Back to Journals » Journal of Inflammation Research » Volume 14
Hydrogen Sulfide Contributes to Uterine Quiescence Through Inhibition of NLRP3 Inflammasome Activation by Suppressing the TLR4/NF-κB Signalling Pathway
Authors Chen Z, Zhang M, Zhao Y, Xu W, Xiang F, Li X, Zhang T , Wu R, Kang X
Received 26 February 2021
Accepted for publication 4 June 2021
Published 25 June 2021 Volume 2021:14 Pages 2753—2768
DOI https://doi.org/10.2147/JIR.S308558
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Zixi Chen,1,* Mengzhe Zhang,1,* Yunzhi Zhao,2 Wenjuan Xu,2 Fenfen Xiang,1 Xiaoxiao Li,1 Tao Zhang,1 Rong Wu,1 Xiangdong Kang1
1Department of Laboratory Medicine, Putuo Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 2Department of Obstetrics and Gynecology, Putuo Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiangdong Kang; Rong Wu
Department of Laboratory Medicine, Putuo Hospital, Shanghai University of Traditional Chinese Medicine, No. 164 Lanxi Road, Shanghai Email [email protected]; [email protected]
Background: The NLRP3 inflammasome plays a critical role in inflammatory responses in various diseases. Our previous study showed that NLRP3 expression was significantly increased in human pregnancy tissue during term labour. Therefore, we explored whether NLRP3 participated in inflammatory responses of preterm and term labour and whether this process could be relieved by H2S, one anti-inflammatory gasotransmitter.
Methods: Human myometrium was obtained from non-labouring and labouring women. Mouse myometrium was obtained from LPS-induced infectious preterm labour. Uterine smooth muscle cells were isolated from non-labouring women’s myometrial tissues, transfected with siRNA, and treated cells with IL-1β, H2S donor NaHS, NF-κB inhibitor BAY 11– 7082 and TLR4 inhibitorTAK-242. The NLRP3 inflammasome, CSE, CBS, TLR4, uterine contraction-associated proteins (CAPs), NF-κB activation and inflammatory cytokine expression were assessed by Western blotting and RT-PCR.
Results: The NLRP3 inflammasome, TLR4 and activated NF-κB expression were upregulated in human term labour, mouse preterm labour and human uterine smooth muscle cells treated with IL-1β. NLRP3 levels were negatively correlated with CSE and CBS expression. Treatment with the H2S donor NaHS delayed LPS-induced preterm birth in mice and inhibited NLRP3 inflammasome activation. In siNLRP3-transfected cells, there was a significant decrease in the expression of CAPs and inflammatory cytokines compared with IL-1β stimulation. In addition, treatment with the H2S donor NaHS inhibited NLRP3 inflammasome activation, reduced the expression of uterine contraction-associated proteins and inflammatory cytokines and reduced the activation of TLR4 and NF-κB compared with stimulation with IL-1β in human uterine smooth muscle cells. Furthermore, treatment of uterine smooth muscle cells with BAY 11– 7082 and TAK-242 found that NLRP3 activation was regulated by the TLR4 and NF-κB pathways.
Conclusion: H2S suppresses CAP expression and the inflammatory response and contributes to uterine quiescence by inhibiting the TLR4/NF-κB signalling pathway and downstream NLRP3 inflammasome activation. Thus, H2S contributes to uterine quiescence through inhibition of NLRP3 inflammasome activation by suppressing the TLR4/NF-κB signalling pathway.
Keywords: hydrogen sulfide, uterine quiescence, NLRP3 inflammasome, inflammation, TLR4/NF-κB
Introduction
Recent studies have found that NLRP3 expression is associated with spontaneous term labour of the chorionic amniotic membrane. Specifically, NLRP3 protein expression was higher in the chorionic amniotic tissue of women who delivered at term than in those who did not give birth at term.1 Significantly, NLRP3 expression in chorionic tissue was higher in women with chorionic amniotic disease who delivered naturally to term than in women without chorionic amniotic disease.2 However, it is unknown if NLRP3 is also increased in the labouring myometrium, and furthermore, whether the TLR4-NF-κB-NLRP3 pathway is involved in the labouring myometrium remains unknown.
Preterm labor (PTL) is a major cause of morbidity and mortality in newborns worldwide.3,4 Compared with those born at term, survivors of PTL have an increased risk for cerebral palsy, neurodevelopmental delays and respiratory problems that can spread to adult life.5 Moreover, high medical costs place a huge burden on families and society.6 However, there is still no effective way to prevent or predict preterm birth in the world.
Spontaneous preterm labors account for 70% of preterm labors,7 and premature rupture of membranes accounts for 30% of spontaneous preterm births, in addition, the causes of up to 45% of preterm labors are unclear.8 According to previous studies, 40% of all preterm labors are associated with infection and/or inflammation.9 Specifically, the number of leukocytes and the contents of chemokines or inflammatory factors in uterine muscle tissue increased significantly. In addition, the chemotaxis of maternal peripheral blood leukocytes to uterine tissue was enhanced. Infection and/or inflammation activate the maternal immune system and promote myometrial contraction, cervical maturation, and rupture of the membranes, leading to spontaneous preterm labor. The transformation of the human uterus from a quiescent state to a contractile state results from the coordinated expression of proinflammatory cytokines and chemokines and the expression of contraction-associated proteins such as gap junctions, prostaglandins, and oxytocin receptors;10 however, the mechanisms underlying the modulation of the inflammatory response in the uterus remain unknown.
Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs) are pattern recognition receptors, both of which play an important role in the activation of innate and adaptive immune responses.11 Activation of TLRs triggers a signalling cascade that releases several inflammatory cytokines.12 Our previous study showed that there is a significant increase in TLR4 expression in human pregnancy tissue during preterm and term labour. Knockdown of TLR4 can attenuate NF-κB activity and the expression of the inflammatory cytokines IL-1β, IL-6, TNF-α and CAPs.13
NLR pyrin domain containing 3 (NLRP3), which is a key component of the NLRP3 inflammasome, is activated by connecting to cytokine or TLRs.14,15 NF-κB regulates the transcription of the NLRP3 gene, and when the NLRP3 inflammasome is stimulated, it triggers caspase-1 activation, thereby promoting the secretion of the proinflammatory cytokines IL-1β and IL-18.16,17 Studies have shown that knockdown of NLRP3 can prevent inflammation in various animal disease models, such as age-related inflammation animal model, acute kidney injury (AKI) animal model, etc.18–20 Furthermore, abnormal activation of NLRP3 was found to be related to the pathogenesis of a variety of inflammatory diseases, such as intestinal inflammation disease and Behcet’s disease (BD).21,22
Hydrogen sulfide (H2S), the third living system gas signal molecule in addition to NO and CO, is synthesized from cysteine by cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS).23 H2S is involved in many pathological and physiological processes, including oxidative stress, angiogenesis, inflammation, vasodilation and carcinogenesis.24 Studies have confirmed that both CBS and CSE are expressed in human pregnant tissues, such as the placenta, foetal membrane and myometrium.25,26 Our previous study found that the expression levels of the H2S-generating enzymes CBS and CSE and the production of H2S are decreased with the onset of labour and preterm labour in human chorionic tissues and the myometrium.27,28 Furthermore, our studies have demonstrated that H2S produced by CSE and CBS contributes to uterine quiescence during pregnancy,28 which suggests that H2S is involved in the initiation of labour. However, the mechanism by which H2S contributes to uterine quiescence and the regulation of labour remains largely unknown.
Therefore, based on the above background, in this study, we investigated whether H2S contributes to uterine quiescence by inhibiting NLRP3 inflammasome activation and suppressing the TLR4/NF-κB signalling pathway.
Materials and Methods
Human Myometrium Collection
This study was successfully approved by the Ethics Administration Office of Pu Tuo Hospital Affiliated with Shanghai University of Traditional Chinese Medicine (No. PTEC-R-2021-28-1). All patients included in this study provided informed consent, in accordance with the Declaration of Helsinki. Human myometrium was collected from pregnant women with singleton pregnancy during caesarean section: term in labour (TL, n=12) and term no labour (TNL, n=12). TL and TNL group myometritis data were collected from pregnant women with normal pregnancies. Women who had evidence of hypertension, preeclampsia, diabetes, or other diseases were not included in this study. The myometritis tissues were washed with cold saline, snap-frozen in liquid nitrogen overnight, and then stored at −80°C for the following Western blot and RT-PCR detection analyses.
Animal Experiment and Tissue Collection
Six- to eight-week-old female and male C57BL/6 mice were purchased from Shanghai SLAC Laboratory Animal Co., Ltd., housed in the Laboratory Animal Centre of Putuo Hospital, Shanghai University of Traditional Chinese Medicine, and maintained in a specific pathogen-free environment with the following conditions: 22–25°C, 12 h light/dark cycle and humidity of 50–60%. All animal treatments were considered humanely, and procedures were approved by the Ethics Committee for animal experiments at Pu Tuo Hospital Affiliated with Shanghai University of Traditional Chinese Medicine (No.0018) in compliance with the recommendation of the principles of laboratory animal care (NIH publication 85–23, revised 1985).
Female mice were mated with males, and a vaginal plug was observed on gestation day (GD) 0.5. Previous research has reported that intrauterine lipopolysaccharide (LPS) administration dose-dependently induces PTL in a mouse model.29 As reported, in our study, pregnant females at GD15.5 were subcutaneously injected with LPS at a concentration of 0.5 mg/kg twice to construct a preterm labour model. In addition, H2S donor NaHS (10 mg/kg) and LPS were injected subcutaneously on GD15.5 twice with an interval of 12 hours. The delivery time and foetal weight were recorded, and myometrium tissue was collected on preterm labour. All myometrium samples were washed with cold saline, snap-frozen in liquid nitrogen overnight, and then stored at −80°C for Western blot and RT-PCR analysis.
