Back to Journals » Biologics: Targets and Therapy » Volume 17
Hypoxia Inducible Factor-1α Through ROS/NLRP3 Pathway Regulates the Mechanism of Acute Ischemic Stroke Microglia Scorching Mechanism
Authors Ma X, Jiao J, Aierken M, Sun H, Chen L
Received 30 October 2023
Accepted for publication 13 December 2023
Published 19 December 2023 Volume 2023:17 Pages 167—180
DOI https://doi.org/10.2147/BTT.S444714
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Shein-Chung Chow
Xin Ma,* Junxia Jiao,* Mayila Aierken,* Hong Sun, Li Chen
Department of Clinical Laboratory, Urumqi Friendship Hospital, Urumqi, Xinjiang Uygur Autonomous Region, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong Sun; Li Chen, Department of Clinical Laboratory, Urumqi Friendship Hospital, No. 558 Shengli Road, Tianshan District, Urumqi, Xinjiang Uygur Autonomous Region, 830049, People’s Republic of China, Tel +86-13899816208 ; +86-13579401192, Email [email protected]; [email protected]
Purpose: In vitro experiments explored how the hypoxia-induced factor-1α (HIF-1α) regulates the regulation of pyroptosis in microglial cells (BV 2) in acute ischemic stroke through ROS/NLRP3 pathway.
Methods: The microglia acute phase oxygen-glucose deprivation/reoxygenation (OGD/R) was established, CCK-8 was applied to determine the optimal timing of intervention modeling. HIF-1α was overexpressed with stabilizer GF-4592 and HIF-1α small molecule interfering RNA (HIF-1α-siRNA), which was divided into group A (blank group), group B (OGD/R model group), group C (model+FG-4592 intervention group), group D (model+siRNA negative control group) and group E (model+HIF-1α-siRNA group). Cell proliferation of different groups was measured by CCK-8 assay. Pyroptosis and intracellular ROS levels were measured by flow cell technology. IL-18, IL-1β levels were measured by ELISA. HIF-1α, GSDMD-D, GSDMD-N, clean-Caspase-1 and NLRP3 protein expression levels were measured by Western blot. On the above experiments, ROS and NLRP3 response experiments were performed to explore how HIF-1α regulates pyroptosis through ROS/NLRP3 pathway.
Results: Hypoxia for 6 h then reoxygenation for 12 h was the optimal intervention time. Compared with groups B and D, cell proliferation in group C was significantly enhanced, pyroptosis, intracellular levels of ROS, IL-18, IL-1β and the expression of GSDMD-D, GSDMD-N, clean-Caspase-1, and NLRP3 proteins were significantly decreased in group C (P < 0.05). However, in group E, the performance of these test indicators were exactly the opposite, and the difference was statistically significant (P < 0.05). Through ROS and NLRP3 response experiments, it was found that HIF-1α Inhibition of Pyroptosis by inhibiting ROS/NLRP3 pathway.
Conclusion: Overexpression of HIF-1α factor can inhibit microglia pyroptosis. HIF-1α factor has an inhibitory effect on the ROS/NLRP 3 pathway, which can inhibit the pyroptotic process in microglia.
Keywords: hypoxia-inducible factor-1α, acute ischemic stroke, microglial pyroptosis, reactive oxygen species, nucleotide-binding oligomerization domain-like receptor protein 3
Introduction
Ischemic stroke is a disease with a high fatality rate. Due to its poor prognosis, it puts great pressure on global health management.1 Ischemic stroke in the acute phase involves pale tissue with mild enlargement of brain tissue in the hypoxic cell necrotic area between 1 and 24 hours after onset, and significant changes in both microglia and endothelial cells.2 The hypoxia-inducible factor-1α (HIF-1α) is an important transcription factor regulating the complex cellular stress response of the body.3 The HIF-1α factor plays an important role in regulating the intracellular environment in the ischemic and hypoxic environment and activating the transcription of various downstream target genes.4,5
Microglia are the earliest and fastest innate immune cells with stress response after brain tissue injury, and researchers pay attention to the inflammatory response of ischemic stroke.6–8
In the early stages of brain injury, microglia undergo rapid activation, a process that leads to the release of various cytokines, which in turn causes persistent inflammatory damage to the brain tissue.9–11 Pyroptosis, a special form of cell death, is a manifestation of cell swelling with condensed chromatin reaches plasma membrane permeability, subsequent lysis of swollen cells releases inflammatory factors involved in the inflammatory response after brain injury.12–14 Microglial pyroptosis is closely related to the inflammatory cascade of ischemic stroke. The nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) formed by microglial activation participates in the occurrence of pyroptosis and constitutes the classical pyroptosis pathway.15,16
Therefore, this study intends to deeply explore the regulation mechanism of HIF-1α on microglial pyroptosis in acute cerebral ischemia and hypoxia through in vitro cell modeling, which will help to deepen the clinical understanding of acute ischemic stroke and provide potential targets for clinicians in the precise treatment of acute ischemic stroke.
