Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Hyperinsulinemia Influences the Short-Term Efficiency of Laparoscopic Sleeve Gastrectomy for Patients with Obesity and Insulin Resistance
Authors Yue Z, Qian L, Jin Y, Xia Y, Sha H, Wu Q, Hu K
Received 7 March 2023
Accepted for publication 2 June 2023
Published 13 June 2023 Volume 2023:16 Pages 1745—1753
DOI https://doi.org/10.2147/DMSO.S411440
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gian Paolo Fadini
Zilong Yue,1,* Long Qian,2,* Yan Jin,1,* Yabin Xia,1 Hui Sha,1 Qin Wu,1 Kaifeng Hu1
1Department of Gastrointestinal Surgery, The First Affiliated Yijishan Hospital of Wannan Medical College, Wuhu, Anhui, People’s Republic of China; 2General Surgery Department, Wuhu Hospital of Traditional Chinese Medicine, Wuhu, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kaifeng Hu, Department of Gastrointestinal Surgery, The First Affiliated Yijishan Hospital of Wannan Medical College, No. 2, Zheshan West Road, Wuhu, Anhui, 241001, People’s Republic of China, Tel +86-13655537677, Email [email protected]
Purpose: The effect of hyperinsulinemia on short-term outcomes after laparoscopic sleeve gastrectomy (LSG) in patients with obesity combined with insulin resistance is unclear.
Material and Methods: This was a retrospective analysis of patients who underwent LSG at our center between January 1, 2020, and December 31, 2021. Patients were divided into hyperinsulinemia (HINS) and nonhyperinsulinemia (NHINS) groups based on fasting insulin levels. The primary endpoint was weight change. Metabolic disease outcomes, postoperative complications, and quality of life score changes were secondary endpoints.
Results: A total of 92 patients were included in this study, with 59 in the HINS group and 33 in the NHINS group. At 6 months postoperatively, the median (P25, P75) %EWL was 76.01 (64.40, 86.99)% in the HINS group and 92.02 (86.78, 100.88)% in the NHINS group (P< 0.001). The mean %TWL (SD) was 23.26 (7.14)% in the HINS group and 26.80 (6.55)% in the NHINS group (P=0.021). The remission of dyslipidemia and hypertension in the NHINS group and the HINS group were not significantly different (P> 0.05 for all). The differences in QOL between groups were not statistically significant (P=0.788). In terms of postoperative complications, there was no statistically significant difference between the groups (P> 0.05 for all).
Conclusion: HINS negatively influences weight change in patients with obesity and insulin resistance, and the NHINS group had better postoperative weight loss. In terms of hypertension, dyslipidemia, and postoperative complications, there was no significant effect of HINS.
Keywords: laparoscopic sleeve gastrectomy, hyperinsulinemia, insulin resistance
Obesity ranks 5th on the list of 84 risk factors affecting human health published by the Global Burden of Disease Study (GBD).1 It is difficult for patients with obesity to lose weight through lifestyle changes and behavioral interventions alone. Apart from a few drugs that can be used clinically to treat morbid obesity, bariatric surgery is currently the most effective treatment for morbid obesity.2 According to IFSO’s 2018 bariatric surgery data, approximately 696,191 bariatric surgeries were performed in 57 countries, and laparoscopic sleeve gastrectomy (LSG) was the most commonly used surgery in the world, accounting for 55.4% of all bariatric surgeries.3 Several studies have demonstrated the efficacy and safety of LSG.4,5 With the large-scale application of LSG in clinical practice, the factors affecting surgical efficacy have become a new research hotspot to obtain better surgical outcomes.
Insulin resistance (IR) is closely related to obesity, and only a fraction of patients with IR exhibit hyperinsulinemia (HINS), while another fraction have normal fasting insulin, ie, nonhyperinsulinemia (NHINS). HINS was defined as an excess of insulin relative to that required to maintain normal glucose (FINS>15 mU/L).6 The current widely accepted view is that hyperinsulinemia and nonhyperinsulinemia are two different periods in the development of IR, and nonhyperinsulinemia appears earlier than hyperinsulinemia, which can be understood as IR of two different severities. HINS is not a reflection of insulin resistance but a result of hypersecretion of basal INS relative to the degree of IR.7 A study by Templeman et al found that the inhibition of HINS in mice led to the development of protection against obesity8 and that HINS can contribute to the development of obesity. Therefore, we designed this study to determine whether HINS affects the results of LSG surgery for patients with obesity combined with IR. In addition, we provided some suggestions for future clinical work.
