Back to Journals » Breast Cancer: Targets and Therapy » Volume 7
Hypofractionated whole breast radiotherapy: current perspectives
Authors Koulis T, Phan T, Olivotto I
Received 12 June 2015
Accepted for publication 21 August 2015
Published 27 October 2015 Volume 2015:7 Pages 363—370
DOI https://doi.org/10.2147/BCTT.S81710
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Pranela Rameshwar
Theodora A Koulis, Tien Phan, Ivo A Olivotto
Department of Oncology, University of Calgary, Tom Baker Cancer Centre, Calgary, AB, Canada
Abstract: Adjuvant radiotherapy (RT) is an important part of breast cancer management but the dose and fractionation schedules used are variable. A total of 50 Gy in 25 daily fractions delivered over 5 weeks is often considered the "standard" adjuvant RT prescription. Hypofractionated regimes such as 42.5 Gy in 16 daily fractions or 40 Gy in 15 daily fractions following breast-conserving surgery have proven to be equally effective and achieve similar or better cosmetic and normal tissue outcomes for both invasive and in situ diseases and when treating the regional nodes. Hypofractionation is more convenient for patients and less costly. However, certain patients at higher risk of RT late effects may benefit from a less intense, even more extended fractionation schedule. This review describes the indications for whole breast hypofractionated adjuvant RT for patients with breast cancer following breast-conserving surgery and proposes that hypofractionation should be the new "standard" for adjuvant breast cancer RT.
Keywords: fractionation, breast cancer, cosmesis, radiotherapy
Introduction
Adjuvant treatment of breast cancer is one of the most common indications for radiotherapy (RT) in western countries due to the high incidence of breast cancer and the multiple indications for RT in this disease.1–3 The meta-analyses of RT by Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) and surgery for early stage breast cancer have shown that breast-conserving surgery (BCS) followed by whole breast RT is equivalent to mastectomy and that BCS + RT is superior to BCS alone in terms of local control and survival.4,5 In randomized trials, the relative risk reductions from RT after BCS were independent of the dose/fractionation used. However, “standard” post-BCS fractionation has come to mean 5–6 weeks of daily treatments of 1.8–2 Gy/d, with or without a boost.1,6–8 This is in part because 50 Gy in 25 daily fractions to the whole breast was used in the earliest published randomized trials that validated the use of BCS + RT.9,10
Over the past two decades, evidence has been accumulated from well-conducted, large, prospective randomized trials, comparing shorter RT courses to 50 Gy in 25 daily fractions.11–13 These trials and institutional series have confirmed that shorter courses of RT are equally effective compared to longer RT schedules for women with invasive11–16 or in situ breast cancer,17–20 provided the total dose of RT is appropriately reduced. Shorter RT courses also result in improved quality of life,21 convenience, and lower treatment delivery resource requirements. This has led to the suggestion that short fractionation should be the new standard following BCS for early stage breast cancer.22
Whole breast RT schedules using 15–16 daily treatments following BCS have become widely accepted in parts of Canada and the UK. In Canada, 75%–85% of patients22–24 and in the UK, 91% of patients in 2014 (I Locke, Lead for Clinical Oncology, Royal Marsden Hospital, UK, personal communication, April 29, 2015) receiving whole breast RT after BCS were treated with short fractionation but it has been adopted more slowly in the USA.25–27 This review discusses the safety and efficacy of short fractionation as compared to longer courses of adjuvant RT following BCS for invasive or in situ breast cancer and for locoregional RT for patients with node-positive disease following BCS or mastectomy. The role of accelerated partial breast irradiation is not addressed.
