Back to Journals » Infection and Drug Resistance » Volume 17
Identification of Mycobacterium tuberculosis Resistance to Common Antibiotics: An Overview of Current Methods and Techniques
Authors Xiong XS , Zhang XD, Yan JW, Huang TT , Liu ZZ, Li ZK, Wang L , Li F
Received 14 January 2024
Accepted for publication 26 March 2024
Published 12 April 2024 Volume 2024:17 Pages 1491—1506
DOI https://doi.org/10.2147/IDR.S457308
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xue-Song Xiong,1,2,* Xue-Di Zhang,3,* Jia-Wei Yan,3 Ting-Ting Huang,1,2 Zhan-Zhong Liu,4 Zheng-Kang Li,5 Liang Wang,5 Fen Li1,2
1Department of Laboratory Medicine, The Affiliated Huai’an Hospital of Yangzhou University, Huai’an, Jiangsu Province, People’s Republic of China; 2Department of Laboratory Medicine, The Fifth People’s Hospital of Huai’an, Huai’an, Jiangsu Province, People’s Republic of China; 3Department of Laboratory Medicine, Xuzhou Infectious Diseases Hospital, Xuzhou, Jiangsu Province, People’s Republic of China; 4Department of Pharmacy, Xuzhou Infectious Diseases Hospital, Xuzhou, Jiangsu Province, People’s Republic of China; 5Department of Laboratory Medicine, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, Guangdong Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liang Wang; Fen Li, Email [email protected]; [email protected]
Abstract: Multidrug-resistant tuberculosis (MDR-TB) is an essential cause of tuberculosis treatment failure and death of tuberculosis patients. The rapid and reliable profiling of Mycobacterium tuberculosis (MTB) drug resistance in the early stage is a critical research area for public health. Then, most traditional approaches for detecting MTB are time-consuming and costly, leading to the inappropriate therapeutic schedule resting on the ambiguous information of MTB drug resistance, increasing patient economic burden, morbidity, and mortality. Therefore, novel diagnosis methods are frequently required to meet the emerging challenges of MTB drug resistance distinguish. Considering the difficulty in treating MDR-TB, it is urgently required for the development of rapid and accurate methods in the identification of drug resistance profiles of MTB in clinical diagnosis. This review discussed recent advances in MTB drug resistance detection, focusing on developing emerging approaches and their applications in tangled clinical situations. In particular, a brief overview of antibiotic resistance to MTB was present, referred to as intrinsic bacterial resistance, consisting of cell wall barriers and efflux pumping action and acquired resistance caused by genetic mutations. Then, different drug susceptibility test (DST) methods were described, including phenotype DST, genotype DST and novel DST methods. The phenotype DST includes nitrate reductase assay, RocheTM solid ratio method, and liquid culture method and genotype DST includes fluorescent PCR, GeneXpert, PCR reverse dot hybridization, ddPCR, next-generation sequencing and gene chips. Then, novel DST methods were described, including metabolism testing, cell-free DNA probe, CRISPR assay, and spectral analysis technique. The limitations, challenges, and perspectives of different techniques for drug resistance are also discussed. These methods significantly improve the detection sensitivity and accuracy of multidrug-resistant tuberculosis (MRT) and can effectively curb the incidence of drug-resistant tuberculosis and accelerate the process of tuberculosis eradication.
Keywords: MTB, antibiotic resistance, Raman spectroscopy, rapid detection
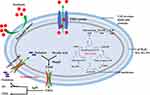
Graphical Abstract:
Introduction
Just as the 2023 global tuberculosis report released by the World Health Organization (WHO), 7.5 million tuberculosis patients were newly diagnosed globally in 2022, with an estimated 1.3 million deaths, nearly twice the rate of HIV/AIDS. At present, tuberculosis, a class A infectious disease aroused by Mycobacterium tuberculosis (MTB), has turned into the first cause of death of a single infectious disease after novel coronavirus pneumonia. Although the clinical detection rate of MTB has increased, over 90% of the pathogenic bacteria of human pulmonary tuberculosis are MTB. Its main transmission routes are air and contact, and it is easy to form an explosive epidemic. The main high-risk places include schools, nursing homes, and other vital social places, which cause potential significant harm to public security. The treatment cycle of tuberculosis and multidrug-resistant tuberculosis is time-losing and expensive, resulting in a substantial social and economic burden on countries and individual patients.1 Then, the incidence data of multidrug-resistant tuberculosis (MRT) and rifampicin-resistant tuberculosis in China is the fourth in the world, and China is one of the high-burden countries with drug-resistant tuberculosis in the world. So, timely and effective identification of MTB infection diagnosis and its drug resistance can effectively realize the early detection, early control, and precise treatment of tuberculosis, an effective tuberculosis prevention and control measure. Therefore, rapid identification of tuberculosis infection and drug resistance types is crucial to curb the current high incidence rate, high mortality, and high economic burden of tuberculosis. In a word, the treatment situation is dire.2
Moreover, the treatment of tuberculosis is facing pain points such as long cycles, high drug resistance rate, and prolonged drug sensitivity reporting time, which brings great trouble to the diagnosis and treatment of tuberculosis. This study aims to explore an immediate method for rapid drug sensitivity diagnosis of MTB and to provide tuberculosis patients with an efficient and reliable laboratory basis for treatment. The diagnosis of tuberculosis mainly includes smear staining, gamma interferon test, bacterial culture, metagenome sequencing, targeted DNA sequencing technology, mass spectrometry technology, and other emerging technologies. The diversity of diagnostic methods provides more selectivity for diagnosing and treating tuberculosis, but the help for tuberculosis still needs to be improved. To realize the early diagnosis and treatment of tuberculosis, rapid results of drug sensitivity diagnosis of tuberculosis must be required. Therefore, we have summarized the latest progress in drug resistance detection of MTB and attempted to find a fast, low-cost, and easy-to-operate drug sensitivity detection system for MTB.
Antibiotic Resistance of MTB
The drug resistance mechanism of MTB is complex. Currently, the partially elucidated mechanism is divided into intrinsic and acquired resistance. Inherent drug resistance mainly includes reduced cell wall permeability, efflux pump function, and cell metabolism. Mutations in target genes mainly cause acquired drug resistance and are the primary mechanism in MTB. The main resistance mechanisms of MTB are shown in Figure 1, and one of these acquired resistance is exemplified by the inhA gene mutations that caused resistance to isoniazid.
 |
Table 1 Summary of Antibiotic Resistance Genes in Drug-Resistant MTB |
Inherent Drug Resistance
The inner layer of the cell wall of MTB was made up of peptidoglycan, arabinogalactan, and mycolic acid, which are jointed to form the mycolic acid–peptidoglycan–arabinogalactan (MA-AG-PG) complex, constituting a permeable barrier.31 Then, the outer cell wall is mainly covered by lipids and proteins. Therefore, the reduced permeability of the cell wall of MTB will interfere with the transport of nutrients or drugs. At the same time, the low permeability plays a protective role in the mycobacteria survival in the host, resulting in the drug resistance of MTB. The cell wall barrier makes MTB impermeable to most antibiotics.32 The survival and pathogenicity of MTB depend on the trehalose, the critical ingredient of glycolipids, with the help of type I ATP-binding cassette (ABC) transporter LpqY-SugABC, the only pathway for trehalose to enter MTB.33,34 Studies have shown that trehalose metabolism is related to drug tolerance in MTB.35 Meantime, cellular metabolism is also an extremely important mechanism of drug resistance in MTB. Then, the efflux pump system also plays a specific role in developing drug resistance in MTB. The efflux pump is a transport protein located in the bacterial plasma membrane. When antibiotics enter the bacteria to act, the gene-associated efflux pump can be overexpressed so that the expression of efflux pump-related proteins is up-regulated, and the antibiotics in the body will be actively removed so that the reduction of drug concentration in the bacterium, resulting in the formation of drug resistance. There are currently 6 types of efflux pumps discovered in bacteria, including major facilitator superfamily (MFS), ATP-binding cassette family (ABC), protein bacterial antibacterial compound efflux family (proteobacterial antimicrobial compound efflux family (PACE), small multidrug resistant family (SMR), resistance–nodulation–division family (RND), and multidrug-resistant and toxic compound efflux Family (Multidrug and toxic compound extrusion, MATE).36 Previous studies showed verapamil, one of the efflux pump inhibitors, could effectively inhibit drug efflux.37 At the same time, studies have also demonstrated that combining verapamil with isoniazid or rifampicin (RIF) can cause the MIC of isoniazid (INH) or RIF reduction and even reverse the resistance of MTB.38 About 20 genes in the genus MTB have been identified as related genes encoding efflux pumps, mainly concentrated in MFS, RND, and SMR.39 It was shown that most of the exocytosis pump gene expression levels were upregulated in isoniazid-resistant MTB strains exposed to low concentrations of antibiotics.
