Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Imaging-Derived Biomarkers Integrated with Clinical and Laboratory Values Predict Recurrence of Hepatocellular Carcinoma After Liver Transplantation
Authors Hoang TPT , Schindler P, Börner N, Masthoff M, Gerwing M, von Beauvais P, De Toni EN, Lange CM, Trebicka J, Morgül H, Seidensticker M, Ricke J, Pascher A, Guba M, Ingrisch M, Wildgruber M, Öcal O
Received 22 August 2023
Accepted for publication 22 November 2023
Published 18 December 2023 Volume 2023:10 Pages 2277—2289
DOI https://doi.org/10.2147/JHC.S431503
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Thi Phuong Thao Hoang,1 Philipp Schindler,2 Nikolaus Börner,3 Max Masthoff,2 Mirjam Gerwing,2 Philippa von Beauvais,2 Enrico N De Toni,4 Christian M Lange,4 Jonel Trebicka,5 Haluk Morgül,6 Max Seidensticker,1 Jens Ricke,1 Andreas Pascher,6 Markus Guba,3 Michael Ingrisch,1 Moritz Wildgruber,1,* Osman Öcal1,*
1Department of Radiology, University Hospital, LMU Munich, Munich, Germany; 2Clinic for Radiology, University Hospital Muenster, Muenster, Germany; 3Department of General, Visceral and Transplantation Surgery, University Hospital, LMU Munich, Munich, Germany; 4Department for Internal Medicine II, University Hospital, LMU Munich, Munich, Germany; 5Department for Internal Medicine B, Universitätsklinikum Münster, Münster, Germany; 6Department of General, Visceral and Transplant Surgery, Universitätsklinikum Münster, Münster, Germany
*These authors contributed equally to this work
Correspondence: Osman Öcal, Department of Radiology, University Hospital – LMU Munich, Marchioninistrasse 15, D-81377, München, Germany, Email [email protected]
Purpose: To investigate the prognostic value of computed tomography (CT) derived imaging biomarkers in hepatocellular carcinoma (HCC) recurrence after liver transplantation (LT) and develop a predictive nomogram model.
Patients and Methods: This retrospective study included 178 patients with histopathologically confirmed HCC who underwent liver transplantation between 2007 and 2021 at the two academic liver centers. We evaluated dedicated imaging features from baseline multiphase contrast-enhanced CT supplemented by several clinical findings and laboratory parameters. Time-to-recurrence was estimated by Kaplan–Meier analysis. Univariable Cox proportional hazard regression and multivariable Least Absolute Shrinkage and Selection Operator (LASSO) regression were used to assess independent prognostic factors for recurrence. A nomogram model was then built based on the independent factors selected through LASSO regression, to predict the probabilities of HCC recurrence at one, three, and five years.
Results: The rate of HCC recurrence after LT was 17.4% (31 of 178). The LASSO analysis revealed six independent predictors associated with an elevated risk of tumor recurrence. These predictors included the presence of peritumoral enhancement, the presence of over three tumor lesions, the largest tumor diameter greater than 3 cm, serum alpha-fetoprotein (AFP) levels exceeding 400 ng/mL, and the presence of a tumor capsule. Conversely, a history of bridging therapies was found to be correlated with a reduced risk of HCC recurrence. In addition, Kaplan-Meier curves showed patients with irregular margin, satellite nodules, or small lesions displayed shorter time-to-recurrence. Our nomogram demonstrated good performance, yielding a C-index of 0.835 and AUC values of 0.86, 0.88, and 0.85 for the predictions of 1-year, 3-year, and 5-year TTR, respectively.
Conclusion: Imaging parameters derived from baseline contrast-enhanced CT showing malignant behavior and aggressive growth patterns, along with serum AFP and history of bridging therapies, show potential as biomarkers for predicting HCC recurrence after transplantation.
