Back to Journals » Drug Design, Development and Therapy » Volume 12
Hydralazine HCl rapidly disintegrating sublingual tablets: simple dosage form of higher bioavailability and enhanced clinical efficacy for potential rapid control on hypertensive preeclampsia
Authors Genedy S, Khames A, Hussein A, Sarhan H
Received 7 May 2018
Accepted for publication 3 September 2018
Published 5 November 2018 Volume 2018:12 Pages 3753—3766
DOI https://doi.org/10.2147/DDDT.S173326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Samar Genedy,1 Ahmed Khames,2,3 Amal Hussein,4 Hatem Sarhan4
1Department of Clinical Pharmacy, Faculty of Pharmacy, Minia University, Minia, Egypt; 2Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Beni-Suef University, Beni-Suef, Egypt; 3Department of Pharmaceutics and Pharmacy Technology, College of Pharmacy, Taif University, Taif, Kingdom of Saudi Arabia; 4Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Minia University, Minia, Egypt
Background: Hypertensive disorders are the most common complication in pregnancy which can even lead to maternal mortality. Hydralazine hydrochloride (HHC), a direct-acting vasodilator, is intravenously used as the first-line therapy in controlling hypertension in pregnancy (preeclampsia). It suffers poor oral bioavailability (26%–50%) due to first-pass metabolism.
Objective: This work aims for the preparation of HHC rapidly disintegrating sublingual tablets of higher absorption rate, short onset of action, and higher bioavailability for rapid control on blood pressure (BP) in hypertensive emergencies especially preeclampsia.
Methods: HHC sublingual tablet mixtures were prepared using starch sodium glycolate and Pharmaburst as super disintegrants at three different levels by direct compression and were subjected to full in vitro evaluation; the drug bioavailability from the optimized sublingual tablet formula was assessed in comparison to conventional oral tablets in rabbits, and the clinical efficacy on controlling BP in induced preeclampsia like mouse model was also studied.
Results: The results indicated compatibility of the prepared tablet mixtures, good flow, and acceptable mechanical strength. Sublingual tablet formula containing Pharmaburst (7%) that showed fastest disintegration (21 seconds) and 100% drug release within 5 minutes was selected for further bioavailability and pharmacodynamic studies. The drug bioavailability was significantly increased with Cmax = 28.2767±4.61 µg/mL, AUC(0–α) = 52.85±3.18 µg.h/mL, and Tmax = 0.33±0.011 hour in comparison to 18.0633±23.2 µg/mL, 33.18±5.18 µg.h/mL, and 0.75±0.025 hour for conventional oral tablets. Results of pharmacodynamic studies proved significant rapid control on both systolic and diastolic BP to normal values within only 30 minutes without any significant difference from intravenous data.
Conclusion: These results confirm the suitability of the prepared HHC sublingual tablets for use in rapid control on hypertensive crisis especially in pregnant women as an alternate to parenteral administration.
Keywords: pre-eclampsia like mouse model, absorption from buccal cavity, preeclampsia, sublingual tablets, hypertension, L-NAME
Introduction
Elevated blood pressure (BP) occurs when the force of the blood pumping through arteries is too strong that can cause damage of blood vessels and body organs. Uncontrolled elevated BP can result in severe complications including heart attack, stroke, kidney failure, and other serious problems.1,2
Hypertension may be classified as primary of unknown cause (95% of these hypertensive cases) and secondary which is related to other disease (only 5% of registered cases).3,4 The goal of treatment is usually to decrease systolic BP (SBP) to <140 mmHg and diastolic BP (DBP) to >90 mmHg to prevent complication. If SBP reached 180 and/or DBP ≥120, the case is described as hypertensive crisis which is usually associated with acute end-organ damage. Hypertensive disorders have been proven to be one of the most common pregnancy complications that can lead to maternal mortality.5–7
Hypertensive emergency in pregnant women (preeclampsia) is defined as elevated SBP ≥140 mmHg or DBP ≥90 mmHg and usually associated with proteinuria (≥0.3 g in 24 hours), with expected end-organ damage after 20 weeks of gestation in a previously normotensive women; when preeclampsia develops to seizures it is called as eclampsia.8 Treatment of severe preeclampsia includes intravenous labetalol and hydralazine as first-line therapy. Nifedipine is also used as an alternative to parenteral labetalol or hydralazine.9
