Back to Journals » Infection and Drug Resistance » Volume 16
Identification of Talaromyces marneffei Infection in an HIV-Negative Patient by ITS Sequencing
Authors Sun A, Gou X, Zhu Y, Lv H, Ge Y
Received 8 May 2023
Accepted for publication 2 August 2023
Published 14 August 2023 Volume 2023:16 Pages 5275—5282
DOI https://doi.org/10.2147/IDR.S418174
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Aihua Sun,1,2,* Xiaoyu Gou,1,2,* Yongze Zhu,1 Huoyang Lv,1 Yumei Ge1– 3
1Laboratory Medicine Center, Department of Clinical Laboratory, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital), Hangzhou Medical College, Hangzhou, Zhejiang, 310014, People’s Republic of China; 2School of Basic Medical Sciences and Forensic Medicine, Hangzhou Medical College, Hangzhou, Zhejiang, 310059, People’s Republic of China; 3Key Laboratory of Biomarkers and in vitro Diagnosis Translation of Zhejiang Province, Hangzhou, Zhejiang, 310053, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yumei Ge, Laboratory Medicine Center, Department of Clinical Laboratory, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, Zhejiang, 310014, People’s Republic of China, Email [email protected]
Abstract: Disseminated ankle mycosis is a life-threatening systemic infection caused by the emerging opportunistic and lethal fungal pathogen Talaromyces marneffei which is more common in HIV-positive patients. However, an increasing number of infections are occurring in HIV-negative patients. Here, we report a case of Talaromyces marneffei infection in HIV-negative patient. A 50s HIV-negative male patient with fever, cough, bloody sputum expectoration, pulmonary sarcoidosis and body rashes was hospitalized at Zhejiang Provincial People’s Hospital. CT scanning showed pulmonary multiple nodules with apical bronchial occlusion, patchy infiltration and pathological biopsy demonstrated bronchiolitis obliterans with organized pneumonia and chronic active inflammation of lung tissue with infiltration of numerous lymphocytes, plasma cells, phagocytes and neutrophils. Laboratory tests revealed significantly increased white blood cells count 18.3 × 109/L, neutrophil count 15.34 × 109/L, monocyte count 0.66 × 109/L, platelet count 517 × 109/L, C-reactive protein 116 mg/L, erythrocyte sedimentation rate 112mm/h. The β-D-glucan test was negative (33.06 pg/mL) while fungal culture of broncho alveolar lavage fluid revealed colonies with temperature-dependent dimorphic growth character and Talaromyces marneffei was confirmed by ITS sequencing of the colonies. The patient exhibited radiological improvement and clinical recuperation after intravenously guttae of voriconazole. Talaromycosis in immunocompetent and HIV-negative individuals is relatively rare and is characterized by an insidious onset, various clinical manifestations, and is clinically challenging. Fungal culture and ITS sequencing are warranted for diagnosis Talaromyces marneffei infection. This is the first report on identification of Talaromyces marneffei infection in an HIV-negative patient with skin involvement by ITS sequencing in Zhejiang.
Keywords: Talaromyces marneffei, pulmonary sarcoidosis, HIV-negative, voriconazole, ITS sequencing
Introduction
Disseminated talaromycosis is a life-threatening systemic infection caused by emerging opportunistic and fatal fungal pathogen Talaromyces marneffei (formerly Penicillium marneffei) (T. marneffei), which is frequently consistent with immunosuppressive and immunodeficient hosts including human immunodeficiency virus (HIV)-infected patients, autoimmune diseases, malignant tumors, and organ transplant patients and is prevalent in epidemic regions involving south and southeast Asia.1–4 T. marneffei was first isolated from the liver of bamboo rat in 1956.5 At present, bamboo rats and other rodents have been widely reported to be the reservoirs of T. marneffei infection.6–8 In addition, dogs have also been considered as a possible reservoir of talaromycosis.9 Talaromycosis is generally considered to be an acquired immune deficiency syndrome (AIDS) defining disease.10 However, the increase in the number of T. marneffei infection in HIV-negative individuals suggests that we should not underestimate talaromycosis during clinical visits.11,12 Therefore, we report the clinical manifestation and prognosis of a 50s patient in Geyang, Jiangxi province, where bamboo rats are widely bred and popularized. This patient is HIV-negative and does not have the situation of impaired cell-mediated immunity or received immunosuppressive therapy, but the pulmonary nodules caused by T. marneffei infection.