Keywords: hepatocellular carcinoma, transplantation, imaging, recurrence
Introduction
Liver transplantation is recommended as first-line treatment for hepatocellular carcinoma (HCC) being within Milan criteria but unsuitable for surgical resection.1 Five-year survival rates after transplantation of patients within Milan criteria have been reported ranging from 65 to 80%.2,3 Due to the increased risk of recurrence in patients beyond Milan criteria, transplantation is restricted for these patients in many European countries. Yet, patients beyond Milan criteria can also be considered for liver transplantation by downstaging using locoregional therapies.4
Additional criteria, like UCSF or up-to-7, for patient selection have been described and shown to be non-inferior to Milan criteria regarding post-transplantation survival.5–7 However, most criteria utilize only the number and size of the lesions, a similar concept as the Milan criteria. Although tumor size and number are correlated with tumor recurrence in the transplant organ, tumor biology, such as tumor grade or microscopic vascular invasion, is the ultimate prognostic factor for disease recurrence and transplant failure.8 Therefore, the EASL guideline recommends for evaluation of composite criteria with surrogates of tumor biology and predicts the replacement of conventional criteria for patient stratification towards transplantation.1 However, since HCC can be diagnosed without pathological analysis in the presence of typical perfusion characteristics on imaging, tumor biology is not known for most patients at the time of evaluation for transplantation. Furthermore, biopsy is associated with a major sampling error which may lead to missing microvascular invasion or underestimation of tumor grade.9 Imaging parameters have been shown to correlate with many histopathological characteristics of HCC lesions, which could guide patient stratification for liver transplantation by non-invasive means.10,11 Also, considering that many patients receive locoregional treatments for bridging or downstaging during the period between listing and transplantation, these imaging markers can be used to allocate patients with high risk of recurrence to more aggressive treatments, such as adjuvant therapies. Some studies used pre-transplant images to identify imaging-based risk factors for recurrence after transplantation. However, identifying patients at high recurrence risk at this point can only guide the follow-up imaging intensity. Some studies have evaluated the prognostic value of baseline imaging features in HCC patients who receive transplantation after a certain type of bridging therapy;12 however, a comprehensive analysis of all listed patients using the images at the time of listing is lacking.
The aim of this study was to evaluate the potential role of imaging biomarkers in baseline (prior to any treatment) CT images and clinical parameters for HCC recurrence after liver transplantation.
Methods
Study Population
This retrospective study was conducted at the University Hospital of LMU Munich and University Hospital Munster, and institutional review board approvals were obtained. This study was conducted in accordance with both the Declarations of Helsinki and Istanbul. Due to retrospective nature of the study, informed consent was waived. Using electronic medical records, we retrospectively enrolled adult patients with HCC who underwent liver transplantation between 2007 and 2021 at both institutions. All patients signed informed consent for the transplantation in accordance with the Declaration of Istanbul, and for the use of organs from rescue allocation according to Eurotransplant. All patient data were pseudoanonymized before the analysis. LT evaluation is done according to center standards. Patients eligible for liver transplantation had no extrahepatic tumor manifestation or macrovascular invasion. The size and number of tumor lesions were considered for treatment allocation, but there was no predefined upper limit. All patients were referred to neoadjuvant (bridging) locoregional or systemic therapy with the goal of downstaging. Downstaging was quantified using mRECIST. Patients primarily within the Milan criteria were eligible for standard exception points. Patients outside the Milan criteria were considered eligible if they responded or had stable disease for more than 6 months on neoadjuvant therapy. As these patients were not prioritized, they received primarily livers from extended criteria donors.
The study included patients who met the following criteria: (1) age 18 years or above, (2) HCC diagnosed either histopathological or using EASL criteria, (3) available CT scan within six months before LT for patients without preoperative treatment or baseline CT scan for the initial bridging therapy prior to LT, (4) no previous history of other malignant tumors. Patients with mixed HCC-cholangiocarcinoma, suboptimal image quality for analysis, missing arterial or portal phase images, and follow-up less than 1 month after transplantation were excluded from the study (Figure 1).
 |
Figure 1 Study flow diagram. |
Imaging Examinations and Follow-Up Protocol
All patients underwent a multiphase contrast enhanced liver CT examination, with the arterial and portovenous phases performed at 20–35s and 60–70s after injection, respectively. Delayed phase (120s) images were not mandatory. Routine post-LT surveillance was conducted every 3–6 months during the first two years, consisting of measuring AFP levels and ultrasound. Afterward, clinical assessments and imaging studies were carried out once or twice per year. Diagnosis of the HCC recurrence post-LT was confirmed by imaging according to EASL criteria and/or histopathologic examination.
Data closure and end of follow-up were completed by the 1st of March, 2023. The primary outcome parameter, time-to-recurrence, was defined as the time interval from the date of LT until the date of recurrence that refers to intrahepatic recurrence or extrahepatic metastasis, and patients without recurrence at the date of death or the last clinical follow-up, were censored.
