Back to Journals » Infection and Drug Resistance » Volume 13
Identification and Treatment of Tuberculosis in Pediatric Recipients of Allogeneic Hematopoietic Stem Cell Transplantation: Case Series and Review of the Literature
Authors Wang X, Yu U , Li X, Wang C, Zhang Q, Yang C, Zhang X, Zhang Y, Wang Y, Zheng Y , Deng J, Yang W, Liu G, Deng G , Liu S, Wen F
Received 31 March 2020
Accepted for publication 17 July 2020
Published 31 July 2020 Volume 2020:13 Pages 2641—2648
DOI https://doi.org/10.2147/IDR.S256298
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Xiaodong Wang1,2 ,* Uet Yu2 ,* Xiaonan Li,3 Chunjing Wang,2 Qian Zhang,2 Chunlan Yang,2 Xiaoling Zhang,2 Yu Zhang,2 Ying Wang,2 Yuejie Zheng,3 Jikui Deng,4 Weiguo Yang,5 Guosheng Liu,1 Guofang Deng,6 Sixi Liu,2 Feiqiu Wen1,2
1Department of Pediatrics, First Affiliated Hospital of Jinan University, Guangzhou, People’s Republic of China; 2Department of Hematology and Oncology, Shenzhen Children’s Hospital, Shenzhen, Guangdong, People’s Republic of China; 3Department of Respiratory Diseases, Shenzhen Children’s Hospital, Shenzhen, Guangdong, People’s Republic of China; 4Department of Infectious Diseases, Shenzhen Children’s Hospital, Shenzhen, Guangdong, People’s Republic of China; 5Pediatric Intensive Care Unit, Shenzhen Children’s Hospital, Shenzhen, Guangdong, People’s Republic of China; 6Guangdong Key Laboratory for Emerging Infectious Diseases & Shenzhen Key Laboratory of Infection and Immunity, Shenzhen Third People’s Hospital, Shenzhen, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Sixi Liu; Feiqiu Wen Email [email protected]; [email protected]
Background: Tuberculosis is a rare but life-threatening complication in patients who received hematopoietic stem cell transplantation. Early identification and intervention are essential to prevent severe complications.
Case Presentation: We report two pediatric patients who developed tuberculosis after receiving hematopoietic stem cell transplantation for thalassemia major among 330 recipients between January 2012 and August 2019. Patient A presented with pulmonary tuberculosis and patient B presented with lymph node tuberculosis mimicking post-transplantation lymphoproliferative disorder associated with Epstein–Barr virus reactivation. Patient B’s condition was deteriorated, and shortly after the initiation of anti-tuberculosis therapy, the patient was found to have disseminated pulmonary tuberculosis. Patient B was also found to have tuberculous granulomas, an uncommon manifestation of tuberculosis causing severe airway obstruction. Both patients developed critical respiratory failure and required mechanical ventilation; however, they recovered with almost full resolution of pulmonary lesions after multiple treatment adjustments.
Conclusion: Tuberculosis must be carefully evaluated in all pediatric patients that receive hematopoietic stem cell transplantation, regardless of the identification of other pathogens. Prophylactic tuberculosis therapy should be considered for high-risk pediatric hematopoietic stem cell transplantation recipients from tuberculosis-endemic regions.
Keywords: tuberculosis, hematopoietic stem cell transplantation, thalassemia, pediatric
Background
Recipients of hematopoietic stem cell transplantation (HSCT) are at a high risk of being infected with opportunistic pathogens, among which Mycobacterium tuberculosis (Mtb) is one of the most life-threatening pathogens.1 The incidence of tuberculosis (TB) ranges from 0.4% to approximately 6% in both HSCT as well as solid organ transplant recipients.2–4 Typically, HSCT recipients in endemic regions are reported to have a significantly higher TB incidence than those in the non-endemic regions. Risk factors associated with TB in HSCT recipients, such as pre-transplant human leukocyte antigen (HLA) status, the presence of acute or chronic graft versus host disease (GVHD), the type of conditioning regimens, and the use of total body irradiation have been evaluated in previous studies.5–7 However, most of these studies were based on small adult patient cohorts. Reports focusing on TB in pediatric HSCT recipients are limited.
The diagnosis of TB in HSCT recipients could be challenging because of the uncharacteristic symptom presentation, especially when Mtb co-exists with other pathogens. In this case report, we present the cases of two pediatric patients with thalassemia major (TM) who had undergone HSCT and developed TB with differing manifestations.