Acquired Drug Resistance
Acquired resistance may occur through mutations or horizontal gene transfer. However, studies on the horizontal transfer of resistance genes through plasmids or removable genetic elements in MTB have yet to be reported. All currently known acquired resistance to MTB is mediated by chromosome mutations in the presence of antibiotics.40 RIF resistance in MTB occurs due to mutations in the rpoB gene encoding the β subunit of RNA polymerase with 1178 amino acids. Mutations in the gene cause a change in the amino acids, resulting in a conformational change in the RIF binding site on the RNA polymerase molecule, which results in a loss of the ability to bind RIF, leading to the development of resistance.3 Mutations usually occur at codons 531, 526, and 516. Then, MIC assays have shown that mutations in codons 531 and 526 are associated with high levels of resistance to RIF. In contrast, mutations in codons 511, 516, 518, and 522 can only lead to low resistance levels to RIF.3 The inhA gene encodes an enoyl lipid acyl carrier protein reductase that binds to the NADH and fatty acid synthase II (FAS-II) complexes and forms a covalent 4-subunit complex with the β-ketolipid acyl carrier protein synthase (kasA) and the acyl carrier protein AcpM. The activated INH attacks the target site, prevents the production of mature mycolic acid, and depletes the mature mycolic acid.41,42
It has been shown that Ald gene deletion increases MTB resistance to cycloserine by testing and comparing MTB MICs of sensitive MTB strains, resistant strains, and Ald knockout strains.10 The Ald gene encodes L-alanine dehydrogenase, which catalyzes the conversion of pyruvate to L-alanine with the participation of NADH, hindering the biosynthesis of peptidoglycan. Thereby, the cell wall of MTB is damaged.43 Moreover, the mechanism of resistance to capreomycin (CPM) is mainly due to mutations in the tea and rrs genes, leading to changes in the target of drug action. Mutations in the tlya gene result in a lack of methylation of bacterial ribosomes, leading to the development of drug resistance.12,13 And the tlya mutations primarily cause low-level resistance to CPM.44 The rrs gene encodes 16S rRNA, and mutations in this gene lead to alterations in the CPM action target, resulting in drug resistance.12,14 In addition, mutations in the eis gene can lead to CPM resistance.15 The standard mutant gene segments and functions of the main anti-tuberculosis drugs are shown below (Table 1).
As is known to all, antibiotics work by binding to the target protein on the cell wall, cell membrane, or intracellular to inhibit the synthesis of nucleic acid, protein, and the associated metabolic pathway (Figure 2).
 |
Figure 2 Schematic diagram of MTB drug resistance mechanism. Four major modes of anti-MTB drug mechanism, as follows: work on cell wall proteins (isoniazid, ethambutol, bedaquiline, delamanid, and clofazimine); work on DNA replication machinery (fluoroquinolones); work on translational machinery (streptomycin, capreomycin, linezolid) and work on metabolic pathways (pyrazinamide and cycloserine). InhA, KasA, EmbABC: the target protein on the cell wall to corresponding antibiotics. Note: Reprinted from Life Sciences, 274, Aditi Chauhan, Manoj Kumar, Awanish Kumar, Kajal Kanchan, Comprehensive review on mechanism of action, resistance and evolution of antimycobacterialdrugs, 119301, Copyright 2021, with permission from Elsevier.25 |
Phenotypic Drug Sensitivity Test
Currently, chemotherapy for MTB follows a “one dose fits all” principle. Yet, the individual differences of the anti-tuberculosis drug were neglected, referring to absorption, distribution, metabolism, and drug excretion. High-performance liquid chromatography mass was used to monitor the first- and second-line antibiotic concentrations in plasma or serum and was successfully applied in two clinical patients.45 The drug resistance of MTB is mainly about the peripheral lipids of the MTB cell envelope and the increased amounts of nonpolar lipids.46 The classic laboratory examination method for MTB includes an E-test, nitrate reductase assay, bacterial culture method, and molecular diagnostic technique, with different features and limitations. Although E-tests could successfully rapidly determination of drug resistance to streptomycin (STM), INH, RIF, and ethambutol (EMB), it existed many limitations, such as the high risk of cross-contamination, laboratory infection, expense, high false-positive rate.47
RocheTM Solid Ratio Method
The RocheTM solid ratio method is a classic method for detecting MTB susceptibility. It is to directly inoculate sputum, alveolar lavage fluid, hydrothorax, and ascites of tuberculosis patients and other samples onto Lǒwenstein-Jensenada (L-J) medium, which contains different anti-tuberculosis drugs. The classic culture method of MTB is the gold standard for drug sensitivity detection. It can detect resistance levels to more than a dozen first and second-line antituberculosis drugs simultaneously, has well-established commercial reagents, and is inexpensive to test. However, it requires a long culture cycle, specific instruments, and a laboratory environment (P3 laboratory), which significantly inconveniences the treatment of tuberculosis.48–50
Nitrate Reductase and Indirect Proportion Methods
The nitrate reductase test is used to determine the growth of MTBs by taking advantage of the fact that most MTBs produce an active nitrate reductase enzyme that breaks down nitrate during growth. The test operation has the advantages of simplicity, rapidity, and low cost. Studies have shown that nitrate reductase assay analyses MTB sensitivity testing to six common anti-tuberculosis drugs, which achieved high specificity and sensitivity.51,52 In addition, drug sensitivity testing can be performed directly on microscopy-positive clinical specimens53,54, with a shorter cycle time compared to other phenotyping methods. DST by indirect proportion methods on L-J media was performed at the final drug concentrations. In brief, two appropriate dilutions of the bacilli, 10−2 and 10−4 dilutions (undiluted =106-108 colony-forming units/mL) were inoculated on a drug-containing and drug-free media, to obtain countable colonies on both media. The drug resistance was taken 28–42 days.55
Liquid Culture Method
The BACTEC MGIT 960 system is BD’s fully automated, continuously monitorable, non-invasive liquid culture system that employs a fluorescent substance as a mycobacterial growth indicator and hence is known as the Mycobacteria growth indicator tube (MGIT) method. The main liquid susceptibility test method used in tuberculosis laboratories today is the modified proportional susceptibility test of BD MGIT960. MGIT960 system may test many tuberculosis drugs, such as isoniazid, rifampicin, ethambutol, streptomycin, levofloxacin, moxifloxacin, bedaquiline, and linezolid.56 The turnaround time with the BACTEC MGIT 960 system (7.5 ± 1.8 days) was significantly shorter than the indirect proportion method (28 days or 42 days).57 But both liquid- and solid-based media and indirect proportion methods have fair sensitivity and specificity, compared to the Xpert MTB/RIF and PCR and other molecular methods.58
Genotype Drug Sensitivity Test
Fluorescence Quantitative Real-Time PCR
Multi-Fluorescence quantitative Real-Time PCR (MF-qRT-PCR) assay was an efficient, accurate, reliable, and easy-to-operate method for detecting drug resistance in RIF and INH. It can be used to distinguish MTC and NTM from clinical isolates. Compared with DNA sequencing, the sensitivity and specificity of MF-qRT-PCR to RIF resistance were 97.2% and 100%, and the sensitivity and specificity to INH resistance were 97.9% and 96.4%, respectively. The limitation of this study is that other anti-TB drug genes or regions could not be tested on time.59 Another study showed genotypic predictions of the susceptibility of MTB to first-line drugs were found to be associated with phenotypic susceptibility to these drugs.60 However, there were still many aspects to improve.
