Back to Journals » Nature and Science of Sleep » Volume 14
Identification of Novel Genetic Variants Associated with Insomnia and Migraine Comorbidity
Authors An YC , Tsai CL, Liang CS , Lin YK, Lin GY, Tsai CK, Liu Y, Chen SJ , Tsai SH, Hung KS, Yang FC
Received 11 March 2022
Accepted for publication 1 June 2022
Published 7 June 2022 Volume 2022:14 Pages 1075—1087
DOI https://doi.org/10.2147/NSS.S365988
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 1
Editor who approved publication: Dr Sarah L Appleton
Yu-Chin An,1 Chia-Lin Tsai,2 Chih-Sung Liang,3 Yu-Kai Lin,2 Guan-Yu Lin,2 Chia-Kuang Tsai,2 Yi Liu,2 Sy-Jou Chen,1 Shih-Hung Tsai,1 Kuo-Sheng Hung,4 Fu-Chi Yang2
1Department of Emergency Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan, Republic of China; 2Department of Neurology, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan, Republic of China; 3Department of Psychiatry, Beitou Branch, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan, Republic of China; 4Center for Precision Medicine and Genomics, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan, Republic of China
Correspondence: Fu-Chi Yang, Department of Neurology, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan, Republic of China, Tel +886-2-87923311 # 88078, Fax +886-2-87927174, Email [email protected]
Purpose: Although insomnia and migraine are often comorbid, the genetic association between insomnia and migraine remains unclear. This study aimed to identify susceptibility loci associated with insomnia and migraine comorbidity.
Patients and Methods: We performed a genome-wide association study (GWAS) involving 1063 clinical outpatients at a tertiary hospital in Taiwan. Migraineurs with and without insomnia were genotyped using the Affymetrix Axiom Genome-Wide TWB 2.0. We performed association analyses for the entire cohort and stratified patients into the following subgroups: episodic migraine (EM), chronic migraine (CM), migraine with aura (MA), and migraine without aura (MoA). Potential correlations between SNPs and clinical indices in migraine patients with insomnia were examined using multivariate regression analysis.
Results: The SNP rs1178326 in the gene HDAC9 was significantly associated with insomnia. In the EM, CM, MA, and MoA subgroups, we identified 30 additional susceptibility loci. Multivariate regression analysis showed that SNP rs1178326 also correlated with higher migraine frequency and the Migraine Disability Assessment (MIDAS) questionnaire score. Finally, two SNPs that had been previously reported in a major insomnia GWAS were also significant in our migraineurs, showing a concordant effect.
Conclusion: In this GWAS, we identified several novel loci associated with insomnia in migraineurs in a Han Chinese population in Taiwan. These results provide insights into the possible genetic basis of insomnia and migraine comorbidity.
Keywords: insomnia, migraine, GWAS, SNP, gene, comorbidity
Introduction
Insomnia and migraine are both important worldwide health problems. According to the Global Burden of Disease report, migraine is the sixth most troublesome disease worldwide and the most common neurological disease.1 Migraine attacks are episodic headaches often associated with nausea, vomiting, and sound and light sensitivity that severely impair the quality of life of migraineurs and can even lead to disability. In addition, migraine is associated with several comorbidities, such as anxiety, depression, vascular accidents, epilepsy, restless legs syndrome, stress, and sleep disorders.2,3 Insomnia, defined as the inability to initiate or maintain sleep, is nowadays the most common sleep disorder. It affects one-third of the adult population and remarkably reduces life satisfaction.4 In addition, it is associated with various complications, such as heart disease, diabetes, gastrointestinal problems, and neurological disorders.5 Numerous studies reported that migraineurs have worse sleep quality than non-migraineurs and high migraine frequency is considered related to a higher prevalence of poor sleep quality.2 In an epidemiologic study, primary headaches, including migraine and tension headaches, were significantly associated with insomnia comorbidities with an odds ratio (OR) of 1.4–1.7.6 Moreover, it has been reported that sleep interruptions can trigger migraine attacks. Besides epidemiologic evidence, migraine and insomnia might share some pathophysiological mechanisms in a bidirectional relationship. Migraine and insomnia could be associated due to the dysregulation of nervous system pathways involved in both pathologies, such as in cortical spreading depression, the trigeminovascular system, hypothalamic orexinergic system, and several kinds of neurotransmitters which play a role as mediators.7–9
Recent studies have shown evidence of genetic contributions to migraine and insomnia respectively, but questions remain about the shared framework of genetic influence. A recent meta-analysis study comprising 22 genome-wide association studies (GWAS) identified that the genetic factors associated with a higher risk of migraine were enriched in genes expressed in vascular and smooth muscle tissues, supporting a vascular involvement in the etiology of migraine.10 Eising et al integrated migraine GWAS data with high-resolution spatial gene expression data and identified five modules involved in migraine pathophysiology.11 Furthermore, several genetic studies on pediatric migraine have reported polymorphisms associated with migraine.12,13 Similarly, numerous studies have shown novel susceptibility genes associated with insomnia. Hammerschlag et al identified three loci and seven genes associated with insomnia.14 Stein et al showed that single-nucleotide polymorphism (SNP)-based heritability for insomnia disorder significantly correlated with other psychiatric and physical disorders.15 Furthermore, Jansen et al reported 202 loci identifying 956 genes associated with insomnia that highlighted key brain areas implicated in this disease.16
Although the co-occurrence of migraine and insomnia is widely known, to the best of our knowledge, limited studies have investigated the shared genetic variants between them, especially in the Han Chinese population. Therefore, this study aimed to identify susceptibility loci associated with insomnia and migraine in the Han Chinese population in Taiwan. In addition, we stratified patients into the following subgroups: chronic migraine (CM) versus episodic migraine (EM), and migraine with aura (MA) versus migraine without aura (MoA). To date, whether migraine with and without aura have different genetic components remains controversial, as well as whether migraine chronification is associated with specific genetic variants and with more frequent and severe insomnia symptoms. Hence, our second aim was to investigate whether independent genetic variants are associated with insomnia in the migraine subgroups.