Case Presentation
Patient A
An 8-year-old girl with TM received granulocyte colony stimulating factor mobilized peripheral blood stem cells (PBSCs) from an unrelated human leukocyte antigen (HLA) 9/10 matched donor. The myeloablative conditioning regimen consisted of cyclophosphamide (CTX), busulfan (BU), fludarabine (FLU), and rabbit anti-thymocyte globulin (ATG). Graft-versus-host disease (GVHD) prophylaxis included cyclosporine (CSA), low dose methotrexate (MTX), and mycophenolate (MMF). Neutrophil engraftment was achieved by day 14 post-transplantation. The donor cell chimerism was >99%. Patient A was discharged on day 25 post-transplantation without severe complications.
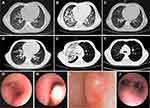
The patient was readmitted for persistent high fever and cough on day 73 post-transplantation. Chest computed tomography (CT) scans suggested mild pneumonia without severe pulmonary lesions (Figure 1A). Despite the administration of broad-spectrum antibiotics and antifungal treatments, the patient developed severe respiratory distress requiring ventilatory support starting on day 82 post-transplantation. A bronchoscopy was performed at this time. Bronchoalveolar lavage (BAL) fluid tested positive for Mtb DNA (2.79 × 103 copies/mL), cytomegalovirus (CMV) DNA (2.65 × 105 copies/mL), and Epstein-Barr virus (EBV) DNA (8.68 × 104 copies/mL) by real-time PCR analysis. Acid-fast bacilli (AFB) testing of the BAL fluid was negative. Thus, diagnoses of pulmonary TB, CMV, and EBV were considered. Ten days after the patient was hospitalized, TB treatment consisting of isoniazid, rifampin, and pyrazinamide, and antiviral therapy consisting of ganciclovir and intravenous immunoglobulin (IVIG) were initiated. The patient was removed from ventilator support 90 days post-transplantation.
Four days after the removal of the ventilator, the patient developed hematemesis and hemoptysis with continued high fever and persistent cough. A chest CT scan revealed the progression of pneumonia with massive pulmonary infiltrates (Figure 1B). The patient was again placed on a ventilator, and pneumorrhagia was observed during intubation. Streptomycin, linezolid, and levofloxacin were immediately added to the patient’s treatment regime and antiviral treatment was changed to ganciclovir and foscarnet sodium. Empiric antibiotic and antifungal treatments were ongoing. The patient developed signs of hemophagocytic lymphohistiocytosis syndrome, including significant enlargement of the liver and spleen, pancytopenia, sudden elevated serum ferritin (18298 mmol/L), and delayed coagulation time. Methylprednisolone was added to the patient’s treatment regime. The patient was removed from the ventilator support after showing progress. On day 110 post-transplantation, the patient was discharged and continued on a modified TB treatment regime of oral isoniazid, rifampin, and pyrazinamide. Chest CT scans performed on day 326 post-transplantation revealed almost full resolution of pulmonary infiltrates (Figure 1C). The patient was continued on oral isoniazid, rifampin, and pyrazinamide treatment for a year before the discontinuation of anti-TB treatment.
Patient B
A 9-year-old boy received a haploidentical bone marrow (BM) and GCSF mobilized PBSC transplant from his biological father for TM treatment, and an HLA-mismatched umbilical cord blood (UCB) transplant from an unrelated donor 6 days later. The patient’s preparation regime consisted of CTX, BU, FLU, ATG, and thiotepa (TT). GVHD prophylaxis included post-transplant CTX, MMF, and tacrolimus (FK506). Neutrophil engraftment, delayed owing to UCB engraftment, was observed by day 40 post-transplantation with an absolute neutrophil count greater than 0.5 × 109 cells/L. On day 79 post-transplantation, the patient was treated for CMV reactivation with IVIG. Critical complications, including severe GVHDs, refractory pancytopenia, and life-threatening infections were not observed.
