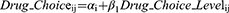Back to Journals » Patient Preference and Adherence » Volume 17
I-PreFer Study: A Discrete Choice Experiment to Explore Patient, Caregiver and Pulmonologist Preferences of Idiopathic Pulmonary Fibrosis Pharmacological Treatment Options
Authors Hollmen M, Wijsenbeek M, Bromilow T , Smith AB, Mealing S , Lewis D , Galvin L, Jones S, Asijee G, Soulard S, Froidure A
Received 23 February 2023
Accepted for publication 5 July 2023
Published 4 August 2023 Volume 2023:17 Pages 1895—1906
DOI https://doi.org/10.2147/PPA.S409767
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Maria Hollmen,1 Marlies Wijsenbeek,2 Tom Bromilow,3 Adam B Smith,3 Stuart Mealing,3 Damian Lewis,3 Liam Galvin,4 Steve Jones,4 Guus Asijee,5 Stéphane Soulard,5 Antoine Froidure6,7
1Faculty of Medicine, University of Helsinki, Helsinki, Finland; 2Centre of Excellence for Interstitial Lung Diseases and Sarcoidosis, Department of Respiratory Medicine, Erasmus MC, University Medical Center, Rotterdam, the Netherlands; 3York Health Economics Consortium (YHEC), York, UK; 4European Pulmonary Fibrosis & Related Disorders Federation, Overijse, Belgium; 5Boehringer Ingelheim, Amsterdam, the Netherlands; 6Service de Pneumologie, Cliniques Universitaires Saint-Luc, Brussels, Belgium; 7Institut de Recherche Expérimentale et Clinique, UCLouvain, Belgium
Correspondence: Maria Hollmen, Faculty of Medicine, University of Helsinki, Haartmaninkatu 8 P.O. Box 63, Helsinki, 00014, Uusimaa, Finland, Email [email protected]
Purpose: Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, and ultimately fatal lung disease that, while rare, has seen incidence rise over time. There is no cure for IPF other than a lung transplant, though two antifibrotic (AF) drugs do exist to slow disease progression. While these drugs are efficacious, they are both associated with differing profiles of adverse events. This study aimed to elicit patient, caregiver and pulmonologist preferences on the treatment profiles of AFs via a discrete choice experiment (DCE).
Patients and Methods: The DCE and associated survey were distributed across 7 European countries, and bespoke DCEs were developed for patients/caregivers and pulmonologists. After collaboration with European Pulmonary Fibrosis & Related Disorders Federation (EU-PFF) and expert pulmonologists, respectively, a patient/caregiver DCE with 5 attributes and a pulmonologist DCE with 6 attributes were finalized. The DCEs had a blocked approach to reduce participant burden and were distributed on an online survey platform. Preferences were estimated through conditional multinomial logit regression analysis.
Results: Ninety-five patients, 22 caregivers and 115 pulmonologists fully completed their respective DCEs. Overall, patients and caregivers preferred management of treatment-related adverse events over both survival benefits and disease progression. Nearly all preference levels were found to be significantly different from their reference level. In contrast, pulmonologists showed a greater preference for control of lung function and exacerbations over adverse events. Although there were relative differences between the univariate subgroups in terms of the preference weights, most of these were not statistically significant.
Conclusion: The outcomes from this study suggest that while patients and caregivers had similar preferences for characteristics of IPF treatments, pulmonologists did not share those same preferences. Patients and caregivers preferred safety, while pulmonologists preferred efficacy. These differences should be considered by clinicians to better involve the patient in treatment decision-making for IPF.
Keywords: treatment preferences, online survey, outcomes research, lung disease
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common form of pulmonary fibrosis. It is a chronic, progressive, and ultimately fatal lung disease. Whilst considered rare, the incidence of IPF has risen over time.1,2 There is currently no cure for IPF, except for lung transplantation in a small minority of patients. Without treatment, the median survival from diagnosis is three to five years.3,4 There are two main antifibrotic pharmacotherapies currently available for IPF: pirfenidone and nintedanib. Both treatments aim to slow the process of scarring, thereby delaying lung degeneration and are recommended by the latest clinical practice guidelines on IPF.5–7 Whilst efficacious, both pharmacotherapies are associated with frequent adverse events, such as diarrhea and sun sensitivity (nintedanib and pirfenidone respectively).8,9 Therefore, in daily practice, treatment choices should, preferably, be jointly decided between healthcare professionals and patients to ensure that the chosen treatment is appropriate for the patient’s preferences, lifestyle and values.