Materials and Methods
Participants
The study protocol was approved by the Institutional Review Board of the Tri-Service General Hospital (TSGH) (TSGHIRB No.: 2-108-05-038) and performed strictly following the Declaration of Helsinki. The study was performed between October 2018 and March 2021 in a cohort of 1063 patients recruited from the neurology outpatient department at the TSGH (Figure 1). All patients provided written informed consent prior to enrollment. Each study participant completed a screening questionnaire and was subsequently interviewed by a board-certified neurologist and headache specialist (FCY). The study sample was then divided into a group with CM (≥ 15 episodes per month; n = 189) or EM (< 15 episodes per month; n = 874). In addition, 298 of the 1063 study participants had MA, and 765 had MoA.
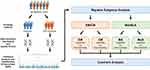 |
Figure 1 Flowchart of a two-step workflow of the phenotype association analysis. |
Participant Evaluation
Migraine
Migraine was diagnosed according to the criteria in the third edition of the International Classification of Headache Disorders (ICHD-3).17 Patients with secondary or other concomitant primary headache disorders were excluded. The clinical characteristics of all participants diagnosed with migraine, including aura symptoms, migraine duration (years), frequency (headache day/month), family history, and headache intensity, were documented.
All patients completed a standardized demographic questionnaire and the Migraine Disability Assessment questionnaire (MIDAS),18 Beck Depression Inventory (BDI),19 and Hospital Anxiety and Depression Scale (HADS)20 questionnaires. The MIDAS is a questionnaire with five items that assess headache-related disabilities over the previous three months. The four-point grading system was as follows: grade I (scores ranging from 0 to 5), little or no disability; grade II (scores ranging from 6 to 10), mild disability; grade III (scores ranging from 11 to 20), moderate disability; and grade IV (21 or greater), severe disability. The BDI scores range from 0 to 63, and individuals with scores ≥ 18 are classified as depressed. The HADS has seven items related to anxiety and depression and has a maximum individual subscale score of 21.
Insomnia
Primary insomnia disorder was diagnosed according to the criteria in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)21 after an evaluation during a clinical interview. The clinical characteristics of all participants diagnosed with primary insomnia, including insomnia duration and insomnia severity, were documented. Medical and psychiatric disorders were evaluated via structured diagnostic interviews, physical examinations, blood tests (blood cell count, thyroid, renal, and hepatic function), and urine drug testing. Patients with secondary insomnia (eg, history of heart disease, stroke, nephritis, psychiatric disorders, hypersomnia, parasomnia, brain tumor, hematoma, drug- or alcohol-related, etc.) were excluded from the study.
All participants completed two brief self-rated questionnaires to assess their perception of insomnia severity using the Pittsburgh Sleep Quality Index (PSQI)22 and the Insomnia Severity Index (ISI).23 The PSQI estimates sleep quality over the previous month, including 19 self-rated items combined into seven components. It has a score range of 0–21, and a final score ≥ 6 indicates sleep disturbance. The ISI score is a seven-item self-rated questionnaire that evaluates the severity and impact of insomnia symptoms in the past month. Each ISI item is rated on a scale of 0–4. The total ISI score is divided into four categories: 0–7, no clinically significant insomnia; 8–14, subthreshold insomnia; 15–21, moderate insomnia; 22–28, severe insomnia.