Clinical and Laboratory Parameters
Pre-transplantation clinical and demographic data were gathered, including gender, age at liver transplantation, etiology of liver disease (chronic alcoholism, hepatitis C, hepatitis B, nonalcoholic steatohepatitis, cryptogenic, other), laboratory parameters (including AFP), the time interval from listing to transplantation, follow-up period, and history of bridging therapies.
Imaging Analysis
For imaging analysis, CT images were independently reviewed by two abdominal radiologists specialized in liver imaging for more than five years, who were blinded to the clinicopathologic data and follow-up results. Several radiological parameters were examined as potential prognostic factors for tumor recurrence after transplantation. First, we assessed all lesions in terms of their enhancement pattern. A typical HCC showed arterial hypervascularity (defined as arterial phase hyperenhancement according to LI-RADS [Liver Imaging Reporting and Data System] classification) and subsequent washout on portovenous or delayed phase images.13 Next, the size and number of HCC lesions (≥1 cm) were recorded. To determine the tumor size, we measured the largest outer-edge to outer-edge dimension, including capsule enhancement, on phase and plane where borders were most visible. The largest viable tumor was considered the representative one (index lesion), and several tumor-related findings on this lesion were evaluated for further analysis. Additionally, we measured the Hounsfield unit (HU) value of the lesion both on the arterial and portal phase on the same slice as the diameter measurement, with the region of interest (ROI) placed at homogeneous solid components. The tumor margin was classified as either smooth (characterized by simple nodular tumors with smooth contours) or non-smooth (marked by irregular contours with a budding portion protruding into the liver parenchyma adjacent to it).14 Peritumoral enhancement was defined as a detectable arterial-enhancing area around the tumor on arterial phase images, which then becomes isodense with the background liver parenchyma on venous images.15 The nodule-in-nodule appearance and the enhancing peripheral rim (capsule) were assessed in the portal phase. We also evaluated the presence of lesions <1cm in size with arterial enhancement (small hypervascular lesions), and satellite nodules, which was defined as small nodules ≤2cm in size and located ≤2cm from another HCC lesion.16 Other relevant radiological features were also recorded, such as biliary dilatation, gastroesophageal varices, ascites, pleural effusion, and cirrhotic liver morphology.
Statistical Analysis
To summarize patient characteristics, continuous data were expressed as either mean with standard deviation or median with interquartile range, while categorical data were presented as numbers with percentages. Categorical data were compared with Chi-square and Fisher’s exact tests, and t-test or Wilcoxon test were used to compare two continuous variables between two centers. For investigating prognostic factors of HCC recurrence, we used univariable Cox proportional hazard regression for image-based, clinical and laboratory variables. The “p.adjust” function with Benjamini Hochberg correction was used to account for multiple testing. Features with p-value less than 0.05 (after correction) were considered significant. For multivariable analysis, 10-fold cross-validation and Least Absolute Shrinkage and Selection Operator (LASSO) were applied using the R package glmnet. This algorithm allowed the selection and regularization of optimal variables by shrinking down to zero coefficient weights for features unrelated to the outcome. A nomogram model for predicting 1-, 3-, and 5-year TTR probabilities was constructed based on independent factors identified from LASSO regression. For model validation, we utilized bootstrap resampling methods based on internal dataset. Calibration curves were plotted to evaluate the consistency between predicted and observed results. C-index calculation, ROC curves and AUC analysis were performed to assess the discriminative efficiency of the model. Time-to-recurrence rates were calculated using the Kaplan–Meier method and the difference in time-to-recurrence between groups was compared using the Log rank test. Cohen’s K-statistic and Intraclass Correlation Coefficients (ICC) were applied to assess interobserver agreement for imaging features of all lesions. A kappa value of 0–0.20 indicated slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement; and 0.81–1.00, almost perfect agreement. All statistical analyses were conducted in R statistical software (R version 4.3.0).