Although treatment options are limited, different stakeholders may have different preferences regarding treatment. Preferences in treatment options may be elicited directly (ie, directly asking the preference between existing treatment options), however this opens up the possibility of potential bias towards certain treatments. One way to elicit stakeholder preferences on treatment options whilst avoiding this bias is through discrete choice experiments (DCE). DCEs are a method to indirectly elicit preferences from participants. Instead of directly asking participants whether they would prefer one real-life treatment or another, a DCE asks participants to decide between numerous hypothetical scenarios where the levels of different factors (attributes) are varied. These decisions are analyzed to understand which attributes (and levels within each attribute) most significantly affect patient choice.10
IPF treatment preferences have previously only been collected from a patient perspective in the United States.11 The aim of this study was to use discrete choice experiments to collect a broader range of preferences (patient, caregiver and pulmonologist) across seven European countries where preferences had not been collected before. Collecting preferences from different stakeholders allows us to compare them and identify areas of discord. In addition, the study aimed to examine the effect of patient characteristics on preferences. This information can then be used to improve the patient experience in the management of IPF by informing pulmonologists on the issues patients and caregivers value.
Materials and Methods
The study entitled “I-PreFer” (Preferences for Treatment Options in Idiopathic Pulmonary Fibrosis) covered seven countries: Belgium, Finland, France, Greece (pulmonologists only), the Netherlands, the Republic of Ireland (ROI) and the United Kingdom (UK). Three surveys were developed (one for patients, caregivers and pulmonologists) and each survey contained a DCE, and a questionnaire identifying patient characteristics. In this manuscript, we describe the development of the DCEs and their results after international administration. The results from the questionnaire portion of the surveys can be found in Hollmen et al.12 The DCEs were developed in English and translated using a medical translation firm (TransPerfect Translations). The organization running the study, York Health Economics Consortium, is wholly owned by the University of York and seeks ethical approval for studies involving human subjects from the University. As such, the I-PreFer study and associated surveys were granted ethical approval by the University of York Health Sciences Research Governance Committee (HSRGC/2021/448/D: I-PreFer, letter dated 14 April 2021) and conformed to all relevant pharmacovigilance rules and reporting standards. A study information page was included at the start of the survey to inform stakeholders of the aims of the research, any potential risks of participation, the anonymous nature of the study and where the data would be stored. This was followed by a question asking for their voluntary consent to participate. If consent was given, the survey continued. Otherwise, the survey ended. This study was conducted in accordance with, and complies with, the principles laid out by the 18th World Medical Assembly in the Declaration of Helsinki (Helsinki, 1964) and all subsequent amendments.
DCE Design
Two DCEs were developed, one for patients and caregivers, and one for pulmonologists. In the initial stage of the study, the aim was to identify the key treatment benefits and adverse effects that would influence decision-making and treatment preferences, hereafter referred to as “attributes”. An initial list of 19 potential attributes was generated via a desk-based literature review that identified a previous DCE in IPF (Hollin et al)11 and the summary of product characteristic documents (SmPC) for pirfenidone13 and nintedanib.14
Three advisory boards were organized to review and refine the potential list of attributes into the final attributes for each DCE. The patient/caregiver board was attended by a patient and caregiver who were both representatives from the European Pulmonary Fibrosis & Related Disorders Federation (EU-PFF). The pulmonologist boards were attended by three external clinicians with experience in managing IPF. Due to sample size considerations, the patient/caregiver was planned to have six attributes while the pulmonologist DCE was planned to have five attributes.