Genotyping and Quality Control
Peripheral blood samples from patients with migraine were isolated in 5-mL EDTA vacutainers (BD, Plymouth, UK). Genomic DNA was extracted using the QIAamp DSP DNA Mini Kit on the QIAsymphony platform (Qiagen, Hilden, Germany). DNA quality was measured using a NanoDrop One spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The DNA samples were applied to the Affymetrix Axiom Genome-Wide TWB 2.0 arrays, which contain approximately 752,921 probes for a total of 686,463 SNPs. Among these SNPs, approximately 446,000 SNPs are associated with the characteristics of background genotypes in Taiwanese; approximately 105,000 SNPs are clinically relevant, whereas the rest are associated with disease features, drug response, and metabolism. The signal CEL files generated from the Axiom TWB 2.0 SNP array were transformed to genotyping data (tped and tfam files) using a Genotyping Console (Affymetrix).
Statistical Analysis
All patients with migraine were grouped into different subsets using our standard demographic questionnaire. In order to evaluate the genetic association between migraine risk and insomnia risk, we performed a genotype-association analysis using PLINK based on the migraine with insomnia and migraine without insomnia groups. The P-value and odds ratio (OR) of the phenotype association study were calculated to assess the variant relationship using the chi-square allelic test with one degree of freedom. To investigate how gender may affect the relationship between migraine and insomnia, we also analyzed the association between gender factors in all migraine cohorts. Additionally, migraineurs were stratified into four groups: EM, CM, MA, and MoA, and patients in each group were further divided into subgroups according to the presence or absence of insomnia. In addition, to validate the effect direction of the variants, we selected 255 variants from a major insomnia GWAS16 present in our SNP microarray. Among these, we genotyped 25 variants in all patients to check the trend (Supplementary Table 1). Finally, the significant P-values lower than 10−6 were retrieved. Intergenic variants were excluded in the subsequent analyses. In addition, variants with a Minor Allele Frequency MAF (TWB) < 0.25 and OR = 0, which represent common variants among the Taiwanese, were excluded. The remaining variants were annotated with NCBI based on the RefSeq database using ANNOVAR.
Results
Demographics
Table 1 shows the demographic metadata of all the participants, as well as those of the migraine subgroups with or without insomnia. There were no significant differences in the proportion of EM/CM, body mass index, and years of education among the groups of migraineurs with insomnia and migraineurs without insomnia. Nevertheless, all other parameters analyzed differed significantly between the two subgroups analyzed (P < 0.05).
 |
Table 1 Demographic and Clinical Data |
Association of Insomnia in All Migraine Cohorts
We then conducted GWAS on migraineurs stratified depending on the presence or absence of the comorbidity insomnia. The analysis yielded one significant intronic variant with a P-value < 1E-06, rs1178326 (P = 5.43E-07) (Table 2). The variant allele frequency in the insomnia group was 0.50%, whereas it was 3.94% in the non-insomnia group (OR = 0.12, Figure 2).
 |
Table 2 Association Between All Migraine Patients Grouped by the Presence or Absence of Insomnia |
When analyzing gender-associated factors, we found that in both gender groups there was only one variant associated between gender and migraine: rs145888117 (P = 7.00E-07) for the male group and rs28535526 (P = 5.12E-07) for the female group (Supplementary Table 2). The OR trend is similar to our finding (rs1178326) in all migraine cohorts; however, the variants are not in the same position.
Association in the Subgroups in EM/CM
Additionally, we performed an association analysis to identify the potential genetic differences between migraineurs suffering from EM and CM with and without insomnia. Two genome-wide significant (P < 10−6) SNPs were identified in the EM group (Figures 2 and 3), and 14 variants we identified in the CM group (Table 3, Figure 3). Most of these variants were intronic and presented a MAF < 5%. The frequency of the significant variants in the EM and CM groups was lower in the insomnia group than that in the non-insomnia group. The OR of all these variants was lower than one.
 |
Table 3 Association Between Insomnia Subgroups: EM, CM, MA, and MoA |
 |
Figure 3 Variant frequency and odds ratio (OR) in the subgroups episodic migraine (EM), chronic migraine (CM), migraine with aura (MA), and migraine without aura (MoA). The x-axis shows the genome-wide significant variants found in the association analysis of EM vs CM (A) and MA vs MoA (B), also reported in Table 2. The y-axis shows the variant allele frequency in the insomnia group. The diameter of the circles represents the OR of each variant. |
Association in the Subgroups in MA/MoA
Moreover, we performed association analyses between the migraineurs with and without aura and with and without insomnia. In the MA group, 13 variants were genome-wide significant, whereas only one variant was significant in the MoA group (Table 3, Figures 2 and 3). The patterns found in the MA/MoA group were similar to those in the EM/CM group, with odds ratios lower than one. Additionally, these variants related to migraineurs tended to occur more frequently in the non-insomnia group as compared to those in the insomnia group.