Hypofractionated RT following BCS is safe and effective
Table 1 summarizes the long-term outcomes of four carefully conducted, randomized trials, involving 7,095 patients, which compared 13–16 fraction RT regimes to a 25-fraction schedule.11–13 With >10-year follow-up, the overall conclusion from the Standardization of Breast Radiotherapy (START) A trial13 and the Canadian trial12 was that 39 Gy in 13 daily fractions over 5 weeks13 and 42.5 Gy in 16 daily fractions over 3.5 weeks12 provided equivalent local control, survival, cosmetic outcome, and normal tissue toxicity compared to 50 Gy in 25 daily fractions. In contrast, 41.6 Gy in 13 daily fractions delivered over 5 weeks was somewhat more intense and 40 Gy in 15 daily fractions delivered over 3 weeks was somewhat less intense in terms of normal tissue toxicity.13 Furthermore, patients randomized to 40 Gy in 15 daily fractions had significantly fewer locoregional recurrences and deaths compared to patients treated with 50 Gy in 25 daily fractions.13 The biological effects of various RT schedules can be estimated using a linear quadratic formula based on the dose delivered each day, the number of treatments, the interval over which the treatment was delivered, and a tissue end point-specific constant called the α/β ratio.28 The α/β ratios are lower for slowly responding tissues, including late fibrosis effects in normal tissues, whereas α/β ratios are higher for more rapidly proliferating tissues, including many, but not all, tumors. Two approaches to estimate the equivalence of different RT schedules have been proposed, the relative biological effective dose (BED) model and the equivalent dose at 2 Gy per fraction (EQD2) model.28
  | Table 1 Characteristics and outcomes of randomized hypofractionation trials |
Tables 2 and 3 present the BEDs and the EQD2s, respectively, for adjuvant breast RT regimens across several estimates of the α/β ratio. At α/β=2, BED and EQD2 values are equivalent for regimens that have been demonstrated in randomized trials to have clinically equivalent normal tissue effects. For example, 42.5 Gy in 16 daily fractions and 50 Gy in 25 daily fractions have been shown to be clinically equivalent.12 For those regimens, using α/β=2, the BEDs are 99 Gy and 100 Gy, and EQD2s are 49.5 Gy and 50 Gy, respectively. Previously, it has been assumed or calculated that normal tissue effects in the breast respond according to an α/β ratio of 3.4.13,29 However, there is greater discrepancy in the BED and EQD2 values when the earlier regimes are compared, assuming an α/β ratio of 3.4 or higher (Tables 2 and 3).
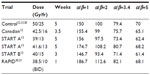  | Table 2 BED of different hypofractionated regimes compared to standard at different α/β (time factor not included) |
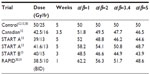  | Table 3 EQD2 of reported hypofractionation regimes compared to standard at different α/β |
Concerns that hypofractionation may increase fibrosis and worsen cosmetic outcomes have limited its adoption. In the Canadian12 and START trials,13 normal tissue effects were not worse when compared to 50 Gy in 25 daily fractions. However, in the Randomized Trial of Accelerated Partial Breast Irradiation (RAPID) trial, 38.5 Gy in 10 fractions BID accelerated partial breast RT resulted in greater cosmetic deterioration.30,31 The excess adverse fibrosis and cosmetic effects may be explained by the BEDs and EQD2s of 38.5 Gy in 10 daily fractions which are 112.6 Gy and 56.3 Gy, respectively, compared to 100 Gy and 50 Gy, respectively, for 50 Gy in 25 daily fractions (Tables 2 and 3). The biological effect may have been even more intense due to the BID treatment used in the RAPID trial.31 The radiobiological explanations for varying normal tissue and tumor responses to alterations in dose fractionation are an area of ongoing research.32
The long-term safety of short RT fractionation has been investigated using health service administrative data. Examining outcomes across many thousands of patients, it has been observed that adjuvant breast RT is associated with a small but statistically significant increased risk of cerebrovascular and cardiac hospitalizations or deaths,33–36 and second malignacies,37 but that these risks were not higher among patients treated with hypofractionation (>2 Gy/d) as compared to ≤2 Gy/d RT schedules. One report suggested that severe hypofractionation (43 Gy in 10 daily fractions) may increase the risk of cardiac injury.36 However, in the START A and B trials, the rate of confirmed ischemic heart disease in patients with left-sided breast cancer was not different between short and longer fractionation, although follow-up at 10 years, somewhat early for this end point.13 To avoid cardiac injury, every effort should be made to exclude the heart from the therapeutic beam, no matter what fractionation is used.