Participants of the advisory boards were first asked to review the initial list of 19 attributes, then add any potential attributes that were missed in the literature review and finally, remove any attributes that they considered irrelevant to the DCE. Then, the participants were asked to rank the remaining attributes in terms of those which were most important in treatment decision-making. The top six (five for the pulmonologist DCE) were then taken as the final attributes for the DCEs. The participants were then asked to develop the levels for each of the final attributes using their experience of the attribute. It was planned for most attributes to have three levels, however the participants reached a consensus for two and four levels on certain attributes. These collaborative steps led to the systematic creation of a patient/caregiver DCE and a pulmonologist DCE which comprised six and five attributes, respectively. The final designs of the two DCEs are shown in Table 1 and 2. A list of the original 19 attributes and their final ranking for each DCE and an example choice set for each DCE are included in Tables S1–S3.
 |
Table 1 Patient and Caregiver DCE |
 |
Table 2 Pulmonologist DCE |
DCE Implementation and Administration
After the DCEs were finalized, they were transferred onto an online survey platform for data collection (Qualtrics LLC). To complete the DCE, the participating pulmonologists, patients and caregivers were required to grade their preferences based on pre-defined attribute levels.
Participants were recruited between November 2021 and January 2022. The method of recruitment varied across participant type and country; however, all methods involved potential participants being presented with a study summary and a link to an online survey. The patient and caregiver surveys were distributed by EU-PFF and local patient groups. In Belgium and the Netherlands, the survey was also distributed by local nurses. There was no patient or caregiver survey for Greece since it was advised that the surveys would have to be paper-based and this was not feasible due to project constraints. There were no exclusion criteria implemented for the patient and caregiver surveys; the only requirements were that the patient currently had IPF, and caregivers were treating, or had been treating, a patient with IPF. No incentives were offered to patients or caregivers. The pulmonologist surveys were distributed by a third-party market research company (IQVIA). In addition, the three expert clinicians who participated in the advisory boards shared the study summary and link with their colleagues. To be eligible to complete the survey, pulmonologists were required to be reviewing at least three IPF patients per year. Pulmonologists were compensated for their time by the study sponsor at a fair market rate to incentivize participation, although the study sponsor was not involved in the recruitment of pulmonologists.
Statistical Analysis
The DCE was designed and analyzed in R Studio (version 4.1.1) statistical software using the following packages: idefix, support.CEs and apollo.10,15–17 The DCE was designed so that participants were presented with two hypothetical drugs: Drug A and Drug B. For each attribute, the hypothetical drug was assigned one of the attribute levels. The participant was asked to read through each scenario (or “choice set”) and choose which drug they would prefer. The combination of attributes and levels meant that a simple orthogonal design (where all participants complete the same choice sets) was not possible as this would result in too many questions and a high burden on the participant. A blocked design was therefore presented with the patients and caregivers divided into 6 blocks of 12 choice sets and the healthcare professionals (HCPs) into 4 blocks (12 choice sets). Each participant was randomly allocated to complete one of the blocks (12 choice sets) using a randomizer that ensured each block was shown an even number of times. The nature of the DCEs meant that only participants who completed the entire DCE were included in the final analyses.
The relative importance of the attribute levels in driving drug choice was estimated through conditional multinomial logit (MNL) regression analysis. This model represents a variation of the logistic regression that can be used for matched, clustered, or longitudinal data. The same MNL regressions were run separately using DCE data from patients, caregivers, and pulmonologists. Dummy coding was applied to the models. The model (shown in Equation 1) only included DCE choice data. The beta coefficients in the equation represent the participants’ preference weights for each individual attribute level, relative to a reference level (eg, the beta coefficient for SUN_2 represents the preference for SUN_2 over SUN_1). Once the beta value for each attribute level was determined, it was used to calculate an odds ratio for preference over the baseline level. This odds ratio represented how much more preferred a level was over the baseline level. Statistical significance was defined as p ≤ 0.05. The aim was to conduct multivariate analyses that included other predictors such as age and sex. Several univariate analyses were also conducted, with the cohorts based on answers from the questionnaire portion of the survey. The following subgroups are age (patients aged ≤70 versus those >70 years), gender (male versus female) and disease severity (self-reported mild or moderate versus self-reported severe) and current treatment (of the cohort who is currently treated, patients who self-reported currently taking nintedanib versus patients who self-reported currently taking pirfenidone).