Parenteral route suffers significant risks including difficulty/impossibility of drug removal or reversal, risks of infection, emboli and hypersensitivity reaction, patient incompliance, high cost, and the need for a health care provider to be an administer.10
Rapidly disintegrating tablets are those that disintegrate or dissolve rapidly in the small volume of saliva available in the patient’s oral cavity. The medication can then be absorbed partially or entirely from blood vessels in the sublingual mucosa.11
Sublingual delivery describes the systemic drug delivery through the mucosal membranes lining the floor of the mouth to the systemic circulation.12 The sublingual route usually produces a faster onset of action than oral tablets (three to ten times greater than oral route), the portion absorbed bypasses the hepatic first-pass metabolism, fast dissolution or disintegration without the need for water or chewing, therapy can be easily discontinued, and it also shows high patient compliance.13,14
Hydralazine hydrochloride (HHC), is a white to off-white, odorless crystalline powder. It is soluble in water, slightly soluble in alcohol, and very slightly soluble in ether. It melts at about 275°C, with decomposition, and has a molecular weight of 196.64.15 Its main antihypertensive effect occurs due to direct relaxing vasodilator action on smooth muscles of arteries and arterioles hence decrease peripheral resistance, thereby lowering BP.16 It reaches peak plasma concentration within 20–80 minutes, its average plasma half-life is 2–4 hours with low oral bioavailability (26%–50%) due to considerable first-pass metabolism by acetylation. It is the first-line therapy for hypertension in pregnancy with methyldopa.17
The small dose requirement, small molecular weight, and absence of objectionable taste and odor make it a suitable candidate for buccal administration. So this study aims for the preparation of HHC fast-disintegrating tablets for sublingual administration as a potential emergency treatment of hypertension especially in pregnancy (preeclampsia).
Materials and methods
Materials
HHC was kindly given as a gift from El-Nasr Company, Cairo, Egypt. Avicel PH 102 (Merck, Darmstadt, Germany), Pharmaburst (kindly given as a gift from EPCI Co., Beni Suef, Egypt), and saccharin sodium (Caesar and Loretz, Hilden, Germany) were obtained from the respective companies. Starch sod-glycolate (SSG), N(omega)-nitro-L-arginine methyl ester (L-NAME), magnesium stearate, talc, citric acid, methyl red and mannitol, and all other solvents and chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA).
Methodology
Compatibility studies
Samples of plain HHC, tested excipient, and their (1:1) physical mixture were prepared and subjected to the following studies.
Differential scanning calorimetric (DSC) studies
Samples (2–4 mg) were weighed into an aluminum pan of differential DSC (STA 449 F3 Jupiter, Nietzsche, Germany) calibrated with purified indium standard (99.9%) and continuously purged with nitrogen gas over a temperature range of (25°C–350°C) at a heating rate of 10°C/min. The DSC thermograms were recorded and analyzed.
Infrared (IR) spectroscopy
Samples (2–4 mg) were directly loaded into IR spectrophotometer (Shimadzu IR-435, Kyoto, Japan) without previous mixing with potassium bromide or compression, and scanning in the range of 4,000–500 cm−1 at ambient temperature. IR spectra were recorded and analyzed.
Preparation of HHC sublingual tablet mixtures
According to the formula composition shown in Table 1, HHC sublingual tablets were prepared by the direct compression. All additives were weighed and gently mixed in mortar with HHC (10 mg) using pestle for 10 minutes. The mixtures were directly compressed on 8-mm punch/die set using a manual single punch tableting machine (ERWEKA single punch Machine EP-1, Apparatebau, Germany) without granulation. Sufficient compression was applied to produce tablet hardness in the range of 6–7 KP. A batch of 50 tablets was prepared.
  | Table 1 Composition and precompression evaluation of HHC sublingual tablet mixtures |
Evaluation of the prepared HHC sublingual tablet mixtures
Precompression evaluation
The flow and compression properties of tablet mixtures were assessed by measuring angle of repose, Carr’s index, and Hausner ratio.18 Each recorded value is an average of three determinations.