Fluorescent PCR Probe Melting Curve Method
Some studies have used the fluorescent PCR probe melting curve method to detect RIF, isoniazid, and fluoroquinolone drug sensitivity in tissue samples of tuberculosis patients, which has high sensitivity and specificity compared to phenotypic drug sensitivity testing results.61 High-resolution melt curve analysis assay showed a high level of concordance with DNA sequencing, without phenotypic drug susceptibility testing data, and could directly detect fluoroquinolone resistance in sputum samples, including direct smear-negative samples.62
GeneXpert MTB/RIF and GeneXpert-Ultra
The Xpert MTB/RIF Assay System developed by Cepheid is a stand-alone assay that uses integrated microfluidics technology in combination with fully automated nucleic acid analysis to detect the rpoB gene fragment of MTB in samples. It is highly integrated and automated, significantly reducing the potential for contamination and human error in the assay.63 Studies have shown the Xpert MTB/RIF Assay to be 95% of sensitivity and 98% of specificity.64 GeneXpert Ultra uses fully closed nested multiplexed real-time fluorescence PCR to detect the “core” region of the RIF resistance-associated rpoB gene (RRDR) and two additional target genes (IS1081 and IS6110). The GeneXpert MTB/RIF Ultra system is more sensitive than the first generation because it dramatically increases the sensitivity of MTB strains from 112.6 to 15.6 CFU/mL, optimizes the interpretation of silent mutations, and reduces the false-positive rate of the RFP resistance assay at low levels of MTB in samples and mixed infections.65
PCR Reverse Dot Hybridization Method
The main principle of the PCR reverse dot hybridization method consists of both amplification and hybridization. The unlabeled oligonucleotide probe is first immobilized on a solid-phase membrane, and then the labeled PCR product is hybridized with the solidified probe.66 It is a gene diagnostic technique that combines probe, nucleic acid hybridization, and enzyme-linked chromatography. It seems superior in sensitivity and specificity, easy operation, fast results, and large detection throughput. Line Probe Assays (LPA), a kind of PCR-single-stranded probe reverse hybridization assays, were used to detect isoniazid and rifampicin resistance, based on the mutations in rpoB, inhA, and katG genes. It could turn around and detect samples within a few hours. LPAs apply biological amplification of target nucleic acids (DNA) by applying specific primers of resistance genes. Then, the product of LPA is denatured and hybridized to a specific oligonucleotide probe immobilized on a nylon membrane, and multiple target sequences can be detected in one hybridization. For the diagnosis of rifampicin mono-resistance, LPA had a perfect agreement with a sensitivity of 90.0% and a specificity of 99.1%. Considering the advantage of LPA, WHO approved the method for the diagnosis of MDR-TB and RIF-resistant TB in smear-positive TB.67–69
Compared with the phenotypic drug sensitivity assay using this technique by Guo et al,70 the sensitivity and specificity of RIF resistance were 91.2% and 98.3%, respectively. It detects whether MTB is drug-resistant and resistant to which drugs and provides insight into the specific mutation sites or regions of the drug-resistant genes. This method is expensive in terms of reagents and instruments and has low detection of mutant loci in other gene fragments not designed on the membrane. Moreover, the interpretation of the spots on the membrane after color development is affected by subjectivity, causing false negatives of MTB.
Digital PCR
Digital PCR combines microfluidic technology with PCR to quantify individual DNA copies accurately and achieve accurate quantification of target DNA with high sensitivity and specificity. It does not require the construction of a standard curve. Zhang et al used digital PCR to detect the susceptibility of MTB to RIF, isoniazid, and streptomycin. The existence of subgroups with different sensitivities to drugs is known as hetero-resistance. Studies have shown that 8.5% and 14.2% of tuberculosis patients are hetero-resistant to INH and RIF,71 respectively. MeltPro TB/INH only detects 20% to 40% of the INH resistance heterogeneity.72 ddPCR can see mutant sequences as low as 0.01%.73 So, digital PCR offers significant advantages in detecting and quantifying heterologous drug resistance in MTB populations.74,75
Next-Generation Sequencing
Next-generation sequencing (NGS), also termed high-throughput or massively parallel sequencing, is used for pathogen detection and monitoring the hospital microbiome and its drug resistance. It is a technology genre that allows thousands to billions of DNA/RNA fragments to be tested and independently sequenced.76,77 Metagenomic next-generation sequencing (NGS) detected MTB with high specificity and sensitivity but overlooked the drug resistance information.78,79 Then, targeted next-generation sequencing (NGS) may be an early detection method for drug resistance directly from MTBC-positive specimens, with the resistance mutations by 100%. The turnaround time of the tNGS assay showed less than culture and Whole Genome Sequencing (WGS) workflows with a two-week and a similar cost.80 The other studies also showed that tNGS could detect various antibiotic resistance genes and related mutations and has great potential in predicting MTB drug resistance.81 The performance of nanopore-targeted sequencing in bronchoalveolar lavage fluid and metagenomic next-generation sequencing for diagnosing pneumonia pathogens and both methods show higher sensitivity than conventional microbial testing. Nanopore-targeted sequencing can be considered a reliable method for diagnosing pneumonia pathogens.82,83 However, its application in MTB research remains limited because of less experience and the lack of sequence data in the bioinformatics analysis of MTB.84 Thus, next-generation sequencing is a comfortable tool to predict MTB drug resistance, but the limitations make its implementation in monitoring drug resistance of clinical pathogens premature.
Gene Chips
Gene chips work by applying known nucleic acid sequences as probes immobilized on a substrate, such as glass, and then hybridizing with the DNA or RNA complementary target nucleotide sequences of the sample to be tested to obtain information about the sample. Gene chip technology can detect and analyze many sequences of a sample simultaneously due to the simultaneous immobilization of many probes on the support. Thus, it solves the shortcomings of traditional nucleic acid blot hybridization technology, such as cumbersome operation, low automation, a small number of operated sequences, and low detection efficiency. Moreover, by designing different probe arrays, the use of specific analysis methods can give the technology a variety of other applications. It was shown that gene microarrays have high sensitivity and specificity in the detection of resistance to RIF, isoniazid, fluoroquinolones, and streptomycin with the advantages of rapidity, accuracy, and high throughput but lower sensitivity in the detection of ethambutol.85 Current gene-core technology can detect wild-type and 13 mutations at six sites of rpoB, wild-type at one site of katG, and wild-type and mutations in the promoter region of the inhA gene.86,87 Therefore, it can be seen that the gene chip technology cannot detect all the drug-resistant genotypes of MTB, so it cannot completely replace the traditional drug sensitivity test. Still, its sensitivity and efficiency can complement the conventional drug sensitivity test.
Novel Drug Sensitivity Test
Metabolism Testing Assay
In recent years, the assay based on bacterial metabolism to the drug or exterior inhibitor provides a new direction for detecting MTB drug resistance. JHU083 is a glutamine (Gln) metabolic antagonist, when administered in a TB mouse model, leading to a decrease in immunosuppressive bone marrow cell levels, an increase in effector T cell levels, and an increase in citrulline and NO production levels.88 According to another study, about 150 MTB metabolomics of ethambutol (ETH) and ethionamide (ETO) phenotypically resistant subgroups, associated with 54 pre-XDR, 63 XDR-TB, and 33 pan-susceptible, two metabolites (meso-hydroxyheme and itaconic anhydride) were found to distinguish the pre-XDR and XDR-TB groups from the pan-susceptible group with 100% sensitivity and 100% specificity.89 Pyrazinamide (PZA) is a first-line anti-MTB regimen, which could be converted to pyrazinoic acid (POA) by the bacterial enzyme pyrazinamidase. Then, POA is excreted into the extracellular medium, with a cycle of protonation, cell influx, and efflux established. Thus, the presence of POA can be used as a marker to assess the PZA resistance.90 RIF, a first-line anti-tubercular agent, has been widely used to treat TB for many years. It inhibits the MTB transcription, restraining protein synthesis and mediating bacterial killing. Many studies have previously reported that the RNA polymerase gene rpoB could lead to MTB resistance against RIF. The RIF-mediated metabolic changes associated with pyrimidine, purine, arginine, phenylalanine, tyrosine, and tryptophan metabolic pathways could exploit potential treatment agents of MTB.91,92 In summary, metabolism analysis of anti-MTB drug or drug-resistant tuberculosis may provide a new orientation for detecting MTB drug resistance and effective treatment references for clinical doctors.
