Back to Journals » Cancer Management and Research » Volume 12
Hypoxia-Induced Aquaporin-3 Changes Hepatocellular Carcinoma Cell Sensitivity to Sorafenib by Activating the PI3K/Akt Signaling Pathway
Authors Malale K, Fu J, Qiu L, Zhan K, Gan X, Mei Z
Received 30 December 2019
Accepted for publication 12 May 2020
Published 9 June 2020 Volume 2020:12 Pages 4321—4333
DOI https://doi.org/10.2147/CMAR.S243918
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
This paper has been retracted.
Kija Malale, 1 Jili Fu, 2 Liewang Qiu, 1 Ke Zhan, 1 Xiuni Gan, 3 Zhechuan Mei 1
1Department of Gastroenterology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 2Department of Endocrinology and Metabolism, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 3Department of Nursing, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Correspondence: Xiuni Gan
Department of Nursing, The Second Affiliated Hospital of Chongqing Medical University, 76, Linjiang Road, Yuzhong District, Chongqing 400010, People’s Republic of China
Tel +86 138 83309318
Fax +86 023 63711527
Email [email protected]
Zhechuan Mei
Department of Gastroenterology, The Second Affiliated Hospital of Chongqing Medical University, 76, Linjiang Road, Yuzhong District, Chongqing 400010, People’s Republic of China
Tel +86 136 08338519
Fax +86 236 3711527
Email [email protected]
Purpose: Hypoxia-induced changes are primarily activated in patients with hepatocellular carcinoma (HCC) and long-term sorafenib exposure, thereby reducing the sensitivity to the drug. Aquaporin-3 (AQP3), a member of the aquaporin family, is a hypoxia-induced substance that affects the chemosensitivity of non-hepatocellular tumors. However, its expression and role in the sensitivity of hypoxic HCC cells to sorafenib-induced apoptosis remain unclear. The purpose of this study was to detect changes in AQP3 expression in hypoxic HCC cells and to determine whether these changes alter the sensitivity of these cells to sorafenib.
Materials and Methods: Huh7 and HepG2 hypoxic cell models were established and AQP3 expression was detected using quantitative real-time polymerase chain reaction (qPCR) and Western blotting. Furthermore, the role of AQP3 in cell sensitivity to sorafenib was evaluated via flow cytometry, Western blotting, and a CCK-8 assay.
Results: The results of qPCR and Western blotting showed that AQP3 was overexpressed in the Huh7 and HepG2 hypoxic cell models. Furthermore, AQP3 protein levels were positively correlated with hypoxia-inducible factor-1α (HIF-1α) levels. Compared with cells transfected with lentivirus-GFP (Lv-GFP), hypoxic cells transfected with lentivirus-AQP3 (Lv-AQP3) were less sensitive to sorafenib-induced apoptosis. However, the sensitivity to the drug increased in cells transfected with lentivirus-AQP3RNAi (Lv-AQP3RNAi). Akt and Erk phosphorylation was enhanced in Lv-AQP3-transfected cells. Compared with UO126 (a Mek1/2 inhibitor), LY294002 (a PI3K inhibitor) attenuated the AQP3-induced insensitivity to sorafenib observed in hypoxic cells transfected with Lv-AQP3. Combined with LY294002-treated cells, hypoxic cells transfected with Lv-AQP3RNAi were more sensitive to sorafenib.
Conclusion: The study results show that AQP3 is a potential therapeutic target for improving the sensitivity of hypoxic HCC cells to sorafenib.
Keywords: AQP3, hypoxia, hypoxic HCC cells, hypoxia-inducible factor 1α, PI3K/Akt and Erk signaling pathways, sorafenib resistance
Introduction
Despite improvements in the treatment for hepatocellular carcinoma (HCC) over the past few decades, it remains one of the deadliest cancers in the world.1,2 HCC is the fifth most common cancer worldwide and the third leading cause of cancer death.2 The prognosis of HCC is poor, most of which are in advanced stage. There are no reliable treatment methods for advanced stage.,2,3 and the existing systemic treatment is limited by drug resistance.2,4 New treatment focus is needed to improve patient prognosis. The increased HCC sensitivity to targeted therapy suggests that the prognosis of patients with advanced HCC can be improved.5–7
Sorafenib is an oral multikinase inhibitor that blocks the RAF signaling pathway as well as inhibits vascular endothelial growth factor (VEGF), platelet-derived growth factor, and KIT expressions. Sorafenib exerts anti-proliferative and anti-angiogenic effects; however, its mechanism of action remains unclear.2 It is the first and only promising targeted therapy for HCC and is currently the treatment of choice for patients with advanced disease.8–11 However, HCC’s long-term response to treatment is unsatisfactory, suggesting the presence of acquired resistance.8,12-14 Extensive cumulative data from preclinical and clinical studies indicate that long-term sorafenib use, among other factors, aggravates tumor hypoxia, which is the main driving factor of the resistance against this drug.3,15,16 During severe hypoxia, certain survival changes associated with tumor progression, metastasis, and drug resistance are activated;16–18 however, to date, the complex mechanism underlying these changes remains unclear. Therefore, an improved understanding of this phenomenon is crucial to ameliorate the HCC sensitivity to sorafenib.