Postcompression evaluation
Ten tablets samples of each formula was randomly selected and tablet dimensions, weight variation, hardness (TBH 325, Erweka, Germany), and friability (TAR, Erweka, Germany) were determined according to standardized pharmacopeial (USP) conditions.19
Content uniformity
Five tablets samples of each formula were separately crushed in a mortar and transferred to 100-mL volumetric flask. The flasks were brought to volume by phosphate buffer with pH 6.8 and sonicated for 10 minutes. A 1 mL of the prepared solution was filtered through a 0.45-μm filter and diluted to 25 mL with phosphate buffer. The absorbance of the solution was then measured spectrophotometrically at λmax 272 nm using a UV/Vis double beam spectrophotometer (Shimadzu, Tokyo, Japan) and the drug content was calculated using K obtained from the slope of the constructed calibration curve of the drug in phosphate buffer with pH 6.8.20
In vitro disintegration time
The in vitro disintegration time (in seconds) of the prepared sublingual tablets was measured according to USP modified disintegration test, where the conventional tablet disintegration tester (Electrolab ED-2L; Electrolab India Pvt Ltd., Mumbai, Maharashtra, India) was used without the covering plastic disks with the maximum acceptable disintegration time <2 minutes to fulfill the official requirements. Phosphate buffer (pH 6.8) maintained at 37°C±0.5°C was applied as disintegration medium and the test results were presented as an average of six determinations.21
In vitro dissolution studies
The drug release rate from the prepared sublingual tablet formulations was investigated in the USP XXIV dissolution testing apparatus II (paddle method) in 500 mL phosphate buffer (pH 6.8) as the dissolution medium maintained at 37°C±0.5°C at 50 rpm. At predetermined time intervals (2, 5, 7.5, 10, 15, and 20 minutes), 5 mL of dissolution medium was withdrawn with replacement. The absorbance of the drug in each sample was measured spectrophotometrically at λmax 272 nm using a Shimadzu UV/Vis double beam spectrophotometer, after filtration on 0.45 membrane filter. The cumulative percentage of drug release was calculated using an equation obtained from previously constructed standard calibration curve. The mean of six determinations was considered.22
Bioavailability and bioequivalence studies
The study protocol was reviewed and approved by the animal use committee of the Faculty of Pharmacy, Minia University (approval # 019-17). All experimental procedures in animals complied with Minia University ethical guidelines for biomedical research.
Depending on the results of the previous in vitro evaluation studies, HHC sublingual tablet formula (F6) was selected and subjected to further in vivo evaluation in comparison to commercial HHC tablets (10 mg) and oral tablets to a group of six healthy male albino rabbits weighing 2.5–3.0 kg. Rabbits were kept on the same diet during the study period and overnight fasting before drug administration with free access to water. All rabbits were cannulated through marginal ear vein for blood sample collection.
Study design
A randomized crossover single dose bioavailability study was performed on two phases with washout period of 7 days in between, where each rabbit received both treatments of selected HHC sublingual tablet formula (A), and conventional (10 mg) oral tablet (B) in suitable animal dose of drugs as calculated with reference to Paget and Barnes table.23 A blood sample (2 mL) was withdrawn from each rabbit before drug administration as control.
For treatment (A), rabbits were anesthetized and positioned on a flat table, the lower jaw was strongly supported in a horizontal level on the table. Using rounded tip stainless tweezers, rabbit’s tongues were carefully raised and the sublingual tablet was placed beneath the tongue. Anesthesia was maintained for 3 hours after drug administration to keep the tablet in sublingual position and prevent escaping down through the gastrointestinal tract.24
For treatment (B), accurately weighed amounts of conventional tablet equivalent to the calculated animal dose was crushed and given orally to the animal with the aid of gastric gavage after suspension in minimum volume of water.
Sample collection
In small stoppered heparinized tubes, 2 mL blood samples were collected from the cannulated ear vein at prespecified time intervals (ie, 0, 2, 5, 7, 10, 15, 20, 30, 45, 60, 90, 120, 180, and 240) minutes post dose. Samples were centrifuged at 2,500 rpm for 20 minutes and the supernatant plasma was separately transferred into polypropylene screw cap tubes and refrigerated at −20°C until analysis.
Determination of HHC in plasma
For the determination of unchanged HHC, the collected plasma samples were analyzed using a validated, sensitive, reproducible, and accurate HPLC method25 briefly described as follows.
Frozen blood samples were thawed at room temperature and prepared for analysis as follows: in a borosilicate glass-stoppered tube, 1 mL EDTA (0.02 m), 1 mL HCl (0.5 M), and 1 mL 2-hydroxy-1 naphthaldehyde (0.01 m) were added to 1 mL plasma sample and vortexed for 1 minute; the mixture was kept in 25°C water bath for 90 minutes. A 7 mL of dichloromethane and 50 μL of internal standard (methyl red) were then added and the mixture was centrifuged at 5,000 rpm for 15 minutes. The organic layer was separated and evaporated to dryness at 50°C under a gentle nitrogen stream. Finally, the residue was reconstituted in 100 μL of mobile phase [acetonitrile: phosphate buffer pH =3 (80:20 v/v)] and (10 μL) was injected onto liquid chromatograph (Shimadzu, SPD-6AV, UV/Vis Detector, Tokyo, Japan) at 0.7 mL/min flow rate (25°C) and HHC concentration was spectrophotometrically calculated at 406 nm with reference to the data obtained from the constructed calibration curve in plasma.