Results
Patient and Baseline Imaging Characteristics
A total of 178 patients, consisting of 140 men, were included in the study. Baseline characteristics of overall cohort are summarized in Table 1, and comparison of two centers is given in Supplementary Table 1. The overall median age at transplantation was 59.0 years (range, 21–73 years). The common etiology of liver disease was hepatitis C in 53 (29.8%), followed by chronic alcoholism in 52 (29.2%) and hepatitis B in 25 (14.0%) patients. The median last pre-transplantation AFP level of the study population was 7.2 ng/mL, and the median diameter of the largest lesion was 31.6 mm. At the time of imaging, 113 (63.4%) patients were within Milan criteria. The majority of the patients (75.8%) underwent bridging therapies. The baseline image read showed parameters in following rates: biliary dilatation (11.8%), varices (60.7%), ascites (39.3%), pleural effusion (6.2%), small hypervascular lesions (34.8%), atypical HCC enhancement pattern (6.1%), capsule (16.3%), irregular margin (56.7%), satellite nodules (8.4%), peritumoral enhancement (11.8%) and nodule-in-nodule appearance (19.1%). The interreader agreement for the individual imaging features in CT ranged from fair to almost perfect (Supplementary Table 2).
 |
Table 1 Summary of Baseline Characteristics and Imaging Features of Patients |
Among the entire cohort, 31 (17.4%) patients experienced recurrence during a median follow-up of 55 months after transplantation. 74.1% of the recurrences were seen in patients beyond Milan criteria at the time of imaging. Specifically, six patients had only local recurrence, ten had only extrahepatic metastasis, and 15 experienced both intra- and extrahepatic recurrence. Kaplan–Meier analysis revealed cumulative recurrence rates of 8.7%, 13.7%, and 18.9% at one, three, and five years, respectively.
Univariable Analysis of Risk Factors for HCC Recurrence
Kaplan Meier curves with Log rank test identified significantly shorter time-to-recurrence in patients with an irregular margin of index lesion (p=0.023), satellite lesions (p=0.02), small (<1cm) hypervascular lesions (p=0.031), peritumoral enhancement of index lesion (p<0.001), index lesion >3cm (p<0.001), presence of more than three lesions (p<0.001), patients who did not receive bridging treatments (p<0.001) (Figure 2).
Table 2 shows the results of univariable Cox proportional hazards regression analysis with p-value adjusted with Benjamini Hochberg correction. Significant risk factors for recurrence were peritumoral enhancement (HR, 6.374; 95% CI, 3.088–13.160; p < 0.001), index lesion >3cm (HR, 5.156; 95% CI, 1.979–13.430; p = 0.003), presence of more than three lesions (HR, 5.455; 95% CI, 2.613–11.390; p < 0.001), and AFP greater than 400 ng/mL (HR, 4.685; 95% CI, 2.009–10.930; p = 0.002). Furthermore, a history of bridging therapies showed a correlation with a lower risk of recurrence after transplantation (HR, 0.257; 95% CI, 0.127–0.521; p = 0.001).
 |
Table 2 Univariable Cox Proportional Hazards Regression Analysis for Prediction of the Recurrence of HCC After Transplantation |
Multivariable Analysis of Risk Factors for HCC Recurrence Using LASSO
In the multivariable analysis with LASSO-penalized logistic regression, six variables were identified as independent prognostic factors for post-transplantation tumor recurrence (Figure 3). The coefficient weights for these factors are listed in Supplementary Table 3. In descending order of hazard ratio, the factors associated with an increased risk of recurrence were peritumoral enhancement (exponentiated LASSO coefficient, 4.13), presence of more than three lesions (2.95), AFP greater than 400 ng/mL (1.92), index lesion >3cm (1.86), presence of capsule (1.03). Furthermore, a history of bridging therapies (0.34) was correlated with a lower risk of recurrence.
 |
Figure 3 Multivariable Cox proportional hazards regression analysis of variable selected by LASSO for prediction the recurrence of HCC after transplantation. |
Construction and Evaluation of Prediction Model Based on Independent Prognostic Factors Identified from LASSO Regression
Building on the foundation of the Cox regression model, we developed a nomogram for the prediction of 1-, 3-, and 5-year time-to-recurrence probabilities. Figure 4 visually presents this nomogram, which incorporates six independent factors selected through LASSO regression. The cumulative score for each patient, obtained by summing the individual scores for all variables in the nomogram, provides an estimate of their probability of recurrence.
 |
Figure 4 The nomogram for predicting 1-, 3-, and 5-year time-to-recurrence probabilities. |
To assess the model’s validity, we created calibration plots and ROC curves from 100 bootstrapped samples, as illustrated in Figures 5 and 6, respectively. The calibration curves aligned closely with the actual outcomes, demonstrating strong consistency between predictions and observations. With AUC values of 0.86, 0.88, and 0.85 for the 1-, 3-, and 5-year TTR predictions, respectively, the model effectively distinguished between those at risk of experiencing early recurrence and those likely to experience recurrence later or not at all. Discrimination ability was further assessed through C-index calculation, yielding a result of 0.835, indicative of a model with very good predictive value. In summary, all these evaluations suggest that this nomogram model provides an effective and reliable tool for predicting the probability of HCC recurrence after transplantation.
