Back to Journals » Journal of Inflammation Research » Volume 16
Identification of Key Genes for Pyroptosis-Induced Salivary Gland Inflammation in Sjogren’s Syndrome Based on Microarray Data and Immunohistochemistry Analysis
Authors Zhang K, Luo Z , Zhu X , Yao X , Lu D , Chen L, Hong T , Ren Y, Wang X
Received 11 August 2023
Accepted for publication 28 November 2023
Published 5 December 2023 Volume 2023:16 Pages 5865—5879
DOI https://doi.org/10.2147/JIR.S435008
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Kaiyuan Zhang,1,* Ziyue Luo,1,* Xinchao Zhu,1 Xinyi Yao,1 Dingqi Lu,2 Liying Chen,1 Tao Hong,1 Yating Ren,1 Xinchang Wang3
1Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang Province, 310053, People’s Republic of China; 2First Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang Province, 310053, People’s Republic of China; 3Department of Rheumatology, The Second Affiliated Hospital, Zhejiang Chinese Medical University, Hangzhou, Zhejiang Province, 310053, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinchang Wang, The Second Affiliated Hospital, Zhejiang Chinese Medical University, Hangzhou, Zhejiang Province, People’s Republic of China, Tel +86 13065714635, Email [email protected]
Purpose: Sjogren’s Syndrome (SS) is a systemic autoimmune disease primarily characterized by dysfunction of the exocrine glands. Research into the etiology and pathogenesis of salivary glands (SG) inflammation of SS is very limited. The aim of this study was to identify potential pyroptosis-related genes in SG inflammation through bioinformatics analysis and validation of the SG in SS.
Methods: GSE157159 dataset and GSE159574 dataset were downloaded from Gene Expression Omnibus (GEO). Differentially Expressed Genes (DEGs) analysis was used to screen DEGs from SS and non-SS SG samples. Pyroptosis-related genes were obtained from GeneCards. After intersecting DEGs with pyroptosis-related genes, the pyroptosis-related DEGs in SS were obtained. Subsequently, ClueGO enrichment analysis, Kyoto Encyclopaedia of Genes and Genomes (KEGG) enrichment analysis, Protein-protein Interaction (PPI), and identification and co-expression analysis of hub genes were performed. Subsequently, we collected SG samples from 17 SS patients and 17 non-SS patients and validated the expression of two hub genes (GZMA, GBP1) and characteristic genes (GSDMD) of pyroptosis through immunohistochemistry. The accuracy of hub genes as biomarkers for predicting SS was evaluated by receiver operating characteristic (ROC) curve.
Results: 834 DEGs were selected from the GSE157159 dataset, and a total of 39 pyroptosis-related DEGs were obtained. Functional analysis showed that these DEGs were significantly enriched in some inflammatory signaling pathways. Through the intersection of seven algorithms proposed by CytoHubba and validation using the GSE159574 dataset, 11 hub genes were identified, including IL18, AIM2, CCL5, CD274, GBP1, GBP5, GZMA, GZMB, TLR8, TNFS13B, and ICAM1. Finally, the results of immunohistochemistry showed that GSDMD, GZMA and GBP1 were all significantly highly expressed in SG from SS. And ROC analysis showed a high combined diagnostic value of the 3 genes (AUC=0.8858).
Conclusion: Our study revealed enhanced levels of pyroptosis in the SS. GZMA and GBP1 were identified as candidate genes for pyroptosis-induced inflammation of the SG in SS, which may be used as biomarkers or potential therapeutic targets for SS.
Keywords: Sjogren’s syndrome, pyroptosis, bioinformatics analysis, salivary gland, immunology
Introduction
Sjögren’s Syndrome (SS) is an autoimmune disease characterized by chronic inflammation. This is marked by the development of dryness in the oral and ocular mucosa due to chronic inflammation of the salivary and lacrimal glands.1,2 The clinical manifestations of SS exhibit significant heterogeneity and can affect various organ systems, including the respiratory system, cardiovascular system, and hematopoietic system. This complexity presents substantial challenges in the diagnosis, management, and treatment of SS.3–5 Epidemiological reports showed that the prevalence of primary Sjogren’s Syndrome (pSS) in the Chinese population is 0.3% −0.7%. It is indisputable that SS significantly impacts the overall well-being of society. Consequently, there is a pressing need to delve into the potential mechanisms underlying the pathogenesis of SS, pinpoint biomarkers or therapeutic targets for the condition, and establish a robust theoretical basis for the diagnosis and treatment of SS.