Multivariate Association Study
We then performed a multivariate regression analysis on migraine frequency, MIDAS, ISI, BDI, HADS-anxiety, and HADS-depression scores. In all migraine cohorts, SNP rs1178326 showed a significant association between migraine frequency and MIDAS (P = 0.006, 0.004; OR = 0.73, 1.16; 95% confidence interval = 0.59–0.91, 1.05–1.28, respectively).
Replication Study
Finally, we aimed to validate the results of a previous major GWAS on insomnia. For this purpose, we selected 25 loci present in a TWB2 SNP array (Supplementary Table 1) and analyzed them in all migraineurs. Among the SNPs tested, rs10947428 and rs728017 were significantly different between insomnia and non-insomnia migraineurs (Table 4). The odds ratios associated with both markers were lower than one (0.04 and 0.19, respectively), indicating that the variant allele seemingly appeared more frequently in the non-insomnia group rather than that in the insomnia group.
 |
Table 4 Replication of Findings in a Previous Major Insomnia GWAS |
Discussion
Here, we analyzed 1063 migraineurs stratified into patients with and without insomnia and found that the rs1178326 variant in the HDAC9 gene was significantly associated with insomnia. Additionally, we stratified the migraineurs depending on the frequency of the symptoms (EM vs CM) and the presence of an aura (MA vs MoA), further comparing differences between patients with and without insomnia in each subgroup. Two, 14, 13, and 1 SNP in the EM, CM, MA, and MoA groups, respectively, were significantly associated with insomnia. Multivariate regression analysis indicated that the SNP rs1178326 was also significantly associated with migraine frequency and MIDAS scores. Moreover, we replicated the association and the direction of the effects of two SNPs (rs10947428 in ITPR3 and rs728017 in NKAIN2) that were significant in a previous insomnia GWAS.
In the demographic and clinical data (Table 1), the MIDAS, ISI, PSQI, HADS, and BDI questionnaire responses differed significantly between the two groups. Our results are consistent with previous studies, which have indicated that the co-existed comorbidity of anxiety and depression significantly correlates with a higher prevalence of poor sleep quality in migraineurs.2,24,25
HDAC9 encodes histone deacetylase 9 (HDAC9), a member of the class II HDAC family that plays key roles in numerous tissues by regulating histone phosphorylation, thereby shaping the transcriptional landscape. The human HDAC9 gene is located on chromosome 7p21 and is highly expressed in the heart, muscles, and brain. HDAC9 has been implicated in numerous pathophysiological processes, including neurological disorders, cardiac growth, T-regulatory cell function, muscle differentiation, and cancers.26 Several reports investigated the association between HDAC9 and the risk of ischemic stroke or coronary artery disease in the Han Chinese population.27,28 HDAC9 gene deficiency may attenuate atherosclerosis and increase risk by altering ischemic brain responses and neuronal survival.27 Recently, HDAC9 was reported as a potential causal link between insomnia and coronary artery disease.29 Besides, a Mendelian randomization analysis showed that short sleep duration and frequent insomnia symptoms are associated with a subtype of ischemic stroke.30 Sleep deprivation may increase HDAC expression in the hippocampus, adversely affecting structural and functional synaptic plasticity and memory formation, leading to spatial memory decline, which could be reversed by HDAC inhibition.31 Therefore, we hypothesized that HDAC9 is functionally involved in migraine and insomnia through the dysregulation of the cardiovascular system. Further research is necessary to elucidate the specific mechanism of the insomnia-related HDAC9 variant in migraineurs.
In the EM group, we found two insomnia-related SNPs (rs17082263 in SCFD2 and rs143607943 in IQCG). SCFD2 encodes Sec1 family domain-containing protein 2, a protein involved in protein transport and vesicle docking during exocytosis. Despite previous studies that implicated SCFD2 as a susceptibility gene for insomnia,14 later studies failed to fully replicate these results.29 IQCG encodes the IQ motif-containing G protein, which interacts with several proteins and contributes to regulating calcium and calmodulin-dependent protein kinase IV activity, neuronal polarized growth and plasticity, fertilization, mitosis, and cytoskeletal organization.32 The SNP in rs9880989 in IQCG was identified among the top ten susceptibility loci associated with migraine in bipolar disorder.33 Although IQCG may also be involved in insomnia and migraine, further studies are warranted to unravel underlying mechanisms.
