Back to Journals » OncoTargets and Therapy » Volume 14
Icotinib versus Cisplatin Plus Docetaxel as Adjuvant Chemotherapy in Patients with Stage II (N1+) Non-Small Cell Lung Cancer Harboring Positive EGFR Mutations: A Single-Center Retrospective Study
Authors Pan S, Wang S, Li W, Chai Y
Received 11 November 2020
Accepted for publication 26 January 2021
Published 16 February 2021 Volume 2021:14 Pages 1083—1091
DOI https://doi.org/10.2147/OTT.S290636
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Saibo Pan,* Shijie Wang,* Wenshan Li, Ying Chai
Department of Thoracic Surgery, The Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, 310009, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ying Chai
Department of Thoracic Surgery, The Second Affiliated Hospital, College of Medicine, Zhejiang University, # 88 Jiefang Road, Hangzhou, 310009, People’s Republic of China
Tel +86-571-87783642
Fax +86-571-87068001
Email [email protected]
Purpose: The superior efficacy of first-line treatment with icotinib over that of standard chemotherapy has been well demonstrated in patients with advanced non-small cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) mutation. However, whether icotinib is superior to cisplatin plus docetaxel as adjuvant chemotherapy in patients with stage II (N1+) NSCLC selected by EGFR mutation is controversial.
Methods: A total of 43 patients with completely resected stage II (T1-2N1M0) NSCLC and proven sensitive EGFR mutation (19Del or L858R) between January 2010 and December 2019 were included in our study. The disease-free survival (DFS) and overall survival (OS) were analyzed in 22 patients treated with icotinib and 21 patients treated with cisplatin plus docetaxel. Factors affecting DFS and OS were assessed by the Kaplan-Meier (KM) estimator and univariate Cox regression analysis.
Results: Our cohort included 22 icotinib patients and 21 cisplatin plus docetaxel patients with a median follow-up of 35.5 months and 38 months, respectively. Survival time was significantly longer in the icotinib group than in the chemotherapy group, with a median DFS of 47 months (95% CI, not reached) versus 18 months (95% CI, 12.4– 23.6; HR 0.16; 95% CI, 0.07– 0.35; log-rank p< 0.0001). In the icotinib group, the most common adverse effects (AEs) were skin rash (40.9%) and elevated alanine aminotransferase (22.7%), whereas in the cisplatin plus docetaxel group, the most common AEs were nausea or vomiting (90.5%), anorexia (71.4%), and fatigue (71.4%). No deaths were treatment-related.
Conclusion: In this study, we demonstrated that in EGFR mutation-positive patients with completely resected stage II (T1-2N1M0) NSCLC, icotinib might provide DFS benefits, and reduced drug toxicity compared to cisplatin plus docetaxel. Thus, icotinib may be a reasonable option for adjuvant chemotherapy in patients with pathological stage II (N1+) NSCLC with EGFR mutation.