Pyroptosis is a form of programmed cell death mediated by Gasdermins (GSDMs). During pyroptosis, cells release a plethora of inflammatory mediators at the culmination of their life cycle, subsequently triggering a robust inflammatory response.6 Serving as the bridge between innate and adaptive immunity, the excessive amplification of pyroptosis has been demonstrated to play a pivotal role in the onset and progression of autoimmune diseases.7 For example, it has been found that Punicalagin can ameliorate Rheumatoid Arthritis (RA) by inhibiting pyroptosis and thereby reducing the pathological inflammatory response.8 In addition, it has also been demonstrated that Gasdermin D (GSDMD)-mediated monocyte/macrophage pyroptosis has an essential role in the pathogenesis of Systemic Lupus Erythematosus (SLE).9 In a mouse model of salivary gland (SG) inflammation, the injection of the P2X7 receptor subtype (P2X7R) antagonist A438079 enhances saliva flow and reduces lymphocyte infiltration in the submandibular gland. This suggests the involvement of the P2X7R/NOD-like Receiver Pyrin Domain Containing Protein 3 (NLRP3) inflammasome/caspase-1/ interleukin (IL)-1β and IL-18 axis in the pathogenesis of Sjögren’s Syndrome.10 In general, the pyroptosis process in salivary gland epithelial cells (SGECs) results in a reduction in their quantity, leading to a significant decline in salivary secretion. Furthermore, the release of proinflammatory cytokines, such as IL-1β and IL-18, by pyroptosis SGECs may induce the infiltration and activation of immune cells in the SG, potentially causing dysfunction in neighboring normal SGECs. Nonetheless, research on the association between SS and pyroptosis remains relatively limited. Therefore, this study aims to delve deeper into the potential mechanisms underlying SS and pyroptosis and to investigate pyroptosis-related genes in the context of SS.
With the rapid advancement of second-generation sequencing technology, an increasing number of studies have shifted their attention towards investigating potential hub genes and targets of interest in the disease. Numerous studies have since delved into the identification of hub genes in the progression of SS and validated their potential as diagnostic biomarkers for SS.11,12 Therefore, the present study intends to explore the candidate genes of pyroptosis-induced SG inflammation in SS through bioinformatics analysis and further validation by obtaining clinical minor salivary gland biopsy, aiming to explore the mechanism of action associated with pyroptosis in SS and provide scientific basis for diagnosis and treatment of SS. Figure 1 illustrates the flowchart of this study.
Methods
Microarray Data and the Exploration of Pyroptosis-Related Genes
From GEO (https://www.ncbi.nlm.nih.gov/) two microarray datasets [GSE15715913 and GSE15957414] were downloaded. The GSE157159 dataset contains SG samples from 8 pSS samples and 9 non-SS samples. The GSE159574 dataset includes 16 pSS samples and 13 non-SS SG samples. Pyroptosis-related genes were downloaded from the GeneCards database (https://www.genecards.org/).
Acquisition of Pyroptosis-Related Differentially Expressed Genes (DEGs)
The easy Visualization and Inference Toolbox for Transcriptome Analysis (eVITTA6)15 was used for DEG analysis. P-values < 0.05 and log | FC | ≥ 1 were considered as screening criteria for DEGs from the GSE157159 and GSE159574 datasets. By using the online Venn tool (https://bioinfogp.cnb.csic.es/tools/venny/index.html), the pyroptosis-related genes obtained from the GeneCards database were overlapped with the DEGs of GSE157159 to obtain pyroptosis-related DEGs in SS and validated in GSE157594. Finally, an online tool (https://www.bioinformatics.com.cn) was used to visualize volcanic maps and hierarchical clustering heatmaps of SS-related pyroptosis genes.
Protein–Protein Interaction (PPI) and Enrichment Analysis
PPI analysis was performed on pyroptosis-related DEGs using STRING (http://www.string-db.org)16, and the results were imported into Cytoscape (http://www.cytoscape.org)17 for visualization. Gene Ontology (GO) enrichment analysis was performed by the ClueGO. The Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was performed by the DAVID database (https://david.ncifcrf.gov/). The results were visualized using the free online platform (https://www.bioinformatics.com.cn). P < 0.05 were considered significant terms.