Pharmacokinetics calculation
Plasma concentration–time curves were constructed and the pharmacokinetic parameters [namely: Cmax (μg/mL), Tmax (hour), AUC0–4, and AUC0–∞ (μg·h/mL), K (hour−1), and t1/2 (hour)] for each rabbit were calculated and manipulated using WinNonlin Professional 4.0.1 software (Pharsight Corp., Cary, NC, USA).
Statistical analysis of pharmacokinetic data
The mean pharmacokinetic parameters were statistically analyzed using independent sample Student’s t-test at P-value>0.01 and the confidence intervals were also calculated (IBM-SPSS, Inc., Chicago, IL, USA).
Pharmacodynamic study
The antihypertensive effect of HHC in the prepared sublingual tablets was compared with that of conventional oral tablets and intravenous (IV) solution using induced preeclampsia animal models26,27 as follows.
Animals
Thirty six age-matched female Wistar albino rats (230–270 g, 10–12 weeks old) were housed individually under standardized conditions of temperature (22°C) and lighting (12-hour light/dark cycle), with free access to food (standard mouse chow pellet diet) and tap water ad libitum. Animals were mated with fertile males for 4 days; the plug day was assigned as day 0 of pregnancy. Pregnant animals were transferred to individual cages during the period of pregnancy.
Study design
L-NAME-induced preeclampsia-like mouse model was applied. For that, standard L-NAME saline solution (30%) was prepared and stored in a freezer at −20°C during the experiment. The animals received the suitable dose (50 mg/kg/day) subcutaneously from day 7 to day 16 of gestation to induce preeclampsia-like symptoms.
Animals were divided into six groups (n=6): oral control group (OC) received placebo oral suspension, sublingual control group (SC) received placebo sublingual tablet, IV control group (IVC) received IV sterile saline solution, oral test group (OHHC), received oral HHC suspension, sublingual test group (SHHC) received the prepared sublingual HHC tablet, and IV test group (IVHHC) received sterile aqueous HHC solution (10 mg). For dosage administration, the previously described schemes for sublingual and oral drug administration in bioavailability study were applied.
Measurement of BP
A noninvasive tail-cuff BP manometer with automated sphygmomanometer (CODA™ tail-cuff BP System; Kent Scientific, Torrington, CT, USA) was used to measure BP. The protocol used by Jacques Podjarny et al,28 to measure BP was applied as follows.
Animals were kept at rest during all measurements in separate restraining cages. The SBP and DBP were measured eight times in each rat, the first three readings were discarded, and the mean of the last five was recorded as the result. For each animal, the BP was recorded at predetermined time intervals (5, 10, 15, 20, 30, 45, 60, 90, 120, and 180 minutes). Results were recorded and compared.
Statistical analysis of pharmacodynamic data
The collected data were statistically analyzed using post hoc one-way ANOVA test (Tukey mode) to declare the significance of the recorded difference in antihypertensive drug action in the prepared sublingual tablets in comparison to conventional oral tablet at P-value>0.05 (IBM-SPSS, Inc.).
Results and discussion
Compatibility studies
Results of DSC studies are shown in Figures 1 and 2. The DSC thermogram of HHC showed a broad endothermic melting peak at about 277.6°C;29–31 this peak was retained in the same position with less intensity in physical mixture sample with D-Mannitol (Figure 1B), Pharmaburst (Figure 2A), and Saccharin (Figure 2C) due to drug dilution within the mixture. In case of drug physical mixture with SSG (Figure 1C), the drug melting peak was also retained with appearance of other sharp melting exothermic peak at 234°C. The thermogram of drug physical mixture with talc (Figure 2B) was considered indiscriminative, since the thermogram of talc showed a sharp endothermic peak at the same melting range of HHC (274.9°C). The DSC thermogram of drug with Avicel (Figure 1A) showed significant changes, where the broad melting peak of Avicel at 51.1°C and the sharp large peak at 328°C completely disappeared, while the drug melting peak was lowered to 240°C, indicating possibility of interaction between HHC and Avicel. These results indicate compatibility of the drug with D-Mannitol, Pharmaburst, and Saccharin mixture, while the compatibility with other excipients requires further investigation by IR to declare the possibility of any chemical interaction within these mixtures.
