Back to Journals » Journal of Pain Research » Volume 13
Hyperalgesia and Reduced Offset Analgesia During Spinal Anesthesia
Authors Sitsen E , van Velzen M , de Rover M, Dahan A , Niesters M
Received 16 April 2020
Accepted for publication 11 August 2020
Published 24 August 2020 Volume 2020:13 Pages 2143—2149
DOI https://doi.org/10.2147/JPR.S258533
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Elske Sitsen, Monique van Velzen, Mischa de Rover, Albert Dahan, Marieke Niesters
Department of Anesthesiology, Leiden University Medical Center, Leiden, RC 2300, the Netherlands
Correspondence: Marieke Niesters
Department of Anesthesiology, Leiden University Medical Center, H5-022, Leiden, RC 2300, the Netherlands
Email [email protected]
Introduction: Spinal anesthesia induces short-term deafferentation and causes connectivity changes in brain areas involved in endogenous pain modulation. We determined whether spinal anesthesia alters pain sensitivity and offset analgesia. Offset analgesia is a manifestation of endogenous pain modulation and characterized by profound analgesia upon a small decrease in noxious stimulation.
Methods: In this randomized controlled crossover trial, static thermal pain responses and offset analgesia were obtained in 22 healthy male volunteers during spinal anesthesia and control conditions (absence of spinal anesthesia). Pain responses and offset analgesia were measured on a remote skin area above the upper level of anesthesia (C8/Th1).
Results: Following spinal injection of the local anesthetic, the average maximum anesthesia level was Th6. Static pain scores at C8/Th1 were higher during spinal anesthesia compared to control: 59.1 ± 15.0 mm (spinal anesthesia) versus 51.7 ± 19.7 mm (control; p = 0.03). Offset analgesia responses were decreased during spinal analgesia: pain score decrease 79 ± 27% (spinal anesthesia) versus 90 ± 17% (control; p = 0.016).
Discussion: We confirmed that spinal anesthesia-induced deafferentation causes hyperalgesic responses to noxious thermal stimulation and reduced offset analgesia at dermatomes remote and above the level of deafferentation. While these data suggest that the reduction of offset analgesia has a central origin, related to alterations in brain areas involved in inhibitory pain control, we cannot exclude alternative (peripheral) mechanisms.
Trial Registration: Dutch Cochrane Center under identifier (www.trialregister.nl) NL3874.
Keywords: spinal anesthesia, analgesia, pain responses, hyperalgesia, deafferentation
Introduction
Deafferentation or the traumatic or anesthetic disruption of afferent input from the peripheral to the central nervous system results in cortical, subcortical or brainstem reorganization and alters neuronal connectivity.1–7 Using resting-state functional magnetic resonance imaging (fMRI), we previously showed that short-term spinal anesthesia-induced deafferentation is associated with functional connectivity changes in the thalamus in relation to the thalamocortical network and in the anterior cingulate cortex and insula in relation to the thalamo-parietal network.5 In general, deafferentation causes adaptive plasticity, such as cortical expansion of brain areas adjacent to deafferentated areas, due to a rebalancing of excitatory and inhibitory neuronal modulators involved in plasticity.6 Deafferentation may be associated with behavioral changes. While some studies show improved acuity of motor or sensory function related to cortical plasticity,3,8 it is well known that deafferentation may additionally have negative behavioral effects related to maladaptive plasticity, as may occur in traumatic deafferentation, including phantom limb pain or pain after spinal cord injury.1,2,6
Spinal and epidural anesthesia are forms of short-term deafferentation. There is evidence that both types of neuraxial blockade are associated with sensory distortions or pain.9–11 In patients that require a subarachnoid or epidural block for surgery, illusionary limb position, pain in the deafferented limbs, or paradoxical heat perception upon application of a cold stimulus on the transition from normal to deafferentated skin are often observed.9–11 Additionally, in healthy volunteers, we showed that spinal deafferentation is associated with hyperalgesic responses above the level of deafferentation.5
The current study was performed to first determine whether we could replicate the observation of hyperalgesic responses above the level of spinal anesthesia-induced deafferentation. Next, we studied the effect of spinal deafferentation on offset analgesia (OA), a manifestation of endogenous pain modulation. OA is characterized by profound analgesia after a slight decrease in noxious stimulation and is considered an expression of temporal filtering of nociception related to post-stimulus inhibition.12–14 Both peripheral and central mechanisms are suggested to regulate OA.12,15-24 We tested the effect of the spinal anesthesia deafferentation paradigm on OA, and hypothesized that OA responses would be decreased during spinal deafferentation, possibly due to plastic changes in areas of the brain involved pain modulation.