Analysis of Hub Genes
The hub genes were found by the cytoHubba plug-in of Cytoscape. 7 algorithms including MCC, MNC, Degree, Stress, Closeness, DMNC, and EPC were used to obtain the Top 20 hub genes. The genes obtained from these 7 algorithms were intersected to obtain the final hub genes. We constructed a co-expression network of these hub genes through GeneMANIA (http://www.Genemania.org/)18. Finally, we conducted GO and KEGG analysis on 13 hub genes.
Validation of Hub Genes Expression
The mRNA expression of the identified hub genes was validated in the GSE159574 dataset. Comparisons between groups were performed by t-test. P-value < 0.05 was considered significant.
Validation of Clinical Minor Salivary Gland Biopsy (MSGB) Samples
We primarily followed the methods of MSGB to obtain samples from the patients’ SG.19 34 MSGB samples, including 17 pSS patients and 17 non-SS patients (Table 1), were obtained from patients. SS patients were diagnosed by the 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for pSS.20 The use of patient information and MSGB was sanctioned by the Ethics Committee of The Second Affiliated Hospital, Zhejiang Chinese Medical University (Ethics Approval No.2020-KL-011-01).
 |
Table 1 Patients Basis Information |
Immunohistochemical Staining and Validation of Hub Genes
After obtaining SG through MSGB, it underwent staining and diagnosis by specialized pathologists. Following this, the SG was preserved using paraffin embedding. We sectioned the preserved SG paraffin-embedded tissues and affixed the sections onto glass slides. Subsequently, we proceeded with the subsequent immunohistochemical procedures. The main antibodies used for IHC were as follows: A GBP1 IgG Mouse monoclonal antibody (ab119236), An Anti-Granzyme Rabbit IgG monoclonal antibody (ab209205) both from Abcam (Cambridge, 1:100; UK), and GSDMD Rabbit IgG Polyclonal antibody (AF4012) from Affinity (Cincinnati, 1:100; USA). Salivary gland samples were fixed in 10% formalin, embedded in paraffin blocks, and processed as continuous sections (4 µm thick). They underwent an alcohol gradient rehydration process before being H&E stained. They were treated with citrate buffer solution (pH 6.0) for antigen retrieval, and then endogenous peroxidase activity was blocked for 15 minutes with 3% H2O2. The sections were subjected to goat anti-rabbit antibodies at 37 °C for 60 minutes after being incubated with GBP1, GZMA, and GSDMD antibodies overnight at 4 °C. To assess antigen expression, each section was photographed at ×200x magnification, and five images were taken over the entire section. Aperio ImageScope (Leica Biosystems, Germany) was used to assess nuclear and membrane expressions based on the immunohistochemistry biomarkers’ staining properties. Positive pixel count v9 algorithm of Aperio ImageScope software was used for analysis (positive rate of immunohistochemical results = number of positives + number of strong positives)/total area). Receiver operating characteristic (ROC) curves between the two groups were created in order to further establish the prediction values of the positive expression rate of three genes for SS. The plot area under the curve (AUC) was calculated through the numerical integration of the ROC curves. The genes with the highest AUC values were the most diagnostic for SS. Three hub genes were used to create model an integrated diagnostic utilizing logistics regression analysis.
Immune Cell Infiltration Analysis
CIBERSORTx (https://cibersortx.stanford.edu/) was used to analyze the differences in immune infiltration between SS and non-SS groups to evaluate the role of the immune microenvironment in SS and non-SS formation. The results were then visualized using box-line plots.
Statistical Analysis
Numerical results were presented as the means ± standard deviations (SD). Differences between the two groups were analyzed using an unpaired t-test with Welch’s correction. All analyses were performed using GraphPad Prism (v 8.0.2) and statistical software package SPSS (v 22.0). P<0.05 was considered statistically significant.