Materials and Methods
Material
BV2 Mouse Microglia Cell Strain
BV 2 mouse microglia cells were obtained from Wuhan Prosai Biotechnology Co., LTD. (stock number: CM-0493), and the cells were introduced to the library for less than 5 generations.
2. Reagents and consumables
2.1 Reagents and consumables are detailed in Table 1.
2.2 Details of the experimental instruments are shown in Table 2.
 |
Table 1 Reagent Schedule |
 |
Table 2 Experimental Instruments |
Method
1. Cell line cultivation Cell culture conditions were MEM medium + 10% FBS + 1%PS, 37℃, 5% CO2 with saturated humidity. The cells were grown continuously for 8 days and detected the OD values by a microplate reader every 24 hours.
2. In vitro experiments
2.1 To model oxygen-glucose deprivation reoxygenation in the acute phase (OGD/R)
After 24 h incubation, BV 2 cells were replaced. Cells were placed in a 37℃ saturated gas incubator with 95% nitrogen plus 5% oxygen for 3 h and 6 h of hypoxia culture and replaced with fresh MEM complete medium, and cells were replaced into a CO2 incubator for normal incubation for 12 hours. The control group was always cultured in the CO2 incubator for the same time.
2.2 CCK-8 detects cell viability to determine the optimal timing of intervention
3. Investigate the effect of HIF-1α on cell pyroptosis in the OGD/R model
3.1 Experimental grouping
(1) Blank group: The BV 2 cells were routinely cultured.
(2) OGD/R model group: BV2 cells were subjected to hypoxia for 6 hours in sugar-free basal medium, followed by reoxygenation for 12 hours in normal medium.
(3) OGD/R model + FG-4592 intervention group: After BV2 cells were cultured for a certain period of time, FG-4592 (50 μM) was added intervention for 1 hour, hypoxia for 6 hours in a sugar-free basal medium, followed by reoxygenation in a normal medium for 12 hours.
(4) Model + siRNA negative control group: After transfection of siRNA NC into BV2 cells for 20 hours, hypoxia was observed in sugar-free basal medium for 6 hours, followed by reoxygenation in normal medium for 12 hours.
(5) Model + HIF-1α -siRNA group: Transfection of siRNA-HIF-1α-835 into BV2 cells for 20 hours, hypoxia was observed in sugar-free basal medium for 6 hours, followed by reoxygenation in normal medium for 12 hours.
3.2 Cell proliferation and pyroptosis were detected in the different groups
Different grouped cell proliferation was assessed by CCK-8. Cell pyroptosis was determined in different groupings by flow cytometry.
3.3 Detection of intracellular reactive oxygen species (ROS) levels by flow cytometry
3.4 The level of IL-1β, IL-18 concentration in the cell supernatants was determined by ELISA
3.5 Western Blot detected the expression of HIF-1α, GSDMD-D, GSDMD-N, cle-Caspase-1, and NLRP 3 proteins in different groups
3.6 On the above experimental basis further explored the role of ROS in HIF-1α regulation of microglia pyroptosis
Using ROS inhibitor N-acetylcysteine (NAC) to further explore the role of ROS in HIF-1α Regulation of cell apoptosis. The ROS inhibitor NAC combined with HIF-1α intervention could be divided into blank group, blank + ROS + NAC group, OGD/R model + FG-4592 intervention group, OGD/R model + FG-4592 intervention+ROS+NAC group, model + HIF-1α-siRNA group, and model+HIF-1α-siRNA+ROS+NAC group. Intracellular ROS activity, NLRP3 protein expression, LDH activity, IL-1 β, and IL-18 were examined again.