AQP3 is one of the aquaglyceroporins, which are a subclass of aquaporins that afford permeability to water and glycerol.19 Although all aquaglyceroporins (AQP3, 7, 9, and 10) are expressed in the human liver, the AQP3 subtype is overexpressed mostly in HCC.20–23 AQP3’s role in tumor progression, prognosis, and treatment has recently garnered attention. In addition to promoting tumor progression and metastasis,24–28 AQP3 is involved in drug resistance in various malignant tumors.29,30 AQP3 knockdown or knockout studies have demonstrated its role in various tumor types.29,31–33 Although the exact mechanism is unclear, AQP3 is an hypoxia-induced substance and has been linked to hypoxia-induced tumor progression, metastasis, and drug resistance.23,28,31 Studies have shown that AQP3 expression is altered in hypoxic HCC cells; however, its effect on sorafenib sensitivity is unclear.
We aimed to detect changes in AQP3 expression in hypoxic HCC cells and to determine whether these changes alter hypoxic cell sensitivity to sorafenib.
Materials and Methods
Cell Culture, Reagents, and Antibodies
Human HCC cell lines Huh7 and HepG2 were purchased (Chinese Academy of Sciences, Shanghai, China). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Shanghai, China) supplemented with 10% fetal bovine serum (Gibco, Shanghai, China) and 1% penicillin/streptomycin and maintained in an incubator with a humidified atmosphere of 5% CO2 at 37°C for the normoxic cell model or 1% O2, 5% CO2 at 37°C for the hypoxic cell model. Sorafenib, UO126 and LY294002 (Sigma-Aldrich, St. Louis, Missouri, USA) were purchased and dissolved in 100% DMSO to prepare a 10mM stock solution, which was then diluted with DMEM to the desired concentration, with a final concentration of 0.1% DMSO, recommended for in vitro studies. Unless otherwise stated, all antibodies were obtained from Life Span BioSciences (LSBIO, Seattle, Washington, USA). The enhanced chemiluminescence detection kit was purchased from Amersham Pharmacia Biotech (APB, Buckinghamshire, UK). The primers for AQP3 and GAPDH were obtained from Sangon Biotech (Sangon Biotech, Shanghai, China). SYBR® Premix Ex Taq™ (Tli RNaseH Plus) was purchased from Takara Bio Inc (Takara, Beijing, China).
Cytotoxicity Assay
The difference in the sensitivity toward sorafenib treatment was examined using a cell counting kit-8 assay (CCK-8), as per the Sigma-Aldrich (Sigma-Aldrich, St. Louis, Missouri, USA) CCK-8 protocol. Briefly, Huh7 and HepG2 cells were seeded in a 96-well plate at a density of 5000 cells/well and incubated for 48 h under normoxic or hypoxic conditions, followed by replacement of the medium with culture medium with the indicated sorafenib concentration (0–24μM). After 24 h, 10μL of CCK-8 solution was added to each well of the plate, followed by 2 h incubation before detecting absorbance at 450nm using a microplate reader. Cell viability (as a percentage) was determined in relation to the average absorbance of the untreated cells from three replicate samples.
Annexin V Apoptosis Assay
The differences in apoptosis were examined using an Annexin V apoptosis assay. Briefly, Huh7 and HepG2 cells were seeded in 6-well plates at 2 × 105 per well for 48 h under normoxic or hypoxic conditions prior to treatment. After sorafenib treatment/transfection, cells were harvested via trypsinization, washed twice with Phosphate buffered saline (PBS), and stained with a fluorescein isothiocyanate Annexin V Apoptosis Detection kit (BD Pharmingen, Franklin Lakes, NJ, USA). Flow cytometry (Thermo Fisher Scientific, Waltham, Massachusetts, USA) was used to determine the cell apoptosis ratio.
Protein Extraction and Western Blotting
Protein was extracted from HCC cell lines using RIPA lysis buffer as per the protocol (Pierce Biotechnology Inc, Rockford, Illinois, USA). Protein concentration was determined via the bicinchoninic acid protein assay as per the Bio-Protocol (bio-protocol, Philadelphia, Pennsylvania, USA). An equivalent of 30μg of the protein extract was resolved via sodium dodecyl sulfate polyacrylamide gel electrophoresis and electro transferred (wet) to polyvinylidene difluoride membranes (EMD Millipore Corporation, Billerica, Massachusetts, USA). The membranes were initially blocked with 5% BSA in TBST (137mM NaCl, 20mM Tris HCl [pH 7.6], and 0.1% [v/v] Tween 20) for 1 h, followed by incubation overnight at 4°C with primary antibodies (1:1000) against AQP3, Erk, phospho-Erk, Akt, phospho-Akt, HIF-1α, LC3B, p62, cleaved caspase-3, and GAPDH/beta-tubulin (as loading controls) and horseradish peroxidase (HRP)-conjugated secondary antibodies (1:2000). Immunodetection was performed using an ECL Western Blotting Detection Kit (Beyotime, Shanghai, China). The relative protein expression levels were quantified by densitometric measurement of ECL reaction bands and normalized to GAPDH/beta-tubulin levels.