USMC Cultures
Uterine smooth muscle cells (USMCs) were isolated and cultured as described previously.13,28 Approximately 50 g of fresh myometrium tissues obtained from TNL patients were cut into pieces with sterile scissors and then dispersed with 0.125% trypsin (Invitrogen, Carlsbad, CA, USA) and DMEM containing collagenase type II and deoxyribonuclease I (1 mg/mL) at 37°C with shaking for 45 min. A purified fraction of USMCs was obtained following gradient centrifugation at 600 g for 10 min and then suspended in DMEM (Invitrogen, Carlsbad, CA, USA) containing 10% (vol/vol) foetal bovine serum (Invitrogen-Gibco, Thermo Fisher Scientific, Waltham, MA, USA), 100 µ/mL penicillin, and 100 mg/mL streptomycin. Cells were then seeded into 25 cm2 culture bottles and kept at 37°C in a 5% CO2-95% air incubator for one week and the medium was changed every 48 hours. Cell purity was assessed by immunocytochemistry using an α-actin monoclonal antibody (Sigma-Aldrich) as described previously.28 Cells were seeded into six-well plates at a density of 30×104/well and grown in DMEM containing 10% foetal serum at 37°C in 5% CO2-95% air overnight. Then, the cells were treated with the following drugs for 24 h: NaHS (1×10−4 M), interleukin (IL)-1β (1 ng/mL), NF-κB inhibitor BAY-117082 (1×10−5 M) and TLR4 inhibitor TAK-242 (5×10−6 mol/L). Vehicle control was set without additive. The concentrations of the drugs were determined based on our previous studies, and all drugs were purchased from Sigma Aldrich (St. Louis, MO, USA).
RNA Interferences and Transfection
To explore whether the activation of NLRP3 is involved in IL-1β-induced pro-inflammatory and pro-labour mediators, we knocked down NLRP3 by NLRP3-siRNA and vehicle with a nontargeting siRNA. For shRNA plasmids used in lentivirus-mediated interference, complementary sense and antisense oligonucleotides (sense:5ʹ-gatccGCTTCATCCACATGACTTTCCTTCAAGAGAGGAAAGTCATGTGGATGAAGCTTTTTTg-3ʹ, antisense: 5ʹ-aattcAAAAAAGCTTCATCCACATGACTTTCCTCTCTTGAAGGAAAGTCATGTGGATGAAGCg-3ʹ) encoding shRNAs targeting NLRP3 were synthesized, annealed and cloned into RNAi pLenti hU6-MCS-CMV-ZsGreen1-PGK-Puro vector (LncBio Co. Shanghai, China), Negative control shRNA was scrambled sequence without any specific known target. The above plasmids along with the related scrambled controls were obtained from Lncbio (Shanghai) Technologies, China. Transfection of USMCs with siRNA was performed by using Lipofectamine TM 3000 as previously described.28 Briefly, NLRP3 siRNA (siNLRP3) and negative control siRNA (siCONT) were transfected into USMCs at 50% confluence with Lipofectamine 3000 transfection reagent (Life Technologies; Mulgrave, Victoria, Australia) according to the manufacturer’s guidelines. After being cultured for twenty-four hours, cells were collected for analyses.
ELISA for Detection of IL-1β and IL-18
The concentrations of IL-1β (Mouse IL-1 beta Quantikine ELISA Kit, SMLB00C, Human IL-1 beta Quantikine ELISA Kit, SLB50) and IL-18 (Human Total IL-18 DuoSet ELISA, DY318-05, Mouse IL-18 DuoSet ELISA, DY7625-05) in the human and mouse myometrium were determined by specific ELISA (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer’s instructions.
Measurement of Caspase-1 Activity
The caspase-1 enzyme level was measured with a Caspase-1 Activity Assay Kit (Beyotime, Shanghai, China) following the manufacturer’s recommendations. Briefly, the uterine tissues and cells were lysed at 4°C. The supernatants were incubated with a substrate of caspase-1 (Ac-YVAD-pNA) to produce the yellow formazan product p-nitroaniline (pNA) at 37°C for 2 h. The pNA levels were detected at 405 nm by an Epoch 2 microplate spectrophotometer (Biotek, VT, USA).
Western Blot Analysis
Western blotting was performed as described previously.13 Briefly, 50 µg of total protein extracted from human and mouse myometrial tissues and USMCs was separated by 10% SDS-PAGE and subsequently transferred to nitrocellulose membranes (EMD Millipore, Billerica, MA, USA). After incubation for 2 h with blocking buffer, the membranes were incubated with the following specific primary antibodies: NLRP3 (#15101, 1:1000), ASC (#151700, 1:1000), TLR4 (#14358, 1:1000), phospho-p65 (#3033, 1:1000), total p65 (#8242, 1:1000), CBS (#14782, 1:1000), GAPDH (#2118, 1:10000) (purchased from Cell Signalling Technology, Beverly, MA, USA), Connexin 43 (Cx43, ab235585, 1:1000, Abcam), oxytocin receptor (OTR, ab181077, 1:1000, Abcam), CSE (sc-365381, 1:100, purchased from Santa Cruz Biotechnology, Dallas, TX, USA), overnight at 4°C. Then, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. Then, imaging software was used to analyse band densitometry.