3.7 On the above experimental basis, we further explored the role of NLRP3 protein in the regulation of HIF-1α in microglia pyroptosis
In this study, the NLRP3 inhibitor MCC950 was used to further validate the role of NLRP3 in regulating cell pyroptosis by HIF-1α. The NLRP3 inhibitor MCC950 combined with HIF-1α intervention could be divided into blank, blank+NLRP3+MCC950, OGD/R+FG-4592 intervention group, OGD/R + FG-4592 intervention + NLRP3 + MCC950, model + HIF-1α -siRNA, and model+HIF-1α-siRNA+NLRP3+MCC950 group. Intracellular ROS activity, NLRP3 protein expression, LDH activity, IL-1 β, and IL-18 were then again examined.
Statistical Analysis
All data were represented by mean ± standard deviation (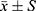 ). Spss19.0 software was used to analyze the data of each group. One-way ANOVA was used to analyze the data between groups, P<0.05 showed significant differences.
). Spss19.0 software was used to analyze the data of each group. One-way ANOVA was used to analyze the data between groups, P<0.05 showed significant differences.
Results
The BV 2 Cell Growth Curve
Cultivate BV2 cells in vitro, detect OD values at the wavelength of 450 nm using an enzyme-linked immunosorbent assay every 24 hours, and plot growth curves. From the growth curve, it can be seen that cells are in a slow growth latent period from 1 to 3 days, and the slope of the growth curve is relatively large from 3 to 5 days. The curve of slow growth from 5 to 7 days presents a plateau like flat top period (Figure 1).
 |
Figure 1 BV 2 cell growth curves. (A) Growth plot of BV 2 cells. (B1–B7) Plot of BV 2 cell proliferation at days 1–7. |
Determine the Optimal Intervention Time as 12 Hours of Reoxygenation After 6 Hours of Hypoxia
Compared with the blank group of the BV 2 cell lines, the BV2 cell line showed a decrease in cell viability after 3 hours of hypoxia then 12 hours of reoxygenation, but the difference was not statistically significant (P>0.05). The cell viability of the model significantly decreased after 6 hours of hypoxia then 12 hours of reoxygenation, the difference was statistically significant (P<0.05) (Table 3). The optimal intervention timing was ultimately determined to be 6 hours of hypoxia and 12 hours of reoxygenation.
 |
Table 3 Results of BV 2 Cell Proliferation Detection in Two Groups Under Hypoxia for 3 Hours and 6 Hours ( |
Cells Proliferation Was Significantly Higher in OGD/R Model + FG-4592 Intervention Group but Significantly Decreased in Model + HIF-1α-siRNA Group Cells
The analysis of variance showed that compared with the blank group, the cell proliferation in the other four groups was significantly decreased (P<0.05). Compared with the OGD/R model group, cell proliferation was significantly higher in the OGD/R model + FG-4592 intervention group but significantly decreased in the model + HIF-1α-siRNA group (P<0.05). Compared with the OGD/R model + FG-4592 intervention group, cell proliferation was significantly reduced in the model + siRNA negative control group and model + HIF-1α-siRNA group (P<0.05). Compared with the model + siRNA negative control group, cell proliferation was significantly reduced in the model + HIF-1α-siRNA group (P<0.05) (Table 4).
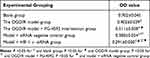 |
Table 4 Results of BV 2 Cell Proliferation Detection in 5 Different Experimental Groups ( |
The OGD/R model+FG-4592 Intervention Group Significantly Reduced the Cell Apoptosis Rate, While the Cell Death Rate Significantly Increased in the model+HIF-1α-siRNA Group
Analysis of variance was used. Compared with the OGD/R model group, the OGD/R model+FG-4592 intervention group significantly reduced the cell apoptosis rate, while the cell death rate significantly increased in the model+HIF-1α-siRNA group (P<0.05). Compared with the OGD/R model + FG-4592 intervention group, the cell pyroptosis rate was significantly increased in the model + siRNA negative control group and model + HIF-1α -siRNA group (P<0.05). Compared with the model + siRNA negative control group, the cell pyroptosis rate was significantly increased in the model + HIF-1α-siRNA group (P<0.05) (Figure 2).