Figures 3 and 4 show the IR spectra of HHC in comparison to its (1:1) physical mixtures with different sublingual formulae excipients. IR spectrum of plain HHC shows the main characteristic functional groups32 which can be summarized as follows: N−H stretch (3,255.84 cm−1), N−H2 stretch (3,427.51 cm−1), aromatic C−H stretch (2,908.56 cm−1), and C=C stretch (1,597.06 cm−1) that all retained in the IR spectra of drug physical mixtures with different excipients eliminating the possibility of any chemical interaction in the prepared sublingual tablet formulae mixtures.
Preparation and evaluation of HHC sublingual tablet mixtures
Different HHC sublingual tablet formulation mixtures were prepared using different super disintegrants (SSG and Pharmaburst) in different concentrations (3%, 5%, and 7%). Detailed formulae composition is presented in Table 1.
Free flowing formula mixture is necessary to insure uniform weight content, accurate dosage, and elegant product of high quality accepted by the market, and the flow properties of powder mixture are mainly controlled by inter particulate friction, particle size, shape, surface texture, size distribution, and other surface characteristics including moisture content. Other factors including bulk density, compaction degree (porosity), equipment geometry, and storage conditions also have an impact on powder flow.33
Angle of repose, compressibility index, and Hausner ratio represent quantitative parametric indicators and describe the powder flow. All prepared sublingual tablet mixtures can be considered to have acceptable flow properties, where the angle of repose was ranging from 29° to 34°, percentage of compressibility of maximum 21.1%, and Hausner ratio values close to unity (Table 1). This result can be correlated to the good flow properties of formula mixture which overcome the poor flow of the amorphous drug.34
Characterization of the prepared sublingual tablets
According to tablet characterization results presented in Table 2, the prepared HHC sublingual tablets showed acceptable mechanical properties with good breaking strength. The values of hardness ranged from 59 to 81 N with percentage of weight loss less than 0.72%, which conforms to the acceptable pharmacopeia limits. It could be also noted that the Pharmaburst-based formulae showed lower friability values with higher hardness and this could be correlated to the binding properties of Pharmaburst. The drug content was within the acceptable weight variation pharmacopeial limit in all formulations (97.89%–100.75%). Pharmaburst-based tablet formulae showed shorter disintegration time (<34 seconds) in comparison to SSG-based formulae (up to 47 seconds), while they all were still within the acceptable pharmacopeial range for sublingual tablets and this could be explained by the highly swelling and water absorbing characteristics of Pharmaburst formula composition.
  | Table 2 Characterization of the prepared HHC sublingual tablets |
In vitro dissolution
Results of HHC release from the prepared sublingual tablet formulations in phosphate buffer (pH 6.8) are shown in Figure 5. The high solubility nature of the drug was highly observed in the dissolution results, where the percentage drug release reached 100% in less than 10 minutes from all prepared formulations and the release time was dependent on super disintegrant concentration and type. In SSG-based formulae (Figure 5A), percentage drug released was 87.38% and 94.09% of the labeled dose after 5 minutes at super disintegrant concentration of 3% and 5%, respectively and reached 100 at 7%. Regarding Pharmaburst-based formulae (Figure 5B), percentage drug released reached 96.98% of labeled dose after 5 minutes at 3% super disintegrant level in comparison to 100 at 5% and 7% level within the same time. These results confirm the suitability of the prepared sublingual formulae for use in emergency cases.
  | Figure 5 Dissolution profiles of HHC from sublingual tablets prepared using (A) SSG and (B) Pharmaburst in comparison to plain drug. |
In vivo studies
Depending on the results of previous in vitro characterization studies, HHC sublingual formula (F6) that was prepared using 7% Pharmaburst was selected and subjected to further bioavailability studies.
Rabbits were selected as an animal model for the bioavailability study mainly due to the nonkeratinized nature of mucosal membranes which are in close similarity to human sublingual mucosa.35
The main characteristic pharmacokinetic parameters are presented in Table 3, while the mean plasma concentration time curve is presented in (Figure 6) for HCC from selected sublingual tablets formula in comparison to conventional tablets.