Materials and Methods
Subjects and Ethics
Twenty-two volunteers were recruited after approval of the protocol by the local institutional review board (Commissie Medische Ethiek, Leiden University Medical Center). None of the subjects had participated in earlier studies. Participants were enrolled in the study and they gave oral and written informed consent. All subjects were right-handed healthy males, aged 18 years or older. Exclusion criteria included: body mass index >30 kg/m2, (history of) any medical or psychiatric disease, use of any medication in the last 3 months that could interfere with pain perception, (history of) illicit drug use in the last 3 months, consumption of more than 3 alcohol units/day, or any condition that could interfere with the placement of a spinal needle. This study is part of a large project on the influence of spinal deafferentation on central pain processing as measured by fMRI in healthy male volunteers. We here report on the influence of spinal deafferentation on offset analgesia. The study was registered at the trial register of the Dutch Cochrane Center (www.trialregister.nl) under identifier NL3874. All procedures were performed in compliance with the latest version of the Declaration of Helsinki and Good Clinical Practice guidelines. The study was performed from June 2013 to February 2014. All subjects received a small monetary compensation for their participation.
Study Design
The study had a randomized, placebo-controlled, single-blind, crossover design. Randomization relates to the fact that the occasions were randomized, the single-blind design relates to the fact that an independent pain researcher (LO, not further involved in the trial) performed the static and offset analgesia pain tests in a fully blinded fashion; the subjects could not be blinded. Additionally, the data analysis was performed on a blinded data set. Participants were randomized to receive an intrathecal injection with a local anesthetic on occasion A and “no intervention” on occasion B, with one week in between the two occasions. Randomization was performed using a computer-generated randomization list. All subjects were requested not to eat or drink during the 8-hours prior to their visit to the research unit and none received any prehydration prior to administration of spinal anesthesia or no intervention.
Prior to the spinal injection, the skin was infiltrated with 1 mL lidocaine (10 mg/mL; AstraZeneca, Zoetermeer, The Netherlands) to induce local anesthesia after which a pencil point 27G spinal needle (Vygon, Valkenswaard, The Netherlands) was inserted in the skin at the interspace between vertebrae L3 and L4 and advanced towards the intrathecal space. After the needle point had reached the spinal space, 3 mL bupivacaine (5 mg/mL; AstraZeneca) was slowly injected. In control experiments subjects were positioned in the lateral position but the skin was not punctured and no medication was injected. Dermatome evaluation was by cold sensation. All procedures were performed by a single anesthetist (ES) with ample experience in the placement of spinal anesthesia under strict sterile conditions. Emergency medication, ephedrine 5 mg or 0.5 mg atropine could be administered intravenously if deemed necessary.
Static Pain and Offset Analgesia Tests
Heat pain was applied on the left volar forearm (dermatome C8-Th1) using a 3 cm2 thermal probe of the Pathway Neurosensory Analyzer (Medoc Ltd., Ramat Yishai, Israel). The temperature of the thermode was computer-controlled and could be set at any desired value between 12°C and 50°C. To quantify the pain intensity during the static pain tests, the subjects rated their pain on a 100-mm paper visual analogue scale (VAS) ranging from 0 (no pain) to 100 mm (most intense pain imaginable). To quantify the pain intensity in the offset analgesia tests, the subjects rated their pain scores on a slider of an electric 100-mm long potentiometer that was connected to the computer; the slider ranged from 0 (no pain) to 100 mm (most intense pain imaginable). This enabled the continuous monitoring of the electronic visual analogue scale (eVAS) during noxious stimulation.