Patient B returned to the hospital with intermittent fever and enlargement of the lymph nodes of the neck and groin on day 89 post-transplantation. An ultrasound revealed that largest lymph node in the neck was 2.6 cm × 1.0 cm. Chest CT scans showed enlarged mediastinal and bronchial lymph nodes without significant pulmonary lesions (Figure 1D). PCR revealed plasma EBV-DNA (1.08 × 105 copies/mL). Post-transplant lymphoproliferative disorder (PTLD) was suspected but could not be confirmed owing to the refusal of the patient’s parents to provide consent for a lymph node biopsy. A positron emission tomography-CT (PET-CT) scan revealed enlarged lymph nodes with increased uptake of fluorodeoxyglucose in the neck, mediastinum, abdomen, and groin, supporting a diagnosis of PTLD. Consequently, the patient was administered foscarnet sodium, and rituximab once weekly for 3 weeks, a course of CTX, and a donor-derived EBV-specific cytotoxic T lymphocyte infusion between day 89 and 114 post-transplantation. The patient also received broad-spectrum antibiotics and prophylactic antifungals. The patient tested negative for EBV-DNA after receiving the first dose of rituximab.
Two weeks after the onset of symptoms, the patient had accelerated chest pain and breathing difficulties. On day 114 post-transplantation, biopsies of the lymph nodes in the neck were performed showing granulomas with caseous necrosis, indicating lymph node TB. On day 116 post-transplantation, AFB testing confirmed TB, and treatment with isoniazid, rifampin, pyrazinamide, levofloxacin, and linezolid was started immediately. The patient did not improve and developed severe chest pain, tachypnea, hypoxemia, and orthopnea within 5 days of the initiation of anti-TB treatment. Chest CT scans revealed bronchial stenosis in both bronchial tubes and worsening lymphadenectasis. A bronchoscopy revealed multiple granulomas severely restricting the primary bronchi (Figure 1G, H). AFB testing was negative; however, BAL fluid tested positive for Mtb by PCR.
Patient B developed type 1 respiratory failure after a week. Chest CT scans revealed massive infiltration, consolidation, and atelectasis of the right upper lobe of the lung, suggesting pulmonary TB (Figure 1E). The patient was then put on a ventilator after 3 days. Cryosurgery was performed via bronchoscopy to remove the granulomas and open the airways (Figure 1I). After a second cryosurgery, the patient showed gradual improvement. On day 155 post-transplantation, a bronchoscopy showed clearance of granulomas from the bronchi (Figure 1J). On day 166 post-transplantation, chest CT scans revealed the dissolution of the majority of pulmonary infiltrates, and the patient was discharged (Figure 1F). He was prescribed oral rifapentine, isoniazid, pyrazinamide, and linezolid for a week, and then changed to rifapentine, isoniazid, and pyrazinamide treatment for the following 4 weeks. Pyrazinamide was discontinued after a month owing to elevated liver enzyme levels and as of the writing of this case report, the patient continues to receive oral rifapentine and isoniazid.
Discussion and Conclusions
TB is one of the most severe post-transplant complications.4,8 Twelve pediatric TB cases in HSCT recipients were reported in literature from 1990 to 2019 (Table 1).2,6,7,9-12 Mortality associated with pediatric post-HSCT TB may reach 50%.10 Between January 2012 and August 2019, our center reported only two pediatric TB cases out of 330 children who had undergone HSCT (0.60%). This is far lower than the number reported from other TB-endemic regions, potentially owing to China’s national bacilli Calmette-Guerin (BCG) vaccination program.13,14 In our center, nearly all patients that received HSCT were BCG vaccinated except a few patients with primary immunodeficiencies. In China, the incidence of pulmonary TB in children less than 15 years of age was 0.00244% between 2004 to 2015.15 Extrapulmonary TB was not included in this survey, and the incidence of TB may be much higher in pediatric HSCT recipients than that in the general pediatric population. Therefore, our patients were carefully screened for TB before HSCT and high-risk patients received prophylactic TB treatment.