Cell-Free DNA Probe Assay
Cell-free DNA (cfDNA) is a cell-free nucleic acid fragment found in human blood and other body fluids.93 It often exists as DNA-protein complexes in 70–200 bp short fragments or 21 kb long fragments.94 The cfDNA was released into the blood during the decomposition of dead cells and microorganisms.95 Alternatively, DNA breaks during apoptosis and nuclear consolidation and is released into the bloodstream.96 The cfDNA enters the body fluids with the blood circulation in various fractions and can be detected and analyzed by different techniques such as PCR or sequencing. CfDNA can be detected in a wide range of body fluid samples and is of great significance for children and adults who have difficulty retaining respiratory specimens and for extra-pulmonary tuberculosis such as osteoarticular tuberculosis. It has been shown that the growth of bacterial populations can be estimated by measuring the skewness of the bacterial genome coverage of cfDNA and that drug resistance can be assessed by sequencing cfDNA against a defined spectrum of drug-resistance genes. The detection of MTB-cfDNA in the blood of people with latent tuberculosis infection (LTBI) suggests that the detection of MTB-cfDNA in the blood can be used to screen people with LTBI, which is a breakthrough in LTBI detection.97 The cfDNA in the patient’s body is quickly cleared by immune cells after treatment.98 Therefore, the detected cfDNA is more likely to be newly released into the bloodstream, which reflects the current disease and facilitates monitoring of the condition. Previous studies demonstrated that the intracellular pathogens’ genomic DNA fragments could be tested in human plasma and could be potential biomarkers for finding the presence of pathogenic organisms.99 Wu et al showed that cell-free DNA of bronchoalveolar lavage fluid could diagnose MTB and resistance to the first-line anti-MTB drug (RIF and INH).100 Despite significant progress, the analysis of cfNAs remains challenging, mainly due to contamination from cells degrading after sampling and releasing more nucleic acids (NAs) into the sampled body fluids. cfNAs have a short half-life, low concentration, and high degree of fragmentation. Therefore, standard pre-treatment of samples is essential to improve specificity and sensitivity. In addition, large sample sizes are needed for clinical validation.
CRISPR Assay
Clustered Regularly Interspaced Short Palindromic repeats (CRISPR) and CRISPR-associated proteins (Cas) are immune defense systems derived from bacteria and archaea to cope with the invasion of exogenous nucleic acids.101 CRISPR-Cas systems are next-generation pathogen methods that detect single nucleotide polymorphisms with high sensitivity and specificity. With its superiority, it has now been developed as a strong tool for genome editing and is widely used in various research fields such as biology, medicine, and the environment. A study was conducted to detect streptomycin-resistant strains of MTB in one hour by a rapid fluorescent assay based on the CRISPR-CAS12a system, with 100% sensitivity and specificity compared with sequencing results.102 Bai et al realized a significant distinction between fluoroquinolone-resistant and sensitive strains of MTB using the CRISPR-CAS13a system, with specificity and sensitivity of 100% and 91.4%.103 In addition, CRISPR assay can be used to explore the mechanisms of drug resistance. Li et al developed a CRISPR-interfering chemical genetics platform to discover multiple drug resistance mechanisms in MTB, such as ettA mutations causing low-level resistance to various drugs, and find that MTB in the Southeast Asian lineage with loss-of-function whiB7 allele is highly sensitive to macrolides, which provides theoretical support for clarithromycin treatment of this sub-lineage.104 Mei-Yi Yan group used CRISPR assay to show the antitubercular drug bedaquiline (BDQ) mechanisms and the possibility of optimizing tuberculosis therapy.105 Additionally, the CRISPR assay could successfully prognosis the coordination action of cyclomarin A with isoniazid or RIF.106 CRISPRi assay could provide a new thinking for anti-MTB drug development. Another group found an application of the Next-Generation CRISPR for Finding Low Abundance Sequences by Hybridization (FLASH), a technique for directly identifying MTB drug resistance from sputum samples.107 CRISPR assay requires a small sample volume and short operational time compared to the Xpert method.
Spectral Analysis Technique
Spectral analysis is a statistical method used to analyze a time series dataset. It identifies statistically meaningful frequencies in a time series to see whether they contain periodic or cyclical components. Spectral analysis is a powerful tool with many applications in numerous fields, such as environmental monitoring and traditional Chinese medicine. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF) is a rapidly developing and new type of soft ionized biomass spectrum, which was first used for MTB identification in 2004. Compared with traditional identification methods, MALDI-TOF MS simplifies the identification process, reduces the identification time, and has the advantages of accuracy, speed, sensitivity, and high throughput. MALDI-TOF MS can also identify different drug resistance types of MTB with other mutations and be a rapid and effective method to scan the drug resistance of MTB. It shows acceptable clinical application values in patients with relapsed tuberculosis.108–110
Raman scattering is an inelastic scattering phenomenon triggered by light irradiation on the surface of a substance. The enhancement effect associated with rough metal surfaces such as gold, silver, and copper was known as the surface-enhanced Raman scattering effect, and the resulting spectra were known as surface-enhanced Raman spectroscopy (SERS). Nucleic acids, proteins, lipids, and carbohydrates in bacteria exhibit different spectral peaks on Raman spectra due to their different molecular vibration frequencies, which provides a basis for applying SERS detection technology in the field of bacteria.111 With no need for labeling, non-invasive, easy operation, and short detection time, SERS has recently become a research hotspot in microbiology. Tang et al112 used two deep learning methods (CNN and LSTM) to detect 117 staphylococcal strains of nine staphylococcal genera with SERS, and the accuracies reached 98.21% and 94.33%. SERS technology also shows excellent potential in the detection of MTB. Perumal et al113 used SERS technology to detect Mycobacterium acid for rapid and effective detection of tuberculosis. Dastgir et al used the SERS technique combined with PCR to obtain spectra from the PCR products of MTB to differentiate RFP resistance using principal component analysis and least discriminant analysis.113 Raman spectroscopy was combined with deep learning for the early diagnosis and to distinguish accurately in the three situations: 1) smear-positive and smear-negative sputum samples to MTB; 2) pulmonary and extra-pulmonary origin of MTB strains; and 3) different antibiotic resistance groups to RIF and INH for pulmonary MTB strains.114 The classification of MTB with different resistance to RFP and INH was predicted by applying SERS technology; its accuracy was 99.59%, precision was 99.64%, recall was 99.61%, F1 score was 99.67%, and the accuracy of 5-fold cross-validation was 99.59%. Raman signals are closely related to the vibrations of multiple components and groups of bacteria. The collected Raman spectra are highly similar, and the data are complex.114 Thus, efficient data processing analysis methods are required to process and analyze Raman spectra. Deep learning is to extract learning features from large-scale raw data and build predictive models to make predictions.115 Many deep learning algorithms have emerged, including convolutional neural networks, fully connected neural networks, residual neural networks, etc.116 Deep learning performance is evaluated by concentrating on the data’s local features and obtaining the data’s global features. Classifying and identifying the data is more efficient than the traditional multivariate statistical analysis algorithms.
Limitations, Challenges, and Perspectives
From traditional testing techniques to emerging methods, all have demonstrated distinct advantages in detecting MTB drug resistance. Phenotypic drug sensitivity testing methods such as tuberculosis culture are accurate and inexpensive. They can test multiple first and second-line anti-tuberculosis drugs simultaneously. However, the operation is complicated, and the culture cycle is long. Many live bacterial detectors have high technical requirements for experimental sites, personnel, and laboratory biosafety risks. Traditional molecular detection techniques are mature and sensitive, with short detection time and low biosafety risk. However, there are areas for improvement in the following aspects: challenging and complex to detect heterogeneous resistance; 2) synonymous and silent mutations that do not affect the phenotypes; 3) all phenotypically resistant strains cannot be detected. Traditional molecular diagnostic techniques are widely used in the clinic as complementary to phenotypic drug-sensitivity testing techniques.
Compared with classical detection methods, emerging detection technologies show great potential for application and need to be more mature and sophisticated in many aspects. The emerging digital PCR does not rely on standard curves and has higher sensitivity. No pre-amplification DNA treatment is required.117 However, amplifying the RNA gene may contain an incompletely reverse-transcribed template, causing off-target phenomena; false positives exist in a few reactions units.118,119 NGS enables a more comprehensive analysis of genomic drug resistance-related variants. Meanwhile, NGS can be used for epidemiological surveillance and exploration of drug resistance mechanisms. At present, genome sequencing technology needs a unified testing procedure. Laboratory operations are prone to exogenous nucleic acid contamination, producing false positive results. Nucleic acid molecules from non-pathogenic pathogens with low sequence counts or residual nucleic acid molecules from dead pathogens can be detected at the same time. These can easily interfere with proper clinical diagnosis and treatment. So, there are still challenges in establishing a unified quality control process and interpretation guidelines, addressing interference from background organisms, and reducing the cost of testing.