RT-PCR Analysis
Total RNA was extracted with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions. The PrimeScript® RT Reagent Kit (Takara Biotechnology Co., Ltd., Dalian, China) was used to transcribe RNA into cDNA according to the manufacturer’s protocol. Then, quantitative real-time PCR was carried out on a Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with a mix including cDNA and SYBR (Takara Biotechnology Co., Ltd.), specific forward/reverse primers and sterile water. The amplification conditions were as follows: denaturation at 95°C for 30 sec, then denaturation and annealing/extension at 60°C for 1 min with 40 cycles. The mRNA levels were analysed by 2−∆∆Cq method. The primers for IL-1β, IL-6, TNF-α and β-actin are listed in Table 1.
 |
Table 1 Primer Sequence for Each Gene |
Statistical Analysis
All statistical analyses were undertaken using SPSS 21.0 software (Chicago, IL, USA). The results are presented as the mean ± SEM. One-way ANOVA and the Student-Newman-Keuls test were performed to assess individual comparisons. Pearson’s correlation was used to investigate the relationships between NLRP3 and the levels of CSE, CBS or CAPs. Significance was set at P value <0.05.
Results
NLRP3 Expression Levels Correlate with the Levels of CBS and CSE in Human Myometritis
First, we wanted to characterize the expression of NLRP3 in the human myometrium from the TNL and TL groups. The protein expression of NLRP3 was assessed by Western blotting, and the results are presented in Figure 1. NLRP3 protein expression was significantly higher in the human TL myometrium than in the TNL myometrium (**P < 0.0001, Figure 1A and C). In this study, we characterize the protein expression of CSE, CBS and CAPs, this results presented that CSE and CBS protein expression were significantly downregulated in human TL myometrium when compared to TNL (**P < 0.0001, P = 0.002, Figure 1B and D–E), the protein levels of CAPs were upregulated in TL myometrial tissues compared with TNL tissues (**P < 0.0001, Figure F-H), and the correlation analysis showed that the NLRP3 expression levels correlate to the levels of CAPs (**P < 0.0001), and negative correlated with that of CBS, CSE. (*P = 0.018, **P = 0.006, Figure J–M). We also examined the p-p65 levels in TNL or TL myometrial tissues. As shown in Figure 1, the p-p65 level was significantly increased in TL myometrial tissues compared with TNL tissues (**P = 0.0016, Figure 1I).
Administration of LPS Induces Preterm Labour and Neonatal Mortality
In our study, we administered LPS at a concentration of 0.5 mg/kg twice to pregnant mice at GD15.5 to induce a preterm labour model. The control group was treated with 1× PBS (200 µL), and mice were monitored until delivery. Compared to PBS-injected controls all delivered at term (8/8), all LPS-treated mice were delivered preterm (8/8); consequently, the gestational length of mice injected with LPS was lower than that of PBS-injected controls. (12.76±0.28h, 89.19±0.70h **P < 0.0001, Figure 2A). The neonatal weight preterm in the LPS-induced models was significantly lower than that of controls injected with PBS delivered at term (0.44±0.01g, 1.47±0.01g **P < 0.0001, Figure 2C). In addition, the survival rate preterm in the LPS-induced models was decreased compared to that of the PBS-injected controls (59±3.6%, 95±2.5% **P < 0.0001, Figure 2D). There was no significant difference in the number of pups per litter between the two groups (12.75±0.77, 13.38±0.7 P= 0.56, Figure 2B).
LPS-Induced Preterm Labour Induces NLRP3 Inflammasome and TLR4/NF-κB Activation
In this study, we determined the protein expression of NLRP3 and ASC, the activity of caspase-1, and the mature forms of IL-1β and IL-18. The results showed that the protein levels of NLRP3, ASC, mature IL-1β, and IL-18 were higher in the LPS-induced preterm labour than in the PBS-injected controls (**P < 0.01, Figure 2E–G and 2I–J). The activity of caspase-1 was greater in LPS-induced preterm labour than in PBS-injected controls (**P < 0.01, Figure 2H). TLR4 and NF-κB were both activated by LPS-induced preterm labour (**P < 0.01, Figure 2K–L).
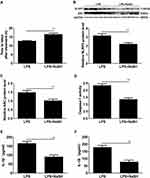
H2S Donor NaSH Delayed LPS-Induced Preterm Labour and Inhibited the Activation of the NLRP3 Inflammasome in Mouse Myometritis
In this study, we characterized the effect of H2S in LPS-induced preterm labour. The results showed that the H2S donor NaHS delayed LPS-induced preterm birth in mice (**P < 0.01, Figure 3A), and NLRP3 and ASC levels, caspase-1 activity and IL-1β and IL-18 concentrations were significantly decreased in the mice treated with NaHS+LPS compared with those treated with LPS (**P < 0.01, Figure 3B–F).
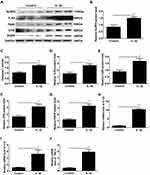
Increased Expression of the NLRP3 Inflammasome, CAPs, TLR4 and NF-κB and Proinflammatory Cytokines in Human Myometrial Cells
As shown in Figure 4, IL-1β treatment led to a remarkable increase in the expression of NLRP3 and ASC and the activity of caspase-1 (**P < 0.01, Figure 4A–C). Our prior studies have shown that the expression of CAPs, such as Cx43 and OTR, and the activation of NF-κB/TLR4 are increased with human labour.13 We therefore examined the protein levels of these proteins and found that the levels of OTR, PGFR, NF-κB and TLR4 were significantly increased by IL-1β in human myometrial cells. (**P < 0.01, Figure 4D–G), and the expression of the proinflammatory cytokines IL-1β, IL-6 and TNF-α was significantly increased in the IL-1β group (*P < 0.05, **P < 0.01, Figure 4H–J).