The target temperature of the pain tests was determined prior to the spinal injection by administering a series of heat stimuli, ranging from 42°C to 50°C in steps of 1°C for 10 s, with 5–10 min interval between stimuli. The lowest temperature evoking an eVAS of 50 mm was used during the remainder of the study. To overcome adaptation the thermode was moved in-between three non-overlapping skin areas between stimuli.12 After this “calibration” process, a first static pain test was obtained at the target temperature applied for 10 s. Thereafter the study started with induction of spinal anesthesia or the “no intervention”, as described above.
Ninety min after the spinal injection or 90 min into the control condition, the static pain test was repeated and 5–10 min later the offset analgesia test was performed on a different part of the skin of the lower arm. Offset analgesia was studied using a three-temperature paradigm (T1-T2-T3). The temperature of the thermode was increased from 32°C to the target temperature at 1.5°C/s and kept constant for 5 s (T1), after which it was increased by 1°C for 5s (T2), then decreased by 1°C and kept constant for 20 s (T3), after which it returned to baseline values (32°C) at 6°C/s.
Data and Statistical Analysis
The study was powered to determine a significant effect of spinal deafferentation on BOLD-signals in brain regions activated by the pain tests (difference between spinal anesthesia and the control condition = 0.25, SD = 0.20, α = 0.05, 1 – β = 0.90). A number of 16 subjects were deemed necessary. To consider any margin of uncertainty in the effect size and SD the group size was enlarged to 22. Previously we tested the effect of spinal deafferentation on static pain perception and observed a significant increase in pain intensity of 6.3 ± 3.8 mm (mean ± SD) in a group of 12 volunteers during spinal anesthesia, while the effect in the control condition treatment was negligible. We therefore argue that our current sample size is sufficient to address the primary end-points of the study.
The eVAS data were averaged over 1-s periods. To quantify OA, the decrease in eVAS from peak eVAS value to the eVAS nadir after the 1°C decrease of the test stimulus was measured (ΔeVAS), corrected for the value of the peak eVAS, ΔeVASC = (ΔeVAS/[peak eVAS]) × 100 (ie, correction for the variation in the peak response among participants).12,25 To compare static pain VAS scores and ΔeVASC between control and spinal anesthesia, a paired two-tailed t-test was performed with p-values <0.05 considered significant (GraphPad Prism 8.3.0 for Mac OS X, GraphPad Software, San Diego, CA). All data are presented as mean ± SD, unless otherwise stated.
Results
Twenty-two males were randomized with median age (interquartile range) 22 years (21–23 years) and body mass index 22.2 ± 1.9 kg.m−2 (Figure 1). All volunteers completed the study without adverse effects. No hemodynamic effects occurred and none of the subjects complained of post-spinal headache. The number of blocked dermatomes was 16 ± 3 at the time of pain testing, corresponding to an average upper block level at thoracic dermatome 6.
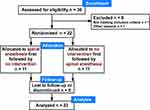 |
Figure 1 Consort flow diagram. |
At baseline (prior to spinal or “no intervention”) the lowest temperature that caused a pain VAS of at least 50 mm on the skin of the lower forearm was 48.0 ± 1.6°C (control) and 48.1 ± 1.2°C (spinal; mean difference +0.07 °C, 95% confidence interval −0.35°C to 0.49°C, p =0.74). The static pain test was performed at the individualized temperatures. Under control conditions, the average pain score at baseline was 52.3 ± 18.0 mm and 90 min later 51.7 ± 19.7 mm (mean difference −0.60 mm, 95% confidence interval −0.64 to 0.52 mm, p = 0.84). On the active treatment visit, the average pain score was at baseline 51.0 ± 15.4 mm and 90 min after the spinal injection 59.1 ± 15.0 mm (mean difference +8.0 mm, 95% confidence interval 1.2 to 14.9 mm, p = 0.02). Pain scores on the arm during spinal anesthesia were higher than 90 min into the control conditions (mean difference 7.4 mm, 95% confidence interval 0.7–14.0, p = 0.03; Figure 2). No correlation was observed between the number of blocked dermatomes and increase in static eVAS scores.