Keywords: icotinib, adjuvant therapy, EGFR-TKI, lung cancer, survival analysis, adverse events
Introduction
Lung cancer is one of the most common cancers worldwide,1 and was diagnosed in approximately 2.1 million patients with an estimated 1.7 million deaths in 2018.2 Non-small cell lung cancer (NSCLC) was the most common subtype, occurring in about 80–85% of all lung cancers. Radical resection (R0) offers the opportunity for cure or better long-term survival in patients with stage I, stage II, and stage IIIA resectable NSCLC; for those who present with advanced disease or poor cardiopulmonary conditions, a combined approach with systemic chemotherapy is generally preferred.3
Previous studies have shown that adjuvant chemotherapy using cisplatin-based regimens is beneficial for patients with R0 resected stage II–IIIA NSCLC, lowering the risk of death at five years by about 5.4%.4 However, cisplatin-based adjuvant chemotherapy still faced a divisive risk-benefit analysis between clinical benefits and severe adverse events (AEs). Grade 3 or 4 neutropenia occurred in approximately 70–80% of patients, and over 25% of patients experienced nausea and vomiting.5,6
More recently, epidermal growth factor receptor (EGFR)-regulated signaling pathways have been shown to play crucial roles in the development and progression of NSCLC. Agents that target these specific molecular pathways, named EGFR tyrosine kinase inhibitors (EGFR-TKIs), are actively being developed in the treatment of certain groups of NSCLC patients. First-generation EGFR-TKIs such as gefitinib and erlotinib have been the standard front-line treatment for EGFR-mutated advanced NSCLC due to their superior efficacy over previous cisplatin-based chemotherapy regimens.7–11
Icotinib is another first-generation EGFR-TKI available in China, which exerts promising anti-neoplastic activity in NSCLC, similar to that of gefitinib and erlotinib but possibly with a better safety profile and lower cost.12 The superior efficacy of icotinib over that of standard chemotherapy has been confirmed in patients with advanced NSCLC possessing sensitive EGFR mutations (19Del or L858R).13–15 A randomized, double-blinded, placebo-controlled, multicenter trial (ICWIP Study), designed to explore the efficacy and safety of icotinib in completely resected stage II–IIIA adenocarcinoma patients with susceptible EGFR mutation who underwent platinum-based doublet chemotherapy, has already begun. The results to be obtained in the near future may provide potential guidance in clinical practice.16 However, whether icotinib is a reasonable option as adjuvant chemotherapy in patients with pathological stage II (N1+) NSCLC selected by EGFR mutation is controversial.
Patients and Methods
Patients
This single-center, retrospective study enrolled patients with stage II (T1-2N1M0) NSCLC harboring a sensitive EGFR mutation (19Del or L858R) who underwent adjuvant chemotherapy with icotinib versus cisplatin plus docetaxel. We reviewed data from 43 screened patients between January 2010 and December 2019 from the clinical database at the Second Affiliated Hospital of Zhejiang University School of Medicine. Clinical staging was performed after guideline-specified staging, which included a preoperative high-resolution chest computed tomography (CT) scan, bone scan, brain scan, abdominal B ultrasound, superficial lymph node ultrasound, and/or positron emission tomography (PET)-CT imaging. All 43 patients underwent lobectomy or pneumonectomy with systematic dissection of the mediastinal, hilar-interlobar, and intrapulmonary lymph nodes (LNs).
The study eligibility criteria included patients with R0 resection, diagnosis of stage II (T1-2N1M0) NSCLC (based on the 8th edition of the TNM staging system17), and the existence of a susceptible EGFR mutation (19Del or L858R). Other important eligibility criteria included adequate hematological, biochemical, and organ function and no severe postoperative complications. We excluded patients who underwent conservative resection, segmentectomy or wedge resection and those with prior neoadjuvant therapy. Patients who had severe postoperative complications, such as heart failure, myocardial infarction, angina, and severe pneumonia were also eliminated. Patients were classified according to sex, age at diagnosis, smoking status, EGFR mutation type, pathology type, stage (T and N stage), and tumor location (central or peripheral).
The study was undertaken according to the principles of the Declaration of Helsinki for medical research involving human subjects, and the consent form of this study was approved by the ethics committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (Approval Number: 2020–254). All patients provided informed consent and accepted treatments.
Procedures
Patients received oral icotinib 125 mg three times daily or intravenous cisplatin (75 mg/m2) plus docetaxel (500 mg/m2) in cycles once every 21 days for 4 cycles after surgery. The interval between the operation and the beginning of adjuvant therapy is shown in supplement Table 1. Treatment continued until progression of disease, development of unacceptable toxicity, or the completion of 4 chemotherapy cycles. The primary endpoint was disease-free survival (DFS). Secondary endpoints included overall survival (OS) and clinical safety.
Patients were screened for EGFR mutations after surgery. Follow-up information was collected every 3 months for the first 2 years, and every 6 months thereafter until the data cut-off point. DFS was assessed from the date of surgery to the earliest sign of disease progression (local relapse and/or distant metastasis) as determined by B-mode ultrasound, CT or magnetic resonance imaging using RECIST criteria, or death from any cause. OS was assessed from the date of surgery until death from any cause or to last known follow-up. Clinical safety was assessed in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 (published in November 2017, effective in April 2018).