Case Presentation
Clinical Features
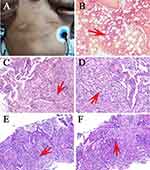
A 50s male patient with cough, bloody sputum expectoration, slight fever and slightly visible body rashes at the thoracoabdominal junction (Figure 1A) presented to Zhejiang Provincial People’s Hospital on July 6, 2021 because of pulmonary shadow found in physical examination of local hospital for more than half a year. The patient had been healthy and had not been infected with HIV in the past but had a 30-year history of smoking. After admission, the physical examination was completed. The body temperature was 37.6°C, pulse rate was 105 times/min, respiration was 20 times/min, blood pressure was 101/68 mmHg, and the breath sound was coarse through auscultation of both lungs. The results of the initial laboratory examination are shown in Table 1. In addition, we also monitored the laboratory test indicators of patients during hospitalization (Figure 2A–D). Chest computed tomography (CT) showed pulmonary solid mass in the right upper lobe with apical bronchial occlusion, patchy infiltration and irregular thickening of local interlobular septum. There were multiple nodules, patchy and cord infiltration in the right lower lobe with fuzzy boundary, irregular thickening of interlobular septum and adjacent pleural thickening. Fibrous foci and tiny nodules were seen in the left lower lobe, presenting with a right pleural effusion (Figure 3A; July 8th).
 |
Table 1 Patient Laboratory Test Results |
Pathological Examination
No cancer cells were found in fibrobronchoscopic fluid, pleural effusion or pulmonary masses and no abnormal cells were seen by bone marrow biopsy. The histopathological results of the right pulmonary masses showed multifocal areas of consolidation with fibrous polypoid tissue, alveolar epithelium and vascular hyperplasia in the alveolar cavity. A large number of lymphocytes, plasma cells, phagocytes and neutrophils were infiltrated in the lung tissue, and the pulmonary septum was widened, which suggested that chronic active inflammation of lung tissue with parenchymal lesions and bronchiolitis obliterans with organized pneumonia (BOOP) (Figure 1B–F).
Etiological Examination
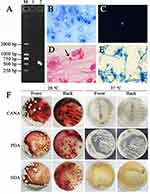
Tuberculosis screening laboratory demonstrated negative acid-fast staining in a suppurative inflammatory background with plentiful neutrophils and macrophages, which preliminarily ruled out the infection of Mycobacterium (Figure 4B). Blue fluorescent fungal spores were observed in the dark field indicating fungal infection by fungal fluorescence staining of sputum (Figure 4C). Gram staining of alveolar lavage fluid showed that deeply stained fungal spores were detected (Figure 4D). Scattered, slightly asymmetric and typical double-helical broom-like fungal hyphae with short, smooth walled, ellipsoidal, mono-verticillate penicillium conidiophores were observed under oil lens after gently touching scotch tape to the colonies and staining with lactophenol cotton blue (Figure 4E). Fungal culture of BALF revealed colonies with temperature-dependent dimorphic growth character, which showed yeast-like fungi producing soluble red pigment at 28°C and mycelia at 37°C in CANA, PDA and SDA (Figure 4F). Single band PCR amplification for internal transcribed spacer (ITS) region in genomic DNA of the clinical isolate was observed after electrophoresis (Figure 4A).13 The nucleotide sequences of the 563-bp ITS including the 5.8S rRNA and 26S rRNA gene were verified and utilized to perform phylogenetic analysis by the Molecular Evolutionary Genetics Analysis (MEGA) software version 11 through the minimum-evolution method as reported in the previous literature.14 A phylogenetic tree was constructed utilizing the sequence of Talaromyces marneffei ZJPPH1 (GenBank number: ON287261.1) from the isolate of this study along with 53 available sequences with the highest homology retrieved from GenBank. The nucleic acid sequence of the present isolate revealed 99.62% similarity with Talaromyces marneffei strain AMC PM002 (Figure 5).
Treatment and Outcome
In the early stage of hospitalization, the cause of infection was unknown, and empirical anti-infection treatment was given. When a clear etiological evidence of T. marneffei infection was obtained, the patient was given voriconazole combined with piperacillin sodium and tazobactam sodium targeted anti-infection therapy and methylprednisolone anti-inflammatory therapy (Figure 3B). The patient still had recurrent fever during treatment (Figure 3C). CT images showed prominent improvement of pulmonary lesion with depauperation of patchy infiltration in original bilateral lung fields (Figure 3A; Aug 26th 2021) after antifungal therapy by voriconazole. In addition, the serum levels of 1-3-β-D glucan and galactomannan were kept at normal levels during hospitalization, and no pathogen were detected in blood culture. This suggested that the patient had not developed further disseminated infection. After 3 months, the patient had no cough and expectoration, recovered and returned to normal life.