Results
Identification of DEGs Associated with Pyroptosis in SS
Based on the conditions, we obtained 834 DEGs from the GSE157159 dataset, including 735 upregulated DEGs and 99 downregulated DEGs (Figure 2A). In addition, we obtained 436 pyroptosis-related genes, and after intersecting DEGs with pyroptosis-related genes, a total of 39 pyroptosis-related DEGs were obtained (Figure 2B). Then, we performed a heat map display of the expression levels of 39 pyroptosis-related DEGs in the GSE157159 dataset (Figure 3A) and the GSE159574 (Figure 3B) validation dataset.
 |
Figure 2 Identification of pyroptosis-related DEGs. (A) The volcano map of GSE157159. (B) The Venn diagram shows the intersection between pyroptosis-related genes and DEGs. |
PPI Analysis and Enrichment Analysis
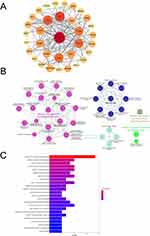
The PPI results showed that genes such as IL1β, IRF1, CCL5, CD274, IL18, ICAM1, and TLR8 exhibited strong levels of interaction in pyroptosis-related DEGs (Figure 4A). The results of ClueGO showed that these 39 DEGs related to pyroptosis were enriched in positive regulation of leukemia promotion, pyroptosis, MyD88 dependent toll-like receptor signaling pathway, response to interference beta, and interference gamma mediated signaling pathway (Figure 4B). The KEGG enrichment results showed that these pyroptosis-related DEGs were enriched in inflammatory signaling pathways such as NOD-like receptor signaling pathway, NF kappa B signaling pathway, TNF signaling pathway, and Toll-like receptor signaling pathway (Figure 4C).
Selection and Analysis of Hub Genes
To further identify hub genes in SS-related pyroptosis genes, we conducted seven algorithms: Stress, EPC, DMCN, Closeness, Degree, MNC, and MCC to obtain the top 20 hub genes (Table 2). Then, we intersected the results of 7 algorithms and finally obtained 13 hub genes related to pyroptosis, including CCL5, IL18, CD274, ICAM1, GZMB, PRF1, TLR8, VCAM1, GZMA, TNFSF13B, AIM2, GBP5, and GBP1 (Figure 5A). Then, we constructed a gene interaction network centered around 13 hub genes. The results show that CCL5, VCAM1, CD274, and IL1B have strong levels of interaction, and their functions involve cellular response to molecular of bacterial origin, response to inter gamma, response to lipopolysaccharides, cellular response to biological stimuli, etc. (Figure 5B).
 |
Table 2 The Top 20 Hub Genes Rank in cytoHubba |
Functional Identification of Hub Genes Related to Pyroptosis
The GO enrichment results showed that 13 hub genes were significantly enriched in positive regulation of T cell proliferation, positive regulation of innate immune response, positive regulation of interleukin-1 beta production, inflammatory positive, and other entries (Figure 6A). The KEGG enrichment results showed that these hub genes were significantly enriched in inflammation-related signaling pathways such as the NOD-like receptor signaling pathway, NF kappa B signaling pathway, and TNF signaling pathway (Figure 6B).
 |
Figure 6 Functional enrichment analysis of pyroptosis-related hub genes. (A) GO enrichment results of hub genes. (B) KEGG enrichment results of hub genes. |
Validation of Hub Genes Expression
To further verify the differential expression of pyroptosis-related hub genes between groups, we conducted inter-group expression differential analysis on the GSE159574 validation set (Figure 7). The results showed that AIM2, CCL5, CD274, GBP1, GBP5, GZMA, GZMB, ICAM1, IL-18, TLR8, and TNFSF13B were significantly overexpressed in the pSS group (P<0.05), while PRF1 and VCAM1 showed no significant difference between the groups (P>0.05).
 |
Figure 7 The expression of hub genes in GSE157594. *P< 0.05; **P< 0.01; ***P< 0.001; P> 0.05, No significance (NS). |
Immunohistochemical Validation of GSDMD, GZMA, and GBP1 as Pyroptosis-Related Hub Genes
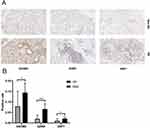
We selected MSGB samples from 17 SS patients and 17 non-SS patients to validate the expression levels of GSDMD, GZMA, and GBP1 using H&E and immunohistochemistry. Compared with non-SS patients, the expression of GSDMD, GZMA, and GBP1 proteins in the SG of SS patients were significantly increased compared to the non-SS group (Figure 8A and B).
