Back to Journals » OncoTargets and Therapy » Volume 9
Icotinib versus docetaxel used in lung adenocarcinoma patients who failed platinum-based chemotherapy: a retrospective study
Authors He W, Zhang Y, Xiong Y, Dai F, Fan Q
Received 29 October 2015
Accepted for publication 1 February 2016
Published 1 July 2016 Volume 2016:9 Pages 4037—4041
DOI https://doi.org/10.2147/OTT.S99434
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Min Li
Wei He, Yan Zhang, Yu Xiong, Feng-juan Dai, Qing-xia Fan
The Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China
Background: The efficacy and safety of epidermal growth factor receptor tyrosine kinase inhibitors have been studied worldwide. However, there are few reports directly comparing the efficacy and safety between icotinib and docetaxel as second-line treatment in lung adenocarcinoma patients who have failed platinum-based chemotherapy. This article offers insight into this field.
Methods: A total of 137 patients with stage III or IV lung adenocarcinoma who had progressed on first-line platinum-based therapies and received icotinib or docetaxel therapy between October 2011 and February 2013 were retrospectively reviewed. Patients in the icotinib group received oral icotinib at a dose of 125 mg tid, while patients in the docetaxel group received infusion docetaxel at a dose of 75 mg/m2 on day 1 of every 21 days (four to six cycles) until disease progression or unacceptable toxicity occurred after which best supportive care was given.
Results: There was no statistically significant difference in the objective response rate (23.3% vs 12.5%, P=0.103), progression-free survival (121 days vs 106 days, P=0.083), and overall survival (307 days vs 254 days, P=0.070) between the two groups. As compared to the docetaxel group, the disease control rate (75.3% vs 54.7%, P=0.011) was significantly better in the icotinib group. In the icotinib group, the most common adverse events were rash (35.62%) and diarrhea (24.66%), whereas in the docetaxel group, elevation of transaminase (37.50%), leukopenia (50.00%), and anemia (54.69%) were the most common.
Conclusion: Icotinib had similar efficacy and a lower adverse events rate in epidermal growth factor receptor-unselected patients as compared to docetaxel, thereby making it an effective second-line therapy option for lung adenocarcinoma.
Keywords: icotinib, docetaxel, second-line therapy, lung adenocarcinoma, EGFR-TKIs
Background
Lung cancer is the leading cause of cancer-related deaths, with a 5-year survival rate of 16.8%, since most patients are diagnosed at an advanced stage.1,2 Lung carcinoma can be classified into non-small-cell lung cancer (NSCLC) and small cell lung cancer according to its histological type. NSCLC accounts for ~85% of all lung cancers and includes adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and other types. Progress in lung cancer treatment has accelerated due to the introduction of new drugs and awareness of histological subtype.3–5
The epidermal growth factor receptor (EGFR) mutations are found in up to 50% of Asian patients and ~10% of non-Asian patients.6,7 These mutations result in activation of the tyrosine kinase domain, which is associated with sensitivity to the small molecule tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib, afatinib, and icotinib. The first three are commonly used in patients with sensitizing EGFR mutations worldwide. Icotinib is an orally administered EGFR-TKI drug that is widely used as second-line treatment in patients with advanced NSCLC in Asia.8 A randomized, double-blind, Phase III, and noninferiority trial (ICOGEN, Ref No 7) proved that icotinib is noninferior to gefitinib in patients with NSCLC. However, there are few reports comparing the efficacy and safety between icotinib and the standard second-line chemotherapy, docetaxel in lung adenocarcinoma patients who have failed first-line platinum-based chemotherapy. This article provides clinical data in this field.
Methods
Patients’ eligibility
We retrospectively reviewed the records of lung adenocarcinoma patients who received icotinib or docetaxel as second-line therapy at the First Affiliated Hospital of Zhengzhou University during October 2011 and February 2013 after relapse or recurrence following prior chemotherapy. All patients had been pathologically confirmed with locally advanced/metastatic or recurrent lung adenocarcinoma that progressed or recurred after previous platinum-based chemotherapy regimen, had Eastern Cooperative Oncology Group (ECOG) performance status 0–2, and had at least one measurable disease by Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1). Patients treated for brain metastases by radiation were eligible, if they were neurologically stable.
Therapeutic schedule
Patients were divided into two groups according to the therapy they received, namely the icotinib group and the docetaxel group. Patients in the icotinib group received oral icotinib at a dose of 125 mg tid, and those in the docetaxel group received infusion docetaxel at a dose of 75 mg/m2 on day 1 of every 21 days (six cycles in total) until disease progression, development of unacceptable toxicity, or patient refusal. Best supportive care was given afterward.