The majority of TB deaths occur in developing countries, and the 30 high-burden TB countries in the world are also mainly developing countries. Studies have shown that the economic burden on multidrug-resistant TB patients has been high and increasing in recent years. SERS technology is inexpensive, simple, and easy to disseminate, significantly reducing the economic pressure on developing countries for outbreak prevention and control and treating individuals with the disease. However, SERS requires a large number of samples for validation. In addition, Raman spectroscopy relies on complex algorithms. There are still significant challenges in the improvement and preparation of substrates to improve the sensitivity of spectra and the quality of spectral data, the establishment of a database of standard maps, the establishment of mature standard operating procedures, the promotion of mutual recognition of results, and the interpretation of consensus, among others.
Conclusion
Currently, there are many techniques for drug sensitivity testing of MTB, and all of them have shown their unique superiority. Under the pressure of emerging technologies, the classical drug-sensitivity testing techniques are still irreplaceable. While emerging technologies offer excellent application prospects, they also face many challenges. Combined drug resistance assays should be performed on a case-by-case basis to provide the clinic with more timely and accurate information, considering the methods’ sensitivity and specificity, the reagents’ cost, the reporting period, and the limitations. Researchers should actively promote the research of emerging technologies and continuously improve them to provide more choices for clinical applications.
Funding
This work was funded by the Research Foundation for Advanced Talents of Guangdong Provincial People’s Hospital (Grant No. KY012023293) and the Fifth People’s Hospital of Huai’an Collaboration Foundation (Grant No. HWY-YL-20230072).
Disclosure
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Wang DM, Liu H, Zheng YL, Xu YH, Liao Y. Epidemiology of nontuberculous mycobacteria in tuberculosis suspects, Southwest of China, 2017–2022. Front Cell Infect Microbiol. 2023;13:1282902. doi:10.3389/fcimb.2023.1282902
2. Yu JJ, Tang SJ. 耐多药/利福平耐药结核病化学治疗年度进展2022 [Annual progress of chemotherapy of multidrug/rifampicin-resistant tuberculosis in 2022]. Zhonghua Jie He He Hu Xi Za Zhi. 2023;46(1):62–66. Chinese. doi:10.3760/cma.j.cn112147-20221030-00853
3. Nusrath Unissa A, Hassan S, Indira Kumari V, Revathy R, Hanna LE. Insights into RpoB clinical mutants in mediating rifampicin resistance in Mycobacterium tuberculosis. J Mol Graph Model. 2016;67:20–32. doi:10.1016/j.jmgm.2016.04.005
4. Seifert M, Catanzaro D, Catanzaro A, Rodwell TC. Genetic mutations associated with isoniazid resistance in Mycobacterium tuberculosis: a systematic review. PLoS One. 2015;10(3):e0119628. doi:10.1371/journal.pone.0119628
5. Bifani P, Mathema B, Campo M, et al. Molecular identification of streptomycin monoresistant Mycobacterium tuberculosis related to multidrug-resistant W strain. Emerg Infect Dis. 2001;7(5):842–848. doi:10.3201/eid0705.010512
6. Spies FS, Ribeiro AW, Ramos DF, et al. Streptomycin resistance and lineage-specific polymorphisms in Mycobacterium tuberculosis gidB gene. J Clin Microbiol. 2011;49(7):2625–2630. doi:10.1128/jcm.00168-11
7. Ramaswamy SV, Amin AG, Göksel S, et al. Molecular genetic analysis of nucleotide polymorphisms associated with ethambutol resistance in human isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2000;44(2):326–336. doi:10.1128/aac.44.2.326-336.2000
8. Brossier F, Sougakoff W, Bernard C, et al. Molecular analysis of the embCAB Locus and embR Gene involved in ethambutol resistance in clinical isolates of mycobacterium tuberculosis in France. Antimicrob Agents Chemother. 2015;59(8):4800–4808. doi:10.1128/aac.00150-15
9. Xu Y, Jia H, Huang H, Sun Z, Zhang Z. Mutations found in embCAB, embR, and ubiA genes of ethambutol-sensitive and -resistant mycobacterium tuberculosis clinical isolates from China. Biomed Res Int. 2015;2015:951706. doi:10.1155/2015/951706
10. Desjardins CA, Cohen KA, Munsamy V, et al. Genomic and functional analyses of Mycobacterium tuberculosis strains implicate ald in D-cycloserine resistance. Nat Genet. 2016;48(5):544–551. doi:10.1038/ng.3548
11. Belanger AE, Porter JC, Hatfull GF. Genetic analysis of peptidoglycan biosynthesis in mycobacteria: characterization of a ddlA mutant of Mycobacterium smegmatis. J Bacteriol. 2000;182(23):6854–6856. doi:10.1128/jb.182.23.6854-6856.2000
12. Maus CE, Plikaytis BB, Shinnick TM. Molecular analysis of cross-resistance to capreomycin, kanamycin, amikacin, and viomycin in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2005;49(8):3192–3197. doi:10.1128/aac.49.8.3192-3197.2005
13. Maus CE, Plikaytis BB, Shinnick TM. Mutation of tlyA confers capreomycin resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2005;49(2):571–577. doi:10.1128/aac.49.2.571-577.2005
14. Jugheli L, Bzekalava N, de Rijk P, Fissette K, Portaels F, Rigouts L. High level of cross-resistance between kanamycin, amikacin, and capreomycin among Mycobacterium tuberculosis isolates from Georgia and a close relation with mutations in the rrs gene. Antimicrob Agents Chemother. 2009;53(12):5064–5068. doi:10.1128/aac.00851-09
15. Malinga L, Brand J, Olorunju S, Stoltz A, van der Walt M. Molecular analysis of genetic mutations among cross-resistant second-line injectable drugs reveals a new resistant mutation in Mycobacterium tuberculosis. Diagn Microbiol Infect Dis. 2016;85(4):433–437. doi:10.1016/j.diagmicrobio.2016.05.010
16. Hillemann D, Rüsch-Gerdes S, Richter E. In vitro-selected linezolid-resistant Mycobacterium tuberculosis mutants. Antimicrob Agents Chemother. 2008;52(2):800–801. doi:10.1128/aac.01189-07
17. Zimenkov DV, Nosova EY, Kulagina EV, et al. Examination of bedaquiline- and linezolid-resistant Mycobacterium tuberculosis isolates from the Moscow region. J Antimicrob Chemother. 2017;72(7):1901–1906. doi:10.1093/jac/dkx094
18. Zong Z, Jing W, Shi J, et al. Comparison of in vitro activity and MIC distributions between the novel oxazolidinone delpazolid and linezolid against multidrug-resistant and extensively drug-resistant mycobacterium tuberculosis in China. Antimicrob Agents Chemother. 2018;62:8. doi:10.1128/aac.00165-18
19. Werngren J, Alm E, Mansjö M. Non-pncA gene-mutated but pyrazinamide-resistant mycobacterium tuberculosis: why is that? J Clin Microbiol. 2017;55(6):1920–1927. doi:10.1128/jcm.02532-16
20. Saeed DK, Shakoor S, Razzak SA, et al. Variants associated with Bedaquiline (BDQ) resistance identified in Rv0678 and efflux pump genes in Mycobacterium tuberculosis isolates from BDQ naïve TB patients in Pakistan. BMC Microbiol. 2022;22(1):62. doi:10.1186/s12866-022-02475-4
21. Nguyen TVA, Anthony RM, Bañuls AL, Nguyen TVA, Vu DH, Alffenaar JC. Bedaquiline resistance: its emergence, mechanism, and prevention. Clin Infect Dis. 2018;66(10):1625–1630. doi:10.1093/cid/cix992
22. Almeida D, Ioerger T, Tyagi S, et al. Mutations in pepQ confer low-level resistance to bedaquiline and clofazimine in mycobacterium tuberculosis. Antimicrob Agents Chemother. 2016;60(8):4590–4599. doi:10.1128/aac.00753-16