Offset analgesia was also tested on dermatome level C8-Th1 but on a skin area adjacent to the area at which the static pain test was performed. Examples of offset analgesia responses of two subjects are given in Figure 3A and B. In both examples the offset analgesia response is less during spinal anesthesia (orange symbols) than in the control condition (blue symbols). In subject A (Figure 3A) the ΔeVASC values were 79% and 57% under the control condition and during spinal anesthesia, respectively, while corresponding values in subject B (Figure 3B) were 100% and 75%. The average ΔeVASC values were 90 ± 17% (control) and 79 ± 27% (spinal anesthesia; mean difference −11% with 95% confidence interval −20 to −2%, p = 0.016; Figure 3C). Individual ΔeVASC values in the two treatment conditions are given in Figure 3D. No correlation was observed between number of blocked dermatomes and changes in OA responses.
Discussion
There is ample evidence that even short-term peripheral deafferentation is associated with behavioral effects, such as referred sensations, pain, bodily illusions or enhanced sensorimotor acuity of non-deafferented areas.8–11 In the current study, we confirm our previous finding that noxious thermal stimuli applied during spinal anesthesia (maximum block level Th6 or 16 blocked dermatomes) are perceived as more painful than similar stimuli applied in the control condition.5 Hyperalgesia, in magnitude a 14% increase in pain intensity, was consistently observed among participants, and in magnitude in close agreement with our previous study. In that study, using resting-state fMRI, we showed that hyperalgesia was correlated to changes in brain networks involved in the sensory discriminative and the affective dimensions of pain perception. Several of the brain areas within these networks are considered essential elements of the pain modulatory system.5 The changes in network connectivity that we observed made us speculate that spinal deafferentation causes a shift of the endogenous pain system from pain inhibition towards pain facilitation. The hyperalgesic response to a static thermal noxious stimulus is then the consequence of such a shift. To test this hypothesis, we assessed whether spinal deafferentation would change offset analgesia, an expression of endogenous pain modulation.
Offset analgesia is defined by the reduction in pain intensity perception observed during noxious thermal stimulation towards hypoalgesia after a small decrease (1°C) in noxious thermal stimulation.12–14 OA is considered the altered spatiotemporal processing of noxious stimuli aimed at amplifying reductions in noxious stimulation. Loss of proper OA engagement has been observed in neuropathic pain patients and in patients with fibromyalgia.12,15,26 We earlier showed that in fibromyalgia patients, reduced OA responses were associated with earlier onset of pain (reduced pain threshold) and increased sensory sensitivity (hyperalgesia) to heat stimuli;26 both phenomena may be involved in the persistence or chronification of pain symptoms. The mechanism of OA is not yet fully understood. The literature indicates various possible offset analgesia mechanisms such as a central mechanism involving activation of descending inhibition from central sites,15–17 spinal mechanisms,23,24 peripheral mechanisms involving the nerves innervating the skin,18,19 and combined central or spinal and peripheral mechanisms.23,24
We observed the reduction of OA responses during spinal anesthesia, in agreement with our hypothesis of the rebalancing of the endogenous pain system during peripheral deafferentation. Although it seems attractive to associate these OA changes to connectivity changes in brain networks (ie, a central mechanism), alternative mechanisms cannot be excluded. We previously tested OA responses in a large group of healthy subjects (without pain) and a group of chronic neuropathic pain patients and calculated a ΔeVASC cutoff of 88% (with sensitivity 90% and specificity 91%) to distinguish abnormal OA from healthy responses.12 Extrapolation of this analysis to our current data set suggests that OA responses are similarly abnormal in chronic pain patients and in individuals without pain during spinal anesthesia. Still, whether the resemblance in responses has a similar mechanistic origin is difficult to determine from our current protocol.
An important limitation of our study was the lack of blinding of the subjects. The nature of the study precludes blinding. Since all subjects were properly instructed prior to participation in the study, all were aware that an anesthetic was present or not. In our previous trial, we performed a sham injection after insertion of a spinal needle into the skin at the interspace of L3 and L4.5 For the current study, the ethics committee decided against such a procedure as the committee reasoned that it would not enhance the blinding of the study. Hence, we cannot exclude some bias of the lack of blinding of the subjects. Pain responses could have been differently affected in the two anesthetic states (real/control) because of more intense unpleasantness, fear or distraction during spinal anesthesia.27 How this affected our study outcome remains unknown. Still, we detected no order effect in either static pain rating or OA response, which suggests that these symptoms were not overwhelmingly influential.