Assessment of response and toxicity
Baseline evaluation included imaging examinations of the chest and upper abdomen, laboratory investigations such as complete blood counts, urinalysis, renal, and liver function tests, and performance status evaluated according to the ECOG criteria. Magnetic resonance imaging of the brain and emission computed tomography of the bone were performed only if metastatic disease was suspected based on the clinical manifestations. Re-evaluation and adverse events data were collected through medical records and follow-up.
Objective tumor response was assessed according to the RECIST 1.1 criteria. Progression-free survival (PFS) was calculated from the start of therapy to the date of disease progression or the last follow-up. Overall survival (OS) was calculated from the start of therapy to the date of patient death or the last follow-up. Adverse events were graded according to the common terminology criteria for adverse events (CTCAE) v4.02.
Statistical analysis
Baseline characteristics of patients, such as sex, smoking history, performance status, and clinical stage, as well as the objective response rate (ORR) and disease control rate (DCR), were compared between the two groups using the chi-square (χ2) test (two-sided test at the 5% significance level; 95% confidence interval [CI]). Patient age was compared between the two groups using the nonparametric test. Survival curves were constructed using the Kaplan–Meier method, and the differences between curves were evaluated by the log-rank test to compare PFS and OS between the two groups. Each analysis was performed by means of IBM SPSS Statistics 19 (SPSS Inc., Chicago, IL, USA). Differences were considered significant if P<0.05.
Ethical approval
Ethical approval was obtained from the medical ethics committee of the First Affiliated Hospital of Zhengzhou University (reference number: 201403). All patients involved allowed us to use their treatment related data for the study anonymously and provided written informed consent to be included.
Results
Patients’ characteristics
A total of 137 eligible patients were reviewed, with 73 patients in the icotinib group and 64 patients in the docetaxel group. Patients’ characteristics are listed in Table 1. In the icotinib group, 30 patients were male and 43 patients were female. The median age was 59 years (range: 32–79 years). Twenty-six patients were ever-smokers, and 47 patients were never-smokers. Twenty patients were stage IIIB, and 53 patients were stage IV. In the docetaxel group, 36 patients were male and 28 patients were female. The median age was 60 years (range: 38–77 years). Thirty-two patients were ever-smokers, and 32 patients were never-smokers. Eighteen patients were at stage IIIB, and 46 patients were at stage IV. The baseline characteristics of patients were compared using the χ2 test and Mann–Whitney test and found to be similar.
Response and survival
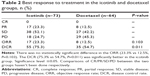
In the icotinib group, 17 (23.3%) cases achieved partial response, 38 (52.1%) had stable disease, and 18 (24.7%) showed progressive disease. In the docetaxel group, eight (12.5%) cases achieved partial response, 27 (42.2%) had stable disease, and 29 (45.3%) showed progressive disease. The two groups had similar ORR (23.3% vs 12.5%, P=0.103), while the DCR (75.3% vs 54.7%, P=0.011) was significantly better in the icotinib group (Table 2).
PFS was similar between the two groups, as median PFS was 121 days (95% CI 94.97–147.03) with icotinib versus 106 days (95% CI 76.08–135.92) with docetaxel (P=0.083; Figure 1).
At the final follow-up, 129 (94.16%) patients had died, while 17 (23.29%) patients in the icotinib group and 22 (34.38%) patients in the docetaxel group with progression had received subsequent therapies. OS in all patients was similar for icotinib and docetaxel (P=0.070). Median OS was 307 days (95% CI 215.84–398.17) in the icotinib group versus 254 days (95% CI 189.57–318.43) in the docetaxel group (Figure 2).
Toxicity
All patients were assessed for drug-related toxicities (Table 3). In the icotinib group, the most common adverse events during treatment were rash and diarrhea. Twenty-three (31.51%) patients had grades 1–2 rash, and three (4.11%) patients had grade 3 or more serious rash. Ten (20.0%) patients had grades 1–2 diarrhea, and three (4.11%) patients had grade 3 or more serious diarrhea. Most events were mild with CTCAE grades 1–2 and occurred in the first 1–3 months of treatment. The symptoms often gradually reduced and minimally influenced follow-up treatment.
In the docetaxel group, the most common adverse events were elevation of transaminase (37.50%), leukopenia (50.00%), and anemia (54.69%). The incidence of CTCAE grade 3 or more serious adverse events was 26.56%, and most were hematological toxicities.
Discussion
Therapy for advanced NSCLC has significantly developed with new drugs and awareness of histological subtype. EGFR mutations are found in up to 50% of Asian patients and ~10% of non-Asian patients. These mutations play an important role as a component of two principal cellular pathways that drive tumor growth and spread. Therefore, EGFR-TKIs are widely studied for the treatment of patients with advanced NSCLC.6,7,9 Several randomized clinical studies, such as INTEREST, V-15-32, ISTANA, and TAILOR,10–15 have investigated the efficacy and safety of gefitinib or erlotinib as compared to standard second-line chemotherapy. However, few studies have compared the efficacy and safety between icotinib and the standard second-line chemotherapy. Hence, we retrospectively studied the efficacy and safety of icotinib as compared to docetaxel.