23. Karmakar M, Rodrigues CHM, Holt KE, Dunstan SJ, Denholm J, Ascher DB. Empirical ways to identify novel bedaquiline resistance mutations in AtpE. PLoS One. 2019;14(5):e0217169. doi:10.1371/journal.pone.0217169
24. Xu J, Wang B, Hu M, et al. Primary clofazimine and bedaquiline resistance among isolates from patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2017;61:6. doi:10.1128/aac.00239-17
25. Chauhan A, Kumar M, Kumar A, Kanchan K. Comprehensive review on mechanism of action, resistance and evolution of antimycobacterial drugs. Life Sci. 2021;274:119301. doi:10.1016/j.lfs.2021.119301
26. Manjunatha UH, Boshoff H, Dowd CS, et al. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2006;103(2):431–436. doi:10.1073/pnas.0508392103
27. Forouhar F, Abashidze M, Xu H, et al. Molecular insights into the biosynthesis of the F420 coenzyme. J Biol Chem. 2008;283(17):11832–11840. doi:10.1074/jbc.M710352200
28. Bashiri G, Rehan AM, Sreebhavan S, Baker HM, Baker EN, Squire CJ. Elongation of the Poly-γ-glutamate Tail of F420 Requires Both Domains of the F420:γ-Glutamyl Ligase (FbiB) of Mycobacterium tuberculosis. J Biol Chem. 2016;291(13):6882–6894. doi:10.1074/jbc.M115.689026
29. Xavier AS, Lakshmanan M. Delamanid: a new armor in combating drug-resistant tuberculosis. J Pharmacol Pharmacother. 2014;5(3):222–224. doi:10.4103/0976-500x.136121
30. Nguyen QT, Trinco G, Binda C, Mattevi A, Fraaije MW. Discovery and characterization of an F(420)-dependent glucose-6-phosphate dehydrogenase (Rh-FGD1) from Rhodococcus jostii RHA1. Appl Microbiol Biotechnol. 2017;101(7):2831–2842. doi:10.1007/s00253-016-8038-y
31. Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis. 2003;83(1–3):91–97. doi:10.1016/s1472-9792(02)00089-6
32. Grover N, Paskaleva EE, Mehta KK, Dordick JS, Kane RS. Growth inhibition of Mycobacterium smegmatis by mycobacteriophage-derived enzymes. Enzyme Microb Technol. 2014;63:1–6. doi:10.1016/j.enzmictec.2014.04.018
33. Liang J, Liu F, Xu P, et al. Molecular recognition of trehalose and trehalose analogues by Mycobacterium tuberculosis LpqY-SugABC. Proc Natl Acad Sci U S A. 2023;120(35):e2307625120. doi:10.1073/pnas.2307625120
34. Kalscheuer R, Weinrick B, Veeraraghavan U, Besra GS, Jacobs WR. Trehalose-recycling ABC transporter LpqY-SugA-SugB-SugC is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2010;107(50):21761–21766. doi:10.1073/pnas.1014642108
35. Lee JJ, Lee SK, Song N, et al. Transient drug-tolerance and permanent drug-resistance rely on the trehalose-catalytic shift in Mycobacterium tuberculosis. Nat Commun. 2019;10(1):2928. doi:10.1038/s41467-019-10975-7
36. Song L, Wu X. Development of efflux pump inhibitors in antituberculosis therapy. Int J Antimicrob Agents. 2016;47(6):421–429. doi:10.1016/j.ijantimicag.2016.04.007
37. de Souza JVP, Murase LS, Caleffi-Ferracioli KR, et al. Isoniazid and verapamil modulatory activity and efflux pump gene expression in Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2020;24(6):591–596. doi:10.5588/ijtld.19.0458
38. Long Y, Wang B, Xie T, et al. Overexpression of efflux pump genes is one of the mechanisms causing drug resistance in Mycobacterium tuberculosis. Microbiol Spectr. 2023:e0251023. doi:10.1128/spectrum.02510-23
39. Ghajavand H, Kargarpour Kamakoli M, Khanipour S, et al. Scrutinizing the drug resistance mechanism of multi- and extensively-drug resistant Mycobacterium tuberculosis: mutations versus efflux pumps. Antimicrob Resist Infect Control. 2019;8:70. doi:10.1186/s13756-019-0516-4
40. Gygli SM, Trauner A, Gagneux S. Antimicrobial resistance in Mycobacterium tuberculosis: mechanistic and evolutionary perspectives. FEMS Microbiol Rev. 2017;41(3):354–373.
41. Slayden RA, Lee RE, Barry CE. Isoniazid affects multiple components of the type II fatty acid synthase system of Mycobacterium tuberculosis. Mol Microbiol. 2000;38(3):514–525. doi:10.1046/j.1365-2958.2000.02145.x
42. Prasad MS, Bhole RP, Khedekar PB, Chikhale RV. Mycobacterium enoyl acyl carrier protein reductase (InhA): a key target for antitubercular drug discovery. Bioorg Chem. 2021;115:105242. doi:10.1016/j.bioorg.2021.105242
43. Bruning JB, Murillo AC, Chacon O, Barletta RG, Sacchettini JC. Structure of the Mycobacterium tuberculosis D-alanine:D-alanine ligase, a target of the antituberculosis drug D-cycloserine. Antimicrob Agents Chemother. 2011;55(1):291–301. doi:10.1128/aac.00558-10
44. Zhang Z, Liu M, Wang Y, Pang Y, Kam KM, Zhao Y. Molecular and phenotypic characterization of multidrug-resistant Mycobacterium tuberculosis isolates resistant to kanamycin, amikacin, and capreomycin in China. Eur J Clin Microbiol Infect Dis. 2014;33(11):1959–1966. doi:10.1007/s10096-014-2144-5
45. Kohler N, Karakose H, Grobbel HP, et al. A single-run HPLC-MS multiplex assay for therapeutic drug monitoring of relevant first- and second-line antibiotics in the treatment of drug-resistant tuberculosis. Pharmaceutics. 2023;15(11). doi:10.3390/pharmaceutics15112543
46. Schami A, Islam MN, Belisle JT, Torrelles JB. Drug-resistant strains of Mycobacterium tuberculosis: cell envelope profiles and interactions with the host. Frontiers in Cellular and Infection Microbiology. 2023;13. doi:10.3389/fcimb.2023.1274175
47. Verma JS, Rawat D, Hasan A, et al. The use of E-test for the drug susceptibility testing of Mycobacterium tuberculosis - a solution or an illusion? Indian J Med Microbiol. 2010;28(1):30–33. doi:10.4103/0255-0857.58725
48. Slail MJ, Booq RY, Al-Ahmad IH, et al. Evaluation of xpert MTB/RIF ultra for the diagnosis of extrapulmonary tuberculosis: a retrospective analysis in Saudi Arabia. J Epidemiol Glob Health. 2023;13(4):782–793. doi:10.1007/s44197-023-00150-z
49. Pai M, Schito M. Tuberculosis diagnostics in 2015: landscape, priorities, needs, and prospects. J Infect Dis. 2015;2(Suppl 2):S21–8. doi:10.1093/infdis/jiu803
50. Denkinger CM, Kik SV, Cirillo DM, et al. Defining the needs for next generation assays for tuberculosis. J Infect Dis. 2015;2(Suppl 2):S29–38. doi:10.1093/infdis/jiu821
51. Kwak M, Lee WK, Lim YJ, Lee SH, Ryoo S. Systematic review and meta-analysis of the nitrate reductase assay for drug susceptibility testing of Mycobacterium tuberculosis and the detection limits in liquid medium. J Microbiol Methods. 2017;141:1–9. doi:10.1016/j.mimet.2017.07.001
52. Coban AY, Deveci A, Sunter AT, Martin A. Nitrate reductase assay for rapid detection of isoniazid, rifampin, ethambutol, and streptomycin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J Clin Microbiol. 2014;52(1):15–19. doi:10.1128/JCM.01990-13
53. Kammoun S, Smaoui S, Marouane C, Slim L, Messadi-Akrout F. Drug susceptibility testing of Mycobacterium tuberculosis by a nitrate reductase assay applied directly on microscopy-positive sputum samples. Int J Mycobacteriol. 2015;4(3):202–206. doi:10.1016/j.ijmyco.2015.04.005
54. Musa HR, Ambroggi M, Souto A, Angeby KA. Drug susceptibility testing of Mycobacterium tuberculosis by a nitrate reductase assay applied directly on microscopy-positive sputum samples. J Clin Microbiol. 2005;43(7):3159–3161. doi:10.1128/jcm.43.7.3159-3161.2005