These results clearly show that the extent of drug absorption from sublingual route was significantly higher than conventional oral route as expressed by increase of both Cmax and AUC (28.28 μg/mL and 52.85 μg.h/mL in comparison to 18.06 μg/mL and 33.18 μg.h/mL, respectively). The results also showed that the drug was rapidly absorbed from sublingual route, where Tmax was lowered by more than one half to be only 0.33 hour (20 minutes) in comparison to 0.75 hour (45 minutes) for conventional oral route.
Hence HHC is a BCS class III drug of high solubility and permeability;36 these results could be explained by its higher solubility and small molecular weight (160.19 g/mol), where the capillary walls show free, easy, and rapid permeability to small molecular weight particles. The salivary glands contain lobular cells that secrete saliva through the salivary ducts into the buccal cavity. Acidic taste (pH), due to the presence of citric acid, stimulates secretion of larger amount of saliva, with increased oxygen consumption within sublingual vessels and secretion of vasodilators; this stimulates higher blood flow within the glandular blood vessel with subsequent facilitated drug absorption and uptake into the circulatory system.37
Statistical analysis of data indicated a significant difference between the calculated pharmacokinetic parameters from both conventional tablet and selected sublingual formula (F6) with P-value of <0.001. Table 4 shows the calculated 95% confidence intervals of the calculated pharmacokinetic parameters within narrow range excluding zero value as an evidence for precision of the results and rejection of null hypothesis.
  | Table 4 CIs of the statistically calculated mean differences of calculated pharmacokinetic parameters |
Pharmacodynamic study
Preeclampsia is a multisystemic maternal syndrome including several pathophysiological changes usually associated with hypertension and proteinuria during late gestation mainly due to insufficient production of nitric oxide (NO), within the vascular endothelium with increased vascular resistance and BP as well as reduced blood perfusion with a hypoxic state and suspected intravascular coagulation. L-NAME is an NO inhibitor that causes pathophysiological symptoms of preeclampsia, if applied during mid to late gestation period.38
The daily subcutaneous L-NAME administration to pregnant animals starting from day 7 led to significant BP rise at the late gestation (14 days), where the basal SBP/DBP reached 142.93/111.7 mmHg in comparison to 104.7/75.6 mmHg for untreated animals, respectively (Figure 7A).
Figure 7B shows the effect of sublingual administration of HHC on induced hypertension in comparison to the oral HHC tablets after 30 minutes. It can be clearly seen that the sublingual administration caused rapid control on hypertension to normal safe level (114.3/85.1 mmHg) for both SBP and DBP values, respectively. The oral route showed a markedly slower effect on hypertension, where the SBP/DBP values reached 135.5/106.1 mmHg, respectively within the same time period. Further tracking of animal BP for 3 hours showed that the BP was still under control in case of the prepared sublingual tablets and also showed the oral tablets started to show significant control on BP within 2 hours, where the SBP/DBP values reached 117.1/87.2 mmHg, respectively.
For comparison, BP was also recorded after IV administration of HHC sterile aqueous solution (10 mg) to the hypertensive animals, and results (Figure 7B) showed that the BP decreased to the normal safe level (113.8/83.2 mmHg) within 20 minutes in comparison to (121.2/91.7 mmHg) after sublingual administration within the same time period that could be considered acceptable values for BP.
Generally, for rapid systemic effect, oral mucosal administration is often expected to be an administration route of choice due to rapid absorption with shorter onset time also due to short liquid residence time in the oral cavity (5–10 minute).39
Several studies showed that the rapid drug disappearance from the oral cavity is faster than its appearance within the systemic circulation and does not necessarily mean rapid drug systemic adsorption. Different models that describe the mucosal absorption from oral cavity explained that by an equilibrium between drug concentration in oral cavity and mucosal membranes; the equilibrium point principally depends on the drug solution in saliva (controlled by drug solubility) and drug transfer from mucosal membranes into systemic circulation. The latter is considered as the rate determining step in buccal absorption as it always occurs with slower rate.40
The sublingual route does not suffer this problem and higher correlation could be shown between drug removal from the oral cavity and its appearance within the systemic circulation. This could be explained in terms of higher permeability of sublingual area than other buccal areas including supralingual or buccal; this is mainly due to high blood supply, relatively thin membrane (100–200 μm), and low degree of keratinization.41 Sublingual administration is considered as an optimal route for obtaining a rapid onset of action. In addition to the higher permeability and solubility of HHC, this clearly describes and explains the short onset time of HHC from the prepared sublingual tablets and its rapid control on induced hypertension in animal model.