Materials and Methods
Study Design and Participants
This was a retrospective analysis of patients who underwent LSG surgery at the First Affiliated Hospital of Wannan Medical College between January 1, 2020, and December 31, 2021. All patients underwent an oral glucose tolerance test (OGTT) before surgery, and insulin levels in venous blood were measured before surgery. Patients included in the study were divided into two groups according to FINS: the HINS group with FINS > 15 mU/L or 2 h OGTT insulin (2hinsulin, 2hINS) > 80 mU/L and the NHINS group with FINS ≦ 15 mU/L or 2 h OGTT insulin (2hinsulin, 2hINS) ≤ 80 mU/L. Then, the surgical outcomes of the two groups of patients were compared. This study was conducted in accordance with the Declaration of Helsinki.
The criteria for inclusion were as follows: (1) 30 kg/m2≤BMI<60 kg/m2; (2) insulin resistance index (HOMA-IR) >2.69,9 HOMA-IR= (fasting glucose×fasting insulin)/22.5; and (3) no history of abdominal surgery. The exclusion criteria were as follows: (1) received surgical treatment during the follow-up period; (2) LSG combined with other abdominal surgery; (3) postoperative alcohol or drug abuse; (4) pregnancy and lactation; and (5) diabetes mellitus.
Surgical Technique
All of our patients were operated on by the same team, and the chief surgeon was experienced. The surgical method was as follows. In the first step, we use the ultrasonic scalpel to free the greater omentum starting from the avascular zone of the gastrocolic ligament, up to the gastroesophageal junction, and completely expose the fundus, and down to 4 cm-6 cm from the pylorus. Then, the anesthesiologist places a 36 Fr calibration bougie into the gastric cavity through the mouth, which is used as a template to perform a vertical partial gastrectomy and to aid in the intraoperative emptying of the stomach. Next, the surgeon uses an articulating endoscopic linear cutter to begin resection of the gastric wall at a distance of approximately 2–6 cm from the pylorus under the guidance of the bougie. During the procedure, we completely resected the fundus of the stomach, which is very important for LSG. At the end of the operation, we removed the bougie, added sutures to the gastric incision margin, removed the specimen, left a drainage tube in the abdomen, sutured the incisions, and completed the operation.
Outcome Measurements
General clinical indicators were measured according to predetermined testing times (1 day before surgery and 1, 3, and 6 months after surgery), which included height (m), weight (kg), BMI (kg/m2), and blood pressure (mmHg). Elbow venous blood drawn in the fasting state was used to measure FINS, fasting glucose (GLU), total cholesterol (CHOL), triglycerides (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL). The criteria for remission of hypertension were systolic blood pressure ≤120 mmHg and diastolic blood pressure ≤80 mmHg when the patient was not taking medication. Dyslipidemia remission criteria were defined as LDL < 2.60 mmol/L, TG < 1.70 mmol/L, HDL-C >1.04 mmol/L (men) or >1.30 mmol/L (women) without pharmacological treatment. QOL was measured by the Moorehead-Ardelt QOL Questionnaire II, with scores ranging from −3 to +3, with higher scores representing higher QOL.10 %EWL and %TWL were calculated from the follow-up data, %EWL = (initial weight - follow-up weight)/(initial weight - 25×height2)×100%11 and %TWL = (initial weight - follow-up weight)/initial weight×100%.
The primary endpoint was the patient’s postoperative weight change, which was compared using percentage excess weight loss (%EWL) and percentage total weight loss (%TWL). Secondary endpoints were remission of patient comorbidities, improvement in quality of life (QOL), and incidence of postoperative complications after surgery.
Statistical Analysis
The Shapiro‒Wilk normality test was used to test the normality of the data, with normally distributed data expressed as the mean ± SD using the t-test and nonnormally distributed data expressed as the median (P25, P75) using the Mann‒Whitney U-test. Differences in categorical variables were assessed using Fisher’s exact test or the chi-square test. We conducted Spearman correlation analysis between preoperative FINS and %EWL/%TWL. All tests were two-sided, and P <0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS statistical software (version 24.0; SPSS Inc.) and R (version 3.6.3; R Foundation for Statistical Computing).