 |
Figure 5 Calibration curves of the nomogram for predicting 1-, 3-, and 5-year time-to-recurrence probabilities. |
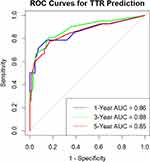 |
Figure 6 Receiver operating characteristic (ROC) curves of 1- (blue line), 3- (green line), and 5-year (red line) time-to-recurrence based on the nomogram. |
Discussion
The herein presented results show that in HCC patients receiving transplantation, in addition to common risk factors like tumor size and number, AFP levels, the presence of peritumoral enhancement, and capsule are independent risk factors for shorter time-to-recurrence. Additionally, bridging therapies before transplantation are associated with a significantly reduced risk of recurrence.
Liver transplantation is the recommended treatment in patients with HCC confined to Milan criteria, considering it is a curative solution not only for HCC lesions but also for the underlying liver disease. However, it is associated with 5-year recurrence rates of up to 20%, which is even higher in patients beyond Milan. Some additional criteria have also been described for patient selection, but they also consider only the lesion size and number, neglecting HCC biology.17,18 Considering its relationship with tumor aggressiveness, AFP has been incorporated into other criteria.19 Still, there is a consensus on room for improvement in patient selection for transplantation. Several studies have evaluated the risk of recurrence using explant histopathology in terms of tumor burden and biology, and scoring systems to identify patients with increased risk have been developed.20 Or some studies have identified imaging-based risk factors using the images just prior to the transplantation.10 However, no adjuvant treatment other than the atezolizumab/bevacizumab combination has been shown to be effective in the adjuvant setting, whose safety is unclear in transplantation patients.21 Thus, high-risk patients need to be identified before transplantation, at best at the time of listing, which might lead to bridging these patients more intensively considering the waiting time.
In our study, in addition to lesion size and number, peritumoral enhancement and presence of capsule were significantly associated with HCC recurrence after transplantation. Peritumoral enhancement has been shown to correlate with microvascular invasion and treatment outcome of HCC patients.14,22,23 It is believed to be the result of occlusion of perilesional small portal branches and a compensatory increase in arterial supply. In our study, peritumoral enhancement was the risk factor with the highest hazard ratio, clearly associating tumor biology with recurrence. This situation is also supported by studies showing its correlation with microvascular invasion in histopathological evaluation.11 For example, exploratory subgroup analysis of our cohort showed the prognostic value of peritumoral enhancement in patients with viral (p=0.05) and non-viral etiology (p<0.001, data not shown). Enhancing capsule develops at later stages of hepatocarcinogenesis, indicating transformation to overt HCC from the high-grade dysplastic nodule or early HCC. Thus, it has been incorporated into LI-RADS as a major imaging feature for HCC.13 Considering patients listed for transplantation usually have smaller lesions, higher recurrence in patients with a capsule could result from more advanced stages of hepatocarcinogenesis. Locoregional therapies are commonly used in patients listed for transplantation, especially when the expected waiting time is above six months. Considering the increased risk of recurrence, patients with peritumoral enhancement and capsule can be assigned to more intensified bridging treatments to improve transplantation outcomes. This idea has also been supported by our results showing significantly lower recurrence in patients receiving bridging treatments. Although most of the imaging parameters evaluated in this publication differ from the ancillary features of LI-RADS criteria, which mainly focus differentiating premalignant lesions from HCC lesions,13 they indicate aggressive behavior of HCC lesions.11,14,24
Similar to the relationship between imaging features and tumor biology, AFP levels also correlate with aggressive tumor behavior. In addition to its role in surveillance for HCC, higher levels of AFP are associated with poorer outcomes after treatment at all tumor stages.1 Furthermore, it has been shown that increased AFP levels are seen in HCC subclasses known for resistance to therapy, high cell proliferation, and poor outcomes,25,26 and incorporated in recently developed additional transplant criteria for patient selection.20,27 This situation was confirmed in our study by higher recurrence in patients with AFP >400 ng/mL. However, in addition to the number of tumors at baseline, the presence of peritumoral enhancement outperformed the pre-transplant AFP levels in predicting tumor recurrence in our study. This underlines the importance of imaging features at the time of listing and the potential benefit to incorporate these imaging features into the organ allocation systems.