 |
Table 1 Case Reports of Tuberculosis in Pediatric Patients Who Received Hematopoietic Stem Cell Transplant Recipients from 1990 to 2019 |
In HSCT recipients, TB typically presents as a pulmonary infection, and TB affecting only the extrapulmonary sites is rare.3,8,16-19 TB in HSCT recipients can present atypically, and diagnoses may take weeks to confirm.5,20 TB diagnoses are complicated by the shortcomings of many testing methods. The gold standard for TB diagnoses is the isolation of Mtb by culture. However, this has a very high rate of false negatives. Most molecular methods involving nucleic acid amplification directly detect Mtb-DNA and can be used for the detection of genetic mutations associated with drug resistance. These methods generally have higher sensitivity and specificity compared to that of Mtb culture. However, the usefulness of molecular amplification methods has been limited because of the variable sensitivities, specificities, quality control issues, and costs.21,22 Genetic sequencing has high specificity, but low sensitivity. Interferon-γ (IFN- γ) assays have higher sensitivity than other methods, but a high rate of false positives.14,23 Radiological examinations often confuse TB with other pathogens. Complicating diagnoses in our TB patients were co-infections, such as those by CMV and EBV. Invasive testing such as bronchoscopy and tissue biopsies were required to confirm the presence of TB. Patient B had lymphoid TB and uncommon presentation that we initially believed to be PTLD owing to EBV infection.24,25 Unconventional, potentially invasive testing for TB should not be delayed in HSCT recipients.1,26,27
Neither patient developed severe GVHD; however, GVHD increases the susceptibility to TB after HSCT, and the patients were receiving immunosuppressive therapies at the time of TB onset.7 HSCT recipients on immunosuppressive therapies are at an increased risk of acquiring TB.28 Patient B received UCB engraftment, which is often associated with delayed immune reconstitution and may result in higher morbidity and mortality in TB patients.29,30
TB status should be evaluated carefully before HSCT. In our center, most children, except those with immunodeficiencies received the BCG vaccination. All potential HSCT recipients were screened for TB prior to transplantation using an IFN-γ release assay (IGRA) by ELISA, tubercular skin test (TST), and chest CT scans. A TST is considered positive when the reaction site is 10 mm in diameter or more. However, the sensitivity of TST is low (less than 50%). In addition, BCG vaccination can lead to a reaction at the TST site and cause false positive results in vaccinated children. Thus, we also performed an IGRA-ELISA assay as a screening test for TB. Suspected cases with positive results using TST or ELISA, and radiological findings suggestive of TB were assigned to an infectious disease specialist. To exclude TB, further examinations included an IGRA via QuantiFERON-TB Gold or T-SPOT assay. Less frequently, patients were assigned for PCR examination or high-throughput sequencing for TB-DNA. Patients suspected of TB infections were administered prophylactic TB treatment approximately 3 months before and after HSCT. It has been suggested that prophylactic TB treatment should be considered in patients from endemic regions regardless of the TB status.31 The recommended prophylactic TB treatment is isoniazid or rifampicin alone or in combination.20 Long-term TB management is challenging owing to the development of resistant TB and hepatotoxicity associated with TB drugs and drug interactions.1 In this study, drug susceptibility test was not performed for resistant Mtb. However, the identification of drug resistance is important for guiding the appropriate treatment. Post HSCT prophylactic TB treatment is another important arm for preventing the occurrence of latent TB, but the decision of initiation and administration of the treatment is hard for clinicians owing to drug toxicity and a higher chance of developing chronic complications such as chronic GVHD.32,33 The choice of long-term TB prophylaxis also varies between transplant centers according to TB prevalence in different counties and regions. In our center, patients suspected of having TB by laboratory or radiological examinations during the pre-HSCT evaluation are prescribed with prophylactic isoniazid treatment for 3 months after transplantation. Patients are closely monitored for their liver, renal, and cardiac functions during the treatment for early identification of drug-related complications.
In conclusion, TB is a rare but potentially devastating complication in pediatric HSCT recipients. Post-HSCT TB is difficult to diagnose, particularly in patients with atypical presentation and co-infections. It is critical to carefully evaluate patients before ruling out TB. Prophylactic TB treatment may be necessary for pediatric HSCT recipients who were previously infected with TB or were in contact with persons with TB.
Abbreviations
AFB, acid-fast bacilli; BAL, bronchoalveolar lavage; CMV, cytomegalovirus; CT, computed tomography; EBV, Epstein–Barr virus; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; IFN- γ, interferon-γ; MTb, Mycobacterium tuberculosis; PCR, polymerase chain reaction; PTLD, post-transplant lymphoproliferative disorder; TB, tuberculosis; TM, thalassemia major; UCB, umbilical cord blood.
Ethical Approval and Consent for Participate
Written informed consents for both the publication of the images and case details had been obtained from parents of both patients. Ethical approvals were not required for case reports.
Consent for Publication
Written consent was obtained from the parents of both patients for the publication of this study.