Combination ROC Analysis
The sensitivity and specificity of these three genes as potential diagnostic biomarkers in SS patients were investigated by ROC analysis. The AUC of GSDMD was 0.7647 (P = 0.0084) (Figure 9A). The AUC of GZMA was 0.8131 (P = 0.0018) (Figure 9B). The AUC of GBP1 was 0.7474 (P = 0.0138) (Figure 9C). To better predict the prognosis, the three hub genes were integrated to constitute a multi-marker diagnosis model through logistics regression analysis, which could efficiently predict the diagnosis of SS (AUC = 0.8858, P=0.0001) (Figure 9D).
 |
Figure 9 ROC curves analysis of Positive rate of immunohistochemical results in SS patients. (A) GSDMD, (B) GZMA, (C) GBP1, (D) Multi-Markers predicted probability including GSDMD, GZMA, and GBP1. |
Immune Cell Infiltration Analysis
SS Compared with non-SS, the proportions of B cells naive, B cells memory, T cells follicular helper, T cells gamma delta, and Macrophages M1 were relatively high, while Plasma cells and mast cells resting were low (Figure 10).
 |
Figure 10 Results of immune infiltration. Box-plot presented the difference of immune cells. |
Discussion
SS, characterized as a chronic, insidious onset diffuse connective tissue disease, exhibits progressive infiltration of lymphocytes into exocrine glands in its histopathology. These infiltrating lymphocytes produce inflammatory cytokines, leading to degeneration and functional impairment of exocrine glands.21 While the precise etiology and pathogenic mechanisms of SS remain unclear, most scholars posit that the inflammatory infiltration of lymphocytes into exocrine glands serves as a pivotal catalyst for the onset and progression of SS. This can trigger inflammation and autoimmune reactions, resulting in the destruction and functional impairment of exocrine gland epithelial cells.22,23 Central to this process is the epithelial cells of SGECs, which play a crucial role in salivary gland function. SGECs are primarily responsible for regulating the secretion of fluids and proteins, and their dysfunction can lead to insufficient saliva production, thereby triggering a range of symptoms associated with dry mouth.24 As widely recognized, cell death is the ultimate outcome of cellular damage. When SGECs undergo cell death, the homeostatic structure between cells is disrupted, impacting cellular function. Current research indicates that various modes of cell death, including pyroptosis, triggered in SGECs under the backdrop of SS, initiate an inflammatory response, disrupting their homeostasis. This, in turn, promotes an inflammatory immune reaction in the SG, compromising their normal secretory function.25–27 Thus, cell death, particularly pyroptosis in SGECs, may be a key factor in the occurrence and progression of immune-mediated inflammation in the SG in SS.
Currently, numerous studies have identified the significant role of pyroptosis in the occurrence and development of various autoimmune diseases, including SS. A substantial body of literature confirms the presence of cell pyroptosis in the exocrine glands of many SS patients.7,28 Literature reports indicated an increased expression of components related to the NLRP3 inflammasome in peripheral blood mononuclear cells (PBMC) or macrophages infiltrating SG in SS patients. This mediated pyroptosis is involved in the secretion dysfunction of exocrine glands in SS.29 Moreover, research has identified a correlation between the expression levels of interferon (IFN)-I characteristic genes and the mRNA levels of caspase-1 and GSDMD in the SG of SS patients. IFN-I has been shown to upregulate the expression of caspase-1 and GSDMD in the SGECs of SS patients, potentially accelerating cell pyroptosis related to NLRP3 or absent in melanoma 2 (AIM2) inflammasomes.30 Furthermore, an animal experimental study has confirmed that pyroptosis of SGECs leaded to a significant reduction in saliva secretion. The inflammatory cytokines released during this process may even cause functional impairment in neighboring normal salivary gland tissues.10,31 Although there is evidence suggesting that pyroptosis can impact SS, research on this topic is still limited. The classical pathways of pyroptosis in autoimmune diseases need further validation in SS, such as GBP1/caspase 4, Granzyme A/GSDMB-dependent pathways, and the GSDMs protein family. More research is needed to elucidate the relationship between cell pyroptosis and the development of SS.