Irregular tumor margin, presence of satellite nodules, and presence of small (<1cm) hypervascular lesions were associated with shorter time-to-recurrence, although they were not significant in the multivariable analysis, probably due to the limited sample size. These imaging features suggest aggressive tumor biology, potentially indicating the presence of tumor cells beyond the lesion or the presence of circulating tumor cells. Presence of small (<1cm) hypervascular nodules needs further evaluation, considering these lesions are currently not considered in patient selection for transplantation according to current guidelines.
Our study has some limitations. Despite being a bi-institutional study, it has a limited sample size, which might have led to the loss of significance for some variables in the multivariable model. Second, patients with various types of bridging therapies have been included. Although this situation causes inhomogeneity, it reflects the daily clinical routine of large transplantation centers. Also, MRI images were not considered, unlike some studies mentioned above, which might have delivered more information on tumor biology. However, it is more susceptible to institutional variations considering the bi-institutional analysis in this study. Furthermore, the histopathological features have not been considered due to many patients reaching complete pathological response after bridging treatments. However, this study aimed to identify prognostic factors which can be determined prior to transplantation and guide the treatment during the waiting period.
Conclusion
In conclusion, baseline CT imaging features supplemented by clinical and laboratory parameters may guide prediction of HCC recurrence after transplantation and the treatment decision-making during the waiting time. Besides, the high-performing nomogram model is an effective and reliable tool for predicting HCC recurrence probability at different time points.
Abbreviations
AFP, Alpha fetoprotein; ALP, Alkaline phosphatase; ALT, Alanine transaminase; AST, Aspartate transaminase; AUC, Area Under the Curve; CT, Computed tomography; EASL, European Association for the Study of the Liver; GGT, Gamma-glutamyl transferase; HBV, Hepatitis B; HCC, Hepatocellular carcinoma; HCV, Hepatitis C; HU, Hounsfield Unit; INR, International normalized ratio; IQR, Interquartile range; LASSO, Least Absolute Shrinkage and Selection Operator; LT, Liver transplantation; MELD, Model for End-Stage Liver Disease; NASH, Non-alcoholic steatohepatitis; ROC, Receiver Operating Characteristic SD, Standard deviation; TTR, Time-to-recurrence.
Ethics Approval and Informed Consent
Institutional review board approvals were obtained at both centers. Due to retrospective nature of the study, informed consent was waived. All patients signed informed consent for the transplantation in accordance with the Declaration of Istanbul, and for the use of organs from rescue allocation according to Eurotransplant.
Funding
There is no funding to report.
Disclosure
Prof. Dr. Enrico De Toni reports personal fees from AstraZeneca, Bayer, BMS, EISAI, Eli Lilly & Co, MSD, Mallinckrodt, Omega, Pfizer, IPSEN, Terumo and Roche; grants from Arqule, AstraZeneca, BMS, Bayer, Celsion and Roche, personal fees from BMS and Falk, Eli Lilly, and Roche, during the conduct of the study. Prof. Dr. Christian Lange reports personal fees, non-financial support from AbbVie, personal fees from AstraZeneca, Falk, CSL Behring, Boston Scientific, Eisai, Roche, Norgine, Shionogi, Sobi, outside the submitted work. Prof. Dr. Max Seidensticker reports personal fees from Bayer, during the conduct of the study; grants, personal fees from Sirtex medical, personal fees from Cook Medical, Siemens Healthineers, Balt, Astra Zeneca, LIAM; grants from Bayer, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Liver EAFTSOT. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
2. Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13(1):e11–22. doi:10.1016/S1470-2045(11)70175-9
3. Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17(Suppl 2):S44–57. doi:10.1002/lt.22365
4. Mazzaferro V, Citterio D, Bhoori S, et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21(7):947–956. doi:10.1016/S1470-2045(20)30224-2
5. Yao FY. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394–1403. doi:10.1053/jhep.2001.24563