Discussion
Talaromycosis is common in HIV-positive patients with cellular immune deficiency caused by CD4+ T cells deficiency.15,16 However, cases analogue to this patient who is HIV-negative and has no obvious underlying immune dysfunction in the past long life are not common. We will continue to pay attention to the mechanism of this type of talaromycosis in our clinical follow-up and research. β-D-Glucan as a biomarker for the fungal infection was often positive in the serum of patients with talaromyces in the past literature reports.17 While in this case, although the patient’s lung was involved in infection of T. marneffei, serum β-D-glucan is negative, which indicated the patient was in the early stage of infection. We should pay attention to the clinical manifestations of this rare case. Recent studies have reported that T. marneffei could escape the recognition and clearance of macrophages, resulting in lung disoperation.18 It suggests that clinicians should pay special attention to the possibility of pulmonary T. marneffei infection.
T. marneffei is a biphasic endemic fungus, which is mainly prevalent in Southeast Asia, India, South China and may be related to the local climate and living habits.19 Bamboo rats are the natural hosts of T. marneffei, and exposure to bamboo rats has been proven to be a risk factor of talaromyces.20 A recent study investigating bloodstream fungal infections in southern China found that T. marneffei was the most commonly isolated mycoemia pathogen (31.4%) in the region, with 70.5% of T. marneffei isolated from HIV-infected individuals. However, HIV-negative patients had a higher mortality rate (40.9%) than HIV-infected patients (17.1%).21 In addition, another survey showed the infection situation of T. marneffei in Jiangxi Province, a total of 241 infected patients were admitted in 10 years, 40.7% of the patients were infected with HIV, and 35.3% of the patients eventually died.22 The patient in this case comes from Jiangxi province in where some local farmers have the habit of eating bamboo rats. Because the patient could not be clearly diagnosed in the local hospital, he came to Zhejiang People’s Hospital for further treatment, suggesting that the local government and CDC should focus on monitoring these epidemic areas with widespread T. marneffei and train the doctor and clinical technicians in these areas.
Although T. marneffei is a highly lethal invasive fungus that can lead to systemic complications, early treatment with antifungal drugs is usually effective. In this case, the accurate diagnosis of T. marneffei infection and timely antifungal treatment have made the patient obtain a satisfactory prognosis. At present, the observation method of colonial growth morphology and microscopy after fungal culture are mainly used in the identification of fungi in clinical laboratories. However, the identification of rare biphasic fungi such as T. marneffei based solely on phenotype is often challenging and difficult and requires experienced technicians in microbiological identification. 5.8S rDNA and 28s rDNA gene spacer (ITS region) is accepted as the primary fungal barcode.23 The length and nucleotide sequence of this region is diversity and polymorphic. Sequencing and analysis of their amplicons can be used for the classification and identification of the fungal strains. The ITS in the nucleotide sequence of fungal ribose is relatively conservative which can be used for fungal species identification, phylogenetic research and epidemiological investigation. Through evolutionary tree analysis, we found that this patient had the highest homology with the sequence uploaded by Assam Medical College & Hospital, barbari, Dibrugarh, Assam 786,002 and India.
Conclusion
Cases of HIV-negative and immunocompetent patients with talaromycosis are relatively rare in clinical practice, especially when the patient’s serum β-D-glucan is negative. It deserves clinicians’ attention because timely antifungal treatment can obtain a satisfactory prognosis. Fungal culture and ITS sequencing are helpful to diagnose T. marneffei infection. Through the evolutionary tree analysis of its ITS, this study provides traceability data for the regional epidemic changes of the clinical strain. Villages of the farmers raising bamboo rats in Jiangxi province are an epidemic area of T. marneffei.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author Yumei Ge on reasonable request.
Ethics Approval and Consent to Participate
This study was supported by the Ethics Committee of Zhejiang People’s Hospital (Ethics Committee Approval of Biomedical Research Involving Humans, Approval No.: 2022JS008) and was carried out in accordance with the ethical standards of the Declaration of Helsinki.
Consent for Publication
Written and informed consent was obtained from the patient for publication of this research and any accompanying images.
Acknowledgments
Aihua Sun and Xiaoyu Gou are co-first authors for this study. We thank all members of the microbiology laboratory of Zhejiang Provincial People’s Hospital for their help in the collection of clinical data and thank the patient for his cooperation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Jung JY, Jo GH, Kim HS, et al. Disseminated penicilliosis in a Korean human immunodeficiency virus infected patient from Laos. J Korean Med Sci. 2012;27(6):697–700. doi:10.3346/jkms.2012.27.6.697
2. Miao X, Ye H, Yang S, et al. Concurrence of Talaromycosis and Kaposi Sarcoma in an HIV-infected patient: a case report. Curr HIV Res. 2021;19(2):195–198. doi:10.2174/1570162X18999201105161137
3. Li X, Hu W, Wan Q, et al. Non-HIV talaromycosis: radiological and clinical analysis. Medicine. 2020;99(10):e19185. doi:10.1097/MD.0000000000019185
4. Pongpech N, Rotjanapan P. Absence of cutaneous involvement in disseminated Talaromyces marneffei infection in an AIDS patient: a case report and literature review. Infect Drug Resist. 2019;12:1493–1499. doi:10.2147/IDR.S207819
5. Capponi M, Sureau P, Segretain G. Penicilliosis de Rhizomys sinensis. Bull Soc Pathol Exot Filiales. 1956;49(3):418–421. PMID: 13364636.