In the CM group, 14 SNPs were associated with insomnia. SLC38A10 encodes a member of the solute carrier (SLC) family. Hundreds of SLC genes have been identified in the brain, contributing to the transport of sugars, amino acids, vitamins, neurotransmitters, and inorganic/metal ions. SLC38A10 acts as a glutamate transporter and can affect neuronal viability by protecting against glutamate toxicity and oxidative stress.34 Mounting evidence suggests that glutamate excitotoxicity contributes to migraine and insomnia.35,36 As glutamate transporters could be novel therapeutic targets, it is necessary to further explore the role of SLC38A10 in these disorders. RELN encodes reelin, an extracellular matrix glycoprotein controlling cell-cell interactions, critical for cell positioning and neuronal migration during brain development. Reelin is reportedly involved in several neuropsychiatric disorders, including schizophrenia, bipolar disorder, major depression, autism, and Alzheimer’s disease.37 Reduced RELN expression may contribute to epilepsy pathogenesis and is considered a shared causal pathway between migraine and epilepsy.38 NRF1 encodes nuclear respiratory factor 1, which is involved in the transcription of oxidative phosphorylation components. NRF1 activates the expression of several metabolic genes and is upregulated during sleep deprivation.39 Zhu et al demonstrated that NRF1 positively regulates numerous circadian genes.40 Furthermore, Li et al identified that NRF1 affects sleep initiation and may regulate the human GABA receptor subtype A β1 subunit gene in neurons, which is associated with epilepsy, autism, bipolar disorder, and schizophrenia.41 Finally, we found three SNPs in LINC01933, encoding a long non-coding RNA. A recent study reported altered expression of LINC01933 in the brain, which was associated with sleeping-related loci 5 in the Neanderthal population.42 Further studies are necessary to investigate the potential involvement of these genes in insomnia and the CM group.
In the MA group, we found 13 SNPs associated with insomnia. Two SNPs were in KIF26B, which encodes the Kinesin Family Member 26 B. Kinesins are transporters of membranous organelles in mammalian neurons.43 In a GWAS of sleep duration, KIF26B was reportedly associated with a short sleep duration of fewer than 6 hours.44 In addition, Hautakangas et al performed a genome-wide meta-analysis of migraine and found a lead SNP in KIF26B, although not significant in the MA subgroup.45
MAP3K4 encodes mitogen-activated protein kinase (MAPK) kinase 4. MAPK pathways are involved in regulating calcitonin gene-related peptide (CGRP) release, a migraine-related neuropeptide secreted by the trigeminal ganglion.46 Suppressing MAPK/NF‑кB signaling could attenuate migraine in a nitroglycerin-induced rat model.47 Furthermore, Zhang et al found that substance P may activate MAPK pathways in satellite glial cells of the trigeminal ganglion, contributing to inflammatory orofacial pain associated with peripheral sensitization.48 Moreover, substance P seemingly initiates and perpetuates cortical spreading depression, an electrophysiological phenomenon associated with MA.49 Additionally, substance P may influence the increase of REM latency and time awake, leading to a central arousing effect.50 Overall, these studies support a potential link between MA and insomnia. Besides KIF26B and MAP3K4, other significant loci did not present a clear link between migraine and insomnia, highlighting the need for further research.
In the MoA group, we identified rs4876117 in DLGAP2 associated with insomnia. DLGAP2 encodes Discs Large Homolog Associated Protein 2. It was initially identified as a candidate gene for mental retardation and post-traumatic stress disorder affecting the hippocampus and was recently associated with schizophrenia and Alzheimer’s disease. DLGAPs are expressed in the postsynapse, interact with several proteins, and are involved in the function of NMDA, AMPA, and glutamate receptors.51 In addition, Catusi et al studied patients with 8p23.2-pter microdeletions, suggesting that DLGAP2 deregulation may influence other families of post-synaptic scaffolding proteins. DLGAP2 is considered a strong candidate for neurodevelopmental/behavioral phenotypes.52 Moreover, DLGAP2 was an affected gene in an established murine model of CM triggered by nitroglycerin. Disruption of glutamatergic and dopaminergic synapses and rhythmic processes in the trigeminal ganglia and the nucleus accumbens may be a mechanism associated with migraine but require further validation.53
When investigating the association between gender effects on migraineurs with insomnia, we found one variant in each gender, rs145888117 in CDC14B for the male group and rs28535526 in TAFA5 for the female group. CDC14B encodes Cell Division Cycle 14B, a member of the dual-specificity protein tyrosine phosphatase family, involved in the exit of cell mitosis and regulation of DNA damage repair.54 Furthermore, CDC14B was shown to exhibit oncogenic characteristics in mammals via Ras-MAPK cascade.55 The association of CDC14B in male migraineurs with insomnia may need more validation via MAPK pathways. TAFA5 encodes TAFA Chemokine Like Family Member 5, a member of the TAFA family, and was found highly expressed in the embryonic and postnatal mouse brain, especially in the hippocampus. Genetic deletion of TAFA5 may contribute to an increase in depressive-like behaviors and significantly reduce glutamate release and neuronal activity in the hippocampus.56 Whether migraine, insomnia, and depression share similar pathogenesis via TAFA5, especially in females, is warranted for further validation.