There are limited data on the use of even shorter, four or five fraction RT regimens following BCS. A small trial from France reported comparable toxicity from 23 Gy in 4 daily fractions over 3 weeks and 45 Gy in 25 daily fractions over 5 weeks.38 In the UK, regimens of whole breast RT, using 27 Gy in 5 daily fractions or 30 Gy in 5 daily fractions both delivered once per week over 5 weeks, were compared to 50 Gy in 25 daily fractions.39 More acute normal tissue side effects were observed with 30 Gy in 5 daily fractions,39 but 27 Gy in 5 daily fractions was tolerable and is being compared to 40 Gy in 15 daily fractions in a randomized trial.40 An early report suggested that 27 Gy in 5 daily fractions results in low rates of acute skin reactions.41
Providing whole breast RT in 15–16 treatment sessions is more convenient and preferred by patients compared to RT extending over 5–7 weeks and has been associated with more prompt recovery and improved quality of life compared to longer RT courses.21 Shorter RT schedules significantly reduce resource utilization. Three patients can be treated with the same treatment delivery resources using hypofractionation (45–48 treatment visits for three patients) as would be used for two patients using a 25-fraction regimen (50 treatment visits for two patients). Shorter RT courses are beneficial to the capacity and sustainability of the health care system.27 Several organizations have adopted short fractionation as preferred,1 recommended,2,27 standard,3 or acceptable8 approach for whole breast RT following BCS. The Choosing Wisely campaign in the USA advocates: “Don’t initiate whole breast radiotherapy as a part of breast conservation therapy in women age ≥50 with early stage invasive breast cancer without considering shorter treatment schedules”.42 This approach is entirely justified and is likely too conservative because it introduces caveats to restrict the use of shorter fractionation based on age, stage, and disease behavior. Such caveats are not evidence based as discussed in the following sections.
Hypofractionation appears to be equally effective in patients with breast ductal carcinoma in situ
Many patients with ductal carcinoma in situ (DCIS) are treated with BCS and whole breast RT. A number of institutions have reported that shorter RT schedules, generally 42.5 Gy in 16 daily fractions, achieved equivalent local control compared to longer fractionation.17–20 A meta-analysis of observational series found no difference in local recurrence rates between hypofractionated and 5-week or longer RT courses.20 The efficacy and safety of hypofractionated RT for patients with DCIS are addressed in an Australian-led, randomized trial that accrued 1,608 patients.43 However, there is no inherent reason that normal tissue side effects should be different following whole breast RT for patients with excised DCIS as compared to excised invasive cancer. A short RT schedule of 40 Gy in 15 daily fractions or 42.5 Gy in 16 daily fractions should be offered as an option to patients with DCIS.
Hypofractionation is effective for higher risk patients
An unplanned subset analysis within the Canadian hypofractionation trial suggested that patients with grade 3 histology had an increased risk of local recurrence when treated with 42.5 Gy in 16 daily fractions.12 However, in the larger START trials, hypofractionation among patients with grade 3 histology was not associated with a higher risk of local recurrence.13,44 Furthermore, a comparative effectiveness evaluation of 1,335 patients with grade 3 histology treated with BCS and whole breast RT in British Columbia between 1989 and 1999, 81% of whom received short fractionation, showed identical local control and survival among patients treated with hypofractionated RT as compared to longer RT courses.14 Short fractionation for whole breast RT should not be restricted to patients with lower grade histology, or by extension, other markers of an increased risk of local or distant relapse. The American Society for Radiation Oncology consensus statement that supports the use of shorter fractionation following BCS for women aged 50 and older with T1-2, pN0 breast cancer not receiving chemotherapy, is likely, overly cautious.8
What boost dose should be used with hypofractionated whole breast RT?