 |
Figure 2 Pyroptosis was determined by flow cytometry. (A) Comparison of pyroptosis analysis, (B–F): flow cytometry results in different groups. Note: ***P < 0.05 was statistically significant. |
The ROS Levels in OGD/R model+FG-4592 Intervention Group Was Significantly Decreased While the model+HIF-1α-siRNA Group Was Significantly Elevated
Analysis of variance: compared with the blank group, the ROS levels of the other four groups were significantly increased (P<0.05). Compared with the OGD/R model group, the ROS levels in OGD/R model+FG-4592 intervention group were significantly decreased, while the model+HIF-1α-siRNA group was significantly elevated (P<0.05). Compared with the OGD/R model + FG-4592 intervention group, the ROS levels were significantly increased in the model + siRNA negative control group and the model + HIF-1α-siRNA group (P<0.05). Compared with the model + siRNA negative control group, the ROS levels were significantly increased in the model + HIF-1α-siRNA group (P<0.05) (Figure 3).
 |
Figure 3 Results of ROS levels in BV 2 cells. (A) Different groups’ cell ROS bar graph, (B1–B5) ROS results by flow cytometry in different groups. Note: **P < 0.05 was statistically significant. |
The Contents of IL-1β and IL-18 in the OGD/R Model + FG-4592 Intervention Group Was Significantly Reduced While the Model + HIF-1α -siRNA Group Was Significantly Increased
Compared with the blank group, IL-1β and IL-18 were significantly increased in the other four groups (P<0.05). Compared with the OGD/R model group, the contents of IL-1β and IL-18 in the OGD/R model + FG-4592 intervention group were significantly reduced while model + HIF-1α -siRNA group’s significantly increased (P<0.05). Compared with the OGD/R model + FG-4592 intervention group, IL-1β and IL-18 were significantly increased in the model + siRNA negative control and model + HIF-1α-siRNA group (P<0.05). Compared with the model + siRNA negative control group, IL-1β and IL-18 content increased significantly in the model + HIF-1α -siRNA group (P<0.05) (Table 5).
 |
Table 5 IL-1 β and IL-18 Content in the Supernatant of Cells in Each Group ( |
The Expression of the Target Proteins Were Significantly Decreased in the OGD/R Model + FG-4592 Intervention Group While Significantly Increased in He Model + HIF-1α -siRNA Group
Comparison of expression levels of different grouped proteins after intervention HIF-1α factor, Compared with the blank group, the expression of the target proteins GSDMD-D, GSDMD-N, cle-Caspase-1, and NLRP3 was significantly increased in the other 4 groups (P<0.05). Compared with the OGD/R model group, the expressions of GSDMD-D, GSDMD-N, cle-Caspase-1, and NLRP3 proteins were significantly lower in the OGD/R model + FG-4592 intervention group, while significantly higher in the model + HIF-1α -siRNA group (P<0.05). Compared with the OGD/R model + FG-4592 intervention group, the four proteins were significantly increased in the model + siRNA negative control group and the model + HIF-1α -siRNA group (P<0.05). Compared with the model + siRNA negative control group, the four proteins were significantly higher in the model + HIF-1α -siRNA group (P < 0.05) (Figure 4).
Inhibition of ROS Would Benefit the Regulation of Cell Pyroptosis by HIF-1α
Compared with the blank group, the blank + ROS + NAC group significantly decreased ROS activity, NLRP3 protein expression, LDH activity, IL-1 β and IL-18 (P<0.05). Compared with the OGD/R model + FG-4592 intervention group, the ROS activity, NLRP3 protein expression, LDH activity, IL-1 β, IL-18 content were significantly reduced in the OGD/R model + FG-4592 intervention + ROS + NAC group (P<0.05). Compared with the model + HIF-1α -siRNA group, the ROS activity, NLRP3 protein expression, LDH activity, IL-1β and IL-18 content in the model + HIF-1α -siRNA + ROS + NAC group were significantly reduced (P<0.05) (Figure 5).
Inhibition of NLRP3 Would Benefit the Regulation of Cell Pyroptosis by HIF-1α
Compared with the blank group, the ROS activity, NLRP3 protein expression, LDH activity, IL-1β and IL-18 were significantly reduced in the blank + NLRP3 + MCC950 group (P<0.05). Compared with the OGD/R model + FG-4592 intervention group, the ROS activity, NLRP3 protein expression, LDH activity, IL-1β and IL-18 content were significantly reduced in the OGD/R model + FG-4592 intervention + NLRP3 + MCC950 group (P<0.05). Compared with the model + HIF-1α -siRNA group, the ROS activity, NLRP3 protein expression, LDH activity, IL-1β and IL-18 content were significantly decreased in the model + HIF-1α -siRNA + NLRP3 + MCC950 group (P<0.05) (Figure 6).