RNA Extraction, Reverse Transcription, and qPCR
Total RNA was extracted from HCC cell lines using an RNAIsoPlus assay kit (Takara, Dalian, China) according to the manufacturer’s instructions. After quantification, RNA was transcribed into cDNA using a two-step reverse transcription kit (Takara, Dalian, China). Subsequently, qPCR was used to detect target gene expression levels using the SYBR-Green qPCR master mix (Takara, Dalian, China). The thermocycling conditions were as follows: initial denaturation at 95°C for 10 min, followed by 35 cycles of a two-step PCR of 95°C for 14 s and 60°C for 1 min. The 2ΔΔCq method was used to quantify the results; the relative expression level of AQP3 was normalized to that of GAPDH. The primers used were as follows: AQP3, forward (F), 5ʹ–GGCTGTATTATGATGCAATCT–3ʹ and reverse (R), 5ʹ–ATATCCAAGTGTCCAGAGG–3ʹ. GAPDH F, 5ʹ–GATCATCAGCAATGCCTCCT–3ʹ and R, 5ʹ–GAGTCCTTCCACGATACCAA–3ʹ. The data have been deposited in a publicly accessible database (GenBank) with accession number NM_004925.5 and NM_002046.7 for AQP3 and GAPDH, respectively.
Cell Transfection
Huh7 and HepG2 cells were plated in 6-well plates until 60% confluence and then infected with lentivirus-AQP3 (Lv-AQP3), lentivirus-AQP3RNAi (Lv-AQP3RNAi), or lentivirus-GFP (Lv-GFP)-control (GenePharma Co. Ltd, Shanghai, China) according to infection value multiplicity. The medium was replaced 14 h later and the expression of the GFP gene observed under a fluorescence microscope. The selection of cells with stable virus integration into the genome was performed by replacing the medium with a fresh medium containing 2μg/ML puromycin at 48 h posttransduction, followed by incubation for 10 days. The effect of transfection on AQP3 expression was assessed via qPCR and Western blotting.
Statistical Analysis
Statistical analyses were performed using SPSS 21.0. Parametric data are presented as the mean±standard deviation (SD). Between-group differences were analyzed using Student’s t-test. Significance was set at P < 0.05(*), < 0.01(**), or < 0.001(***).
Results
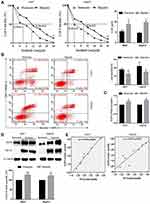
Hypoxia Reduces Sensitivity to Sorafenib and Upregulates AQP3 Expression in HCC Cells
Here, we tested the hypothesis that the expression of AQP3 is upregulated in hypoxic HCC cells.
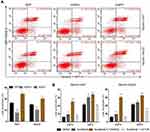
To test the hypothesis, the hypoxic cell model of the Huh7 and HepG2 cells were established based on previous studies17 and HIF-1α expression verified via Western blotting. The changes of IC50 and apoptosis after sorafenib treatment were detected by CCK-8 and flow cytometry, respectively. CCK-8 assay results showed a dose-dependent inhibitory effect of sorafenib on normoxic and hypoxic cell activities; however, the IC50 value of hypoxic cells was significantly higher than that of the normoxic ones (Figure 1A). Flow cytometry showed a significantly lower apoptosis rate in the hypoxic group than in the normoxic group (Figure 1B). These results showed that hypoxia resulted in a decreased HCC cells sensitivity to sorafenib. Conversely, changes in AQP3 mRNA and protein levels in cell models were detected via qPCR and Western blotting, respectively. PCR results showed that the gene expression of AQP3 was significantly higher in the hypoxic cell models than in their normoxic counterparts (Figure 1C). Western blotting showed a similar trend (Figure 1D); additionally, it showed a significant positive correlation between AQP3 and Hif-1α levels in hypoxic cells (Figure 1E). These results show that changes in AQP3 expression are potentially involved in the reduced hypoxic HCC cell sensitivity to sorafenib.
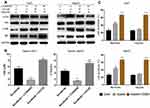
Hypoxia Reduces HCC Cell Sensitivity via PI3K/Akt Signaling Pathway Activation
Considering that the Erk and PI3K/Akt signaling pathways are involved in normal liver cancer cells’ resistance to sorafenib34,35 and that hypoxia induces Erk and PI3K/Akt activation in cells17 here, we determined whether PI3K/Akt or Erk signaling pathway inhibitors could restore this sensitivity. Hypoxic cells were co-treated with 10μM U0126 (Mek1/2 inhibitor)36 or 50μM LY294002 (PI3K inhibitor)37 for 24 h. Subsequently, changes in protein levels, IC50, and apoptotic cell numbers were detected via Western blotting, CCK-8 assay, and flow cytometry, respectively. Western blotting results showed that hypoxia promoted Akt and Erk phosphorylation, suggesting PI3K/Akt and Erk signaling pathway activation in hypoxic HCC cells. This activation was successfully suppressed by LY294002 but not by UO126 (Figure 2A). CCK-8 assay results revealed that LY294002 significantly reduced the IC50 of sorafenib in the cells compared with UO126 (Figure 2B). Flow cytometry results demonstrated that co-treatment with LY294002 attenuated hypoxia-induces insensitivity to sorafenib in Huh7 and HepG2 cells (Figure 2C). These results suggest that the PI3K/Akt signaling pathway is involved in reducing hypoxic HCC cell sensitivity to sorafenib.