Trials from the European Organization for Research and Treatment of Cancer (EORTC)45 and Lyon, France46 showed that a boost dose of RT directed to the primary site significantly improved local control compared to whole breast RT alone following BCS. The EORTC trial used 16 Gy in 8 daily fractions, whereas the French trial used 10 Gy in 5 daily fractions. The absolute differences in local recurrence varied between the trials and among subgroups with different pretreatment risks of local recurrence, but the relative risk reductions in the trials were overlapping.45,46
Common indications for a boost dose include close or focally positive margins and younger age.1–3,7 It seems logical, if treating the whole breast in 15–16 treatments, to also use a shorter boost treatment of 10 Gy in 4–5 daily fractions rather than extending the treatment course by 50% to deliver 16 Gy in 8 daily fractions. We have found that a boost dose of 10 Gy in 4 daily fractions is tolerable (minimal fibrosis, pain, telangiectasia, or decrease in cosmesis) when delivered using a multibeam photon technique or a direct electron field restricted to 6–12 MeV energy and field sizes smaller than 8×8 cm2. Other researches have confirmed this observation.47 We therefore recommend and use 10 Gy in 4 daily fractions if a patient has an indication for a primary site boost and is receiving 42.5 Gy in 16 daily fractions for whole breast RT following BCS.
Which patients should not have hypofractionation after BCS?
Even in jurisdictions where short fractionation for whole breast RT is commonly used, it is not recommended or used for all patients.3,23,24 The primary concern with hypofractionation is that the larger dose per day may induce greater fibrosis or late normal tissue effects. A higher risk of late fibrosis after standard breast RT occurs with greater dose inhomogeneity,28,31,48–51 among current smokers,31,52 with implant reconstruction,53,54 or after postoperative infections.31,48,50 The potential impact of dose inhomogeneities (areas of dose >107%) may be particularly severe with short fractionation.28,54 In addition, patients with significant postoperative breast edema have considerable amounts of protein-rich interstitial fluid in their breast. As that edema fluid resolves it leaves collagen behind and this translates to greater fibrosis over time.
Since 50 Gy in 25 daily fractions produces equivalent late fibrosis and cosmetic deterioration, it is not rational to use this fractionation for patients with an increased risk of later toxicity. A dose/fractionation that is biologically less intense for the whole breast such as 45 Gy in 25 daily fractions at 1.8 Gy/d should be utilized (Table 4). However, that lower dose may be insufficient to achieve optimal local control on its own so, a boost dose to the primary site in all patients needs to be added.3,6,7,55,56
  | Table 4 BED and EQD2 of different fractionation used in regional nodal radiation compared to standard |
We therefore recommend 42.5 Gy in 16 daily fractions (or 40 Gy in 15 daily fractions) for most patients following BCS. If the patient has an indication for a boost, then 10 Gy in 4 daily fractions should be used. If the patient has a higher than average risk of late toxicity due to the factors listed above, 45 Gy in 25 daily fractions plus a boost of 10 Gy in 5 daily fractions is recommended. If the patient has predictors of a greater risk of late toxicity, plus an indication for a boost such as close margins or young age, the boost dose should be increased to 16 Gy in 8 daily fractions or 20 Gy in 10 daily fractions (Figure 1).
Hypofractionation can be used for regional nodal RT after BCS or mastectomy
Institutions where short fractionation for whole breast RT is commonly used also use short fractionation when the regional nodes are part of the target volume.3 There could be two concerns with such an approach: 1) short fractionation may be inadequate to achieve control in the regional nodes or 2) short fractionation may cause more pulmonary fibrosis, brachial plexopathy, or vessel injury, leading to cerebrovascular or cardiac morbidity.