Results
Baseline Characteristics
In this study, a total of 118 patients underwent LSG at our center between 1 January 2020 and 31 December 2021. Thirteen patients were diagnosed with type 2 diabetes before surgery, 3 patients underwent laparoscopic cholecystectomy, 1 patient was pregnant during postoperative follow-up, and 9 patients had imperfect follow-up; the aforementioned patients were not included in this study. A total of 92 patients participated in this study, 59 in the HINS group and 33 in the NHINS group. Figure 1 shows the detailed research process for this study. The mean BMI (SD) of patients in the HINS group was 36.60 (5.05) kg/m2 and that in the NHINS group was 35.14 (3.23) kg/m2 (P=0.098). Baseline characteristics are shown in Table 1. There was no significant difference in baseline characteristics between the study groups before surgery.
 |
Table 1 Baseline Characteristics |
 |
Figure 1 Study Flowchart. |
Primary End Point
At 6 months after LSG surgery, the estimated median (P25, P75) %EWL was 76.01 (64.40, 86.99)% for patients in the HINS group and 92.02 (86.78, 100.88)% for patients in the NHINS group, with a significant difference (P<0.001). There were between-group differences in the mean %TWL (mean [SD], 23.26 [7.14]% in the HINS group vs 26.80 [6.55]% in the NHINS group; P=0.021). %EWL and %TWL were higher in the NHINS group than in the HINS group at 6 months postoperatively, representing more postoperative weight loss in the NHINS group. The differences in mean %EWL and %TWL between the HINS and NHINS groups were statistically significant at all time points (1, 3, and 6 months). The detailed %EWL and %TWL data are listed in Table 2. Figure 2 compares the postoperative %EWL and %TWL of patients in the two groups. Figures 3 and 4 show the correlation analysis of %EWL and %TWL with FINS, respectively. %EWL was negatively associated with FINS at the postoperative follow-up time, and %TWL was not significantly associated with FINS.
 |
Table 2 Excess Weight Loss and Total Weight Loss Mean Differences for the HINS Study Group and NHINS Study Group After Laparoscopic Sleeve Gastrectomy at 1, 3,and 6 Months |
Secondary End Points
Dyslipidemia
At baseline, 27 patients (29.35%) had dyslipidemia, 18 of 59 (30.51%) in the HINS group and 9 of 33 (27.27%) in the NHINS group. At 6 months, 7 of 18 patients (38.89%) in the HINS group and 5 of 9 (55.56%) in the NHINS group met remission criteria, and the difference between the two groups was not statistically significant (P=0.448; Table 3).
 |
Table 3 Hypertension Remission, Dyslipidemia Remission, and QOL Score Reported for the HINS Study Group and NHINS Study Group After LSG at 6 Months |
Hypertension
Preoperatively, the HINS group had 11 patients (18.64%), and the NHINS group had 5 patients (15.15%) with hypertension. At 6 months postoperatively, 6 of 11 patients (54.55%) in the HINS group and 3 of 5 patients (60.00%) in the NHINS group achieved relief from hypertension, and no significant difference was noted between the two groups (P=1.000; Table 3).
Quality of Life
At baseline, the mean (SD) QOL scores of patients in the HINS and NHINS groups were 0.23 (0.79) and 0.19 (0.98), respectively. At 6 months, the mean (SD) QOL score was 1.05 (0.59) in the HINS group and 1.09 (0.68) in the NHINS group, with no significant difference between the study groups (P=0.788; Table 3). All patients had elevated QOL scores at 6 months postoperatively, and the differences were statistically significant when comparing the total patients’ preoperative and postoperative QOL scores (P<0.001).
Complications
The complications are listed in Table 4. For this study, postoperative complications occurred in 4 (6.78%) patients in the HINS group and 2 (6.06%) patients in the NHINS group (P=1.000). A Clavien‒Dindo grade IIIb complication12 (gastric leakage) occurred in 1 patient in the HINS group. No patients died during the postoperative follow-up time.