Discussion
Acute ischemic stroke (AIS) is a central nervous system cerebral infarction caused by cerebral ischemia and hypoxia caused by cerebral vascular occlusion. It is a kind of central nervous system disease with the highest clinical mortality and disability rate at present.17,18 Hypoxia inducible factor-1α (HIF-1α) is an important nuclear transcription factor to maintain the survival of tissues and cells and cell homeostasis after the stimulation of hypoxic environment. It is closely related to poor prognosis such as impaired recovery of nerve cells and severe progression of AIS patients.19,20
HIF-1 is made by α, β Heterodimer of two subunits, HIF-1α can regulate the expression of downstream proteins and regulate cell proliferation, apoptosis, tumor glycolysis and tumor angiogenesis under the stimulation of hypoxic-ischemic environment.21–23 HIF-1α is an important subunit that determines the biological activity of HIF-1, which can induce HIF-1α in the environment of ischemia and hypoxia then start downstream gene expression.24 Under normal oxygen condition, the expression level is very low and mainly exists in the cytoplasm, but in hypoxia, a large aggregation of HIF-1α can transfer from the cytoplasm to the nucleus, and the expression level of HIF-1α increases, with the extension of cell ischemia and hypoxia.25,26 HIF-1α in patients with cerebral hemorrhage, the significantly up-regulated expression has high predictive value for the poor prognosis of patients.27
Abnormal activation of microglia will aggravate the inflammatory response in AIS patients. Microglia are the earliest and fastest innate immune cells after brain injury, and they are also important initiators and participants of other types of glial cells in brain tissue, which lead to persistent inflammatory brain injury.28 Some studies have found that microglia have two-way effects of anti-inflammatory and pro-inflammatory in the process of ischemic stroke.29 When ischemic stroke occurs, microglia are immediately activated and rapidly migrate to the lesion site, while secreting inflammatory cytokines and cytotoxic substances to aggravate brain tissue damage.30 Some small molecule substances produced by activated microglia can protect neurons. Mastering the interaction between neurons and microglia is conducive to clinicians’ understanding of the pathological mechanism of nervous system diseases, thus providing a new direction for patients’ precise treatment and improving prognosis.31–33 Pyroptosis, a newly identified pro-inflammatory disease with a biological characteristic cell death mode unique to cell swelling, plasma swelling cell membrane, inflammatory cytokine secretion, and apoptosis.34 When cell pyroptosis occurs, NLRP3 expression increases sharply along with its activation and converting inactive caspase-1 into catalytically active caspase-1, activated caspase-1 will further cleave and activate Gasdermin domain-containing protein (GSDMD), while activating the expression of inflammatory cytokines IL-1β and IL-18.35,36
This study found that overexpression of HIF-1α can inhibit the production of reactive oxygen species (ROS) in microglia. A large amount of ROS accumulation can activate the NLRP3 inflammasome and promote the development of the associated inflammatory cascade.37 When the formation of excess NLRP3 inflammasome activates caspase-inflammasome, the initiation of caspase-1 will not only lead to the production of IL-1β and IL-18 inflammatory factors, but also produce pro-inflammatory intracellular substances that promote the breakage and release of the cytoplasmic membrane.38 Our cell in vitro experiments show that overexpression of HIF-1α in the OGD/R model would enhance microglial proliferation, and Inhibition of HIF-1α expression would inhibit cell proliferation. Flow cytometry detection of intracellular ROS display, after overexpression of HIF-1α factor, it was verified that HIF-1α could inhibit the production of excessive reactive oxygen species. HIF-1α suppresses mitochondrial oxidation respiration and the generation of reactive oxygen species, thus effectively reducing the ROS content in vivo. The analysis by Western blot experiments showed that the protein content of GSDMD-D, GSDMD-N, cle-Caspase-1 and NLRP3 increased in the OGD/R model. This study showed that the expression of IL-1β, IL-18, NLRP3, GSDMD, and cle-Caspase-1 were all increased in microglial pyroptosis in the OGD/R model, and this conclusion is consistent with the conclusion of Qianjiang.39 Our experimental results suggest that ROS and NLRP3 either rise or fall synchronously in the presence of HIF-1α. Overexpression of HIF-1α will inhibit the increase of ROS in microglia and then inhibit NLRP3 cell pyroptosis. This study found that overexpression of HIF-1α factor would reduce the production of IL-1β and IL-18 inflammatory factors in the cells released by microglial rupture.