Multivariate regression analysis showed that SNP rs1178326 in HDAC9 was also significantly associated with higher migraine frequency and MIDAS scores, a clue that this locus plays an important role in migraine with insomnia. Further larger studies are warranted to investigate HDAC9 in the association of migraine and insomnia with their frequency and intensity.
To validate our results, we selected 255 variants from a major insomnia GWAS16 to the same SNP microarray. Out of 255, 25 SNPs in the TWB2 SNP arrays, SNPs rs10947428 in ITPR3 and rs728017 in NKAIN2 were significantly detected in the migraine cohort of the insomnia group. Furthermore, although the allele frequencies in Taiwan Biobank and UK Biobank differed, our results are consistent with the previous study.
Our study had several strengths. First, we had a carefully chosen population in which migraine and insomnia were diagnosed by qualified neurologists according to a strictly audited protocol. Second, we evaluated individuals using validated questionnaires, sleep quality, migraine frequency, and comorbidities like anxiety and depression. Multivariate regression analysis and stratified analysis of migraine subgroups enabled the investigation of the genetic association of insomnia in migraineurs. Third, Affymetrix’s Axiom Genome-Wide TWB 2.0 array covers a highly representative sample of the gene pool in Taiwan. Our results also showed different allele frequencies in other populations in previous major studies. Nonetheless, this study also had limitations. The statistical power was limited due to the relatively modest sample size. Further studies with larger sample sizes and a more diverse population are warranted to enhance external validity and extend these results in the future.
Conclusions
In conclusion, our study revealed that the SNP rs1178326 located in the HDAC9 gene was significantly associated with insomnia in a cohort of migraineurs from the Han Chinese population in Taiwan. Furthermore, several novel susceptibility loci associated with insomnia were identified in subgroups EM, CM, MA, and MoA. These results provide insights into the possible genetic basis of insomnia and migraine. Further larger studies are warranted to investigate these genes in shared pathogenesis to obtain definitive evidence.
Data Sharing Statement
All data are available from the corresponding author upon request.
Acknowledgments
The authors thank the participants and investigators from Taiwan Precision Medicine Initiative, the Center for Precision Medicine and Genomics of Tri-Service General Hospital, National Defense Medical Center, and Genetics Generation Advancement Corporation for their assistance with genetic testing and statistical analysis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported partly by grants from the Ministry of Science and Technology of Taiwan (grant numbers MOST 108-2314-B-016-023- and MOST 110-2314-B-016 −036 -MY2), Tri-Service General Hospital, Taiwan (grant numbers TSGH-D-109101, TSGH-D-110048, TSGH-C111-091), and Academia Sinica (grant numbers AS-40-05-GMM, AS-GC-110-MD02).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi:10.1016/S0140-6736(15)60692-4
2. Lin YK, Lin GY, Lee JT, et al. Associations between sleep quality and migraine frequency: a cross-sectional case-control study. Medicine. 2016;95(17):e3554. doi:10.1097/MD.0000000000003554
3. An YC, Liang CS, Lee JT, et al. Effect of Sex and adaptation on migraine frequency and perceived stress: a cross-sectional case-control study. Front Neurol. 2019;10:598. doi:10.3389/fneur.2019.00598
4. Zeitlhofer J, Schmeiser-Rieder A, Tribl G, et al. Sleep and quality of life in the Austrian population. Acta Neurol Scand. 2000;102(4):249–257. doi:10.1034/j.1600-0404.2000.102004249.x
5. Byrne EM. The relationship between insomnia and complex diseases-insights from genetic data. Genome Med. 2019;11(1):57. doi:10.1186/s13073-019-0668-0
6. Uhlig BL, Engstrom M, Odegard SS, Hagen KK, Sand T. Headache and insomnia in population-based epidemiological studies. Cephalalgia. 2014;34(10):745–751. doi:10.1177/0333102414540058
7. Tiseo C, Vacca A, Felbush A, et al. Migraine and sleep disorders: a systematic review. J Headache Pain. 2020;21(1):126. doi:10.1186/s10194-020-01192-5
8. Dodick DW, Eross EJ, Parish JM, Silber M. Clinical, anatomical, and physiologic relationship between sleep and headache. Headache. 2003;43(3):282–292. doi:10.1046/j.1526-4610.2003.03055.x
9. Holland PR, Barloese M, Fahrenkrug J. PACAP in hypothalamic regulation of sleep and circadian rhythm: importance for headache. J Headache Pain. 2018;19(1):20. doi:10.1186/s10194-018-0844-4
10. Gormley P, Anttila V, Winsvold BS, et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet. 2016;48(8):856–866. doi:10.1038/ng.3598
11. Eising E, Huisman SMH, Mahfouz A, et al. Gene co-expression analysis identifies brain regions and cell types involved in migraine pathophysiology: a GWAS-based study using the Allen Human Brain Atlas. Hum Genet. 2016;135(4):425–439. doi:10.1007/s00439-016-1638-x
12. Chang X, Pellegrino R, Garifallou J, et al. Common variants at 5q33.1 predispose to migraine in African-American children. J Med Genet. 2018;55(12):831–836. doi:10.1136/jmedgenet-2018-105359
13. Koute V, Michalopoulou A, Siokas V, et al. Val66Met polymorphism is associated with decreased likelihood for pediatric headache and migraine. Neurol Res. 2021;43:1–9.