H2S Protected Against IL-1β-Induced Inflammation and Inhibited Activation of the NLRP3 Inflammasome, Contraction-Associated Proteins, TLR4, NF-κB and Proinflammatory Cytokines
Then, we investigated the effect of H2S on IL-1β-induced activation of the NLRP3 inflammasome, TLR4 and NF-κB and CAP expression. After pretreatment with NaHS for 30 min, the expression of NLRP3, ASC and caspase-1 activity were markedly reduced in the IL-1β+NaHS group compared with the IL-1β group (**P < 0.01, Figure 5A–D). The increase in TLR4 and NF-κB induced by IL-1β was also attenuated by NaHS (**P < 0.01, Figure 5E and F). The expression of the contraction-associated protein OTR was decreased in the presence of NaHS (**P < 0.01, Figure 5G). Furthermore, we examined the effect of H2S on the IL-1β-induced inflammatory response in the IL-1β+NaHS group. In the IL-1β+NaHS group, the expression of the proinflammatory cytokines IL-1β, IL-6 and TNF-α was significantly decreased. (**P < 0.01, Figure 5H–J).
NLRP3 Inflammasome Activation in IL-1β-Treated Human Myometrial Cells Was Suppressed by Gene Silencing
Western blotting was utilized to assess the efficacy of siNLRP3 transfection. The results showed that there was a significant decrease in NLRP3 protein expression in IL-1β+siNLRP3-transfected cells compared to IL-1β+siCONT-transfected human myometrial cells (**P < 0.01, Figure 6A and B). After inhibiting the expression of NLRP3, the high level of ASC and the activity of caspase-1 in human myometrial cells induced by IL-1β were lower after transfection with IL-1β+NLRP3-siRNA than after transfection with vehicle (**P < 0.01, Figure 6C and D).
The NLRP3 Inflammasome is Involved in Inflammation and Myometrial Cell Contractility Induced by IL-1β
The transition of the myometrium from the resting state to the contractility state is the key to the initiation of labour. This transition is associated with an increase in the expression of contraction-associated proteins such as Cx43 and OTR. Thus, to assess whether NLRP3 regulates myometrial contractility and facilitates labour initiation, we first assessed the effect of siNLRP3 on Cx43 and OTR expression. As shown in Figure 6E and F, in IL-1β+siNLRP3 cells, there was a significant attenuation of Cx43 and OTR expression compared with the IL-1β-treated group (**P < 0.01, Figure 6E and F).
Proinflammatory cytokines are involved in leukocyte activation and recruitment into the uterus to promote labour or preterm labour.29–31 Thus, we investigated the effect of siNLRP3 on the expression and secretion of proinflammatory cytokines and chemokines. The results showed that silencing NLRP3 resulted in a significant decrease in the mRNA levels of IL-1β, IL-6 and TNF-α in the IL-1β-treated cells (**P < 0.01, Figure 6G–I).
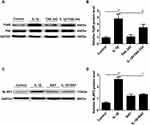
TLR4 and NF-κB Mediated IL-1β-Induced NLRP3 Inflammasome Activation
We determined the association among the NLRP3 inflammasome and TLR4 and NF-κB. As shown in Figure 7A and B, pretreatment of cells with the TLR4 inhibitor TAK-242 inhibited, the protein level of phosphorylated NF-κB was decreased in the IL-1β+TAK-242 group compared to the IL-1β-induced group (**P < 0.01, Figure 7A and B). Moreover, we inhibited the expression of NF-κB by BAY 11–7082, our results showed that inhibition of NF-κB activation by BAY 11–7082 decreased NLRP3 protein expression ((**P < 0.01, Figure 7C and D).
Discussion
The present study has demonstrated for the first time that the H2S-generating enzymes CBS and CSE have a significant negative correlation with the expression of NLRP3 in term labour myometritis obtained from humans and the H2S donor NaHS, can delay LPS-induced mouse preterm birth and decrease the expression of the NLRP3 inflammasome. The NLRP3 inflammasome plays a crucial role in regulating the inflammatory response and uterine activation by upregulating the expression of proinflammatory cytokines and CAPs in cultured USMCs obtained for pregnant myometritis, and spontaneous term labour and LPS-induced preterm labour are also associated with increased NLRP3 inflammasome activation in the human and mouse myometrium. The H2S donor NaHS exhibited suppressive effects on the NLRP3 inflammasome and NF-κB/TLR4 activation in cultured USMCs. Our present study has demonstrated that H2S suppresses the expression of CAPs via inhibition of inflammation and that H2S is one of the key factors in the maintenance of uterine quiescence during pregnancy.28 Collectively, our data show that hydrogen sulfide contributes to uterine quiescence through inhibition of NLRP3 inflammasome activation by suppressing TLR4/NF-κB signalling pathways.