Statistical Analysis
The median value of survival, as well as the range and two-sided 95% confidence intervals (CI), were calculated by the Kaplan-Meier (KM) estimator. The difference of survival time between the 2 treatment groups was tested using Log rank test. KM curves for DFS and OS were generated. To compare the time-to-event outcomes between the icotinib and cisplatin plus docetaxel groups, P-values were calculated using Cox regression. HRs were used to estimate the effect of the covariates. Subgroup analyses between different ages, sex, smoking status, pathological stage, tumor location and EGFR mutation type were conducted using the Cox proportional hazard regression model. The Chi-square test was used to compare proportions. Differences were considered significant at a two-sided p-value of ≤0.05. All statistical analyses were performed using a two-sided 5% significance level and a 95% CI, using SPSS 22.0 software (IBM SPSS, Chicago, IL).
Results
Patient Characteristics
Between January 2010 and December 2019, a total of 43 patients with stage II (T1-2N1M0) NSCLC who underwent R0 resection were enrolled in our study. Of these, 22 received icotinib and 21 received cisplatin plus docetaxel (Figure 1). Notably, the first patient who received cisplatin plus docetaxel was enrolled in April 2010, whereas the first patient who received icotinib was enrolled in September 2014. Forty-one patients underwent lobectomy, and the remainder underwent pneumonectomy. All patients underwent systemic lymph node dissection. Nodes from stations 10, 11, and 12 were dissected during operation, while intrapulmonary lymph nodes (IPLN, defined as stations 13–14) were retrieved by first assist after surgery. Cases were divided into two categories: N1a defined as only IPLN involved (station 13 and/or 14 spread) and N1b defined as single or multiple N1 nodes including at least one of station 10 to 12 spread. The clinical characteristics of these patients are listed in Table 1. The mean ages of the icotinib and cisplatin plus docetaxel groups were 62 (range, 48–77) and 58 (range, 31–75) years, respectively. Overall, 17 of 43 (39.5%) patients were male, and 12 of 43 (27.9%) patients had a history of smoking. The vast majority had adenocarcinoma (42 of 43; 97.7%), with 13 of 22 (61.9%) and 11 of 21 (52.4%) detected at the T1 stage in the icotinib and cisplatin plus docetaxel group, respectively. The two groups were matched and had no significant differences in baseline characteristics. At the data cut-off point (April 10, 2020), the median follow-up period was 35.5 months for the icotinib group and 38 months for the cisplatin plus docetaxel group. For patients who received icotinib, the median duration of treatment was 24 months (range, 6.0–48), and 59.1% of the patients completed 2-year treatment. There was no statistical difference between DFS of patients receiving icotinib for ≤2-year and >2-year (HR = 0.29; 95% CI, 0.04 to 1.97; log-rank P = 0.053; Supplement Figure 1). For those who received cisplatin plus docetaxel, all 21 receiving treatment completed 4 cycles of adjuvant chemotherapy, and 20 of them received icotinib-based combined therapy after progression (Supplement Table 2).
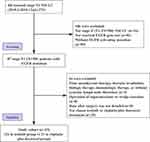 |
Figure 1 Flowchart of screened patients. |
 |
Table 1 Patient Characteristics for 2 Study Groups |
Survival Outcome
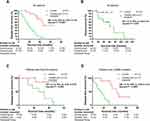
Median DFS was 47 months (95% CI, not reached) in the icotinib group and 18 months (95% CI, 12.4–23.6) in the cisplatin plus docetaxel group (p<0.0001; Figure 2A). Treatment with icotinib resulted in a significantly longer DFS than treatment with cisplatin plus docetaxel (HR=0.16; 95% CI, 0.07–0.35; log-rank p<0.0001). Furthermore, patients in the icotinib group were also observed to have an improved OS compared with patients in the cisplatin plus docetaxel group (HR=0.14; 95% CI, 0.04–0.45; log-rank p=0.027; Figure 2B), although the data were immature, with only 12 patients (27.9%) dead at the cut-off point,1 event (4.5%) in the icotinib group, and 11 events (52.4%) in the chemotherapy group.