Statistical analysis of pharmacodynamic data
For statistical analysis of the variance between more than two groups, post hoc one-way ANOVA test is usually applied. So, it is used to statistically determine the significance of the detected difference between recorded pharmacodynamic data. According to the results presented in Tables 5 and 6, a significant difference between the recorded SBP and DBP data of different groups was determined at the selected probability level (P>0.05). To detect the exact position of the recorded significant difference between the different group means, further data analysis according to post hoc test (Tukey mode) is applied and results showed that IVHHC, OHHC, and SHHC are significantly different from OC, SC, and IVC; also SHHC is significantly different from OHHC, while the recorded difference between SHHC and IVHHC was insignificant at the selected probability level (P>0.05), which indicated suitability of SHHC as a potential alternative to IV route for rapid control on hypertensive preeclampsia.
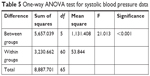  | Table 5 One-way ANOVA test for systolic blood pressure data |
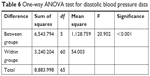  | Table 6 One-way ANOVA test for diastolic blood pressure data |
Conclusion
HHC sublingual tablets were prepared using different super disintegrants at various levels to be used as IV substitute for rapid control on hypertensive emergency, especially in preeclampsia cases. Depending on the evaluation results it could be concluded that the prepared sublingual tablet formulations showed high compatibility without any possibility of chemical interaction as confirmed by DSC and IR studies. Also the prepared tablets showed good mechanical properties with high disintegrating power as indicated by the very short disintegration time. The percentage drug release reached 100% only after 5 minutes (in some formulations). The prepared sublingual tablets showed higher bioavailability as indicated by the significant increase in both rate and extent of drug absorption in comparison to conventional oral route. In induced preeclampsia-like mouse model, sublingual HHC tablets normalized both SBP and DBP within 30 minutes without any significant difference from IV data. All these results lead to the final conclusion that the prepared sublingual tablet formulae of HHC is an optimum formula for rapid control on hypertensive crisis, especially in pregnant women.
It is also worth mentioning that the authors are planning to implement a clinical study of the prepared hydralazine sublingual tablets on pregnant women and patients under hypertensive crisis to insure efficacy of the prepared tablets and explore any unexpected clinical response and/or complications.
Disclosure
The authors report no conflicts of interest in this work.
References
Rey E, Lelorier J, Burgess E, Lange IR, Leduc L. Report of the Canadian Hypertension Society Consensus Conference: 3. Pharmacologic treatment of hypertensive disorders in pregnancy. CMAJ. 1997;157(9):1245–1254. | ||
Weber MA, Schiffrin EL, White WB. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens. 2014;32(1):3–15. | ||
James PA, Oparil S, Carter BL. Evidence-based guideline for the management of high blood pressure in adults. JAMA. 2014;311(5):507–520. | ||
Eckel RH, Jakicic JM, Ard JD, et al. AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;2014(129):S76–S99. | ||
Lewis G, Drife J, Die WM. The Fifth Report of the Confidential Enquiries into. Maternal Deaths in the United Kingdom. Vol. 2001. London: RCOG Press; 1997–1999. | ||
Cooper GM, Lewis G, Neilson J. Confidential enquiries into maternal deaths, 1997–1999. Br J Anaesth. 2002;89(3):369–372. | ||
Roccella EJ. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):1–22. | ||
American College of Obstetricians and Gynecologists; Task force on hypertension in pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;1221122(5):1131. | ||
Magee LA, Pels A, Helewa M, et al. Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416–441. | ||
Thong BY, Tan TC. Epidemiology and risk factors for drug allergy. Br J Clin Pharmacol. 2011;71(5):684–700. | ||
Bhanja SB, Ellaiah P, Roy HK. Formulation and evaluation of perindopril sublingual tablets. Int J Res Pharm Biomed Sc. 2011;2(3):1193–1198. | ||