Based on the above experimental results, we detected ROS activity, NLRP3 protein expression, LDH activity, IL-1β, and IL-18 concentration again, after ROS was inhibited by single factor under the conditions of HIF-1α were unchanged. Statistical analysis found that after the condition of HIF-1α was unchanged and then the inhibition of ROS, the ROS activity, NLRP3 protein expression, LDH activity, IL-1β and IL-18 concentration in cells were significantly decreased to achieve the effect of reducing pyroptosis of microglia. After this experiment, we only inhibited NLRP3 protein under other conditions remained unchanged, and found that the ROS activity was reduced but the difference was not statistically significant, while the NLRP3 protein expression, LDH activity, IL-1β and IL-18 concentrations were reduced and the difference was statistically significant. This response experiment revealed that HIF-1α regulates microglial pyroptosis through inhibition of ROS and NLRP3 protein expression. Reducing the inflammatory response in stroke by inhibiting NLRP3 inflammatory-mediated microglial pyroptosis may be a new direction for the treatment of ischemic stroke.40
In conclusion, our experimental results confirmed that the overexpression of HIF-1α inhibited the increase of ROS in microglia and thus inhibited the inflammasome NLRP3, and ultimately inhibited microglia pyroptosis through the ROS/NLRP3 pathway, thus facilitating the treatment of stroke diseases. The conclusion of this study may provide potential targets for the clinical treatment of ischemic stroke in the acute phase.
There are still shortcomings in this study. First, the recovery test was conducted to detect the Pyroptosis index, which did not involve the detection of related proteins. Second, the related in vivo animal experiments should be further carried out to explore and verify.
Conclusion
In conclusion, our study confirmed that overexpression of HIF-1α factor can effectively inhibit microglial pyroptosis. Secondly, we found that HIF-1α factor inhibits mitochondrial oxidative respiration and reactive oxygen species generation to inhibit the elevation of ROS in microglia and suppress inflammasome NLRP3, thus indicating that HIF-1α factor ultimately inhibits microglia pyroptosis through the ROS/NLRP3 pathway. The conclusion of this study may provide potential targets for the clinical treatment of ischemic stroke in the acute phase.
Data Sharing Statement
The study was approved by the Ethics Committee of Urumqi Friendship Hospital. This study is an in vitro cell experiment with no research risk or adverse effects on patients, and does not involve patient information. The data were anonymized or maintained with confidentiality. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors contributed equally to this manuscript.
Funding
This work was supported by Natural Science Foundation of Xinjiang Uygur Autonomous Region [NO. 2022D01B12].
Disclosure
The authors do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
1. GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):439–458. PMID: 30871944; PMCID: PMC6494974. doi:10.1016/S1474-4422(19)30034-1
2. Ge J. Internal Medicine. People’s Health Publishing House; 2018.
3. Bok S, Kim YE, Woo YK, et al. Hypoxia-inducible factor-1α regulates microglial functions affecting neuronal survival in the acute phase of ischemic stroke in mice. Oncotarget. 2017;8(67):111508–111521. PMID: 29340071; PMCID: PMC5762339. doi:10.18632/oncotarget.22851
4. Sun Y, Chen X, Zhang X, et al. β2-Adrenergic receptor-mediated HIF-1α upregulation mediates blood brain barrier damage in acute cerebral ischemia. Front Mol Neurosci. 2017;10:257. Erratum in: Front Mol Neurosci. 2017 Nov 20;10:392. PMID: 28855859; PMCID: PMC5558520. doi:10.3389/fnmol.2017.00257
5. Xu W, Xu R, Li Z, et al. Hypoxia changes chemotaxis behaviour of mesenchymal stem cells via HIF-1α signalling. J Cell Mol Med. 2019;23(3):1899–1907. PMID: 30628201; PMCID: PMC6378219. doi:10.1111/jcmm.14091