We tested our hypothesis in a group of male volunteers. We did so to have standardized conditions for measurement of the effect spinal anesthesia on the brain connectivity by using resting-state fMRI.5 We previously tested OA responses in 110 men and women over a large age range (6–80 years) and observed no differences in OA responses in healthy volunteers and patients with neuropathic pain.12 Hence, as we assume similar effects of spinal anesthesia on the spinal cord and brain in men and women, we argue that our results may be extrapolated to women but suggest to perform additional studies in a mixed population in future studies to confirm our results.
In conclusion, we observed that spinal deafferentation caused hyperalgesia and reduced offset analgesia responses in dermatomes remote from and above the level of anesthesia. Further studies should address the mechanism of the spinal anesthesia-related reduced offset analgesia responses.
Data Sharing Statement
Upon request, anonymized offset analgesia data are available from the authors.
Author Contributions
All authors made substantial contributions to the study inception and design, collection of data, and analysis and interpretation of data; took part in drafting the article and revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Support was provided solely from institutional and departmental sources. AD reports their research unit received grant funding from MSD, Grunenthal, AMO, Medasense, and Medtronic, all unrelated to the current paper. The authors report no other potential conflicts of interest for this work.
References
1. Bramati IE, Rodrigues EC, Simões EL, et al. Lower limb amputees undergo long-distance plasticity in sensorimotor functional connectivity. Sci Rep. 2019;9(1):2518. doi:10.1038/s41598-019-39696-z
2. Kambi N, Halder P, Rajan R, et al. Large-scale reorganization of the somatosensory cortex following spinal cord injuries is due to brainstem plasticity. Nat Commun. 2014;5(1):3602. doi:10.1038/ncomms4602
3. Kelly MK, Carvell GE, Kodger JM, Simons DJ. Sensory loss by selected whisker removal produces immediate disinhibition in the somatosensory cortex of behaving rats. J Neurosci. 1999;19(20):9117–9125. doi:10.1523/JNEUROSCI.19-20-09117.1999
4. Melton MS, Browndyke JN, Harshbarger TB, Madden DJ, Nielsen KC, Klein SM. Changes in resting-state functional connectivity associated with peripheral nerve block: a pilot study. Anesthesiology. 2016;125(2):368–377. doi:10.1097/ALN.0000000000001198
5. Niesters M, Sitsen E, Oudejans L, et al. Effect of deafferentation from spinal anesthesia on pain sensitivity and resting-state functional brain connectivity in healthy male volunteers. Brain Connect. 2014;4(6):404–416. doi:10.1089/brain.2014.0247
6. Pawela CP, Biswal BB, Hudetz AG, et al. Interhemispheric neuroplasticity following limb deafferentation detected by resting-state functional connectivity magnetic resonance imaging (fcMRI) and functional magnetic resonance imaging (fMRI). Neuroimage. 2010;49(3):
7. Weiss T, Miltner WHR, Liepert J, Meissner W, Taub E. Rapid functional plasticity in the primary somatomotor cortex and perceptual changes after nerve block. Eur J Neurosci. 2004;20(12):3413–3423. doi:10.1111/j.1460-9568.2004.03790.x
8. Petou MA, Jaque FA, Byblow WD, Stinear CM. Cutaneous anesthesia of the forearm enhances sensorimotor function of the hand. J Neurophysiol. 2013;109(4):1091–1096. doi:10.1152/jn.00813.2012
9. Harrison G. “Phantom limb” pain occurring during spinal analgesia. Anesthesia. 1951;6(2):115–116. doi:10.1111/j.1365-2044.1951.tb01357.x
10. Kishikawa H, Wajima Z, Shitara T, Shimizu T, Adachi H, Sakamoto A. Subarachnoid block-induced deafferentation pain successfully treated with pentazocine. J Nippon Med Sch. 2017;84(4):183–185. doi:10.1272/jnms.84.183
11. Melton MS, Browndyke JN, Harshbarger TB, Madden DJ, Nielsen KC, Klein SM. Changes in resting-state functional connectivity associated with peripheral nerve block: a pilot study. Anesthesiology. 2016;125:368–377.