14. Hammerschlag AR, Stringer S, de Leeuw CA, et al. Genome-wide association analysis of insomnia complaints identifies risk genes and genetic overlap with psychiatric and metabolic traits. Nat Genet. 2017;49(11):1584–1592. doi:10.1038/ng.3888
15. Stein MB, McCarthy MJ, Chen CY, et al. Genome-wide analysis of insomnia disorder. Mol Psychiatry. 2018;23(11):2238–2250. doi:10.1038/s41380-018-0033-5
16. Jansen PR, Watanabe K, Stringer S, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. 2019;51(3):394–403. doi:10.1038/s41588-018-0333-3
17. Headache Classification Committee of the International Headache S. The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808. doi:10.1177/0333102413485658
18. Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56(6 Suppl 1):S20–S28. doi:10.1212/WNL.56.suppl_1.S20
19. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi:10.1001/archpsyc.1961.01710120031004
20. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
21. Regier DA, Kuhl EA, Kupfer DJ. The DSM-5: classification and criteria changes. World Psychiatry. 2013;12(2):92–98. doi:10.1002/wps.20050
22. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
23. Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi:10.1016/S1389-9457(00)00065-4
24. Vgontzas A, Cui L, Merikangas KR. Are sleep difficulties associated with migraine attributable to anxiety and depression? Headache. 2008;48(10):1451–1459. doi:10.1111/j.1526-4610.2008.01175.x
25. Yeung WF, Chung KF, Wong CY. Relationship between insomnia and headache in community-based middle-aged Hong Kong Chinese women. J Headache Pain. 2010;11(3):187–195. doi:10.1007/s10194-010-0199-y
26. Yuan Z, Peng L, Radhakrishnan R, Seto E. Histone deacetylase 9 (HDAC9) regulates the functions of the ATDC (TRIM29) protein. J Biol Chem. 2010;285(50):39329–39338. doi:10.1074/jbc.M110.179333
27. Zhou X, Guan T, Li S, et al. The association between HDAC9 gene polymorphisms and stroke risk in the Chinese population: a meta-analysis. Sci Rep. 2017;7:41538. doi:10.1038/srep41538
28. Han Z, Dong X, Zhang C, Wu Y, Yuan Z, Wang X. Polymorphism of HDAC9 gene is associated with increased risk of acute coronary syndrome in Chinese Han population. Biomed Res Int. 2016;2016:3746276. doi:10.1155/2016/3746276
29. Lane JM, Jones SE, Dashti HS, et al. Biological and clinical insights from genetics of insomnia symptoms. Nat Genet. 2019;51(3):387–393. doi:10.1038/s41588-019-0361-7
30. Korostovtseva L. Ischemic stroke and sleep: the linking genetic factors. Cardiol Ther. 2021;10:349–375. doi:10.1007/s40119-021-00231-9
31. Duan R, Liu X, Wang T, Wu L, Gao X, Zhang Z. Histone acetylation regulation in sleep deprivation-induced spatial memory impairment. Neurochem Res. 2016;41(9):2223–2232. doi:10.1007/s11064-016-1937-6
32. Bahler M, Rhoads A. Calmodulin signaling via the IQ motif. FEBS Lett. 2002;513(1):107–113. doi:10.1016/S0014-5793(01)03239-2
33. Knott SV. Investigating Susceptibility to Bipolar Disorder, Migraine and Epilepsy Doctoral dissertation. PhD degree in School of Medicine, Cardiff University; 2016.
34. Tripathi R, Aggarwal T, Fredriksson R. SLC38A10 transporter plays a role in cell survival under oxidative stress and glutamate toxicity. Front Mol Biosci. 2021;8:671865. doi:10.3389/fmolb.2021.671865
35. Gasparini CF, Griffiths LR. The biology of the glutamatergic system and potential role in migraine. Int J Biomed Sci. 2013;9(1):1–8.