6. Toso C, Asthana S, Bigam DL, Shapiro AJ, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the scientific registry of transplant recipients database. Hepatology. 2009;49(3):832–838. doi:10.1002/hep.22693
7. Morgul MH, Felgendreff P, Kienlein A, et al. Milan criteria in the MELD era-is it justifiable to extend the limits for orthotopic liver transplantation? World J Surg Oncol. 2020;18(1):158. doi:10.1186/s12957-020-01932-6
8. Pommergaard H-C, Rostved AA, Adam R, et al. Vascular invasion and survival after liver transplantation for hepatocellular carcinoma: a study from the European liver transplant registry. Hpb. 2018;20(8):768–775. doi:10.1016/j.hpb.2018.03.002
9. Pawlik TM, Gleisner AL, Anders RA, Assumpcao L, Maley W, Choti MA. Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: implications for transplant eligibility. Ann Surg. 2007;245(3):435–442. doi:10.1097/01.sla.0000250420.73854.ad
10. Kim AY, Sinn DH, Jeong WK, et al. Hepatobiliary MRI as novel selection criteria in liver transplantation for hepatocellular carcinoma. J Hepatol. 2018;68(6):1144–1152. doi:10.1016/j.jhep.2018.01.024
11. Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol. 2017;67(3):526–534. doi:10.1016/j.jhep.2017.04.024
12. Ivanics T, Salinas-Miranda E, Abreu P, et al. A pre-TACE radiomics model to predict HCC progression and recurrence in liver transplantation: a pilot study on a novel biomarker. Transplantation. 2021;105(11):2435–2444. doi:10.1097/TP.0000000000003605
13. Chernyak V, Fowler KJ, Kamaya A, et al. Liver imaging reporting and data system (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology. 2018;289(3):816–830. doi:10.1148/radiol.2018181494
14. Öcal O, Ingrisch M, Ümütlü MR, et al. Prognostic value of baseline imaging and clinical features in patients with advanced hepatocellular carcinoma. Br J Cancer. 2022;126(2):211–218. doi:10.1038/s41416-021-01577-6
15. Kim H, Park MS, Choi JY, et al. Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol. 2009;19(7):1744–1751. doi:10.1007/s00330-009-1331-8
16. Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137(3):850–855. doi:10.1053/j.gastro.2009.06.003
17. Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7(11):2587–2596. doi:10.1111/j.1600-6143.2007.01965.x
18. Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35–43. doi:10.1016/S1470-2045(08)70284-5
19. Yang SH, Suh KS, Lee HW, et al. A revised scoring system utilizing serum alpha fetoprotein levels to expand candidates for living donor transplantation in hepatocellular carcinoma. Surgery. 2007;141(5):598–609. doi:10.1016/j.surg.2006.11.006
20. Costentin C, Piñero F, Degroote H, et al. R3-AFP score is a new composite tool to refine prediction of hepatocellular carcinoma recurrence after liver transplantation. JHEP Rep. 2022;4(5):100445. doi:10.1016/j.jhepr.2022.100445
21. Hack SP, Spahn J, Chen M, et al. IMbrave 050: a Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020;16(15):975–989. doi:10.2217/fon-2020-0162
22. Kim BK, Kim KA, An C, et al. Prognostic role of magnetic resonance imaging vs. computed tomography for hepatocellular carcinoma undergoing chemoembolization. Liver Int. 2015;35(6):1722–1730. doi:10.1111/liv.12751
23. Kim KA, Kim MJ, Jeon HM, et al. Prediction of microvascular invasion of hepatocellular carcinoma: usefulness of peritumoral hypointensity seen on gadoxetate disodium-enhanced hepatobiliary phase images. J Magn Reson Imaging. 2012;35(3):629–634. doi:10.1002/jmri.22876
24. Renzulli M, Brocchi S, Cucchetti A, et al. Can current preoperative imaging be used to detect microvascular invasion of hepatocellular carcinoma? Radiology. 2016;279(2):432–442. doi:10.1148/radiol.2015150998
25. Tan PS, Nakagawa S, Goossens N, et al. Clinicopathological indices to predict hepatocellular carcinoma molecular classification. Liver Int. 2016;36(1):108–118. doi:10.1111/liv.12889
26. Nishioka ST, Sato MM, Wong LL, Tiirikainen M, Kwee SA. Clinical and molecular sub-classification of hepatocellular carcinoma relative to alpha-fetoprotein level in an Asia-Pacific island cohort. Hepatoma Res. 2018;4:4. doi:10.20517/2394-5079.2017.50
27. Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154(1):128–139. doi:10.1053/j.gastro.2017.09.025
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