Acknowledgments
We thank Prof Kuang-Yueh Chiang at the Hospital for Sick Children in Toronto for the consultation of both patients. Xiaodong Wang and Uet Yu are co-first authors for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Huaman MA, Brawley R, Ashkin D. Multidrug-resistant tuberculosis in transplant recipients: case report and review of the literature. Transplant Infect Dis. 2017;19(2):2. doi:10.1111/tid.12672
2. Vecino R, Santiago B, Baquero-Artigao F, et al. Tuberculosis in pediatric solid organ and hematopoietic stem cell transplant recipients. Pediatr Infect Dis J. 2012;31(7):774–777. doi:10.1097/INF.0b013e3182564ec7
3. Zhao Z, Leow WQ. Concurrent Hepatic Tuberculosis and Hepatic Graft-versus-host Disease in an Allogeneic Hematopoietic Stem Cell Transplant Recipient: A Case Report. Transplant Proc. 2017;49(7):1659–1662. doi:10.1016/j.transproceed.2017.03.073
4. Yoo JW, Jo KW, Kim SH, et al. Incidence, characteristics, and treatment outcomes of mycobacterial diseases in transplant recipients. Transplant Int. 2016;29(5):549–558. doi:10.1111/tri.12752
5. Benito N, Garcia-Vazquez E, Horcajada JP, et al. Clinical features and outcomes of tuberculosis in transplant recipients as compared with the general population: a retrospective matched cohort study. Clin Microbiol Infect. 2015;21(7):651–658. doi:10.1016/j.cmi.2015.03.010
6. Fan WC, Liu CJ, Hong YC, et al. Long-term risk of tuberculosis in haematopoietic stem cell transplant recipients: a 10-year nationwide study. Int j Tuberculosis Lung Dis. 2015;19(1):58–64. doi:10.5588/ijtld.14.0301
7. Ip MS, Yuen KY, Woo PC, et al. Risk factors for pulmonary tuberculosis in bone marrow transplant recipients. Am J Respir Crit Care Med. 1998;158(4):1173–1177. doi:10.1164/ajrccm.158.4.9712072
8. Collaco JM, Gower WA, Mogayzel PJ. Pulmonary dysfunction in pediatric hematopoietic stem cell transplant patients: overview, diagnostic considerations, and infectious complications. Pediatr Blood Cancer. 2007;49(2):117–126. doi:10.1002/pbc.21061
9. Lee JW, Kwon HJ, Jang PS, et al. Two children with differing outcomes after treatment for pulmonary tuberculosis diagnosed after allogeneic hematopoietic stem cell transplantation. Transplant Infect Dis. 2011;13(5):520–523. doi:10.1111/j.1399-3062.2011.00641.x
10. George B, Mathews V, Viswabandya A, Srivastava A, Chandy M. Infections in children undergoing allogeneic bone marrow transplantation in India. Pediatr Transplant. 2006;10(1):48–54. doi:10.1111/j.1399-3046.2005.00397.x
11. Chen CC, Huang LM, Chang YL, King CC, Lin KH. Acute respiratory distress syndrome due to tuberculosis in a child after allogeneic bone marrow transplantation for acute lymphoblastic leukemia. J Formosan Med Assoc. 1999;98(10):701–704.
12. Biral E, Faraci M, Lanino E, et al. Mycobacterium tuberculosis pneumonia and bacteremia after allogeneic hematopoietic stem cell transplant: report of an instructive pediatric case. New Microbiol. 2012;35(3):353–357.
13. Zhu B, Dockrell HM, Ottenhoff THM, Evans TG, Zhang Y. Tuberculosis vaccines: opportunities and challenges. Respirology. 2018;23(4):359–368. doi:10.1111/resp.13245
14. Russo RL, Dulley FL, Suganuma L, Franca IL, Yasuda MA, Costa SF. Tuberculosis in hematopoietic stem cell transplant patients: case report and review of the literature. Int j Infect Dis. 2010;14(Suppl 3):e187–e191. doi:10.1016/j.ijid.2009.08.001
15. Yang R, Liu M, Jiang H, et al. The epidemiology of pulmonary tuberculosis in children in Mainland China, 2009–2015. Arch Dis Child. 2019.