Patients treated with icotinib had a longer DFS than patients treated with cisplatin plus docetaxel in all subgroup analyses (Figure 3). DFS differed significantly between the 2 groups, irrespective of the EGFR genetic mutation site (p=0.006 for Exon19 deletion, p<0.0001 for L858R mutation; Figure 2C, D, and supplement Figure 2). In our study, never smokers and elderly patients had a more favorable HR (HR=0.09; 95% CI, 0.03–0.22; log-rank p<0.0001; and HR=0.07; 95% CI, 0.02–0.27; log-rank p<0.0001, respectively). Moreover, the DFS for male sex, female sex, former or current smokers (p=0.0006, 0.0004, and 0.0121, respectively), and patients with stage T1, T2, N1a, and N1b differed significantly between the 2 groups (p<0.0001, p=0.0192, p<0.0013, and p<0.0001, respectively; Figure 3).
 |
Figure 3 Hazard ratios for DFS using subgroup analysis in the overall population. |
Exploratory analyses for DFS and overall population using the univariate Cox proportional hazards model showed that, in addition to the treatment group, patients who did not experience nerve invasion had a significantly better prognosis (HR=0.287; 95% CI, 0.084–0.978; p=0.046; and HR=0.211; 95% CI, 0.048–0.936; p=0.041, for DFS and OS, respectively; Table 2).
 |
Table 2 Univariate Cox Proportional Hazards Analysis of Disease-Free Survival and Overall Survival |
Safety
Icotinib not only showed efficacy versus cisplatin plus docetaxel in patients with stage II (T1-2N1M0) EGFR mutation-positive NSCLC, but was also associated with a reduction in the frequency of overall AEs reported. AEs occurring in more than 10% of either of the treatment groups are listed in Table 3. The most common AE in the icotinib group was skin rash (40.9%), followed by elevated alanine aminotransferase (ALT, 22.7%), elevated aspartic transaminase (AST, 18.2%), and fatigue (9.1%). In contrast, the most common AEs in the cisplatin plus docetaxel group, which occurred in over half of the patients, were vomiting (90.5%), nausea (90.5%), anorexia (71.4%), fatigue (71.4%), and myelosuppression (57.1%), followed by anemia (47.6%), thrombocytopenia (42.9%), impaired hepatic function (42.9%), neutropenia (42.9%), and leukopenia (38.1%). Other potential treatment-related toxicities included fever (one patient in the icotinib group, four in the cisplatin plus docetaxel group) and intrapulmonary infection (two patients in the icotinib group, three in the cisplatin plus docetaxel group). Additionally, six patients developed insomnia and four patients developed hypokalemia in the cisplatin plus docetaxel group. No patients in the icotinib group developed grade 3/4 AEs, whereas in the cisplatin plus docetaxel group, the proportion of grade 3/4 AEs was 9.5% (data not shown). There were no treatment-related deaths.
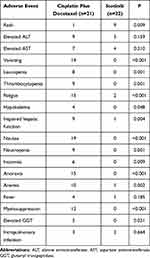 |
Table 3 Patients with Common Adverse Events at Any Grade Within the 2 Study Groups |
Discussion
After the discovery of gefitinib and erlotinib, a novel first-generation EGFR-TKI, icotinib, has recently shown promise in the treatment of NSCLC.9,18,19 This present relatively small retrospective study, to our knowledge, is one of the few trials to compare the antineoplastic effects and safety profiles of front-line icotinib with cisplatin-based chemotherapy as adjuvant chemotherapy in stage II (N1+) NSCLC patients with sensitive EGFR mutations and subjected to complete resection. Benefits of adjuvant chemotherapy using cisplatin-based regimens after surgery clearly exist,20 with an absolute increase in OS of 4% reported at 5 years.21 In our retrospective study, we found that icotinib, a novel first-generation EGFR TKI, in the adjuvant setting may be superior to standard cisplatin-based chemotherapy in R0 resected, EGFR mutation-positive NSCLC.