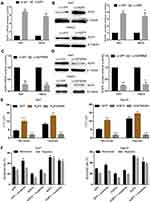
Hypoxia Upregulates AQP3 Expression in HCC Cells Through the PI3K/Akt Signaling Pathway
Based on our previous experiment findings, we hypothesized that hypoxia upregulates AQP3 expression through the Erk and PI3K/Akt signaling pathways. To validate this hypothesis, we examined whether PI3K/Akt or Erk signaling pathway inhibitors could attenuate the hypoxia-induced AQP3 upregulation in HCC cells. Western blotting was used to detect the protein levels of AQP3, p-Akt, Akt, p-Erk, and Erk in Huh7 and HepG2 hypoxic cells treated with 10μM U012637 or 50μM LY29400237 for 24 h. The results showed lower AQP3 protein levels in LY294002-treated cells than in UO126-treated or untreated cells (Figure 3A). The increase in the p-Akt/Akt ratio was similar to that in AQP3, suggesting that PI3K/Akt signaling pathway activation causes AQP3 upregulation in hypoxic HCC cells (Figure 3B). These findings suggest that the PI3K/Akt signaling pathway is involved in AQP3 upregulation in hypoxic HCC cells.
AQP3 Upregulation Renders Hypoxic HCC Cells Insensitive to Sorafenib
We further investigated whether AQP3 upregulation affects hypoxic HCC cell sensitivity to sorafenib. Huh7 and HepG2 cells were transfected with Lv-AQP3, Lv-AQP3RNAi or Lv-GFP (control), and the transfection efficiency was verified via qPCR (Figure 4A and C) and Western blotting (Figure 4B and D). The transfected cells were exposed to hypoxia for 48 h and then incubated with sorafenib at different concentrations (0–24μM) for 24 h. IC50 and apoptosis rate were detected via CCK-8 assay and flow cytometry, respectively. In either case, the IC50 of Lv-AQP3-transfected cells was significantly increased, whereas that of Lv-AQP3RNAi-transfected cells was significantly decreased (Figure 4E). Consistent with CCK-8 assay results, flow cytometry results showed that apoptotic cell proportion was decreased in the Lv-AQP3-transfected group, whereas it was increased in the Lv-AQP3RNAi-transfected group (Figure 4F). These results suggest that AQP3 upregulation reduces hypoxic HCC cell sensitivity to sorafenib.
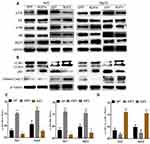
AQP3 Is an Upstream PI3K/Akt and Erk Signaling Pathway Regulator in HCC Cells
We investigated the effect of AQP3 expression on PI3K/Akt and Erk signaling pathway activation. Using Western blotting, p-Akt, Akt, p-Erk, and Erk protein levels were detected in Huh7 and HepG2 hypoxic cells transfected with Lv-AQP3, Lv-AQP3RNAi, or Lv-GFP (control). Western blotting was used to detect the activation of signaling pathways in Huh7 and HepG2 hypoxic cells transfected with Lv-AQP3, Lv-AQP3RNAi, or Lv-GFP (control). The results showed that the signaling pathways were activated after transfection with Lv-AQP3 and inactivated after transfection with Lv-AQP3RNAi (Figure 5A and C). Autophagy is a downstream effector of the PI3K/Akt and Erk signaling pathways. LC3B-I conversion into LC3B-II and p62 marker accumulation indicated that autophagy was inhibited in the Lv-AQP3-transfected group and activated in the Lv-AQP3RNAi group (Figure 5B and D). These results suggest that AQP3 is an upstream PI3K/Akt and Erk signaling pathway regulator.
AQP3 Upregulation Renders Hypoxic HCC Cells Less Sensitive to Sorafenib by Activating the PI3K/Akt Signaling Pathway
Based on our previous experiment results, we hypothesized that AQP3 upregulation reduces hypoxic HCC cell sensitivity to sorafenib via PI3K/Akt signaling pathway activation. Flow cytometry was used to detect the apoptosis of Huh7 and HepG2 hypoxic cells transfected with Lv-AQP3, Lv-AQP3RNAi, or Lv-GFP, followed by incubation with 6.8μM sorafenib or 6.8μM sorafenib + 50μM LY294002/10μM UO126 for 24 h. The results showed a significantly lower apoptosis rate in the Lv-AQP3-transfected group than in the Lv-GFP group (control group). However, the results were reversed in the Lv-AQP3RNAi group (Figure 6A). Co-treatment with LY294002 attenuated the AQP3-induced insensitivity to sorafenib in Lv-AQP3-transfected hypoxic cells. Combined treatment with LY294002 further improved the sorafenib-induced apoptosis of Lv-AQP3RNAi-transfected hypoxic cells (Figure 6B). These results suggest that AQP3 upregulation reduces HCC cell sensitivity to sorafenib by PI3K/Akt signaling pathway activation.
Discussion
In this study, we detected changes in expression of AQP3 in hypoxic HCC cells, and determined whether these changes altered the sensitivity of cells to sorafenib. The results showed that AQP3 is overexpressed in hypoxic HCC cells and altered the sensitivity of these cells to sorafenib via PI3K/Akt signaling pathway activation.