6. Li Y, Dammer EB, Zhang-Brotzge X, et al. Osteopontin is a blood biomarker for microglial activation and brain injury in experimental hypoxic-ischemic encephalopathy. eNeuro. 2017;4(1):ENEURO.0253–16.2016. PMID: 28101531; PMCID: PMC5223053. doi:10.1523/ENEURO.0253-16.2016
7. Ystgaard MB, Scheffler K, Suganthan R, et al. Neuromodulatory effect of NLRP3 and ASC in neonatal hypoxic ischemic encephalopathy. Neonatology. 2019;115(4):355–362. PMID: 30909283. doi:10.1159/000497200
8. Al Mamun A, Yu H, Mirza MA, et al. Myeloid cell IRF4 signaling protects neonatal brains from hypoxic ischemic encephalopathy. Neurochem Int. 2019;127:148–157. PMID: 30586599; PMCID: PMC6579623. doi:10.1016/j.neuint.2018.12.014
9. Ozen I, Ruscher K, Nilsson R, et al. Interleukin-1 beta neutralization attenuates traumatic brain injury-induced microglia activation and neuronal changes in the globus pallidus. Int J Mol Sci. 2020;21(2):387. PMID: 31936248; PMCID: PMC7014296. doi:10.3390/ijms21020387
10. Li Q, Dai Z, Cao Y, et al. Caspase-1 inhibition mediates neuroprotection in experimental stroke by polarizing M2 microglia/macrophage and suppressing NF-κB activation. Biochem Biophys Res Commun. 2019;513(2):479–485. PMID: 30979498. doi:10.1016/j.bbrc.2019.03.202
11. Zhang X, Zhu XL, Ji BY, et al. LncRNA-1810034E14Rik reduces microglia activation in experimental ischemic stroke. J Neuroinflammation. 2019;16(1):75. PMID: 30961627; PMCID: PMC6452518. doi:10.1186/s12974-019-1464-x
12. Chen J, Zhang W, Zhao M, et al. Molecular regulation mechanism of pyroptosis and its research progress in heart failure. J Liaoning Univ Trad Chin Med. 2022;24(2):176–184. doi:10.13194/j.issn.1673-842x.2022.02.039
13. Yu P, Zhang X, Liu N, et al. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6(1):128. PMID: 33776057; PMCID: PMC8005494. doi:10.1038/s41392-021-00507-5
14. Gou X, Xu D, Li F, et al. Pyroptosis in stroke-new insights into disease mechanisms and therapeutic strategies. J Physiol Biochem. 2021;77(4):511–529. PMID: 33942252. doi:10.1007/s13105-021-00817-w
15. Qu Z, Zhou J, Zhou Y, et al. Mycobacterial EST12 activates a RACK1-NLRP3-gasdermin D pyroptosis-IL-1β immune pathway. Sci Adv. 2020;6(43):eaba4733. PMID: 33097533; PMCID: PMC7608829. doi:10.1126/sciadv.aba4733
16. Tian X, Li Y, Li H, et al. Effect of Huanglian Wendan Decoction on the classic pathway of scorch death of skeletal Muscle cell in IGT rats. Chin J Exp Form. 2021;27(18):1–8. doi:10.13422/j.cnki.syfjx.20211704
17. Wang K, Rong L, Wei X, et al. The influencing factors and prognosis of negative diffusion weighted magnetic resonance imaging in patients with acute ischemic stroke.
18. Han Y, Wang S, Huang C, et al. Correlation analysis of serum Lp-PLA2 with HCY, hsCRP, and biochemical markers in patients with acute ischemic stroke. Chin J Emer Med. 2019;28(8):1026–1028.
19. Suzhou, Tian X, Wang Y, et al. Effect of edaravone on the expression of mitogen-activated protein kinase/extracellular regulatory protein kinase signaling pathway proteins in patients with acute ischemic stroke. Chin J Geriatr. 2018;37(12):1372–1375.
20. Zhang D, Li M, Wang Z, et al. Progress in the mechanism of hypoxia-induced factor-1 α mediating neurostem-progenitor cells in an ischemic stroke model. Rehab Theory Pract China. 2017;23(3):319–322.
21. Liu F, Wang G. Related mechanisms and research progress of HIF-1α regulation of glucose metabolism in malignant tumors. Chin Med Innov. 2021;18(27):173–176.
22. Wei Y, Lu B, Jin L, et al. Effects of danquinone on cell proliferation, apoptosis and invasion and HIF-1α expression in HepG 2 under hypoxia. Mod Appl Pharm China. 2022;39(14):1789–1795. doi:10.13748/j.cnki.issn1007-7693.2022.14.001
23. Wang X, Zhao F, Zhang R, et al. Small-molecule inhibitor of anti-tumor hypoxia-inducible factor-1. Chem Prog. 2021;33(12):2259–2269.