i = individual
j = drug choice profile
Drug_Choice_Level = Level of the attribute
α = intercept
β1 = coefficient1
Results
Recruitment and Socio-Demographic Data
A total of 111 patients were recruited into the study; 95 of these completed the DCE in full (85.6%). The majority of patients were men (62%) aged between 61 and 70 years (44%). Ninety-one percent of the patients recruited were white, and 95% had completed secondary or tertiary education. In terms of disease severity, 47% of patients self-reported severe disease, 40% self-reported moderate disease and 13% self-reported mild disease. The breakdown of those currently taking an IPF medicine was 54% taking nintedanib, 42% taking pirfenidone and 4% taking another IPF medicine. Further details of the demographic data for the patients are reported in Hollmen et al.12
There were 22 caregivers recruited to the study, of whom most were from Finland, France and the UK. The majority of caregivers were women aged between 51 and 70 years, living with the patient (82%) (their partner/spouse, 91%). Most caregivers provided three or more hours of care every week. Further details of the demographic data for the caregivers are reported in Hollmen et al.12
There were 140 pulmonologists recruited into the study, of whom 115 (82%) completed the entire survey. The largest number of HCPs was recruited from the UK (51%). The majority of HCPs were in public practice (80%), and 55% had experience in managing IPF for up to ten years. Further details of the demographic data for the pulmonologists are reported in Hollmen et al.12
Patient and Caregiver Preferences
The results of the patient DCE are shown in Table 3 and Figure 1. There is a consistent, logical pattern with “better” outcomes preferred over less beneficial outcomes for five of the 6 attributes. For example, slower disease progression (DIS_2) is “preferred” more than twice as much as disease progression remaining unchanged (odds ratio [OR]: 2.06). Similarly, patients’ preferences for extending life beyond a year are three times greater than no survival benefit (OR: 3.03). Similar preferences are seen for mild or moderate diarrhea relative to severe diarrhea (OR: 3.48) and having no nausea compared with severe nausea (OR: 3.85). The only exception to this pattern is the preference reversal between “disease progression stopped” and “disease progression reversed”. Here, patients seem to prefer disease progression being stopped over disease reversal. Overall, patients preferred management of treatment-related adverse events over both survival benefits and disease progression. Reduction in exacerbations was the least important criteria to patients.
 |
Table 3 DCE Coefficients – Patients |
Multivariate analysis was not possible due to sample size limitations. Although there were relative differences between the univariate subgroups in terms of the preference weights, most of these were not statistically significant. The results of these univariate analyses are shown in Figures S1–S4. Managing nausea and diarrhea was statistically more important to men than women. There were also statistically significant differences between age groups, with those aged ≤70 years valuing efficacy criteria such as reversal of disease progression or survival more than older patients (aged >70 years). There was a statistically significant higher preference for “disease progression stopped” among the nintedanib cohort compared with the pirfenidone cohort, but no statistically significant difference was observed with regard to adverse event management by treatment group. For those with severe disease, there was a lower preference for “number of exacerbations reduced” compared with those without self-reported severe disease.
The results for the caregiver DCE coefficients are shown in Table 4 and Figure 2. There was a consistent, logical pattern for each attribute, and slightly stronger relative magnitudes of preferences for “better” levels than in the patient DCE.
 |
Table 4 DCE Coefficients – Caregivers |
Pulmonologist Preferences
The DCE coefficients for the pulmonologists are shown in Table 5, and Figure 3. This shows a linear progression in terms of preferences with the exception of a reversal between the last two sun sensitivity categories (ie, more extreme levels had more extreme preferences). In contrast to the patients, the pulmonologists showed a greater preference for control of lung function and exacerbations over adverse events (ie, GI and photosensitivity). Titration was not deemed relevant by the pulmonologists.
 |
Table 5 DCE Coefficients – Pulmonologist |
Discussion
The DCE outcomes from the I-PreFer study showed that while patient and caregiver treatment preferences were very similar, pulmonologists did not share those same preferences. Patients and caregivers tended to prefer the control or management of adverse events, whilst pulmonologists tended to prefer improvements in lung function.