Many studies have found that NLRP3 inflammasome activation in the chorioamniotic membranes is associated with spontaneous preterm labour, either in intra-amniotic infection32 or sterile intra-amniotic inflammation.32 The expression of NLRP3 inflammasome-related genes (NLRP3, ASC, Caspase-1, and IL-1β) is upregulated in the chorioamniotic membranes of women who underwent spontaneous preterm labour with acute chorioamnionitis.29,30 In addition to studies in foetal membranes, in our study, spontaneous term labour and LPS-induced preterm labour were also associated with increased NLRP3 inflammasome activation in the myometrium, and the protein expression of NLRP3 inflammasome-related genes (NLRP3, ASC, Caspase-1, IL-1β, and IL-18) was upregulated in the human and mouse myometrium. In addition, the NLRP3 inflammasome was activated after IL-1β stimulation in uterine myometrial cells cultured in vitro.
IL-1β, IL-6, TNF-α, and acute phase proinflammatory cytokines produced by leukocytes that invade the uterus during labour,31,33 play an important role in the process of human labour and pregnancy. Our previous study indicated that the proinflammatory cytokines IL-1β, IL-6, and TNF-α and the chemokine CCL-2 are significantly higher during labour in the myometrium.28 There are research reports that the proinflammatory cytokines IL-1β and TNF-α are important for the induction of uterine contractions by upregulating the expression of contraction-associated proteins (CAPs).34,35 Studies have shown that NLRP3 can induce proinflammatory mediator assembly in non-gestational tissues.36,37 Therefore, in our current study, we used siRNA-NLRP3 to determine the effect of NLRP3 on the genesis of preterm labour induced by IL-1β. We found that the response to IL-1β induction was reduced in siNLRP3-transfected cells. Cells transfected with siNLRP3 showed decreased expression of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) and contraction-associated proteins (CX-43, OTR) when treated with IL-1β compared to siCONT-transfected cells. Collectively, using siRNA to suppress the expression of NLRP3 can significantly reduce inflammation and uterine contractions when human myometrial cells are treated with IL-1β. Overall, our data suggest that NLRP3 inflammasome activation positively regulates the inflammatory response and uterine contractions.
H2S, the third living system gas signal molecule besides NO and CO, has been shown to elicit anti-inflammatory effects in various tissues, such as inhibition of the release of proinflammatory cytokines and the adhesion of leukocytes.38–40 Our previous study showed that exogenous H2S produced by CSE and CBS suppressed the activation of NF-κB and inhibited the production of the proinflammatory cytokines IL-1β, IL-6 and TNF-α in human uterine myometrial cells cultured in vitro.28 Collectively, these findings indicate that H2S is a potential therapeutic agent to suppress the inflammatory response.
Recently, some studies have shown that H2S inhibits the expression of proinflammatory cytokines and prevents the inflammatory response by modulating TLR4/NF-κB activity.41,42 Consistent with these results, we also indicated that H2S inhibited the activity of TLR4 and NF-κB in human myometrial cells cultured in vitro. Our previous results provide evidence that TLR4/NF-κB plays a crucial role in regulating uterine activation and parturition initiation by upregulating the expression of proinflammatory cytokines and CAPs. Furthermore, H2S suppresses spontaneous contraction of the human uterus by inhibiting the expression of contraction-associated proteins (CX-43, FP, OTR).28 Collectively, our data reveal that H2S suppresses the expression of contraction-associated proteins via inhibition of inflammation by regulating the activity of TLR4/NF-κB signalling.
Our current study indicates that the NLRP3 inflammasome plays a crucial role in regulating the inflammatory response and uterine activation by upregulating the expression of proinflammatory cytokines and CAPs in cultured USMCs obtained for pregnant myometritis; thus, it was of interest to determine whether NLRP3 inflammasome activation is involved in the action of hydrogen sulfide. First, we performed statistical analysis of the expression between NLRP3 and hydrogen sulfide, and the results showed that the H2S-generating enzymes CBS and CSE have a significant negative correlation with the expression of NLRP3 in term labour myometritis obtained from humans. Then, the H2S donor NaHS can delay LPS-induced preterm birth in mice and decrease the expression of the NLRP3 inflammasome. Then, we pretreated USMCs with NaHS and found remarkably alleviated activation of the NLRP3 inflammasome compared to that in the IL-1β-induced group. Based on the above results, we show that Scan exert anti-inflammatory effects and contribute to uterine quiescence by suppressing NLRP3 inflammasome activation.
Given the critical role of the NLRP3 inflammasome in the inflammatory response and uterine activation, we tried to identify potential signalling pathways involved in NLRP3 inflammasome activation. Studies have found that NF-κB could increase the expression of NLRP3 and the proinflammatory cytokine IL-1β; moreover, NF-κB binding sites are present in the NLRP3 promoter,43,44 and knockdown of TLR4 with siRNA led to decreased NF-κB activity and IL-1β release.45 Consistent with these reports, in our study, knockout of the TLR4 gene in TLR4 gene knockout mice suppressed the activation of NF-κB.13 In the present study, after pretreatment of cells with the TLR4 inhibitor TAK-242 and NF-κB inhibitor BAY-117082, we found that in IL-1β-treated USMCs, inhibiting TLR4 signalling reduced the expression of NF-κB, while blocking NF-κB activation downregulated NLRP3 expression in IL-1β-treated cells. The data indicated that the TLR4/NF-κB signalling pathway may induce the activation of NLRP3 in IL-1β-treated myometrial cells.