Therefore, this study screened datasets from primary Sjögren’s syndrome (pSS) and non-SS samples, and through bioinformatics analysis, identified 11 key genes associated with pyroptosis in SS salivary glands. Among them, the expression levels of AIM2,30 CCL5,32 CD274,33 ICAM1,34 IL18,30 TLR8,35 TNFSF13B36 have been confirmed to be significantly upregulated in the SG of SS patients. Additionally, numerous studies have validated the close association of these genes with pyroptosis in SS. For instance, research has revealed a significant correlation between the activation of AIM2 inflammasomes in SS ductal epithelial cells and the levels of pyroptosis in salivary glands.37 Additionally, other studies have demonstrated that inflammasomes can promote the maturation of inflammatory cytokines such as IL-18, accelerating the process of cell pyroptosis in the SG of individuals with SS.38 In the case of the genes Guanylate Binding Protein 1 (GBP1), GBP5, Granzyme A (GZMA), and GZMB, GBP1 and GBP5 belong to the GBPs family, while GZMA and GZMB belong to the GZMs family. Current research suggested that GBP1 and GZMA played a more crucial role than GBP5 and GZMB in the occurrence and development of pyroptosis.39,40 The association of GBP1 with pyroptosis can be traced back to as early as 2019, where research found its significant involvement in IFN-γ signaling and cytokine signaling pathways in the immune system. Moreover, GBP1 was identified as a mediator of the release of the pro-inflammatory cytokine IL-18, consequently inducing inflammatory pyroptosis.41,42 GZMA, as a common component necessary for cytotoxic T lymphocytes and natural killer cells to lyse target cells, has been confirmed to influence caspase-independent pyroptosis.43 Additionally, literature indicated a strong correlation between the expression of GZMA and the inflammatory processes in SG of SS patients. However, the specific mechanism of its action remains unclear. Therefore, this study proceeded with clinical validation of GBP1 and GZMA, given their relevance to pyroptosis in SS.44 Furthermore, we also clinically assessed the expression levels of GSDMD to validate the level of pyroptosis in the SG inflammation of SS patients.
Immunohistochemistry results revealed a significant upregulation of GBP1, GZMA, and GSDMD expression in the SG tissues of SS. As indicated by enrichment analysis, genes associated with pyroptosis are prominently enriched in signaling pathways such as NOD-like receptor signaling, NF-kappa B signaling, and TNF signaling. NOD-like NOD-like Receptors (NLRs) have been confirmed to modulate various inflammatory signaling pathways.45 NF-κB is involved in the regulation of numerous inflammatory factors, not only affecting pyroptosis levels but also being closely associated with the inflammatory development of autoimmune diseases.46 Numerous studies have affirmed the significant correlation of GBP1 and GZMA with the aforementioned inflammatory signaling pathways. Hence, it can be inferred that the identified pyroptosis-related genes may participate in the progression of SG inflammation in SS by mediating relevant inflammatory signaling pathways. This research not only confirms the heightened pyroptosis levels accompanying lip gland inflammation in SS but also identifies GZMA and GBP1 as potential candidate genes induced by pyroptosis in SS. In conclusion, the involvement of cell pyroptosis in the development of SS salivary glands is substantial, and targeting regulatory factors associated with pyroptosis may offer novel avenues for future SS treatments.
Conclusion
This study, through bioinformatics analysis, has preliminarily identified hub pyroptosis-related genes in the SG inflammation of SS, such as GZMA and GBP1. Validation of these genes was conducted using the GSE159574 database and immunohistochemistry on MSGBs from SS patients. The findings of this study offered a novel molecular biology insight into the pathogenic mechanisms of SG inflammation induced by pyroptosis in Sjögren’s syndrome.
Data Sharing Statement
The datasets of the GSE157159, and GSE159574 analysed during the current study were retrieved from Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/). All methods were carried out in accordance with relevant guidelines and regulations. The Immunohistochemical images and data used or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was performed in accordance with the Declaration of Helsinki. Approval was obtained from the Institutional Ethics Committee of The Second Affiliated Hospital, Zhejiang Chinese Medical University (No.2020-KL-011-01).
Consent to Participate
Written informed consent for participation was obtained from the patients.
Acknowledgments
We are indebted to all the subjects who took part in this study, and the staff of the Department of Rheumatology, The Second Affiliated Hospital, Zhejiang Chinese Medical University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by National Natural Science Foundation of China (NO.82074341).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Mavragani CP, Moutsopoulos HM. Sjögren’s syndrome: old and new therapeutic targets. J Autoimmun. 2020;110:102364.
2. Seror R, Nocturne G, Mariette X. Current and future therapies for primary Sjögren syndrome. Nature Rev Rheumatol. 2021;17(8):475–486.
3. Luppi F, Sebastiani M, Sverzellati N, Cavazza A, Salvarani C, Manfredi A. Lung complications of Sjogren syndrome. Eur Respir Rev. 2020;29(157):200021.