Patient and Caregivers
Adverse events, especially GI problems, such as diarrhea and nausea, were important to patients. Having no diarrhea was preferred 2.05 times more than having no sun sensitivity based on the OR results. This result is in line with previous research.11 This preference might be influenced by the time spent outside by the patient of the current cohort and the climate and season, and it is also possible that patients were spending less time outside because the DCEs were completed during the Covid-19 pandemic.
It was originally anticipated that patients on pirfenidone would have a higher preference for treatments that minimized photosensitivity, given that the AEs (adverse events) associated with this treatment are skin rash and photosensitivity. However, the results from the univariate analysis showed that current antifibrotic treatment had no significant impact on patient preferences for management of diarrhea or photosensitivity.
In general, the overall preference for reduction in exacerbations (ie, sudden accelerations in disease progression resulting in reduction in lung function) was lower than the preferences for other treatment attributes. This study showed that patients tended to prefer manageable adverse effects more than an impact on mortality or lung function decline. This could be due to the fact that current treatments limit disease progression but do not halt disease nor improve symptoms. The exception to this was that patients who graded their disease as “not severe” had a significantly higher preference for a reduction in exacerbations as shown in Figure S4.
When compared with previous IPF preference studies, it appears that the higher preference for management of diarrhea over improvements in survival in this study, was in contradiction to the findings reported by Hollin et al, where improving lung function was 1.6 times as important as decreasing the risk of GI problems.11 Similarly, results from a survey of IPF treatment preferences conducted by Camponeschi et al revealed that the most important treatment attribute was effect on lung function (35%) followed by the risk of GI problems (23%).18 However, both the Hollin et al and Camponeschi surveys were much smaller than the I-Prefer study (33 and 35 patients respectively) meaning the robustness of the findings from these studies is less certain.11,18 Furthermore, the wording used in these studies was different to that used in the I-Prefer DCE, so a direct comparison cannot be made.
The small sample size and blocked approach of the DCEs led to large confidence intervals in the caregiver DCE. The main trends of the caregiver DCE matched those of the patient DCE. However, drawing strong conclusions from the caregiver DCE should be cautioned.
Pulmonologists
For the pulmonologists, there was a logical, linear pattern observed, as preferences increased for greater control over lung function and amelioration of adverse events. The exception to this pattern was for sun sensitivity causing low impact on HRQoL (OR: 2.4) compared with “no sun sensitivity” (OR 1.96) although there was no statistically significant difference between the two preferences. In general, stabilization or improvement in lung function, as well as no exacerbations, took precedence over management of GI problems and sun sensitivity. Pulmonologists had the strongest preference for lung improvement. Specifically, pulmonologists preferred improvement in lung 12 times more than a slowdown in disease (or large decline, p < 0.05) and almost three times more than preventing a small decline in lung disease. Improvement in lung function was significantly more preferred than management and control of GI symptoms, sun sensitivity and a straightforward medication titration process. Pulmonologists had greater preferences for the management of GI adverse events compared with sun sensitivity adverse events.
Strengths
The I-Prefer study builds on previous results from Hollin et al11 and Camponeschi et al18 by providing preference data from a larger cohort of IPF patients and caregivers. In addition, the I-Prefer study is the first study to collect treatment preference data from pulmonologists and investigate the effect of patient characteristics on patient preferences. The DCE design to elicit stakeholder preferences is advantageous in that participants simply choose one option over another, which is less cognitively complex than providing a free-text response to a question.19 This minimizes the chance of bias.