Conclusions
In conclusion, in our current work, the results show that hydrogen sulfide produced by the enzymes CSE and CBS contributes to uterine quiescence by suppressing NLRP3 inflammasome activation by inhibiting the TLR4/NF-κB signalling pathway. H2S can thus be a potential therapeutic agent to predict or prevent preterm birth.
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of Putuo Hospital, Affiliated to Shanghai University of Traditional Chinese Medicine, and informed consent was obtained from each patient.
Acknowledgments
The authors wish to thank the nursing and medical staff of the delivery suite, and the patients at Putuo Hospital, Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Funding
This work was supported by the National Natural Science Foundation of China (No: 31800988).
Disclosure
None of the authors has any conflict of interests regarding this study.
References
1. Romero R, Xu Y, Plazyo O, et al. A Role for the Inflammasome in Spontaneous Labor at Term. Am J Reprod Immunol. 2018;79(6):e12440. doi:10.1111/aji.12440
2. Gomez-Lopez N, Romero R, Xu Y, et al. A Role for the Inflammasome in Spontaneous Labor at Term with Acute Histologic Chorioamnionitis. Reprod Sci. 2017;24(6):934–953. doi:10.1177/1933719116675058
3. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–440. doi:10.1016/S0140-6736(14)61698-6
4. You S, Cui AM, Hashmi SF, et al. Dysregulation of bile acids increases the risk for preterm birth in pregnant women. Nat Commun. 2020;11(1):2111. doi:10.1038/s41467-020-15923-4
5. Vogel JP, Chawanpaiboon S, Moller AB, et al. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018:523. doi:10.1016/j.bpobgyn.2018.04.003
6. Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med. 2016;21(2):68–73. doi:10.1016/j.siny.2015.12.011
7. Verschueren KJC, Prust ZD, Paidin RR, et al. Childbirth outcomes and ethnic disparities in Suriname: a nationwide registry-based study in a middle-income country. Reprod Health. 2020;17(1):62. doi:10.1186/s12978-020-0902-7
8. Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi:10.1016/S0140-6736(08)60074-4
9. Munoz-Perez VM, Ortiz MI, Carino-Cortes R, et al. Preterm Birth, Inflammation and Infection: new Alternative Strategies for their Prevention. Curr Pharm Biotechnol. 2019;20(5):354–365. doi:10.2174/1389201020666190408112013
10. Sivarajasingam SP, Imami N, Johnson MR. Myometrial cytokines and their role in the onset of labour. J Endocrinol. 2016;231(3):R101–R119. doi:10.1530/JOE-16-0157
11. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi:10.1038/ni.1863
12. Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430(6996):257–263. doi:10.1038/nature02761
13. Chen Z, Liu Q, Zhu Z, et al. Toll-like receptor 4 contributes to uterine activation by upregulating pro-inflammatory cytokine and CAP expression via the NF-kappaB/P38MAPK signaling pathway during pregnancy. J Cell Physiol. 2020;235(1):513–525. doi:10.1002/jcp.28991
14. Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. doi:10.1038/nri2873
15. Spel L, Martinon F. Inflammasomes contributing to inflammation in arthritis. Immunol Rev. 2020;294(1):48–62. doi:10.1111/imr.12839
16. Tong Y, Wang Z, Cai L, et al. NLRP3 Inflammasome and Its Central Role in the Cardiovascular Diseases. Oxid Med Cell Longev. 2020:20204293206. doi:10.1155/2020/4293206
17. Prochnicki T, Latz E. Inflammasomes on the Crossroads of Innate Immune Recognition and Metabolic Control. Cell Metab. 2017;26(1):71–93. doi:10.1016/j.cmet.2017.06.018
18. Youm YH, Grant RW, McCabe LR, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18(4):519–532. doi:10.1016/j.cmet.2013.09.010
19. Komada T, Usui F, Kawashima A, et al. Role of NLRP3 Inflammasomes for Rhabdomyolysis-induced Acute Kidney Injury. Sci Rep. 2015:510901. doi:10.1038/srep10901
20. Kobayashi M, Usui-Kawanishi F, Karasawa T, et al. The cardiac glycoside ouabain activates NLRP3 inflammasomes and promotes cardiac inflammation and dysfunction. PLoS One. 2017;12(5):e0176676. doi:10.1371/journal.pone.0176676
21. Neudecker V, Haneklaus M, Jensen O, et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J Exp Med. 2017;214(6):1737–1752. doi:10.1084/jem.20160462
22. Kim EH, Park MJ, Park S, et al. Increased expression of the NLRP3 inflammasome components in patients with Behcet’s disease. J Inflamm. 2015;12:1241. doi:10.1186/s12950-015-0086-z