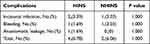 |
Table 4 Complication Reported for the HINS Study Group and NHINS Study Group After LSG at 6 Months |
Discussion
The obesity epidemic is one of the greatest medical challenges of our time, and evidence-based medicine has demonstrated that obesity can increase the incidence of type 2 diabetes, hypertension, and the risk of death from gastrointestinal tumors and kidney tumors, as well as the occurrence of adverse events from related diseases.13 The development of obesity is closely related to a hormone in the body called insulin.14 Insulin is a peptide hormone secreted by pancreatic β cells in the pancreas that are stimulated by endogenous or exogenous substances, and insulin is mainly involved in the metabolism of glucose in the body.15 In addition, insulin is also involved in various anabolic pathways in the body, such as glycogen synthesis, lipid metabolism, DNA synthesis, gene transcription, amino acid transport, protein synthesis and degradation.16
When the sensitivity and responsiveness of insulin target organs or target tissues to endogenous or exogenous insulin are reduced, it can cause systemic or local IR. IR is prevalent in patients with obesity, and existing studies have found that this phenomenon may be associated with the accumulation of specific lipid mediators, abnormal fat deposition, abnormal adipose tissue hormone secretion, abnormalities in linear stereotypic function, and increased emergency activator protein c-Jun N-terminal kinase (JNK) and inflammatory pathways in patients with obesity.17,18 In patients with obesity and IR, FINS is expressed at different levels, both HINS and NHINS. HINS has been shown to promote the development of obesity while producing a protective effect against obesity. Through our study, we found that HINS also had an impact on weight change after LSG in patients with obesity. The %EWL and %TWL of patients in the NHINS group were significantly higher than those in the HINS group at the 6-month postoperative follow-up time. In conjunction with related studies, we believe that HINS is involved in multiple metabolic pathways in the human body and thus has an impact on postoperative weight loss. Lu Guo et al similarly found that patients with HINS lost less weight after treatment with LSG.19 HINS is an important proinflammatory factor in patients with obesity, promoting the development of chronic inflammation in adipose tissue and affecting local or systemic metabolic function in patients with obesity.6,20 Inflammation and impaired metabolism of adipose tissue are closely related to the development of obesity. On the other hand, HINS also promotes the proliferation of adipose tissue while inhibiting the breakdown of adipose tissue and stimulating the expression of genes involved in lipid uptake and storage.21,22 The effect of HINS on the body’s metabolism is caused by higher concentrations of insulin in the circulating blood. In a study by Jansen, H. J. et al23 It was found that patients with type 2 diabetes had a more pronounced inflammatory state in adipose tissue after insulin treatment. Skovso et al showed that the use of long-acting insulin analogs led to weight gain, and Lustig et al demonstrated that the use of drugs that inhibit insulin secretion led to weight loss in people with obesity.24,25 HINS plays a role in weight protection by promoting inflammation, affecting lipid metabolism, and influencing energy metabolism to influence weight loss after LSG, but the main mechanism by which HINS affects postoperative weight loss is unclear. When we further analyzed the correlation of FINS with %EWL and %TWL, we found an interesting phenomenon in which FINS was negatively associated with %EWL and not significantly associated with %TWL. The %TWL was more influenced by the initial weight in the calculation, and we found that the %TWL distribution was more clustered, which may have had an impact on the correlation analysis between FINS and %TWL. Although the correlation analysis between FINS and %TWL was not statistically significant, we observed that there was a significant difference in %TWL between the two groups of patients at the postoperative follow-up time. The effect of HINS on postoperative weight change provides new ideas for our treatment, and we may achieve better surgical outcomes in this group of patients if we use pharmacological interventions for HINS preoperatively.