Limitations
While most results followed a logical preference order, in that “better” outcomes were preferred over “worse” outcomes, there was a reversal of preference order with regard to “disease reversal” and “disease progression stopped” with patients selecting a higher preference for “disease progression stopped” over “disease reversal”. This implies that there may be a misunderstanding of the two terms, or it could be reflective of a pragmatic acknowledgement that there is no curative treatment of IPF. Similarly, the lower preference for reduction in exacerbations may indicate a lack of understanding of what an exacerbation is. Future DCEs could be piloted on a small number of patients and caregivers to ensure that all terms are likely to be understood by participants.
Another limitation is that the patient respondents to the DCE were all based in northern Europe. It is possible that including patients from more southern countries (ie, with greater sun exposure or other dietary habits) would have revealed different preferences around adverse event. Future studies are needed to explore this.
A third limitation is the lack of multivariate analyses. The study had originally planned to include socio-demographic covariates in the DCE MNL models. However, the lower-than-expected sample sizes collected meant that this could not occur without reducing the quality of the models.
Conclusion
This DCE has shown that patients and caregivers have different preferences compared to pulmonologists with regard to the benefits they would most like from their treatment. Among patients and caregivers, the strongest preferences were for control of adverse events, especially gastro-intestinal, whereas among pulmonologists, the strongest preferences were for efficacy, with treatments that could improve lung function or prevent exacerbations. This discord in preference demonstrates that more education by the pulmonologist to the patient is required on the benefits of treatments and on the management of adverse events. Finally, the findings also suggest that clinicians should better involve patients in treatment decision-making for IPF.20
Funding
This manuscript is supported and funded by Boehringer Ingelheim International GmbH (BI). Boehringer Ingelheim was given the opportunity to review the abstract for medical and scientific accuracy as well as intellectual property considerations. EU-PFF received a financial compensation to help support the design of the questionnaire, disseminate the survey and analyse the results. The authors did not receive payment related to the development of the manuscript.
Disclosure
Stephane Soulard and Guus Asijee are employees of Boehringer Ingelheim. Steve Jones is President of EU-PFF and a patient. Liam Galvin is Chief Executive of EU-PFF and was a carer. He also reports grants from Boehringer Ingelheim, The Roche Group, Chiesi Pharmaceuticals, Vicore Pharma, Trevi Therapeutics, Bristol Meyer Squibb, CSL Behring; travel support and/or registration from European Lung Foundation and ERN-Lung, during the conduct of the study. Tom Bromilow, Stuart Mealing, Adam B Smith, and Damian Lewis of York Health Economics Consortium provided writing, editorial support, and formatting assistance, which was contracted and funded by Boehringer Ingelheim. Maria Hollmen and Antoine Froidure received payment from Boehringer Ingelheim for helping to develop and review the questionnaire and DCE and comment on the study results. Antoine Froidure discloses consultancy and speakers fees from GlaxoSmithKline, Roche and Boehringer Ingelheim, outside the submitted work. Antoine Froidure’s institution has received unrestricted research grants from Roche and Boehringer Ingelheim. Marlies Wijsenbeek discloses the following: grants or contracts received from Hoffmann-La Roche, and Boehringer Ingelheim, outside the submitted work. Consulting fees received from Roche, Boehringer Ingelheim, Galapagos, Bristol Myers Squibb, Galecto, and Respivant, outside the submitted work. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events Boehringer Ingelheim, F. Hoffmann-La Roche, and Novartis, outside the submitted work. Support for attending meetings and/or travel received from Boehringer Ingelheim and F. Hoffmann-La Roche, outside the submitted work. Participation on a Data Safety Monitoring Board or Advisory Board for Savara and Galapagos. Leadership or fiduciary role in other board, society, committee or advocacy group; Chair of the Idiopathic Interstitial Pneumonia group of the European Respiratory Society; Member of the board of the Netherlands Respiratory Society; Member of the scientific advisory board of the European Idiopathic Pulmonary Fibrosis and related disorders federation; Chair of the educational committee of the European Reference Network for rare Lung Diseases; Advisory board of the Dutch Lung fibrosis and Sarcoidosis patient associations. She also reports grants paid to his institution from Astra Zeneca, Boehringer Ingelheim, BMS, CLS Behring, Daiichi, Hoffman la Roche, Galapagos, Galecto, Horizon Therapeutics, Kinevant, Molecure, NeRRe Therapeutics, Novartis, Puretech, Thyron, Trevi Therapeutics, and Vicore, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811–1823. doi:10.1056/NEJMra1705751
2. Olson AL, Gifford AH, Inase N, Fernández Pérez ER, Suda T. The epidemiology of idiopathic pulmonary fibrosis and interstitial lung diseases at risk of a progressive-fibrosing phenotype. Eur Respir Rev. 2018;27(150):180077. doi:10.1183/16000617.0077-2018
3. Cottin V, Hirani NA, Hotchkin DL, et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018;27(150):180076. doi:10.1183/16000617.0076-2018
4. Fernández Fabrellas E, Peris Sánchez R, Sabater Abad C, Juan Samper G. Prognosis and follow-up of idiopathic pulmonary fibrosis. Med Sci. 2018;6(2):51. doi:10.3390/medsci6020051
5. Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an Update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18–e47. doi:10.1164/rccm.202202-0399ST
6. Salton F, Ruaro B, Confalonieri P, Confalonieri M. Epithelial-mesenchymal transition: a major pathogenic driver in idiopathic pulmonary fibrosis? Medicina. 2020;56(11):608. doi:10.3390/medicina56110608
7. Takeda Y, Tsujino K, Kijima T, Kumanogoh A. Efficacy and safety of pirfenidone for idiopathic pulmonary fibrosis. Patient Prefer Adherence. 2014;8:361–370. doi:10.2147/PPA.S37233
8. Richeldi L, Du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi:10.1056/NEJMoa1402584
9. Taniguchi EM, Kondoh Y, Ogura T, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):821–829. doi:10.1183/09031936.00005209
10. Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13. doi:10.1016/j.jval.2012.08.2223
11. Hollin IL, Dimmock AE, Bridges JF, Danoff SK, Bascom R. Collecting patient preference information using a Clinical Data Research Network: demonstrating feasibility with idiopathic pulmonary fibrosis. Patient Prefer Adherence. 2019;13:795. doi:10.2147/PPA.S201632
12. Hollmen M, Bromilow T, Smith AB, et al. I-PreFer study: a questionnaire to explore patient, caregiver and pulmonologist preferences of idiopathic pulmonary fibrosis treatment options. Patient Prefer Adherence. 2023;17:1621–1639. doi:10.2147/PPA.S408857
13. Roche Products Limited. Esbriet SmPC, Esbriet 267 mg film-coated tablets - summary of product characteristics (SmPC); 2022. Available from: www.medicines.org.uk/emc/product/2731/smpc.
14. Boehringer Ingelheim Limited. Ofev SmPC, Ofev 100 mg soft capsules - summary of product characteristics (SmPC). Available from: www.medicines.org.uk/emc/product/1786/smpc.
15. Aizaki H. Basic functions for supporting an implementation of choice experiments in R. J Stat Software Code Snippets. 2012;50(2):1–24.
16. Hess S, Palma D. Apollo: a flexible, powerful and customisable freeware package for choice model estimation and application. J Choice Model. 2019;32:100170. doi:10.1016/j.jocm.2019.100170
17. Team RC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020.
18. Camponeschi GF, Bridges JF, Danoff SK, Richardson B. PP4 - measuring treatment preferences of patients diagnosed with idiopathic pulmonary fibrosis using best-worst scaling. Value Health. 2015;18(3):A10. doi:10.1016/j.jval.2015.03.065
19. Xie S, Wu J, He X, Chen G, Brazier JE. Do discrete choice experiments approaches perform better than time trade-off in eliciting health state utilities? Evidence from SF-6Dv2 in China. Value Health. 2020;23(10):1391–1399. doi:10.1016/j.jval.2020.06.010
20. Say RE, Thomson R. The importance of patient preferences in treatment decisions--challenges for doctors. BMJ. 2003;327(7414):542–545. doi:10.1136/bmj.327.7414.542
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.