23. Holwerda KM, Bos EM, Rajakumar A, et al. Hydrogen sulfide producing enzymes in pregnancy and preeclampsia. Placenta. 2012;33(6):518–521. doi:10.1016/j.placenta.2012.02.014
24. Xiao Q, Ying J, Xiang L, et al. The biologic effect of hydrogen sulfide and its function in various diseases. Medicine. 2018;97(44):e13065. doi:10.1097/MD.0000000000013065
25. Hu TX, Wang G, Guo XJ, et al. MiR 20a,-20b and −200c are involved in hydrogen sulfide stimulation of VEGF production in human placental trophoblasts. Placenta. 2016:39101. doi:10.1016/j.placenta.2016.01.019
26. Cindrova-Davies T, Herrera EA, Niu Y, et al. Reduced cystathionine gamma-lyase and increased miR-21 expression are associated with increased vascular resistance in growth-restricted pregnancies: hydrogen sulfide as a placental vasodilator. Am J Pathol. 2013;182(4):1448–1458. doi:10.1016/j.ajpath.2013.01.001
27. Sun Q, Chen Z, He P, et al. Reduced Expression of Hydrogen Sulfide-Generating Enzymes Down-Regulates 15-Hydroxyprostaglandin Dehydrogenase in Chorion during Term and Preterm Labor. Am J Pathol. 2018;188(1):63–71. doi:10.1016/j.ajpath.2017.09.006
28. You X, Chen Z, Zhao H, et al. Endogenous hydrogen sulfide contributes to uterine quiescence during pregnancy. Reproduction. 2017;153(5):535–543. doi:10.1530/REP-16-0549
29. Kim CJ, Romero R, Chaemsaithong P, et al. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S29–52. doi:10.1016/j.ajog.2015.08.040
30. Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015;213(4 Suppl):S21–28. doi:10.1016/j.ajog.2015.05.056
31. Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9(1):41–45. doi:10.1093/molehr/gag001
32. Gomez-Lopez N, Romero R, Panaitescu B, et al. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am J Reprod Immunol. 2018;80(5):e13049. doi:10.1111/aji.13049
33. Young A, Thomson AJ, Ledingham M, et al. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66(2):445–449. doi:10.1095/biolreprod66.2.445
34. Li H, Yu Y, Shi Y, et al. HoxA13 Stimulates Myometrial Cells to Secrete IL-1beta and Enhance the Expression of Contraction-Associated Proteins. Endocrinology. 2016;157(5):2129–2139. doi:10.1210/en.2015-2005
35. Liang Z, Sooranna SR, Engineer N, et al. Prostaglandin F2-alpha receptor regulation in human uterine myocytes. Mol Hum Reprod. 2008;14(4):215–223. doi:10.1093/molehr/gan008
36. Kinoshita T, Imamura R, Kushiyama H, et al. NLRP3 mediates NF-kappaB activation and cytokine induction in microbially induced and sterile inflammation. PLoS One. 2015;10(3):e0119179. doi:10.1371/journal.pone.0119179
37. Wang W, Wang X, Chun J, et al. Inflammasome-independent NLRP3 augments TGF-beta signaling in kidney epithelium. J Immunol. 2013;190(3):1239–1249. doi:10.4049/jimmunol.1201959
38. Zheng D, Dong S, Li T, et al. Exogenous Hydrogen Sulfide Attenuates Cardiac Fibrosis Through Reactive Oxygen Species Signal Pathways in Experimental Diabetes Mellitus Models. Cell Physiol Biochem. 2015;36(3):917–929. doi:10.1159/000430266
39. Yurinskaya MM, Krasnov GS, Kulikova DA, et al. H2S counteracts proinflammatory effects of LPS through modulation of multiple pathways in human cells. Inflamm Res. 2020;69(5):481–495. doi:10.1007/s00011-020-01329-x
40. Bhatia M. H2S and Inflammation: an Overview. Handb Exp Pharmacol. 2015;230:230165. doi:10.1007/978-3-319-18144-8_8
41. Sun L, Chen L, Wang F, et al. Exogenous hydrogen sulfide prevents lipopolysaccharide-induced inflammation by blocking the TLR4/NF-kappaB pathway in MAC-T cells. Gene. 2019:710114. doi:10.1016/j.gene.2019.05.033
42. Zhang GY, Lu D, Duan SF, et al. Hydrogen Sulfide Alleviates Lipopolysaccharide-Induced Diaphragm Dysfunction in Rats by Reducing Apoptosis and Inflammation through ROS/MAPK and TLR4/NF-kappaB Signaling Pathways. Oxid Med Cell Longev. 2018:20189647809. doi:10.1155/2018/9647809
43. Grishman EK, White PC, Savani RC. Toll-like receptors, the NLRP3 inflammasome, and interleukin-1beta in the development and progression of type 1 diabetes. Pediatr Res. 2012;71(6):626–632. doi:10.1038/pr.2012.24
44. Shimada K, Crother TR, Karlin J, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–414. doi:10.1016/j.immuni.2012.01.009
45. Dasu MR, Devaraj S, Zhao L, et al. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 2008;57(11):3090–3098. doi:10.2337/db08-0564
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.