4. Davies K, Ng WF. Autonomic nervous system dysfunction in primary Sjögren’s syndrome. Front Immunol. 2021;12:702505.
5. Atzeni F, Gozza F, Cafaro G, Perricone C, Bartoloni E. Cardiovascular Involvement in Sjögren’s syndrome. Front Immunol. 2022;13:879516.
6. Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6(1):128.
7. You R, He X, Zeng Z, Zhan Y, Xiao Y, Xiao R. Pyroptosis and its role in autoimmune disease: a potential therapeutic target. Front Immunol. 2022;13:841732.
8. Ge G, Bai J, Wang Q, et al. Punicalagin ameliorates collagen-induced arthritis by downregulating M1 macrophage and pyroptosis via NF-κB signaling pathway. Sci Chin Life Sci. 2022;65(3):588–603.
9. Zhuang L, Luo X, Wu S, et al. Disulfiram alleviates pristane-induced lupus via inhibiting GSDMD-mediated pyroptosis. Cell Death Discovery. 2022;8(1):379.
10. Baldini C, Rossi C, Ferro F, et al. The P2X7 receptor-inflammasome complex has a role in modulating the inflammatory response in primary Sjögren’s syndrome. J Intern Med. 2013;274(5):480–489.
11. Li N, Li L, Wu M, et al. Integrated bioinformatics and validation reveal potential biomarkers associated with progression of primary Sjögren’s syndrome. Front Immunol. 2021;12:697157.
12. Chen L, Lu D, Yu K, et al. Bioinformatics analysis for identification of key genes in salivary gland and the potential of a combination of biomarkers for the diagnosis of SS. J Inflamm Res. 2021;14:4143–4153.
13. Oyelakin A, Horeth E, Song EC, et al. Transcriptomic and network analysis of minor salivary glands of patients with primary Sjögren’s syndrome. Front Immunol. 2020;11:606268.
14. Luo J, Liao X, Zhang L, et al. Transcriptome sequencing reveals potential roles of ICOS in Primary Sjögren’s syndrome. Front Cell Dev Biol. 2020;8:592490.
15. Cheng X, Yan J, Liu Y, Wang J, Taubert S. eVITTA: a web-based visualization and inference toolbox for transcriptome analysis. Nucleic Acids Res. 2021;49(W1):W207–w215.
16. Franceschini A, Szklarczyk D, Frankild S, et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41(Database issue):D808–815.
17. Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27(3):431–432.
18. Warde-Farley D, Donaldson SL, Comes O, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38(Web Server issue):W214–220.
19. Kim J, Sun D, Ozl R, et al. A validated method of labial minor salivary gland biopsy for the diagnosis of Sjogren’s syndrome. Laryngoscope. 2016;126(9):2041–2046.
20. Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: a Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017;69(1):35–45.
21. Manfrè V, Cafaro G, Riccucci I, et al. One year in review 2020: comorbidities, diagnosis and treatment of primary Sjögren’s syndrome. Clin Exp Rheumatol. 2020;38(4):10–22.
22. Dela Cruz A, Kartha V, Tilston-Lunel A, et al. Gene expression alterations in salivary gland epithelia of Sjögren’s syndrome patients are associated with clinical and histopathological manifestations. Sci Rep. 2021;11(1):11154.
23. Tseng YC, Yang HY, Lin WT, et al. Salivary dysbiosis in Sjögren’s syndrome and a commensal-mediated immunomodulatory effect of salivary gland epithelial cells. NPJ Biofilms Microbiomes. 2021;7(1):21.
24. Katsiougiannis S, Stergiopoulos A, Moustaka K, et al. Salivary gland epithelial cell in Sjögren’s syndrome: metabolic shift and altered mitochondrial morphology toward an innate immune cell function. J Autoimmun. 2023;136:103014.
25. Tang Y, Zhou Y, Wang X, et al. The role of epithelial cells in the immunopathogenesis of Sjögren’s syndrome. J Leukoc Biol. 2023. doi:10.1093/jleuko/qiad049
26. Colafrancesco S, Barbati C, Priori R, et al. Maladaptive autophagy in the pathogenesis of autoimmune epithelitis in Sjögren’s syndrome. Arthritis Rheumatol. 2022;74(4):654–664.
27. Cao T, Zhou J, Liu Q, et al. Interferon-γ induces salivary gland epithelial cell ferroptosis in Sjogren’s syndrome via JAK/STAT1-mediated inhibition of system Xc(). Free Radic Biol Med. 2023;205:116–128.