55. Kohli A, Bashir G, Fatima A, Jan A, Wani NU, Ahmad J. Rapid drug-susceptibility testing of Mycobacterium tuberculosis clinical isolates to first-line antitubercular drugs by nitrate reductase assay: a comparison with proportion method. Int J Mycobacteriol. 2016;5(4):469–474. doi:10.1016/j.ijmyco.2016.06.006
56. Li S, Tan Y, Deng Y, et al. The emerging threat of fluroquinolone-, bedaquiline-, and linezolid-resistant Mycobacterium tuberculosis in China: observations on surveillance data. J Infect Public Health. 2024;17(1):137–142. doi:10.1016/j.jiph.2023.11.018
57. L-l Z, Xia Q, Lin N, et al. Evaluation of BACTEC MGIT 960 system for the second-line drugs susceptibility testing of Mycobacterium tuberculosis in China. Journal of Microbiological Methods. 2012;91(1):212–214. doi:10.1016/j.mimet.2012.06.010
58. Rafael LL, Raquel MS, Rogelio FA, Miroslava FP, Alejandra-Isabel JG, Paola RS. Discordant results between genotypic and phenotypic assays (Xpert MTB/RIF vs. BACTEC MGIT 960 system) for detection of RIF-resistant Mycobacterium tuberculosis isolates in a high burden region. Infect Genet Evol. 2021;96:105142. doi:10.1016/j.meegid.2021.105142
59. Peng J, Yu X, Cui Z, et al. Multi-fluorescence real-time PCR assay for detection of RIF and INH resistance of M. tuberculosis. Front Microbiol. 2016;7:618. doi:10.3389/fmicb.2016.00618
60. Allix-Beguec C; Consortium CR, the GP. Prediction of susceptibility to first-line tuberculosis drugs by DNA sequencing. N Engl J Med. 2018;379(15):1403–1415. doi:10.1056/NEJMoa1800474
61. Mu J, Liu Z, Zhang C, et al. Performance of the MeltPro MTB assays in the diagnosis of drug-resistant tuberculosis using formalin-fixed, paraffin-embedded tissues. Am J Clin Pathol. 2021;156(1):34–41. doi:10.1093/ajcp/aqaa203
62. Gupta RK, Anthwal D, Bhalla M, Tyagi JS, Choudhary S, Haldar S. Direct detection of fluoroquinolone resistance in sputum samples from tuberculosis patients by high resolution melt curve analysis. Curr Microbiol. 2023;81(1):27. doi:10.1007/s00284-023-03519-2
63. Lawn SD, Nicol MP. Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6(9):1067–1082. doi:10.2217/fmb.11.84
64. Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;2014(1):Cd009593. doi:10.1002/14651858.CD009593.pub3
65. Kolia-Diafouka P, Carrère-Kremer S, Lounnas M, et al. Detection of Mycobacterium tuberculosis in paucibacillary sputum: performances of the Xpert MTB/RIF ultra compared to the Xpert MTB/RIF, and IS6110 PCR. Diagn Microbiol Infect Dis. 2019;94(4):365–370. doi:10.1016/j.diagmicrobio.2019.02.008
66. Saiki RK, Walsh PS, Levenson CH, Erlich HA. Genetic analysis of amplified DNA with immobilized sequence-specific oligonucleotide probes. Proc Natl Acad Sci U S A. 1989;86(16):6230–6234. doi:10.1073/pnas.86.16.6230
67. Lin M, Chen Y-W, Li Y-R, et al. Systematic evaluation of line probe assays for the diagnosis of tuberculosis and drug-resistant tuberculosis. Clinica Chimica Acta. 2022;533:183–218. doi:10.1016/j.cca.2022.06.020
68. Aricha SA, Kingwara L, Mwirigi NW, et al. Comparison of GeneXpert and line probe assay for detection of Mycobacterium tuberculosis and rifampicin-mono resistance at the National Tuberculosis Reference Laboratory, Kenya. BMC Infect Dis. 2019;19(1):852. doi:10.1186/s12879-019-4470-9
69. Nathavitharana RR, Cudahy PG, Schumacher SG, Steingart KR, Pai M, Denkinger CM. Accuracy of line probe assays for the diagnosis of pulmonary and multidrug-resistant tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2017;49(1). doi:10.1183/13993003.01075-2016
70. Guo Q, Yu Y, Zhu YL, et al. Rapid detection of rifampin-resistant clinical isolates of Mycobacterium tuberculosis by reverse dot blot hybridization. Biomed Environ Sci. 2015;28(1):25–35. doi:10.3967/bes2015.003
71. Hofmann-Thiel S, van Ingen J, Feldmann K, et al. Mechanisms of heteroresistance to isoniazid and rifampin of Mycobacterium tuberculosis in Tashkent, Uzbekistan. Eur Respir J. 2009;33(2):368–374. doi:10.1183/09031936.00089808
72. Hu S, Li G, Li H, et al. Rapid detection of isoniazid resistance in Mycobacterium tuberculosis isolates by use of real-time-PCR-based melting curve analysis. J Clin Microbiol. 2014;52(5):1644–1652. doi:10.1128/jcm.03395-13
73. Zheng Y, Xia H, Bao X, Zhao B, He P, Zhao Y. Highly sensitive detection of isoniazid heteroresistance in mycobacterium tuberculosis by droplet digital PCR. Infect Drug Resist. 2022;15:6245–6254. doi:10.2147/idr.S381097
74. Pholwat S, Stroup S, Foongladda S, Houpt E. Digital PCR to detect and quantify heteroresistance in drug resistant Mycobacterium tuberculosis. PLoS One. 2013;8(2):e57238. doi:10.1371/journal.pone.0057238
75. Zhang S, Chen X, Lin Z, et al. Quantification of isoniazid-heteroresistant mycobacterium tuberculosis using droplet digital PCR. J Clin Microbiol. 2023;61(6):e0188422. doi:10.1128/jcm.01884-22
76. Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. 2019;14:319–338. doi:10.1146/annurev-pathmechdis-012418-012751
77. Cason C, D’Accolti M, Soffritti I, Mazzacane S, Comar M, Caselli E. Next-generation sequencing and PCR technologies in monitoring the hospital microbiome and its drug resistance. Front Microbiol. 2022;13:969863. doi:10.3389/fmicb.2022.969863
78. Zhang G, Zhang H, Hu X, et al. Clinical application value of metagenomic next-generation sequencing in the diagnosis of spinal infections and its impact on clinical outcomes. Front Cell Infect Microbiol. 2023;13:1076525. doi:10.3389/fcimb.2023.1076525
79. Xiang ZB, Leng EL, Cao WF, et al. A systematic review and meta-analysis of the diagnostic accuracy of metagenomic next-generation sequencing for diagnosing tuberculous meningitis. Front Immunol. 2023;14:1223675. doi:10.3389/fimmu.2023.1223675
80. Cabibbe AM, Spitaleri A, Battaglia S, et al. Application of targeted next-generation sequencing assay on a portable sequencing platform for culture-free detection of drug-resistant tuberculosis from clinical samples. J Clin Microbiol. 2020;58(10). doi:10.1128/JCM.00632-20
81. Zhang G, Zhang H, Zhang Y, Hu X, Tang M, Gao Q. Targeted next-generation sequencing technology showed great potential in identifying spinal tuberculosis and predicting the drug resistance. J Infect. 2023;87(6):e110–e112. doi:10.1016/j.jinf.2023.10.018
82. Lin Q, Yao Y, Li X, et al. The application of nanopore targeted sequencing for pathogen diagnosis in bronchoalveolar lavage fluid of patients with pneumonia: a prospective multicenter study. Infect Dis. 2024;56(2):128–137. doi:10.1080/23744235.2023.2276785
83. Sun X, Song J, Leng X, et al. A preliminary evaluation of targeted nanopore sequencing technology for the detection of Mycobacterium tuberculosis in bronchoalveolar lavage fluid specimens. Front Cell Infect Microbiol. 2023;13:1107990. doi:10.3389/fcimb.2023.1107990
84. Dippenaar A, Goossens SN, Grobbelaar M, et al. Nanopore sequencing for mycobacterium tuberculosis: a critical review of the literature, new developments, and future opportunities. J Clin Microbiol. 2022;60(1):e0064621. doi:10.1128/JCM.00646-21
85. Wu W, Cheng P, Lyu J, Zhang Z, Xu J. Tag Array gene chip rapid diagnosis anti-tuberculosis drug resistance in pulmonary tuberculosis -a feasibility study. Tuberculosis. 2018;110:96–103. doi:10.1016/j.tube.2018.03.010
86. Torres JN, Paul LV, Rodwell TC, et al. Novel katG mutations causing isoniazid resistance in clinical M. tuberculosis isolates. Emerg Microbes Infect. 2015;4(7):e42. doi:10.1038/emi.2015.42