Several findings have been reported from studies in the adjuvant setting of EGFR TKIs in stage II–IIIA NSCLC. Most earlier studies were unable to demonstrate a significant benefit for TKIs, possibly due to inappropriate patient population selection (ie, no EGFR mutation selection) or early trial termination with no benefit.22,23 Recently, the ADJUVANT trial showed that gefitinib improved DFS in Chinese patients with EGFR mutation-positive stage II–IIIA (N1–N2) NSCLC.24 Median DFS in the intention-to-treat populations was 28.7 months (95% CI, 249–32.5) in the gefitinib group and 18.0 months (95% CI, 13.6–22.3) in the cisplatin plus vinorelbine group (HR 0.60; 95% CI, 0.42–0.87; p=0.0054). Subsequently, in the EVAN trial, the median DFS in the intention-to-treat populations was 42.4 months (95% CI, 31.7–not reached) in the erlotinib group and 21.0 months (12.3–32.4) in the chemotherapy group.25 Erlotinib prolonged survival time compared with cisplatin plus vinorelbine adjuvant chemotherapy in stage IIIA patients with EGFR mutation (HR 0.268; 95% CI, 0.136–0.531; p<0.0001). In our retrospective study, DFS was also significantly longer in the icotinib group than that in the cisplatin plus docetaxel group (47 months [95% CI, not reached] versus 18 months [95% CI, 12.4–23.6]; HR=0.16 [95% CI, 0.07–0.35], log-rank p<0.0001), which was comparable to that reported in the ADJUVANT and EVAN studies.
However, the patient population in our study (stage II [T1-2N1M0] disease) differed from that in the ADJUVANT (stage II–IIIA [N1-N2] NSCLC) and EVAN (stage IIIA–N2 NSCLC) studies. The effect of adjuvant chemotherapy on survival varied by disease stage, and a statistically significant benefit was shown only for patients with stage II and IIIA disease. A greater DFS benefit with EGFR TKIs compared with cisplatin-based adjuvant chemotherapy among patients with N2 disease versus those with N1 disease was observed in previous studies. In comparison to the ADJUVANT trial, which consisted of patients with stage N1 and N2 disease, our study showed a greater DFS benefit of icotinib versus cisplatin plus docetaxel (47 and 18 months vs 28.9 and 18 months, respectively). There are several potential reasons for this discrepancy, such as a smaller sample size and the potential contribution of confounding biases. Furthermore, different EGFR-TKI reagents (gefitinib vs icotinib) were used. In the subgroup analysis, never smokers, female sex, older patients, and stage N1b patients might have derived more benefit from icotinib than former or current smokers, male sex, younger patients, and stage N1a patients, although the analysis between group analyses did not show a significant difference, although our smaller cohort size may have been a factor.
Icotinib was observed to have a superior safety and tolerability profile compared with cisplatin plus docetaxel, with a reduction in the frequency of overall AEs reported. Furthermore, no unexpected safety signals were noted and the AE profile was in line with that profile reported previously. With icotinib treatment, rash, elevated ALT and AST were regarded as the common AEs. Whereas with cisplatin-based chemotherapy, the most common AEs were nausea and vomiting, hematological toxicity, loss of appetite, and fatigue. None of the patients in the icotinib group developed interstitial lung disease, whose incidence was relatively lower compared with previous reports of treatment with gefitinib,26,27 suggesting that treatment with icotinib is safe and tolerable.
Limitations to our study include premature OS data, a relatively small sample size, and the limited potential for generalizability or extrapolation of our results to non-Asian populations with NSCLC. OS was confounded by the crossover design. When disease progression occurred in the chemotherapy group or drug resistance emerged in the icotinib group, most patients chose to receive targeted drug therapy or a third-generation EGFR TKI, respectively, as second-line therapy. Thus, OS data were heterogeneous with too short of a follow-up term to permit analysis. However, DFS has been shown to be a valid surrogate endpoint for OS in the adjuvant setting and is not confounded by treatment crossover,28 despite OS being an important endpoint in appropriate clinical trial designs. The ongoing ALCHEMIST (NCT02193282) and ADAURA (NCT02511106) trials may also help to clarify whether EGFR TKIs do provide an OS benefit in the adjuvant setting. Additionally, because of the smaller cohort size, our study may inevitably be biased, emphasizing the need for future confirmatory studies. Finally, these results may only apply to a part of the general population as our findings derived from a single-center retrospective trial. Nevertheless, the baseline characteristics were well balanced, and allowed for accurate comparison of survival between two treatment groups.