28. Pontarini E, Sciacca E, Grigoriadou S, et al. NKp30 receptor upregulation in salivary glands of Sjögren’s syndrome characterizes Ectopic lymphoid structures and is restricted by rituximab treatment. Front Immunol. 2021;12:706737.
29. Vakrakou AG, Boiu S, Ziakas PD, Xingi E, Boleti H, Manoussakis MN. Systemic activation of NLRP3 inflammasome in patients with severe primary Sjögren’s syndrome fueled by inflammagenic DNA accumulations. J Autoimmun. 2018;91:23–33.
30. Hong SM, Lee J, Jang SG, et al. Type I interferon increases inflammasomes associated pyroptosis in the salivary glands of patients with primary Sjögren’s syndrome. Immun net. 2020;20(5):e39.
31. Khalafalla MG, Woods LT, Camden JM, et al. P2X7 receptor antagonism prevents IL-1β release from salivary epithelial cells and reduces inflammation in a mouse model of autoimmune exocrinopathy. J Biol Chem. 2017;292(40):16626–16637.
32. Cuello C, Palladinetti P, Tedla N, et al. Chemokine expression and leucocyte infiltration in Sjögren’s syndrome. Br J Rheumatol. 1998;37(7):779–783.
33. Kobayashi M, Kawano S, Hatachi S, et al. Enhanced expression of programmed death-1 (PD-1)/PD-L1 in salivary glands of patients with Sjögren’s syndrome. J Rheumatol. 2005;32(11):2156–2163.
34. Aziz KE, McCluskey PJ, Wakefield D. Pattern of adhesion molecule expression in labial salivary glands from patients with primary Sjögren’s syndrome. Ocul Immunol Inflamm. 1995;3(4):221–236.
35. Alexopoulou L. Nucleic acid-sensing toll-like receptors: important players in Sjögren’s syndrome. Front Immunol. 2022;13:980400.
36. Roescher N, Vosters JL, Alsaleh G, et al. Targeting the splicing of mRNA in autoimmune diseases: BAFF inhibition in Sjögren’s syndrome as a proof of concept. Mol Ther. 2014;22(4):821–827.
37. Vakrakou AG, Svolaki IP, Evangelou K, Gorgoulis VG, Manoussakis MN. Cell-autonomous epithelial activation of AIM2 (absent in melanoma-2) inflammasome by cytoplasmic DNA accumulations in primary Sjögren’s syndrome. J Autoimmun. 2020;108:102381.
38. Kong R, Sun L, Li H, Wang D. The role of NLRP3 inflammasome in the pathogenesis of rheumatic disease. Autoimmunity. 2022;55(1):1–7.
39. Zhong X, Zeng H, Zhou Z, et al. Structural mechanisms for regulation of GSDMB pore-forming activity. Nature. 2023;616(7957):598–605.
40. Johns CE, Galam L. Guanylate Binding Protein 1 (GBP1): a Key Protein in Inflammatory Pyroptosis. Cell Biochem Biophys. 2022;80(2):295–299.
41. Yao Q, Song Z, Wang B, Qin Q, Zhang JA. Identifying Key Genes and functionally enriched pathways in Sjögren’s syndrome by weighted gene co-expression network analysis. Front Genetics. 2019;10:1142.
42. Fisch D, Bando H, Clough B, et al. Human GBP1 is a microbe-specific gatekeeper of macrophage apoptosis and pyroptosis. EMBO j. 2019;38(13):e100926.
43. Alpert S, Kang HI, Weissman I, Fox RI. Expression of granzyme A in salivary gland biopsies from patients with primary Sjögren’s syndrome. Arthritis Rheum. 1994;37(7):1046–1054.
44. Jia Y, Cui R, Wang C, et al. Metformin protects against intestinal ischemia-reperfusion injury and cell pyroptosis via TXNIP-NLRP3-GSDMD pathway. Redox Bio. 2020;32:101534.
45. Zhang WJ, Chen SJ, Zhou SC, Wu SZ, Wang H. Inflammasomes and Fibrosis. Front Immunol. 2021;12:643149.
46. Ren C, Chen J, Che Q, et al. IL-37 alleviates TNF-α-induced pyroptosis of rheumatoid arthritis fibroblast-like synoviocytes by inhibiting the NF-κB/GSDMD signaling pathway. Immunobiology. 2023;228(3):152382.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.