12. Niesters M, Hoitsma E, Sarton E, Aarts L, Dahan A. Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. Anesthesiology. 2011;115(5):1063–1071. doi:10.1097/ALN.0b013e31822fd03a
13. Yelle MD, Rogers JM, Coghill RC. Offset analgesia: a temporal contrast mechanism for nociceptive information. Pain. 2008;134(1):174–186. doi:10.1016/j.pain.2007.04.014
14. Yelle MD, Oshiro Y, Kraft RA, Coghill RC. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J Neurosci. 2009;29(33):10264–10271. doi:10.1523/JNEUROSCI.4648-08.2009
15. Zhang S, Li T, Kobinata H, Ikeda E, Ota T, Kurata J. Attenuation of offset analgesia is associated with suppression of descending pain modulatory and reward systems in patients with chronic pain. Mol Pain. 2018;14:1744806918767512. doi:10.1177/1744806918767512
16. Derbyshire SW, Osborn J. Offset analgesia is mediated by activation in the region of the periaqueductal grey and rostral ventromedial medulla. NeuroImage. 2009;47(3):1002–1006. doi:10.1016/j.neuroimage.2009.04.032
17. Nahman-Averbuch H, Martucci KT, Granovsky Y, Weissman-Fogel I, Yarnitsky D, Coghill RC. Distinct brain mechanisms support spatial vs temporal filtering of nociceptive information. Pain. 2014;155:2491–2501.
18. Darian-Smith I, Johnson KO, LaMotte C, Shigenaga Y, Kenins P, Champness P. Warm fibers innervating palmar and digital skin of the monkey: responses to thermal stimuli. J Neurophysiol. 1979;42(5):1297–1315. doi:10.1152/jn.1979.42.5.1297
19. Naugle KM, Cruz-Almeida Y, Fillingim RD, Riley JL. Offset analgesia is reduced in older adults. Pain. 2013;154(11):2381–2387. doi:10.1016/j.pain.2013.07.015
20. Martucci KT, Eisenach JC, Tong C, Coghill RC. Opioid-independent mechanisms supporting offset analgesia and temporal sharpening of nociceptive information. Pain. 2012;153(6):1232–1243. doi:10.1016/j.pain.2012.02.035
21. Niesters M, Proto P, Aarts L, Sarton E, Drewes AM, Dahan A. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anesth. 2014;113(1):148–156. doi:10.1093/bja/aeu056
22. Olesen AE, Nissen TD, Nilsson M, et al. Offset analgesia and the impact of treatment with oxycodone and venlafaxine: a placebo-controlled, randomized trial in healthy volunteers. Basic Clin Pharmacol Toxicol. 2018;123(6):727–731. doi:10.1111/bcpt.13078
23. Sprenger C, Stenmans P, Tinnermann A, Büchel C. Evidence for a spinal involvement in temporal pain contrast enhancement. Neuroimage. 2018;183:788–799. doi:10.1016/j.neuroimage.2018.09.003
24. Ligato D, Petersen KK, Mørch CD, Arendt-Nielsen L. Offset analgesia: then role of peripheral and central mechanisms. Eur J Pain. 2018;22(1):142–149. doi:10.1002/ejp.1110
25. Niesters M, Dahan A, Swartjes M, et al. Effect of ketamine on endogenous pain modulation in healthy volunteers. Pain. 2011;152(3):656–663. doi:10.1016/j.pain.2010.12.015
26. Oudejans LCJ, Smit JM, van Velzen M, Dahan A, Niesters M. The influence of offset analgesia on the onset and offset of pain in fibromyalgia. Pain. 2015;156(12):2521–2527. doi:10.1097/j.pain.0000000000000321
27. Hird EJ, Charalambous C, El-Deredy W, Jones AK, Talmi D. Boundary effects of expectation in human pain perception. Sci Rep. 2019;9(1):9443. doi:10.1038/s41598-019-45811-x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