The INTEREST study enrolled 1,466 patients at 149 centers in 24 countries and maintained comparable follow-up treatment, thereby establishing noninferior survival of gefitinib as compared to docetaxel (hazard ratio 1.020, 95% CI 0.905–1.150), suggesting that gefitinib is a valid treatment for pretreated patients with advanced NSCLC.10 The ICOGEN study showed that icotinib was noninferior to gefitinib for PFS (hazard ratio 0.84, 95% CI 0.67–1.05, P=0.13) and caused less drug-related adverse events in patients than gefitinib (61% vs 70%, P=0.046), especially drug-related diarrhea (19% vs 28%, P=0.033), suggesting that icotinib could be a treatment option for pretreated patients with advanced NSCLC.7
We found no significant difference in ORR (23.3% vs 12.5%, P=0.103), PFS (121 days vs 106 days, P=0.083), and OS (307 days vs 254 days, P=0.070) between the two groups in our study. As compared to the docetaxel group, the DCR (75.3% vs 54.7%, P=0.011) was significantly better in the icotinib group.
However, this study was retrospective and not randomized, so it may have several limitations. Although the baseline characteristics were well-matched between the two groups in terms of sex, age, smoking history, ECOG performance status, and stage, whether the EGFR status was comparable in the two groups was unknown because 90 (64.96%) patients were not examined for genetic mutations. This could be due to the lack of awareness of the importance of EGFR in the efficacy of icotinib at the early stage or financial constraints of patients who refused gene sequencing. In the icotinib group, 24 (32.9%) patients had EGFR-mutated tumors and five (6.8%) had EGFR wild-type tumors, while in the docetaxel group, four (6.3%) patients had EGFR-mutated tumors and 15 (23.4%) patients had EGFR wild-type tumors, which could possibly affect the results. Also, 17 (23.29%) patients in the icotinib group and 22 (34.38%) patients in the docetaxel group (P=0.151) had subsequent chemotherapy after failure of the second-line treatment, which may explain why the better DCR in the icotinib group did not provide survival benefit.
The TAILOR study enrolled patients with metastatic NSCLC, who received prior platinum-based chemotherapy and had wild-type EGFR as assessed by direct sequencing. The results showed that docetaxel was more effective than erlotinib for second-line treatment of these patients. Median OS was 8.2 months (95% CI 5.8–10.9) with docetaxel versus 5.4 months (95% CI 4.5–6.8) with erlotinib (P=0.05). PFS was significantly better with docetaxel than with erlotinib, median PFS was 2.9 months (95% CI 2.4–3.8) with docetaxel versus 2.4 months (95% CI 2.1–2.6) with erlotinib (P=0.02).13
These results suggest that EGFR status should be detected before using EGFR-TKI drugs as second-line therapies. More studies are needed to determine whether Asian patients with wild-type EGFR tumors and stage III or IV lung adenocarcinoma can benefit from icotinib as second-line therapy.
Conclusion
Icotinib had similar efficacy and lower adverse events rate in EGFR-unselected patients as compared to docetaxel, thereby making it an effective second-line therapy option for lung adenocarcinoma.
Acknowledgments
This work was funded by Science and Technology Research Project of Henan Education Department (14A320087), Science and Technology Research Project of Henan Health Department (201303021), and Youth Innovation Fund in the First Affiliated Hospital of Zhengzhou University.
Disclosure
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work; there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, this manuscript. The authors report no other conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. | ||
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. | ||
Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382(9893):709–719. | ||
Forde PM, Ettinger DS. Targeted therapy for non-small-cell lung cancer: past, present and future. Expert Rev Anticancer Ther. 2013;13(6):745–758. | ||
Laurie SA, Goss GD. Role of epidermal growth factor receptor inhibitors in epidermal growth factor receptor wild-type non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1061–1069. | ||
Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. | ||
Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14(10):953–961. | ||
Liang JL, Ren XC, Lin Q. Treating advanced non-small-cell lung cancer in Chinese patients: focus on icotinib. Onco Targets Ther. 2014;7:761–770. | ||
Han W, Lo HW. Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett. 2012;318(2):124–134. | ||
Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372(9652):1809–1818. | ||
Maruyama R, Nishiwaki Y, Tamura T, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol. 2008;26(26):4244–4252. | ||
Lee DH, Park K, Kim JH, et al. Randomized Phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307–1314. | ||
Garassino MC, Martelli O, Broggini M, et al; TAILOR Trialists. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14(10):981–988. | ||
Sun JM, Lee KH, Kim SW, et al; Korean Cancer Study Group. Gefitinib versus pemetrexed as second-line treatment in patients with nonsmall cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234–6242. | ||
Ciuleanu T, Stelmakh L, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13(3):300–308. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.