87. Feng G, Han W, Shi J, Xia R, Xu J. Analysis of the application of a gene chip method for detecting Mycobacterium tuberculosis drug resistance in clinical specimens: a retrospective study. Sci Rep. 2021;11(1):17951. doi:10.1038/s41598-021-97559-y
88. Parveen S, Shen J, Lun S, et al. Glutamine metabolism inhibition has dual immunomodulatory and antibacterial activities against Mycobacterium tuberculosis. Nat Commun. 2023;14(1):7427. doi:10.1038/s41467-023-43304-0
89. Chaiyachat P, Kaewseekhao B, Chaiprasert A, et al. Metabolomic analysis of Mycobacterium tuberculosis reveals metabolic profiles for identification of drug-resistant tuberculosis. Sci Rep. 2023;13(1):8655. doi:10.1038/s41598-023-35882-2
90. Janssen EM, Dy SM, Meara AS, Kneuertz PJ, Presley CJ, Bridges JFP. Analysis of patient preferences in lung cancer - estimating acceptable tradeoffs between treatment benefit and side effects. Patient Prefer Adherence. 2020;14:927–937. doi:10.2147/ppa.S235430
91. Wang L, Ying R, Liu Y, Sun Q, Sha W. Metabolic profiles of clinical isolates of drug-susceptible and multidrug-resistant mycobacterium tuberculosis: a metabolomics-based study. Infect Drug Resist. 2023;16:2667–2680. doi:10.2147/IDR.S405987
92. Xu G, Liu H, Jia X, Wang X, Xu P. Mechanisms and detection methods of Mycobacterium tuberculosis rifampicin resistance: the phenomenon of drug resistance is complex. Tuberculosis. 2021;128:102083. doi:10.1016/j.tube.2021.102083
93. Weerakoon KG, McManus DP. Cell-free DNA as a diagnostic tool for human parasitic infections. Trends Parasitol. 2016;32(5):378–391. doi:10.1016/j.pt.2016.01.006
94. Traver S, Assou S, Scalici E, et al. Cell-free nucleic acids as non-invasive biomarkers of gynecological cancers, ovarian, endometrial and obstetric disorders and fetal aneuploidy. Hum Reprod Update. 2014;20(6):905–923. doi:10.1093/humupd/dmu031
95. Aucamp J, Bronkhorst AJ, Badenhorst CPS, Pretorius PJ. The diverse origins of circulating cell-free DNA in the human body: a critical re-evaluation of the literature. Biol Rev Camb Philos Soc. 2018;93(3):1649–1683. doi:10.1111/brv.12413
96. Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73(4):1907–1916. doi:10.1128/iai.73.4.1907-1916.2005
97. Pan SW, Su WJ, Chan YJ, Chuang FY, Feng JY, Chen YM. Mycobacterium tuberculosis-derived circulating cell-free DNA in patients with pulmonary tuberculosis and persons with latent tuberculosis infection. PLoS One. 2021;16(6):e0253879. doi:10.1371/journal.pone.0253879
98. Labugger I, Heyckendorf J, Dees S, et al. Detection of transrenal DNA for the diagnosis of pulmonary tuberculosis and treatment monitoring. Infection. 2017;45(3):269–276. doi:10.1007/s15010-016-0955-2
99. Casadevall A, Fang FC. The intracellular pathogen concept. Mol Microbiol. 2020;113(3):541–545. doi:10.1111/mmi.14421
100. Wu X, Liang R, Xiao Y, et al. Application of targeted next generation sequencing technology in the diagnosis of Mycobacterium Tuberculosis and first line drugs resistance directly from cell-free DNA of bronchoalveolar lavage fluid. J Infect. 2023;86(4):399–401. doi:10.1016/j.jinf.2023.01.031
101. Barrangou R, Fremaux C, Deveau H, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315(5819):1709–1712. doi:10.1126/science.1138140
102. Liu P, Wang X, Liang J, et al. A recombinase polymerase amplification-coupled Cas12a mutant-based module for efficient detection of streptomycin-resistant mutations in mycobacterium tuberculosis. Front Microbiol. 2021;12:796916. doi:10.3389/fmicb.2021.796916
103. Bai X, Gao P, Qian K, et al. A highly sensitive and specific detection method for mycobacterium tuberculosis fluoroquinolone resistance mutations utilizing the CRISPR-Cas13a system. Front Microbiol. 2022;13:847373. doi:10.3389/fmicb.2022.847373
104. Li S, Poulton NC, Chang JS, et al. CRISPRi chemical genetics and comparative genomics identify genes mediating drug potency in Mycobacterium tuberculosis. Nat Microbiol. 2022;7(6):766–779. doi:10.1038/s41564-022-01130-y
105. Yan MY, Zheng D, Li SS, et al. Application of combined CRISPR screening for genetic and chemical-genetic interaction profiling in Mycobacterium tuberculosis. Sci Adv. 2022;8(47):eadd5907. doi:10.1126/sciadv.add5907
106. Samukawa N, Yamaguchi T, Ozeki Y, et al. An efficient CRISPR interference-based prediction method for synergistic/additive effects of novel combinations of anti-tuberculosis drugs. Microbiology. 2022;168:12. doi:10.1099/mic.0.001285
107. Tram TTB, Ha VTN, Trieu LPT, et al. FLASH-TB: an application of next-generation CRISPR to detect drug resistant tuberculosis from direct sputum. J Clin Microbiol. 2023;61(4):e0163422. doi:10.1128/jcm.01634-22
108. Shi J, He G, Ning H, et al. Application of matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) in the detection of drug resistance of Mycobacterium tuberculosis in re-treated patients. Tuberculosis. 2022;135:102209. doi:10.1016/j.tube.2022.102209
109. Lavigne JP, Espinal P, Dunyach-Remy C, Messad N, Pantel A, Sotto A. Mass spectrometry: a revolution in clinical microbiology? Clin Chem Lab Med. 2013;51(2):257–270. doi:10.1515/cclm-2012-0291
110. Sauer S, Kliem M. Mass spectrometry tools for the classification and identification of bacteria. Nat Rev Microbiol. 2010;8(1):74–82. doi:10.1038/nrmicro2243
111. Strola SA, Baritaux JC, Schultz E, et al. Single bacteria identification by Raman spectroscopy. J Biomed Opt. 2014;19(11):111610. doi:10.1117/1.JBO.19.11.111610
112. Tang JW, Liu QH, Yin XC, et al. Comparative analysis of machine learning algorithms on surface enhanced raman spectra of clinical staphylococcus species. Front Microbiol. 2021;12:696921. doi:10.3389/fmicb.2021.696921
113. Perumal J, Dinish US, Bendt AK, et al. Identification of mycolic acid forms using surface-enhanced Raman scattering as a fast detection method for tuberculosis. Int J Nanomedicine. 2018;13:6029–6038. doi:10.2147/ijn.S171400
114. Wang L, Zhang XD, Tang JW, et al. Machine learning analysis of SERS fingerprinting for the rapid determination of Mycobacterium tuberculosis infection and drug resistance. Comput Struct Biotechnol J. 2022;20:5364–5377. doi:10.1016/j.csbj.2022.09.031
115. Lyu JW, Zhang XD, Tang JW, et al. Rapid prediction of multidrug-resistant Klebsiella pneumoniae through deep learning analysis of SERS spectra. Microbiol Spectr. 2023;11(2):e0412622. doi:10.1128/spectrum.04126-22
116. Mathew A, Amudha P, Sivakumari S. Deep learning techniques: an overview. In: Hassanien AE, Bhatnagar R, Darwish A, editors. Advanced Machine Learning Technologies and Applications. Singapore: Springer Singapore; 2021:599–608.
117. Pavšič J, Žel J, Milavec M. Digital PCR for direct quantification of viruses without DNA extraction. Anal Bioanal Chem. 2016;408(1):67–75. doi:10.1007/s00216-015-9109-0
118. Trypsteen W, Vynck M, De Neve J, et al. ddpcRquant: threshold determination for single channel droplet digital PCR experiments. Anal Bioanal Chem. 2015;407(19):5827–5834. doi:10.1007/s00216-015-8773-4
119. Bosman KJ, Nijhuis M, van Ham PM, et al. Comparison of digital PCR platforms and semi-nested qPCR as a tool to determine the size of the HIV reservoir. Sci Rep. 2015;5:13811. doi:10.1038/srep13811
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.