36. Mormile R, Mazzei G, Vittori G, De Michele M, Squarcia U. Insomnia and shift-work sleep disorder: a crosstalk between glutamate excitotoxicity and decreased GABAergic neurotransmission? Sleep Biol Rhythms. 2012;10:340–341. doi:10.1111/j.1479-8425.2012.00574.x
37. Guidotti A, Grayson DR, Caruncho HJ. Epigenetic RELN dysfunction in schizophrenia and related neuropsychiatric disorders. Front Cell Neurosci. 2016;10:89. doi:10.3389/fncel.2016.00089
38. Eising E, A Datson N, van den Maagdenberg AM, Ferrari MD. Epigenetic mechanisms in migraine: a promising avenue? BMC Med. 2013;11(1):26. doi:10.1186/1741-7015-11-26
39. Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92(3):1087–1187. doi:10.1152/physrev.00032.2011
40. Zhu W. Mechanisms and Functional Roles of Nuclear Respiratory Factor 1 (NRF1) Binding Sites in the Human Genome doctoral dissertation. PhD degree in School of Public Health, University of Pittsburgh; 2011.
41. Li Z, Cogswell M, Hixson K, Brooks-Kayal AR, Russek SJ. Nuclear respiratory factor 1 (NRF-1) controls the activity dependent transcription of the GABA-A receptor Beta 1 subunit gene in neurons. Front Mol Neurosci. 2018;11:285. doi:10.3389/fnmol.2018.00285
42. Dannemann M, Milaneschi Y, Yermakovich D, et al. Neandertal introgression dissects the genetic landscape of neuropsychiatric disorders and associated behavioral phenotypes. medRxiv. 2021;4:54.
43. Aizawa H, Sekine Y, Takemura R, Zhang Z, Nangaku M, Hirokawa N. Kinesin family in murine central nervous system. J Cell Biol. 1992;119(5):1287–1296. doi:10.1083/jcb.119.5.1287
44. Lahtinen A, Puttonen S, Vanttola P, et al. A distinctive DNA methylation pattern in insufficient sleep. Sci Rep. 2019;9(1):1193. doi:10.1038/s41598-018-38009-0
45. Hautakangas H, Winsvold BS, Ruotsalainen SE, et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. medRxiv. 2021;56:e34.
46. Lei L, Yuan X, Wang S, et al. Mitogen-activated protein kinase pathways are involved in the upregulation of calcitonin gene-related peptide of rat trigeminal ganglion after organ culture. J Mol Neurosci. 2012;48(1):53–65. doi:10.1007/s12031-012-9772-y
47. Lai T, Chen L, Chen X, He J, Lv P, Ge H. Rhynchophylline attenuates migraine in trigeminal nucleus caudalis in nitroglycerin-induced rat model by inhibiting MAPK/NF-small ka, CyrillicB signaling. Mol Cell Biochem. 2019;461(1–2):205–212. doi:10.1007/s11010-019-03603-x
48. Zhang Y, Song N, Liu F, et al. Activation of mitogen-activated protein kinases in satellite glial cells of the trigeminal ganglion contributes to substance P-mediated inflammatory pain. Int J Oral Sci. 2019;11(3):24. doi:10.1038/s41368-019-0055-0
49. Richter F, Eitner A, Leuchtweis J, et al. The potential of substance P to initiate and perpetuate cortical spreading depression (CSD) in rat in vivo. Sci Rep. 2018;8(1):17656. doi:10.1038/s41598-018-36330-2
50. Lieb K, Ahlvers K, Dancker K, et al. Effects of the neuropeptide substance P on sleep, mood, and neuroendocrine measures in healthy young men. Neuropsychopharmacology. 2002;27(6):1041–1049. doi:10.1016/S0893-133X(02)00369-X
51. Rasmussen AH, Rasmussen HB, Silahtaroglu A. The DLGAP family: neuronal expression, function and role in brain disorders. Mol Brain. 2017;10(1):43. doi:10.1186/s13041-017-0324-9
52. Catusi I, Garzo M, Capra AP, et al. 8p23.2-pter microdeletions: seven new cases narrowing the candidate region and review of the literature. Genes. 2021;12(5):652. doi:10.3390/genes12050652
53. Jeong H, Moye LS, Southey BR, et al. Gene network dysregulation in the trigeminal ganglia and nucleus accumbens of a model of chronic migraine-associated hyperalgesia. Front Syst Neurosci. 2018;12:63. doi:10.3389/fnsys.2018.00063
54. Lin H, Ha K, Lu G, et al. Cdc14A and Cdc14B redundantly regulate DNA double-strand break repair. Mol Cell Biol. 2015;35(21):3657–3668. doi:10.1128/MCB.00233-15
55. Wei Z, Zhang P. A phosphatase turns aggressive: the oncogenicity of Cdc14B. Cell Cycle. 2011;10(15):2414. doi:10.4161/cc.10.15.15887
56. Huang S, Zheng C, Xie G, et al. FAM19A5/TAFA5, a novel neurokine, plays a crucial role in depressive-like and spatial memory-related behaviors in mice. Mol Psychiatry. 2021;26(6):2363–2379. doi:10.1038/s41380-020-0720-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