Intratumoral hypoxia is a hallmark of poor prognosis and treatment response in many solid cancers, including liver cancer.17,35,38-41 Through changes in gene expression, hypoxia induces, and/or selects for, cells with altered characteristics, including replication potential and stem cell property maintenance, angiogenesis, metabolic reprogramming, metastasis, and radiation and chemotherapy resistance3,17,35 HIF transcription factors mediate the hypoxia response and activate hypoxia response genes in multiple aspects of tumor development, including aggressiveness and drug resistance.16–18 Similarly, we showed that hypoxic HCC cells were less sensitive to sorafenib. Although different underlying mechanisms for this phenomenon have been suggested, the results remain controversial and need further clarification.17,39,41–46
Studies exploring AQP3’s role in the prognosis of various tumors, including HCC, have shown that AQP3 expression is a predictor of poor prognosis.27,33,47–59 AQP3 is the most overexpressed aquaglyceroporin in HCC.20–23 In the current study, we found that AQP3 expression was altered in hypoxic HCC cells. AQP3 protein levels increased proportionally to HIF-1α protein levels. Furthermore, hypoxia-activated PI3K/Akt signaling pathway leads to up-regulation of AQP3. Similarly, hypoxia increases the expression of AQP3 mRNA in L929 fibrosarcoma cells.23 Wang and colleagues also found that AQP3 protein levels correlated with HIF-1α in a matrix-grown Madin–Darby canine kidney cyst model.60 Therefore, we investigated whether changes in AQP3 expression affected hypoxic HCC cell sensitivity to sorafenib. We found that hypoxic cells transfected with lentivirus expressing AQP3 were less sensitive to sorafenib-induced apoptosis. Additionally, hypoxic cell transfection with Lv-AQP3RNAi restored the sensitivity to sorafenib. These results indicate that AQP3 upregulation is a key hypoxic pro-survival change and contributes to HCC cell adaptation and resistance to sorafenib-induced apoptosis. Our results are consistent with the findings of a study reporting that siRNA inhibition of AQP3 increases prostate cancer cells’ susceptibility to cryotherapy.29
Furthermore, we investigated the mechanism through which changes in AQP3 expression reduced hypoxic HCC cell sensitivity to sorafenib. Various mechanisms have been reported;8,13,61–66 however, we focused on the PI3K/Akt and Erk signaling pathways for several reasons, including crosstalk between them that leads to sorafenib resistance,67 their involvement in AQP3 expression regulation in multiple tumors,58,68,69 and their involvement in acquired sorafenib resistance in patients with liver cancer.13,61,62,66 Our results show that both the PI3K/Akt and Erk signaling pathways are downstream effectors of AQP3. The use of LY294002 but not UO126 successfully attenuated the AQP3-induced insensitivity to sorafenib in hypoxic cells transfected with lentivirus overexpressing AQP3. Hypoxic cells with reduced AQP3 levels were more sensitive to sorafenib; this effect was further exacerbated by co-treatment with LY294002. These results showed that the hypoxia-induced PI3K/Akt signaling pathway activation led to AQP3 upregulation, affording a positive feedback regulation on the pathway. Although the exact underlying mechanism is unclear, our findings suggest that the positive feedback by AQP3 upregulation to the PI3K/Akt signaling pathway leads to changes in hypoxic HCC cell sensitivity to sorafenib. These results also suggest a mechanism for further discussion in the literature on how the combined treatment with sorafenib and a PI3K/Akt inhibitor successfully increases HCC cell sensitivity to sorafenib.
Conclusion
Changes in AQP3 expression modulate hypoxic HCC cell sensitivity to sorafenib; the hypoxia-induced AQP3 expression reduces HCC cell sensitivity to sorafenib via PI3K/Akt signaling pathway activation. Therefore, AQP3 is a potential therapeutic target for improving hypoxic HCC cell sensitivity to sorafenib. Further in vivo studies are needed to confirm our findings.
Acknowledgments
This work was supported by the grants from the Ministry of Science and Technology of the People’s Republic of China (Grant No. 2017ZX10203202-004-005). The funder played no role in the study design, data collection or analyses, the decision to publish, or manuscript preparation. The English language, grammar, punctuation, and spelling of the manuscript was edited by Enago, the editing brand of Crimson Interactive Consulting Co. Ltd. During the preparation of the manuscript, Jennie Van Schindel gave her unconditional support.
Disclosure
The authors declare no conflicts of interest regarding this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
3. Mendez-Blanco C, Fondevila F, Garcia-Palomo A, Gonzalez-Gallego J, Mauriz JL. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med. 2018;50:134. doi:10.1038/s12276-018-0159-1