Regarding efficacy, the British Columbia randomized trial of postmastectomy RT (PMRT) used a 16-fraction treatment schedule. This trial randomly allocated 318 premenopausal women with node-positive breast cancer treated with modified radical mastectomy, to full locoregional RT including the chest wall, axilla, supraclavicular fossa and a direct field over the internal mammary nodes, or no adjuvant RT.57 Patients were treated from 1978 to 1986 and 20-year follow-up was reported in 2005.58 Patients received a chest wall dose of 37.5 Gy in 16 daily fractions and the mid-axilla received 35 Gy in 16 daily fractions. These dose/fractionation schedules are biologically, substantially less intense compared to 45–50 Gy in 25 daily fractions (Table 4). In spite of this, the PMRT used in the British Columbia trial achieved similar reductions in the risk of locoregional recurrence, distant metastases, and death compared to the concurrently reported Danish trials that used a more conventional dose, 50 Gy in 25 daily fractions.59,60 These data demonstrate that a hypofractionated RT schedule is sufficient to achieve cancer control end points.
For many decades, the British Columbia Cancer Agency has used a PMRT prescription of 40 Gy in 16 daily fractions through a tangent pair with 0.5 cm bolus to the chest wall and dose of 37.5 Gy in 16 daily fractions to the mid-axilla.4,33–35,61 These doses are 7% higher than the doses shown to be effective in their randomized PMRT trial and are biologically less intense than 50 Gy in 25 daily fractions (Table 4). This PMRT prescription has also been used by others.15 When combining regional RT with hypofractionated whole breast RT following BCS, the same nodal dose of 37.5 Gy in 16 daily fractions can be used.3
Regarding safety, Powell et al reported that a higher dose/day fractionation schedule increased the brachial plexopathy rate from 1% to 6%.62 This was based on a retrospective comparison of 45 Gy/15 fr and 54 Gy/30 fr, both delivered over 6 weeks. The 45 Gy/15 fr schedule has a much higher BED and EQD2 compared to 37.5 Gy/16 fr or 50 Gy/25 fr using a ratio of α/β=1 or α/β=2 (Table 4), which are commonly attributed to the brachial plexus.63 Among patients treated in the START trials, 8%–14% of patients had nodal RT and no differences were noted in arm or shoulder function between the shorter and longer RT prescriptions.13 A single case of brachial plexopathy (<0.1% of cases) was reported with the use of 41.6 Gy/13 fr in the START A trial.13 Adjuvant RT irrespective of dose per fraction results in a small but statistically significant increase in cardiac and cerebrovascular toxicity as compared to no RT but is not increased with hypofractionation.33–36 Short fractionation for PMRT and for the regional nodal RT component after BCS is safe, provided the total dose is reduced sufficiently to avoid late normal tissue effects. Short fractionation does not compromise locoregional control.58,63
Summary
Two decades of observations from randomized trials and institutional series have demonstrated that following BCS, whole breast doses of 40 Gy/15 fr or 42.5 Gy/16 fr are as safe and effective as 50 Gy/25 fr. Evidence has been obtained from randomized trials and institutional series that hypofractionation is also effective and safe for adjuvant treatment of the regional lymph nodes. A shorter RT course should be the new standard and offered to most women because it is more convenient and cost-effective. However, short fractionation is not appropriate for all patients. Patients with postoperative complications, those with large breasts for whom a maximum dose of <107% is not achievable, or patients with implants for augmentation or reconstruction, have an increased risk for late fibrosis or cosmetic deterioration following RT. They should receive a whole breast or chest wall dose that is biologically less intense. For such patients, we recommend 45 Gy/25 fr to the whole breast and a primary site boost of at least 10 Gy/5 fr to achieve an equivalent antineoplastic effect at the primary site. For all others, a 15–16 treatment prescription is safe, effective, and recommended.
Disclosure
All authors declare that this is an original work and has not been submitted elsewhere for publication, and they report no conflicts of interest in this work.
