, Frosch A. The effectiveness of psychoanalytic psychotherapy: the role of treatment duration, frequency of sessions, and the therapeutic relationship. J Am Psychoanal Assoc. 1999;47(3):741–772.
33. Cliffe B, Croker A, Denne M, Stallard P. Clinicians’ use of and attitudes towards technology to provide and support interventions in child and adolescent mental health services. Child Adolesc Ment Health. 2020;25(2):95–101.
34. Kolenik T, Gams M. Intelligent Cognitive Assistants for Attitude and Behavior Change Support in Mental Health: state-of-the-Art Technical Review. Electronics. 2021;10(11):1250.
35. Kolenik T, Gams M. Persuasive Technology for Mental Health: one Step Closer to (Mental Health Care) Equality? IEEE Technol Soc Mag. 2021;40(1):80–86.
36. Madathil KC, Rivera-Rodriguez AJ, Greenstein JS, Gramopadhye AK. Healthcare information on YouTube: a systematic review. Health Informatics J. 2015;21(3):173–194.
37. Miralles I, Granell C, Díaz-Sanahuja L, et al. Smartphone Apps for the Treatment of Mental Disorders: systematic Review. JMIR mHealth uHealth. 2020;8(4):e14897.
38. Takagaki K, Okamoto Y, Jinnin R, et al. Behavioral characteristics of subthreshold depression. J Affect Disord. 2014;168:472–475.
39. Kageyama K, Kato Y, Mesaki T, et al. Effects of video viewing smartphone application intervention involving positive word stimulation in people with subthreshold depression: a pilot randomized controlled trial. J Affect Disord. 2021;282:74–81.
40. Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1.
41. Ejiri H, Uchida H, Tsuchiya K, Fujiwara K, Kikuchi S, Hirao K. Effects of Smartphone-Delivered Positive-Word Stimulation on Depressed Mood in People with Subthreshold Depression: protocol for a Pilot Randomized Controlled Trial. Neuropsychiatr Dis Treat. 2021;17:2739–2748.
42. Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239.
43. Furukawa TA, Horikoshi M, Kawakami N, et al. Telephone cognitive-behavioral therapy for subthreshold depression and presenteeism in workplace: a randomized controlled trial. PLoS One. 2012;7(4):e35330.
44. Takagaki K, Okamoto Y, Jinnin R, et al. Behavioral activation for late adolescents with subthreshold depression: a randomized controlled trial. Eur Child Adolesc Psychiatry. 2016;25(11):1171–1182.
45. Kojima M, Furukawa TA, Takahashi H, Kawai M, Nagaya T, Tokudome S. Cross-cultural validation of the Beck Depression Inventory-II in Japan. Psychiatry Res. 2002;110(3):291–299.
46. Otsubo T, Tanaka K, Koda R, et al. Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin Neurosci. 2005;59(5):517–526.
47. Upton J. Beck Depression Inventory (BDI). In: Gellman MD, Turner JR, editors. Encyclopedia of Behavioral Medicine. New York: Springer New York; 2013:178–179.
48. Furukawa TA. Assessment of mood: guides for clinicians. J Psychosom Res. 2010;68(6):581–589.
49. Apostolou M, Lambrianou R. What Motivates People to Do and Watch Sports? Exploring the Effect of Sex, Age, Partner Status, and Parenthood. Evolutionary Psychol Sci. 2017;3(1):20–33.
50. Tsuji T, Kanamori S, Watanabe R, et al. Watching sports and depressive symptoms among older adults: a cross-sectional study from the JAGES 2019 survey. Sci Rep. 2021;11(1):10612.
51. Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31(5):635–643.
52. Takimoto H, Yokoyama T, Yoshiike N, Fukuoka H. Increase in low-birth-weight infants in Japan and associated risk factors, 1980-2000. J Obstet Gynaecol Res. 2005;31(4):314–322.
53. Akhtar PC, Haw SJ, Currie DB, Zachary R, Currie CE. Smoking restrictions in the home and secondhand smoke exposure among primary schoolchildren before and after introduction of the Scottish smoke-free legislation. Tob Control. 2009;18(5):409–415.
54. Nishi N, Yoshizawa T, Okuda N. Effects of rapid aging and lower participation rate among younger adults on the short-term trend of physical activity in the National Health and Nutrition Survey, Japan. Geriatr Gerontol Int. 2017;17(10):1677–1682.
55. Heuchert J, McNair D. POMS 2. J North Tonawanda, NY: Multi-Health Systems Incorporated; 2012.
56. Yokoyama K, Watanabe K. Japanese Translation of POMS 2: Profile of Mood States Second Edition. Tokyo: Kaneko Shobo; 2015.
57. Lin S, Hsiao -Y-Y, Wang M. Test Review: the Profile of Mood States 2nd Edition. J Psychoeduc Assess. 2014;32(3):273–277.
58. Hidano T, Fukuhara M, Iwawaki S, Soga S, Spielberger C. State-Trait Anxiety Inventory-Form JYZ. Tokyo: JITSUMUKYOIKU-SHUPPAN Co. Ltd.; 2000.
59. Spielberger C, Gorsuch R, Lushene R. STAI Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1970.
60. Spielberger C. State–Trait Anxiety Inventory for Adults. Sampler Set, Manual Set, Scoring Key. Palo Alto CA: Consulting Psychologists Press; 1983.
61. Nomura T, Kanda T, Suzuki T, Yamada S. Do people with social anxiety feel anxious about interacting with a robot? AI Soc. 2020;35(2):381–390.
62. Nakano Y, Akechi T, Furukawa TA, Sugiura-Ogasawara M. Cognitive behavior therapy for psychological distress in patients with recurrent miscarriage. Psychol Res Behav Manag. 2013;6:37–43.
63. Charles P, Giraudeau B, Dechartres A, Baron G, Ravaud P. Reporting of sample size calculation in randomised controlled trials: review. BMJ. 2009;338:b1732.
64. Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol. 2010;10:67.
65. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180–191.
66. Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61(3):310–317.
67. Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. New York: Academic Press; 1985.
68. Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Statistics. 1981;6(2):107–128.
69. Durlak JA. How to select, calculate, and interpret effect sizes. J Pediatr Psychol. 2009;34(9):917–928.
70. Cohen J. Statistical Power Analysis for the Behavioral Sciences.
71. Meyer JD, Koltyn KF, Stegner AJ, Kim JS, Cook DB. Influence of Exercise Intensity for Improving Depressed Mood in Depression: a Dose-Response Study. Behav Ther. 2016;47(4):527–537.
72. Briel M, Speich B, von Elm E, Gloy V. Comparison of randomized controlled trials discontinued or revised for poor recruitment and completed trials with the same research question: a matched qualitative study. Trials. 2019;20(1):800.
73. McKay FH, Cheng C, Wright A, Shill J, Stephens H, Uccellini M. Evaluating mobile phone applications for health behaviour change: a systematic review. J Telemed Telecare. 2018;24(1):22–30.
74. Neugebauer EAM, Rath A, Antoine SL, et al. Specific barriers to the conduct of randomised clinical trials on medical devices. Trials. 2017;18(1):427.
75. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