4. Jiao Q, Bi L, Ren Y, Song S, Wang Q, Wang YS. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol Cancer. 2018;17(1):1–12. doi:10.1186/s12943-018-0801-5
5. Huynh H, Ong R, Goh KAHY, et al. Sorafenib/MEK inhibitor combination inhibits tumor growth and the Wnt/β ‑ catenin pathway in xenograft models of hepatocellular carcinoma. 2019:1123–1133. doi:10.3892/ijo.2019.4693
6. Ye L, Mayerle J, Ziesch A, Reiter FP, Gerbes AL, De TEN. The PI3K inhibitor copanlisib synergizes with sorafenib to induce cell death in hepatocellular carcinoma. Cell Death Discov. 2019;5(86):1–12. doi:10.1038/s41420-019-0165-7
7. Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Evers BM. PKI-587 and sorafenib targeting PI3K/AKT/mTOR and Ras/Raf/MAPK pathways synergistically inhibit HCC cell proliferation. J Surg Res. 2012;176(2):542–548. doi:10.1016/j.jss.2011.10.045
8. Geier A, Macias RIR, Bettinger D, et al. The lack of the organic cation transporter OCT1 at the plasma membrane of tumor cells precludes a positive response to sorafenib in patients with hepatocellular carcinoma. Oncotarget. 2017;8(9):15846–15857. doi:10.18632/oncotarget.15029
9. Desai JR, Ochoa S, Prins PA, He AR. Systemic therapy for advanced hepatocellular carcinoma: an update. J Gastrointest Oncol. 2017;8(2):243–255. doi:10.21037/jgo.2017.02.01
10. Finn RS, Zhu AX, Farah W, et al. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic: a systematic review and meta-analysis. Hepatology. 2018;67(1):422–435. doi:10.1002/hep.29486
11. Ray EM, Sanoff HK. Optimal therapy for patients with hepatocellular carcinoma and resistance or intolerance to sorafenib: challenges and solutions. J Hepatocell Carcinoma. 2017;4:131–138. doi:10.2147/JHC.S124366
12. Niu L, Liu L, Yang S, Ren J, Lai PBS, Chen GG. New insights into sorafenib resistance in hepatocellular carcinoma: responsible mechanisms and promising strategies. Biochim Biophys Acta - Rev Cancer. 2017;1868(2):564–570. doi:10.1016/j.bbcan.2017.10.002
13. Zhai B, Hu F, Jiang X, et al. Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol Cancer Ther. 2014;13(6):1589–1598. doi:10.1158/1535-7163.MCT-13-1043
14. Van MH, Dekervel J, Verslype C, et al. Long-term exposure to sorafenib of liver cancer cells induces resistance with epithelial-to-mesenchymal transition, increased invasion and risk of rebound growth q. Cancer Lett. 2013;329(1):74–83. doi:10.1016/j.canlet.2012.10.021
15. Semenza GL. Interpreting intratumoral hypoxia: can it guide therapy? Ovarian Cancer Oncol J. 2007;21(3):1–3.
16. Qiu Y, Shan W, Yang Y, et al. Reversal of sorafenib resistance in hepatocellular carcinoma: epigenetically regulated disruption of 14-3-3η/hypoxia-inducible factor-1α. Cell Death Discov. 2019;5(1):120. doi:10.1038/s41420-019-0200-8
17. Zhao D, Zhai B, He C, et al. Upregulation of HIF-2α induced by sorafenib contributes to the resistance by activating the TGF-α/EGFR pathway in hepatocellular carcinoma cells. Cell Signal. 2014;26(5):1030–1039. doi:10.1016/j.cellsig.2014.01.026
18. Won C, Kim B, Yi EH, et al. Signal transducer and activator of transcription 3-mediated CD133 up-regulation contributes to promotion of hepatocellular carcinoma. Hepatology. 2015;62(4):1160–1173. doi:10.1002/hep.27968
19. Yang B. Aquaporins. In: Yang B, editor. Aquaporins. The Netherlands: Springer Science+Business Media B.V; 2017:6. doi:10.1007/978-94-024-1057-0
20. Méndez-Giménez L, Rodríguez A, Balaguer I, Frühbeck G. Role of aquaglyceroporins and caveolins in energy and metabolic homeostasis. Mol Cell Endocrinol. 2014;397(1–2):78–92. doi:10.1016/j.mce.2014.06.017
21. Peplowski MA, Vegso AJ, Iablokov V, et al. Tumor necrosis factor α decreases aquaporin 3 expression in intestinal epithelial cells through inhibition of constitutive transcription. Physiol Rep. 2017;5:19. doi:10.14814/phy2.13451
22. Wang J, Gui Z, Deng L, et al. C-Met upregulates aquaporin 3 expression in human gastric carcinoma cells via the ERK signalling pathway. Cancer Lett. 2012;319(1):109–117. doi:10.1016/j.canlet.2011.12.040
23. Hoogewijs D, Vogler M, Zwenger E, Krull S, Zieseniss A. Oxygen-dependent regulation of aquaporin-3 expression. Hypoxia. 2016;4:91–97. doi:10.2147/HP.S97681
24. Dong X, Wang Y, Zhou Y, Wen J, Wang S, Shen L. Aquaporin 3 facilitates chemoresistance in gastric cancer cells to cisplatin via autophagy. Cell Death Discov. 2016;2(1):19–24. doi:10.1038/cddiscovery.2016.87
25. Satooka H, Hara-Chikuma M. Aquaporin-3 controls breast cancer cell migration by regulating hydrogen peroxide transport and its downstream cell signaling. Mol Cell Biol. 2016;36(7):1206–1218. doi:10.1128/mcb.00971-15
26. Wang W, Geng X, Lei L, et al. Aquaporin-3 deficiency slows cyst enlargement in experimental mouse models of autosomal dominant polycystic kidney disease. FASEB J. 2019;3(9):1–12. doi:10.1096/fj.201801338RRR
27. Guo X, Sun T, Yang M, Li Z, Li Z, Gao Y. Prognostic value of combined aquaporin 3 and aquaporin 5 overexpression in hepatocellular carcinoma. Biomed Res Int. 2013;2013. 10.1155/2013/206525.