References
NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, Version 2.2015. National Comprehensive Cancer Network. Available from: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed May 3, 2015. | |
NICE Clinical Guideline 80. National Institute for Health and Care Excellence; 2009. Available from: http://pathways.nice.org.uk/pathways/early-and-locally-advanced-breast-cancer#path=view%3A/pathways/early-and-locally-advanced-breast-cancer/early-and-locally-advanced-breast-cancer-adjuvant-therapy.xml&content=view-node%3Anodes-radiotherapy. Accessed May 3, 2015. | |
BCCA Clinical Management Guidelines: Breast Cancer; Stage I and II Invasive Breast Cancer RT section; 2013. Available from: http://www.bccacncer.bc.ca/health-professionals/professional-recources/cancer-management-guidelines/breast/breast#Management. Accessed May 3, 2015. | |
Clarke M, Collins R, Darby S, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. | |
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. | |
Morrow M, Strom EA, Bassett LW, et al; American College of Radiology; American College of Surgeons; Society of Surgical Oncology; College of American Pathology. Standard for breastconservation therapy in the management of invasive breast carcinoma. CA Cancer J Clin. 2002;52:277–300. | |
White JR, Halberg FE, Rabinovitch R, et al. American College of Radiology appropriateness criteria on conservative surgery and radiation: stages I and II breast carcinoma. J Am CollRadiol. 2008;5: 701–713. | |
Smith BD, Bentzen SM, Correa CR, et al. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;81:59–68. | |
Veronesi U, Saccozzi R, Del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305: 6–11. | |
Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312:665–673. | |
Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7:467–471. | |
Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362: 513–520. | |
Haviland JS, Owen JR, Dewar JA, START Trialists’ Group, et al. The UK Standardisation of breast radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. | |
Herbert C, Nichol A, Olivotto I, et al. The impact of hypofractionated whole breast radiotherapy on local relapse in patients with Grade 3 early breast cancer: a population-based cohort study. Int J Radiat OncolBiol Phys. 2012;82:2086–2092. | |
Ko DH, Norriss A, Harrington CR, Robinson BA, James ML. Hypofractionated radiation treatment following mastectomy in early breast cancer: the Christchurch experience. J Med Imaging Radiat Oncol. 2015;59:243–247. | |
Shelley W, Brundage M, Hayter C, Paszat L, Zhou S, Mackillop W. A shorter fractionation schedule for postlumpectomybreast cancer patients. Int J Radiat OncolBiol Phys. 2000;47:1219–1228. | |
Wai ES, Lesperance ML, Alexander CS, et al. Effect of radiotherapy boost and hypofractionation on outcomes in ductal carcinoma in situ. Cancer. 2011;117:54–62. | |
Lalani N, Paszat L, Sutradhar R, et al. Long-term outcomes of hypofractionation versus conventional radiation therapy after breast-conserving surgery for ductal carcinoma in situ of the breast. Int J Radiat OncolBiol Phys. 2014;90:1017–1024. | |
Hathout L, Hijal T, Théberge V, et al. Hypofractionated radiation therapy for breast ductal carcinoma in situ. Int J Radiat OncolBiol Phys. 2013;87:1058–1063. | |
Nilsson C, Valachis A. The role of boost and hypofractionation as adjuvant radiotherapy in patients with DCIS: a meta-analysis of observational studies. Radiother Oncol. 2015;114:50–55. | |
Versmessen H, Vinh-Hung V, Van Parijs H, et al. Health-related quality of life in survivors of stage I-II breast cancer: randomized trial of post-operative conventional radiotherapy and hypofractionatedtomotherapy. BMC Cancer. 2012;12:495. | |
Holloway C, Panet-Raymond V, Olivotto IA. Hypofractionation should be the new ‘standard’ for radiation therapy after breast conserving surgery. Breast. 2010;19:163–167. | |
Ashworth A, Kong W, Whelan T, Mackillop WJ. A population-based study of the fractionation of post-lumpectomy breast radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:51–57. | |