In conclusion, icotinib provided statistically significant DFS and OS benefits and reduced toxicity in EGFR mutation-positive patients with completely resected stage II (T1-2N1M0) NSCLC compared to patients receiving cisplatin plus docetaxel in our single-center retrospective study. However, OS data were premature, and our results require further validation by prospective randomized trials.
Acknowledgments
The authors thank the members of the laboratory for their helpful comments. The study was supported by fund from Education Foundation of Zhejiang Province (Y201636498). Saibo Pan and Shijie Wang are co-first authors for this study.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020.. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
3. Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–649.
4. Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J clin oncol. 2008;26(21):3552–3559.
5. Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719–727.
6. Pepe C, Hasan B, Winton TL, et al. Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. J clin oncol. 2007;25(12):1553–1561.
7. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a Phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J clin oncol. 2011;29(21):2866–2874.
8. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957.
9. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised Phase 3 trial. Lancet Oncol. 2012;13(3):239–246.
10. Wu Y-L, Zhou C, Liam C-K, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883–1889. doi:10.1093/annonc/mdv270
11. Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, Phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol. 2015;26(9):1877–1883.
12. Liu D, Zhang L, Wu Y, et al. Clinical pharmacokinetics, safety, and preliminary efficacy evaluation of icotinib in patients with advanced non-small cell lung cancer. Lung Cancer. 2015;89(3):262–267.
13. Shen YW, Zhang XM, Li ST, et al. Efficacy and safety of icotinib as first-line therapy in patients with advanced non-small-cell lung cancer. Onco Targets Ther. 2016;9:929–935.
14. Xue ZX, Wen WX, Zhuang Y, Hua ZJ, Xia YN. Comparison of the efficacy of icotinib in patients with non-small-cell lung cancer according to the type of epidermal growth factor receptor mutation. Mol clin oncol. 2016;5(3):265–268.
15. Qin N, Yang X, Zhang Q, et al. Efficacy of Icotinib treatment in patients with stage IIIb/IV non-small cell lung cancer. Thoracic Cancer. 2014;5(3):243–249.
16. Liu YT, Hao XZ, Liu DR, et al. Icotinib as adjuvant treatment for stage II-IIIA lung adenocarcinoma patients with egfr mutation (ICWIP study): study protocol for a randomised controlled trial. Cancer Manag Res. 2020;12:4633–4643.
17. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thoracic Oncol. 2016;11(1):39–51.
18. Hu X, Zhang L, Shi Y, et al. The efficacy and safety of icotinib in patients with advanced non-small cell lung cancer previously treated with chemotherapy: a single-arm, multi-center, prospective study. PLoS One. 2015;10(11):e0142500.
19. Shao L, Zhang B, He C, et al. Efficacy and safety of icotinib in Chinese patients with advanced non-small cell lung cancer after failure of chemotherapy. Chin Med J. 2014;127(2):266–271.
20. Pisters KM, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J clin oncol. 2007;25(34):5506–5518.
21. Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Sys Rev. 2015;3:CD011430.
22. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J clin oncol. 2013;31(27):3320–3326.
23. Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind, phase III trial. J clin oncol. 2015;33(34):4007–4014.
24. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19(1):139–148.
25. Yue D, Xu S, Wang Q, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): a randomised, open-label, Phase 2 trial. Lancet Respir Med. 2018;6(11):863–873.
26. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. oncologist. 2003;8(4):303–306.
27. Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med. 2008;177(12):1348–1357.
28. Chen YP, Sun Y, Chen L, et al. Surrogate endpoints for overall survival in combined chemotherapy and radiotherapy trials in nasopharyngeal carcinoma: meta-analysis of randomised controlled trials. Radiother Oncol. 2015;116(2):157–166.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.