28. Galán-cobo A, Ramírez-lorca R, Serna A, Echevarría M. Overexpression of AQP3 modifies the cell cycle and the proliferation rate of mammalian cells in culture. PLoS One. 2015;10(9):e0137692. doi:10.1371/journal.pone.0137692
29. Ribatti D, Ranieri G, Annese T, Nico B. Aquaporins in cancer. BBA - Gen Subj. 2014;1840(5):1550–1553. doi:10.1016/j.bbagen.2013.09.025
30. Chen X, Chen W, Ding X, Zheng W, Zhang Q, Yang J. Effects of aquaporins on chemosensitivity to cisplatin in ovarian cancer cells. Arch Gynecol Obstet. 2014;290(3):525–532. doi:10.1007/s00404-014-3216-6
31. Hou S-Y, Li Y-P, Wang J-H, et al. Aquaporin-3 inhibition reduces the growth of NSCLC cells induced by hypoxia. Cell Physiol. 2016;38:129–140. doi:10.1159/000438615
32. Chen L, Li Z, Zhang Q, et al. Silencing of AQP3 induces apoptosis of gastric cancer cells via downregulation of glycerol intake and downstream inhibition of lipogenesis and autophagy. Onco Targets Ther. 2017;10:2791–2804. doi:10.2147/OTT.S134016
33. Li Z, Li B, Zhang L, et al. The proliferation impairment induced by AQP3 deficiency is the result of glycerol uptake and metabolism inhibition in gastric cancer cells. Tumor Biol. 2016;37(7):9169–9179. doi:10.1007/s13277-015-4753-8
34. Xia H, Ooi LL, Hui KM. MiR-216a/217-induced epithelial-mesenchymal transition targets PTEN and SMAD7 to promote drug resistance and recurrence of liver cancer. Hepatology. 2014;58(2):629–641. doi:10.1002/hep.26369
35. Nishida N, Kitano M, Sakurai T, Kudo M. Molecular mechanism and prediction of sorafenib chemoresistance in human hepatocellular carcinoma. Dig Dis. 2015;33(6):771–779. doi:10.1159/000439102
36. Evans C, Cook SJ, Coleman MP, Gilley J. MEK inhibitor U0126 reverses protection of axons from wallerian degeneration independently of MEK – ERK signaling. PLoS One. 2013;8(10):e76505. doi:10.1371/journal.pone.0076505
37. Phyu SM, Tseng C, Fleming IN, Smith TAD. Probing the PI3K/Akt/mTOR pathway using 31 P-NMR spectroscopy: routes to glycogen synthase kinase 3. Sci Rep. 2016;6:36544. doi:10.1038/srep36544
38. Zhu B, Lin SS, Liu PF, et al. Desumoylation of hypoxia inducible factor (HIF)-2α by SENP1 is involved in HPPCn-enhanced sorafenib resistance under hypoxia in hepatocellular carcinoma. Hepatology. 2014;60:S83–4. doi:10.1016/S0168-8278(14)60214-2
39. Liu F, Dong X, Lv H, et al. Targeting hypoxia inducible factor 2 α enhances sorafenib antitumor activity via β catenin/C Myc dependent pathways in hepatocellular carcinoma. Oncol Lett. 2015;10(2):778–784. doi:10.3892/ol.2015.3315
40. Sun T, Liu H, Ming L. Multiple roles of autophagy in the sorafenib resistance of hepatocellular carcinoma. Cell Physiol Biochem. 2017;44(2):716–727. doi:10.1159/000485285
41. Liang Y, Zheng T, Song R, et al. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through von hippel-lindau tumor suppressor-dependent HIF-1 a inhibition in hepatocellular carcinoma. Hepatology. 2013;57(5):1847–1857. doi:10.1002/hep.26224
42. Prieto-domínguez N, Méndez-blanco C, Carbajo-pescador S, et al. Melatonin enhances sorafenib actions in human hepatocarcinoma cells by inhibiting mTORC1/p70S6K/HIF-1α and hypoxia- mediated mitophagy. Oncotarget. 2017;8(53):91402–91414. doi:10.18632/oncotarget.20592
43. Ma L, Li G, Zhu H, et al. 2-Methoxyestradiol synergizes with sorafenib to suppress hepatocellular carcinoma by simultaneously dysregulating hypoxia-inducible factor-1 and −2. Cancer Lett. 2014;355(1):96–105. doi:10.1016/j.canlet.2014.09.011
44. Xu M, Xie X, Xie X, et al. Sorafenib suppresses the rapid progress of hepatocellular carcinoma after insufficient radiofrequency ablation therapy: an experiment in vivo. Acta Radiol. 2013;54(2):199–204. doi:10.1258/ar.2012.120249
45. You A, Cao M, Guo Z, et al. Metformin sensitizes sorafenib to inhibit postoperative recurrence and metastasis of hepatocellular carcinoma in orthotopic mouse models. J Hematol Oncol. 2016;9(20):1–9. doi:10.1186/s13045-016-0253-6
46. Xu J, Zheng L, Chen J, et al. Increasing AR by HIF-2 α inhibitor (PT-2385) overcomes the side-effects of sorafenib by suppressing hepatocellular carcinoma invasion via alteration of pSTAT3, pAKT and pERK signals. Cell Death Dis. 2017;8(10):e3095. doi:10.1038/cddis.2017.411
47. Chen J, Wang T, Zhou YC, et al. Aquaporin 3 promotes epithelial-mesenchymal transition in gastric cancer. J Exp Clin Cancer Res. 2014;33(1):1–10. doi:10.1186/1756-9966-33-38
48. Liu S, Zhang S, Jiang H. Co-expression of AQP3 and AQP5 in esophageal squamous cell carcinoma correlates with aggressive tumor progression and poor prognosis. Med Oncol. 2013;30:636. doi:10.1007/s12032-013-0636-2
49. Peng R, Zhang Y, Zhao GX, et al. Differential regulation of the expression of aquaporins 3 and 9 by Auphen and dbcAMP in the SMMC-7721 hepatocellular carcinoma cell line differential regulation of the expression of aquaporins 3 and 9 by Auphen and dbcAMP in the SMMC-7721 hepatocellular c. 2016;20(26). 10.3109/10520295.2016.1168525.