Berrang TS, Truong PT, Tyldesley S, Olivotto IA. A population-based study of the fractionation of post-lumpectomy breast radiation therapy. In regard to Ashworth et al. Int J Radiat Oncol Biol Phys. 2013;87: 632–633. | |
Jagsi R, Falchook AD, Hendrix LH, Curry H, Chen RC. Adoption of hypofractionated radiation therapy for breast cancer after publication of randomized trials. Int J Radiat Oncol Biol Phys. 2014;90: 1001–1009. | |
Wang EH, Mougalian SS, Soulos PR, et al. Adoption of hypofractionated whole-breast irradiation for early-stage breast cancer: a National Cancer Data Base analysis. Int J Radiat Oncol Biol Phys. 2014;90: 993–1000. | |
Rajogopalan MS, Flickinger JC, Heron DE, Beriwal S. Changing practice patterns for breast cancer radiation therapy with clinical pathways: an analysis of hypofractionation in a large, integrated cancer centre network. Pract Radiat Oncol. 2015;5:63–69. | |
Jones B, Dale RG, Deehan C, Hopkins KI, Morgan DAL. The role of biologically effective dose (BED) in clinical oncology. Clin Oncol. 2001;13:71–81. | |
Yarnold J, Ashton A, Bliss J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol. 2005;75:9–17. | |
Olivotto IA, Whelan TJ, Parpia S, et al. Interim cosmetic and toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. J Clin Oncol. 2013;31:4038–4045. | |
Peterson D, Truong PT, Parpia S, et al; RAPID Trial Investigators. Predictors of adverse cosmetic outcome in the RAPID Trial: an exploratory analysis. Int J Radiat Oncol Biol Phys. 2015;91:968–976. | |
Somaiah N, Rothkamm K, Yarnold J. Where do we look for markers of radiotherapy fraction size sensitivity? Clin Oncol. Epub 2015 Jun 21. | |
Stokes EL, Tyldesley S, Woods R, Wai E, Olivotto IA. Effect of nodal irradiation and fraction size on cardiac and cerebrovascular mortality in women with breast cancer treated with local and loco-regional radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80: 403–409. | |
Chan EK, Woods R, McBride ML, et al. Adjuvant hypo-fractionated versus conventional whole breast radiation therapy for early-stage breast cancer: long-term hospital-related morbidity from cardiac causes. Int J Radiat Oncol Biol Phys. 2014;88:786–792. | |
Chan EK, Woods R, Virani S, et al. Long-term mortality from cardiac causes after adjuvant hypo-fractionated vs. conventional radiotherapy for localized left-sided breast cancer. Radiother Oncol. 2015;114: 73–78. | |
Tjessem KH, Johansen S, Malinen E, et al. Long-term cardiac mortality after hypofractionated radiation therapy in breast cancer. Int J Radiat Oncol Biol Phys. 2013;87:337–343. | |
Hamilton SN, Tyldesley S, Li D, Olson R, McBride M. Second malignancies after adjuvant radiation therapy for early stage breast cancer: is there increased risk with addition of regional radiation to local radiation? Int J Radiat Oncol Biol Phys. 2015;91:977–985. | |
Baillet F, Housset M, Maylin C, et al. The use of a specific hypofractionated radiation therapy regimen versus classical fractionation in the treatment of breast cancer: a randomized study of 230 patients. Int J Radiat Oncol Biol Phys. 1990;19:1131–1133. | |
FAST TRIALISTS Group, Agrawal RK, Alhasso A, et al. First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015). Radiother Oncol. 2011;100:93–100. | |
The Institute for Cancer Research, clinical trials, FAST-Forward; 2015. Available from: http://www.icr.ac.uk/our-research/our-research-centres/clinical-trials-and-statistics-unit/clinical-trials/fast_forward_page. Accessed April 25, 2015 | |
Brunt AM, Yarnold J, Wheatley D, et al. Acute skin toxicity reported in the FAST-forward trial (HTA 09/01/47): a phase III randomized trial of 1-week whole breast radiotherapy compared to standard 3 weeks in patients with early breast cancer. In: Proceedings of the 10th National Cancer Research Conference; November 2–5, 2014; Liverpool, UK. | |
American Society for Radiation Oncology. Choose Wisely; 2013. Available from: http://www.choosingwisely.org/clinician-lists/american-society-radiation-oncology-whole-breast-radiotherapy/. Accessed April 23, 2015 | |