At the same time, when we analyzed the treatment effect of dyslipidemia as well as hypertension, we found that both groups obtained good treatment effects after surgery, and there was no significant difference in the treatment effect between the two groups (P>0.05, for all). HINS is closely associated with the development of hypertension, which could mediate the development of hypertension by enhancing the contractile effect of angiotensin II on the thoracic aorta and inhibiting the diastolic effect of acetylcholine on the thoracic aorta, increasing sympathetic excitability, increasing renal sodium reabsorption leading to water and sodium retention, and promoting smooth muscle proliferation leading to vascular remodeling.26–28 However, the results of this study found that HINS did not influence the remission of postoperative hypertension. In the HINS group, patients with improved IR and reduced adipocytokine secretion experienced a significant decrease in FINS in the short term after surgery; at the same time, patients experienced significant weight loss. As the stimulation of blood pressure decreases by FINS, multiple pathways that elevate blood pressure are inhibited, which we believe is the main reason why hypertensive patients in the HINS group can achieve remission in the short term after surgery. On the other hand, according to Goldstein’s study, 64% of hypertensive patients <40 years of age had significantly elevated catecholamines,29 reflecting that activation of the sympathetic nervous system is an important mechanism in the pathogenesis of hypertension in young people. The patients treated at our center were relatively young, with a mean age of 30.29 years, which creates favorable conditions for obtaining the desired therapeutic results in the short term after surgery in both groups. Although the difference in the treatment effect of dyslipidemia between the two groups was not statistically significant (P=0.397), we found that postoperative remission of dyslipidemia occurred in both groups of patients with milder dyslipidemia and that remission of dyslipidemia may be related to the degree of preoperative dyslipidemia, which was not further demonstrated in this study.
In addition, no significant differences were found between the HINS group and the NHINS group in terms of postoperative complications and postoperative QOL, indicating that there was no significant relationship between HINS and the occurrence of postoperative complications and QOL.
There are some limitations of this experiment, most notably the small sample size, which may lead to some selective bias in the statistical results; therefore, we will expand the sample size in subsequent experiments to continue the research and improve the content. This study only investigated the short-term effects of HINS on surgical outcomes, but we are also conducting other related studies, including a study of long-term postoperative outcomes, to supplement this study.
Conclusion
HINS negatively influences weight change in patients with obesity and insulin resistance, and the NHINS group had more postoperative weight loss than the HINS group. In terms of hypertension, dyslipidemia, and postoperative complications, there was no significant effect of HINS.
Ethics Approval Statement
This study was approved by the Ethics Committee of the First Affiliated Yijishan Hospital of Wannan Medical College. Informed consent was obtained from participants in this study.
Acknowledgments
Thank you to all those who participated in this experiment. Zilong Yue, Long Qian and Yan Jin share first authorship. Thanks for the Teaching Case Bank for Postgraduate Professional Degrees in Anhui Province (2022zyxwjxalk152), the Management and Innovation Project of the First Affiliated Hospital of Wannan Medical College (CX2023009), the Key Teaching and Research Project of Anhui Provincial Department of Education (2021jyxm1623) and the Outstanding Young Backbone Teachers of Universities in Anhui Province Department of Education Foreign Visit Training Project (gxgwfx2021040) supported this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stanaway JD, Afshin A, Gakidou E, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1923–1994.
2. Grönroos S, Helmiö M, Juuti A, et al. Effect of laparoscopic sleeve gastrectomy vs roux-en-y gastric bypass on weight loss and quality of life at 7 years in patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA Surg. 2021;156(2):137–146. doi:10.1001/jamasurg.2020.5666
3. Angrisani L, Santonicola A, Iovino P, Ramos A, Shikora S, Kow L. Bariatric surgery survey 2018: similarities and disparities among the 5 IFSO chapters. Obes Surg. 2021;31(5):1937–1948. doi:10.1007/s11695-020-05207-7
4. Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241–254. doi:10.1001/jama.2017.20313
5. Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255–265. doi:10.1001/jama.2017.20897
6. Zhang A, Wellberg EA, Kopp JL, Johnson JD. Hyperinsulinemia in obesity, inflammation, and cancer. Diabetes Metab J. 2021;45(3):285–311. doi:10.4093/dmj.2020.0250
7. Weyer C, Hanson RL, Tataranni PA, Bogardus C, Pratley RE. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes. 2000;49(12):2094–2101. doi:10.2337/diabetes.49.12.2094
8. Templeman NM, Clee SM, Johnson JD. Suppression of hyperinsulinaemia in growing female mice provides long-term protection against obesity. Diabetologia. 2015;58(10):2392–2402. doi:10.1007/s00125-015-3676-7
9. Xiao-yan X, Wwn-ying Y, Zhao-jun Y. The diagnostic significance of homeostasis model assessment of insulin resistance in metabolic syndrome among subjects with different glucose tolerance. Chin J Diabetes. 2004;12(3):182–186.