16. Campos A, Vaz CP, Campilho F, et al. Central nervous system (CNS) tuberculosis following allogeneic stem cell transplantation. Bone Marrow Transplant. 2000;25(5):567–569. doi:10.1038/sj.bmt.1702163
17. Al-Anazi KA, Al-Jasser AM, Evans DA. Infections caused by mycobacterium tuberculosis in patients with hematological disorders and in recipients of hematopoietic stem cell transplant, a twelve year retrospective study. Ann Clin Microbiol Antimicrob. 2007;6(1):16. doi:10.1186/1476-0711-6-16
18. Lam W, Viswabandya A, Hussain S, et al. A unique case of tuberculosis dissemination presenting as cutaneous lesions in a post allogeneic stem cell transplant patient. Bone Marrow Transplant. 2016;51(10):1385–1386. doi:10.1038/bmt.2016.137
19. Venkataramani V, Seif Amir Hosseini A, Schulze MH, et al. Intestinal Pneumatosis Associated with Tuberculosis after Allogeneic Hematopoietic Stem Cell Transplantation. Acta Haematol. 2017;137(1):51–54. doi:10.1159/000452436
20. Cordonnier C, Martino R, Trabasso P, et al. Mycobacterial infection: a difficult and late diagnosis in stem cell transplant recipients. Clin Infect Dis. 2004;38(9):1229–1236. doi:10.1086/383307
21. Oommen S, Banaji N. Laboratory diagnosis of tuberculosis: advances in technology and drug susceptibility testing. Indian J Med Microbiol. 2017;35(3):323–331. doi:10.4103/ijmm.IJMM_16_204
22. Aygun D, Akcakaya N, Cokugras H, Camcıoglu Y. Evaluation of Clinical and Laboratory Characteristics of Children with Pulmonary and Extrapulmonary Tuberculosis. Medicina. 2019;55:8.
23. Lee YM, Lee SO, Choi SH, et al. A prospective longitudinal study evaluating the usefulness of the interferon-gamma releasing assay for predicting active tuberculosis in allogeneic hematopoietic stem cell transplant recipients. J Infect. 2014;69(2):165–173. doi:10.1016/j.jinf.2014.02.019
24. Crombie JL, LaCasce AS. Epstein Barr Virus Associated B-Cell Lymphomas and Iatrogenic Lymphoproliferative Disorders. Front Oncol. 2019;9:109. doi:10.3389/fonc.2019.00109
25. Ru Y, Chen J, Wu D. Epstein-Barr virus post-transplant lymphoproliferative disease (PTLD) after hematopoietic stem cell transplantation. Eur J Haematol. 2018;101(3):283–290. doi:10.1111/ejh.13131
26. Bisero ED, Luque GF, Rizzo CN, Zapata AE, Cuello MS. [Pulmonary actinomycosis and tuberculosis. A comorbidity pediatric case]. Arch Argent Pediatr. 2016;114(4):e233–e236. doi:10.5546/aap.2016.e233. Spanish.
27. Martin-Sanchez G, Drake-Perez M, Rodriguez JL, Yanez L, Insunza A, Richard C. Lymph node tuberculosis after allogeneic haematopoietic stem cell transplantation: an atypical presentation of an uncommon complication. Ecancermedicalscience. 2015;9:535. doi:10.3332/ecancer.2015.535
28. Yang A, Shi J, Luo Y, et al. Allo-HSCT recipients with invasive fungal disease and ongoing immunosuppression have a high risk for developing tuberculosis. Sci Rep. 2019;9(1):20402. doi:10.1038/s41598-019-56013-w
29. Lee YJ, Bacchus M, Schmidt E, Chawla M, Barker JN, Papanicolaou GA. Paradoxical immune reconstitution inflammatory syndrome associated with disseminated tuberculosis infection in an unrelated donor cord blood transplant recipient. Transplant Infect Dis. 2018;20(3):e12889. doi:10.1111/tid.12889
30. Maeda T, Kusumi E, Kami M, et al. Disseminated tuberculosis following reduced-intensity cord blood transplantation for adult patients with hematological diseases. Bone Marrow Transplant. 2005;35(1):91–97. doi:10.1038/sj.bmt.1704740
31. Garces Ambrossi G, Jakubowski A, Feinstein MB, Weinstock DM. Active tuberculosis limited to foreign-born patients after allogeneic hematopoietic stem cell transplant. Bone Marrow Transplant. 2005;36(8):741–743. doi:10.1038/sj.bmt.1705129
32. Cheng MP, Kusztos AE, Bold TD, et al. Risk of Latent Tuberculosis Reactivation After Hematopoietic cell Transplantation. Clin Infect Dis. 2019;69(5):869–872. doi:10.1093/cid/ciz048
33. Park JH, Choi EJ, Park HS, et al. Treatment of latent tuberculosis infection based on the interferon-gamma releasing assay in allogeneic stem cell transplant recipients. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa030
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.