Trans-Tasman Radiation Oncology Group (TROG). Radiation Doses and Fractionation Schedules in Non-Low Risk Ductal Carcinoma In Situ (DCIS) of the Breast. Available from: https://clinicaltrials.gov/ct2/show/NCT00470236?term=ductal+carcinoma+in+situ&rank=24. Accessed April 23, 2015. [ClinicalTrials.gov identifier: NCT00470236]. | |
Haviland JS, Yarnold JR, Bentzen SM. Hypofractionated radiotherapy for breast cancer. N Engl J Med. 2010;362:1843. | |
Bartelink H, Maingon P, Poortmans P, et al; European Organisation for Research and Treatment of Cancer Radiation Oncology and Breast Cancer Groups. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16: 47–56. | |
Romestaing P, Lehingue Y, Carrie C, et al. Role of a 10-Gy boost in the conservative treatment of early breast cancer: results of a randomized clinical trial in Lyon, France. J Clin Oncol. 1997;15:963–968. | |
Chan EK, Tabarsi N, Tyldesley S, et al. Patient-reported long-term cosmetic outcomes following short fractionation whole breast radiotherapy with boost. Am J Clin Oncol. Epub 2014. | |
Vrieling C, Collette L, Fourquet A, et al. The influence of patient, tumor and treatment factors on the cosmetic results after breast-conserving therapy in the EORTC ‘boost vs no boost’ trial. EORTC Radiotherapy and Breast Cancer Cooperative Groups. Radiother Oncol. 2000;55: 219–232. | |
Van Limbergen E, Rijnders A, van der Schueren E, Lerut T, Christiaens R. Cosmetic evaluation of breast conserving treatment for mammary cancer. 2. A quantitative analysis of the influence of radiation dose, fractionation schedules and surgical treatment techniques on cosmetic results. Radiother Oncol. 1989;16:253–267. | |
Barnett GC, Wilkinson JS, Moody AM, et al. The Cambridge breast intensity-modulated radiotherapy trial: patient- and treatment-related factors that influence late toxicity. Clin Oncol. 2011;23: 662–673. | |
Ciammella P, Podgornii A, Galeandro M, et al. Toxicity and cosmetic outcome of hypofractionated whole-breast radiotherapy: predictive clinical and dosimetric factors. Radiat Oncol. 2014;9:97. | |
Ho AL, Bovill ES, Macadam SA, Tyldesley S, Giang J, Lennox PA. Postmastectomy radiation therapy after immediate two-stage tissue expander/implant breast reconstruction: a University of British Columbia perspective. Plast Reconstr Surg. 2014;134:1e–10e. | |
Sbitany H, Wang F, Peled AW, et al. Immediate implant-based breast reconstruction following total skin-sparing mastectomy: defining the risk of preoperative and postoperative radiation therapy for surgical outcomes. Plast Reconstr Surg. 2014;134:396–404. | |
Yarnold J, Bentzen SM, Coles C, Haviland J. Hypofractionated whole-breast radiotherapy for women with early breast cancer: myths and realities. Int J Radiat Oncol Biol Phys. 2011;79:1–9. | |
Rose MA, Olivotto I, Cady B, et al. Conservative surgery and radiation therapy for early breast cancer. Long-term cosmetic results. Arch Surg. 1989;124:153–157. | |
Zissiadis Y, Langlands AO, Barraclough B, Boyages J. Breastconservation: long-term results from Westmead Hospital. Aust N Z J Surg. 1997;67:313–319. | |
Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. | |
Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. | |
Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. | |
Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. | |
Wai ES, Lesperance M, Speers CH, et al. Increased use of regional radiotherapy is associated with improved outcome in a population-based cohort of women with breast cancer with 1–3 positive nodes. Radiother Oncol. 2010;97:301–306. | |
Powell S, Cooke J, Parsons C. Radiation-induced brachial plexus injury: follow-up of two different fractionation schedules. Radiother Oncol. 1990;18:213–220. | |
Badiyan SN, Shah C, Arthur D, et al. Hypofractionated regional nodal irradiation for breast cancer: examining the data and potential for future studies. Radiother Oncol. 2014;110:39–44. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