24. Milner E, Johnson AW, Nelson JW, et al. HIF-1α mediates isoflurane-induced vascular protection in subarachnoid hemorrhage. Ann Clin Transl Neurol. 2015;2(4):325–337. PMID: 25909079; PMCID: PMC4402079. doi:10.1002/acn3.170
25. Zhao H, Li L, Miao C, et al. Effect of tanshinone A on the expression of HIF-1α and VE GF in the brain tissue of rats with ICH. J Stroke Neurol Dis. 2017;34(2):130–132. doi:10.19845/j.cnki.zfysjjbzz.2017.02.009
26. Du C, Li N, Zhao Y, et al. The relationship between the levels of NT-proBNP, IGF-1, and HIF-1α and the prognosis of patients with cerebral hemorrhage. Lab Med Clin Prac. 2017;14(18):2707–2710.
27. Zheng H, Cathy D, Lou X, et al. Correlation between expression of hypoxia-inducing factor-1 α/vascular endothelial growth factor pathway and intracranial pressure and prognosis in patients with hypertensive cerebral hemorrhage. Chin J Hypertens. 2022;30(3):283–286. doi:10.16439/j.issn.1673-7245.2022.03.014
28. Lu Y. The NLRP-3/Caspase-1/GSDMD Signaling Axis Mediates Pyroptosis in Microglia. Suzhou University; 2020. doi:10.27351/d.cnki.gszhu.2020.003571
29. Luo L, Han Z, Feng R, et al. Microglia in a common signaling pathway for activation in ischemic stroke. J Gannan Med Coll. 2021;41(8):844–850.
30. Qin C, Zhou LQ, Ma XT, et al. Dual functions of microglia in ischemic stroke. Neurosci Bull. 2019;35(5):921–933. PMID: 31062335; PMCID: PMC6754485. doi:10.1007/s12264-019-00388-3
31. Xue Y, Enosi Tuipulotu D, Tan WH, et al. Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol. 2019;40(11):1035–1052. PMID: 31662274. doi:10.1016/j.it.2019.09.005
32. Sun R, Peng M, Xu P, et al. Low-density lipoprotein receptor (LDLR) regulates NLRP3-mediated neuronal pyroptosis following cerebral ischemia/reperfusion injury. J Neuroinflammation. 2020;17(1):330. PMID: 33153475; PMCID: PMC7643474. doi:10.1186/s12974-020-01988-x
33. Xu P, Zhang X, Liu Q, et al. Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis. 2019;10(8):555. PMID: 31324751; PMCID: PMC6642102. doi:10.1038/s41419-019-1777-9
34. Sarhan M, Land WG, Tonnus W, et al. Origin and consequences of necroinflammation. Physiol Rev. 2018;98(2):727–780. PMID: 29465288. doi:10.1152/physrev.00041.2016
35. Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 2019;26(1):99–114. PMID: 30341423; PMCID: PMC6294779. doi:10.1038/s41418-018-0212-6
36. Sun L, Ma W, Gao W, et al. Propofol directly induces caspase-1-dependent macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell Death Dis. 2019;10(8):542. PMID: 31316052; PMCID: PMC6637184. doi:10.1038/s41419-019-1761-4
37. Sho T, Xu J. Role and mechanism of ROS scavengers in alleviating NLRP3-mediated inflammation. Biotechnol Appl Biochem. 2019;66(1):4–13. PMID: 30315709. doi:10.1002/bab.1700
38. Kesavardhana S, Malireddi RKS, Kanneganti TD. Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol. 2020;38:567–595. PMID: 32017655; PMCID: PMC7190443. doi:10.1146/annurev-immunol-073119-095439
39. Jiang Q, Geng X, Warren J, et al. Hypoxia inducible Factor-1α (HIF-1α) mediates NLRP3 inflammasome-dependent-pyroptotic and apoptotic cell death following ischemic stroke. Neuroscience. 2020;448:126–139. PMID: 32976985. doi:10.1016/j.neuroscience.2020.09.036
40. Fu X, Zhao Y. Brain protective effect and mechanism of K~ + ATP channel opening agent nicorodidil in a mouse model of ischemic stroke. Chin J Neuroimmunol Neurol. 2023;30(1):25–29+38.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.