6. Vanittanakom N, Cooper CR, Fisher MC, et al. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95–110. doi:10.1128/CMR.19.1.95-110.2006
7. Cao C, Liang L, Wang W, et al. Common reservoirs for Penicillium marneffei infection in humans and rodents, China. Emerg Infect Dis. 2011;17(2):209–214. doi:10.3201/eid1702.100718
8. Chaiwun B, Vanittanakom N, Jiviriyawat Y, et al. Investigation of dogs as a reservoir of Penicillium marneffei in northern Thailand. Int J Infect Dis. 2011;15(4):e236–239. doi:10.1016/j.ijid.2010.12.001
9. Headley SA, Pretto-Giordano LG, Lima SC, et al. Pneumonia due to Talaromyces marneffei in a dog from southern Brazil with concomitant canine distemper virus infection. J Comp Pathol. 2017;157(1):61–66. doi:10.1016/j.jcpa.2017.06.001
10. Supparatpinyo K, Khamwan C, Baosoung V, et al. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344(8915):110–113. doi:10.1016/s0140-6736(94)91287-4
11. Chan JF, Lau SK, Yuen KY, et al. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. 2016;5(3):e19. doi:10.1038/emi.2016
12. Zhao YK, Liu JY, Liu JH, et al. Recurrent Talaromyces marneffei infection presenting with intestinal obstruction in a patient with systemic lupus erythematosus. Mycopathologia. 2020;185(4):717–726. doi:10.1007/s11046-020-00469-2
13. Ben H, Huo J, Yao Y, et al. Stemphylium lycopersici Causing leaf spot of watermelon (Citrullus lanatus) in China. Plant Dis. 2021;105:4157. doi:10.1094/PDIS-05-21-0990-PDN
14. Swain SK, Sahu BP, Panda S, et al. Molecular characterization and evolutionary analysis of Orientia tsutsugamushi in eastern Indian population. Arch Microbiol. 2022;204(4):221. doi:10.1007/s00203-022-02823-y
15. Jiang J, Meng S, Huang S, et al. Effects of Talaromyces marneffei infection on mortality of HIV/AIDS patients in southern China: a retrospective cohort study. Clin Microbiol Infect. 2019;25(2):233–241. doi:10.1016/j.cmi
16. Lin F, Yang Z, Qiu Y, et al. Talaromyces marneffei infection in lung cancer patients with positive AIGAs: a rare case report. Infect Drug Resist. 2021;14:5005–5013. doi:10.2147/IDR.S340694
17. Sunamura EI, Iwasaki M, Shiina S, et al. A novel enzyme immunoassay for the measurement of plasma (1→3)-β-D-glucan levels. J Immunol Methods. 2020;487:112872. doi:10.1016/j.jim.2020.112872
18. Wei W, Ning C, Huang J, et al. Talaromyces marneffei promotes M2-like polarization of human macrophages by downregulating SOCS3 expression and activating the TLR9 pathway. Virulence. 2021;12(1):1997–2012. doi:10.1080/21505594.2021.1958470
19. Hung CC, Chang SY, Sun HY, et al. Cavitary pneumonia due to Penicillium marneffei in an HIV-infected patient. Am J Respir Crit Care Med. 2013;187(2):e3–4. doi:10.1164/rccm.201202-0321IM
20. Qiu Y, Lu D, Zhang J, et al. Treatment of disseminated Talaromyces marneffei with tracheal infection: two case reports. Mycopathologia. 2015;180(3–4):245–249. doi:10.1007/s11046-015-9891-4
21. Chen M, Hu D, Li T, et al. The epidemiology and clinical characteristics of fungemia in a tertiary hospital in Southern China: a 6-year retrospective study. Mycopathologia. 2023;188:353–360. PMID: 37380875. doi:10.1007/s11046-023-00757-7
22. Yu Q, Wei M, Xiao R, et al. Clinical characteristics, course, and long-term outcomes in patients with Talaromyces marneffei infection: a 10-year retrospective cohort study. Infect Dis Ther. 2023;12(5):1283–1297. doi:10.1007/s40121-023-00801-5
23. Chen M, Houbraken J, Pan W, et al. Pulmonary fungus ball caused by Penicillium capsulatum in a patient with type 2 diabetes: a case report. BMC Infect Dis. 2013;13:496. doi:10.1186/1471-2334-13-496
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.