50. Shen Q, Lin W, Luo H, et al. Differential expression of aquaporins in cervical precursor lesions and invasive cervical cancer. Reprod Sci. 2016;23(11):1551–1558. doi:10.1177/1933719116646202
51. Rubenwolf PC, Denzinger S, Otto W. Aquaporin 3 protein expression in transitional cell carcinoma: A potential marker with regard to tumour progression and prognosis? Eur Urol. 2012;61(3):627–628. doi:10.1016/j.eururo.2011.12.023
52. Liu S, Zhang S, Jiang H, Yang Y, Jiang Y. Co-expression of AQP3 and AQP5 in esophageal squamous cell carcinoma correlates with aggressive tumor progression and poor prognosis. Med Oncol. 2013;30:3. doi:10.1007/s12032-013-0636-2
53. Ichiyama T, Nakatani E, Tatsumi K, et al. Expression of aquaporin 3 and 5 as a potential marker for distinguishing dry mouth from Sjögren’s syndrome. J Oral Sci. 2018;60(2):212–220. doi:10.2334/josnusd.17-0150
54. Jin Z, Ho S, Choi Y, Il Y, Choi SA, Geun S. International journal of pediatric otorhinolaryngology expression of CXCL4 and aquaporin 3 and 10 mRNAs in patients with otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2016;81:33–37. doi:10.1016/j.ijporl.2015.11.035
55. Moosavi MS, Elham Y. Aquaporins 1, 3 and 5 in different tumors, their expression, prognosis value and role as new therapeutic targets. Pathol Oncol Res. 2019. doi:10.1007/s12253-019-00646-9
56. Marlar S, Jensen HH, Login FH, Nejsum LN. Aquaporin-3 in cancer. Int J Mol Sci. 2017;18(10):1–12. doi:10.3390/ijms18102106
57. Qiu J, Zhang Y, Chen H, Guo Z. MicroRNA-488 inhibits proliferation, invasion and EMT in osteosarcoma cell lines by targeting aquaporin 3. Int J Oncol. 2018;53(4):1493–1504. doi:10.3892/ijo.2018.4483
58. Huang X, Huang L, Shao M. Aquaporin 3 facilitates tumor growth in pancreatic cancer by modulating mTOR signaling. Biochem Biophys Res Commun. 2017;486(4):1097–1102. doi:10.1016/j.bbrc.2017.03.168
59. Zhao H, Yang X, Zhou Y, et al. Potential role of aquaporin 3 in gastric intestinal metaplasia. Oncotarget. 2015;6(36):38926–38933. doi:10.18632/oncotarget.5370
60. Wang W, Geng X, Lei L, et al. Aquaporin-3 deficiency slows cyst enlargement in experimental mouse models of autosomal dominant polycystic kidney disease. FASEB J. 2019;33(5):6185–6196. doi:10.1096/fj.201801338RRR
61. Chen K-F, Chen H-L, Tai W-T, et al. Activation of phosphatidylinositol 3-Kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2011;337(1):155–161. doi:10.1124/jpet.110.175786.in
62. He C, Dong X, Zhai B, et al. MiR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget. 2015;6(30):28867–28881. doi:10.18632/oncotarget.4814
63. Tomonari T, Takeishi S, Taniguchi T, et al. MRP3 as a novel resistance factor for sorafenib in hepatocellular carcinoma. Oncotarget. 2016;7(6):7207–7215. doi:10.18632/oncotarget.6889
64. Haga Y, Kanda T, Nakamura M, et al. Overexpression of c-Jun contributes to sorafenib resistance in human hepatoma cell lines. PLoS One. 2017;12(3):1–14. doi:10.1371/journal.pone.0174153
65. Chow AK, Ng L, Lam CS, et al. The enhanced metastatic potential of hepatocellular carcinoma (HCC) cells with sorafenib resistance. PLoS One. 2013;8(11):e78675. doi:10.1371/journal.pone.0078675
66. Kim JS, Choi GH, Jung Y, et al. Downregulation of Raf-1 kinase inhibitory protein as a sorafenib resistance mechanism in hepatocellular carcinoma cell lines. J Cancer Res Clin Oncol. 2018:1–15. doi:10.1007/s00432-018-2672-y
67. Zhai B, Sun X. Mechanisms of resistance to sorafenib and the corresponding strategies in hepatocellular carcinoma. World J Hepatol. 2013;5(7):345–352. doi:10.4254/wjh.v5.i7.345
68. Xu H, Xu Y, Zhang W, Shen L, Yang L, Xu Z. Aquaporin-3 positively regulates matrix metalloproteinases via PI3K/AKT signal pathway in human gastric carcinoma SGC7901 cells. J Exp Clin Cancer Res. 2011;30(1):86. doi:10.1186/1756-9966-30-86
69. Chen J, Wang T, Zhou Y, et al. Aquaporin 3 promotes epithelial-mesenchymal transition in gastric cancer. J Exp Clin Cancer Res. 2014;33(38):1–10. doi:10.1186/1756-9966-33-38
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.