10. Moorehead MK, Ardelt-Gattinger E, Lechner H, Oria HE. The validation of the Moorehead-ardelt quality of life questionnaire II. Obes Surg. 2003;13(5):684–692. doi:10.1381/096089203322509237
11. Brethauer SA, Kim J, El Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Obes Surg. 2015;25(4):587–606. doi:10.1007/s11695-015-1645-3
12. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
13. Müller TD, Blüher M, Tschöp MH, DiMarchi RD. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2022;21(3):201–223. doi:10.1038/s41573-021-00337-8
14. Kolb H, Stumvoll M, Kramer W, Kempf K, Martin S. Insulin translates unfavourable lifestyle into obesity. BMC Med. 2018;16(1):232. doi:10.1186/s12916-018-1225-1
15. Vecchio I, Tornali C, Bragazzi NL, Martini M. The discovery of insulin: an important milestone in the history of medicine. Front Endocrinol. 2018;9:613. doi:10.3389/fendo.2018.00613
16. Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022;7(1):216. doi:10.1038/s41392-022-01073-0
17. Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature. 2019;576(7785):51–60. doi:10.1038/s41586-019-1797-8
18. Ye J. Mechanisms of insulin resistance in obesity. Front Med. 2013;7(1):14–24. doi:10.1007/s11684-013-0262-6
19. Guo L, Luo W, Tan T, et al. Early-phase insulin hypersecretion associated with weight loss outcome after LSG: a prospective cohort study in Asian patients with BMI ≥28 kg/m(2). Surg Obes Relat Dis. 2022;18(10):1209–1217. doi:10.1016/j.soard.2022.05.013
20. Pedersen DJ, Guilherme A, Danai LV, et al. A major role of insulin in promoting obesity-associated adipose tissue inflammation. Mol Metab. 2015;4(7):507–518. doi:10.1016/j.molmet.2015.04.003
21. Templeman NM, Skovsø S, Page MM, Lim GE, Johnson JD. A causal role for hyperinsulinemia in obesity. J Endocrinol. 2017;232(3):R173–R183. doi:10.1530/JOE-16-0449
22. Kim MK, Reaven GM, Kim SH. Dissecting the relationship between obesity and hyperinsulinemia: role of insulin secretion and insulin clearance. Obesity. 2017;25(2):378–383. doi:10.1002/oby.21699
23. Jansen HJ, Stienstra R, van Diepen JA, et al. Start of insulin therapy in patients with type 2 diabetes mellitus promotes the influx of macrophages into subcutaneous adipose tissue. Diabetologia. 2013;56(12):2573–2581. doi:10.1007/s00125-013-3018-6
24. Skovsø S, Damgaard J, Fels JJ, et al. Effects of insulin therapy on weight gain and fat distribution in the HF/HS-STZ rat model of type 2 diabetes. Int J Obes. 2015;39(10):1531–1538. doi:10.1038/ijo.2015.92
25. Lustig RH, Greenway F, Velasquez-Mieyer P, et al. A multicenter, randomized, double-blind, placebo-controlled, dose-finding trial of a long-acting formulation of octreotide in promoting weight loss in obese adults with insulin hypersecretion. Int J Obes. 2006;30(2):331–341. doi:10.1038/sj.ijo.0803074
26. Esler M, Lambert G, Schlaich M, Dixon J, Sari CI, Lambert E. Obesity paradox in hypertension: is this because sympathetic activation in obesity-hypertension takes a benign form. Hypertension. 2018;71(1):22–33. doi:10.1161/HYPERTENSIONAHA.117.09790
27. da Silva AA, Do Carmo JM, Li X, Wang Z, Mouton AJ, Hall JE. Role of hyperinsulinemia and insulin resistance in hypertension: metabolic syndrome revisited. Can J Cardiol. 2020;36(5):671–682. doi:10.1016/j.cjca.2020.02.066
28. Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res. 2017;122:1–7. doi:10.1016/j.phrs.2017.05.013
29. Goldstein DS. Plasma catecholamines and essential hypertension. An analytical review. Hypertension. 1983;5(1):86–99. doi:10.1161/01.HYP.5.1.86
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