Saha P, Verma S, Das PS. Sublingual drug delivery: an indication of potential alternative route. Int J Curr Pharm Res. 2017;9(6):5–7. | ||
Birudaraj R, Berner B, Shen S, Li X. Buccal permeation of buspirone: mechanistic studies on transport pathways. J Pharm Sci. 2005;94(1):70–78. | ||
Bind AK, Gnanarajan G, Kothiyal P. A review: sublingual route for systemic drug delivery. Int J Drug Res Tech. 2013;3(2):31–36. | ||
Hetero drugs limited, Hydralazine hydrochloride tablets, USP (2008). Available from: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a2b73026-2451-406f-b866-9dc16177cb44&type=display [online]. Accessed November 8, 2017. | ||
Singh B, Pahuja S, Kapil R, Ahuja N. Formulation development of oral controlled release tablets of hydralazine: optimization of drug release and bioadhesive characteristics. Acta Pharm. 2009;59(1):1–13. | ||
Oates JA, Brown NJ. Antihypertensive Agents and the Drug Therapy of Hypertension, in Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. Hardman JG, Limbird LE, editors. New York: Mc Graw Hill Medical Publishing Division; 2001:871–900. | ||
Khames A. Liquisolid technique: a promising alternative to conventional coating for improvement of drug photostability in solid dosage forms. Expert Opin Drug Deliv. 2013;10(10):1335–1343. | ||
Pharmacopeia US, Formulary N. U.S. Pharmacopeia and National Formulary. USP 25-NF 20. Supplement 1: United States Pharmacopeial Convention, Incorporated. 2002. | ||
Kaur R, Sharma G, Nagpal M, et al. preparation and characterization of fast disintegrating tablets of hydralazine hydrochloride using design of experiment technique. J Biomed Pharm Res. 2014;3(1):1–6. | ||
Prajapati ST, Patel PB, Patel CN. Formulation and evaluation of sublingual tablets containing Sumatriptan succinate. Int J Pharm Investig. 2012;2(3):162–168. | ||
Bhanja SB, Ellaiah P, Roy HK, et al. Formulation and evaluation of perindopril sublingual tablets. Int J Res Pharm Biomed Sci. 2011;2(3):1130–1198. | ||
Khames A. Investigation of the effect of solubility increase at the main absorption site on bioavailability of BCS class II drug (risperidone) using liquisolid technique. Drug Deliv. 2017;24(1):328–338. | ||
Aburahma MH, El-Laithy HM, Hamza Y-S, Hanan M. Preparation and in vitro/in vivo characterization of porous sublingual tablets containing ternary kneaded solid system of vinpocetine with î-cyclodextrin and hydroxy acid. Sci Pharm. 2010;78(2):363–379. | ||
Mañes J, Mari J, Garcia R, Font G. Liquid chromatographic determination of hydralazine in human plasma with 2-hydroxy-1-naphthaldehyde pre-column derivatization. J Pharm Biomed Anal. 1990;8(8–12):795–798. | ||
Motta C, Grosso C, Zanuzzi C, et al. Effect of sildenafil on pre-eclampsia-like mouse model induced by L-NAME. Reprod Domest Anim. 2015;50(4):611–616. | ||
McCarthy FP, Kingdom JC, Kenny LC, Walsh SK. Animal models of preeclampsia; uses and limitations. Placenta. 2011;32(6):413–419. | ||
Podjarny E, Ben-Chetrit S, Rathaus M, et al. Pregnancy-induced hypertension in rats with adriamycin nephropathy is associated with an inadequate production of nitric oxide. Hypertension. 1997;29(4):986–991. | ||
Vanitha K, Varma M, Ramesh A, Mohan Varma AR. Floating tablets of hydralazine hydrochloride: optimization and evaluation. Brazilian J Pharm Sci. 2013;49(4):811–819. | ||
Rekhi G, Hamm S, Sidwell R. Controlled release compositions comprising a combination of isosorbide dinitrate and hydralazine hydrochloride. United States patent US 9308177. 2016 Google Patents. | ||
Ooi T. Chemistry. Heat and light switch a chiral catalyst and its products. Science. 2011;331(6023):1395–1396. | ||
Swamy Kumara S, Nagarjun Reddy L, Agaiah Goud B. Development and in vitro evaluation of bioadhesive buccal tablets of hydralazine hydrochloride. Int J Pharm Educ Res. 2014;1(2):8–16. | ||
Morin G, Briens L. The effect of lubricants on powder flowability for pharmaceutical application. AAPS Pharm Sci Tech. 2013;14(3):1158–1168. | ||
Sarraguça MC, Cruz AV, Soares SO, Amaral HR, Costa PC, Lopes JA. Determination of flow properties of pharmaceutical powders by near infrared spectroscopy. J Pharm Biomed Anal. 2010;52(4):484–492. | ||
Odou P, Barthélémy C, Chatelier D, et al. Pharmacokinetics of midazolam: comparison of sublingual and intravenous routes in rabbit. Eur J Drug Metab Pharmacokinet. 1999;24(1):1–7. | ||
Proposal to waive in vivo bioequivalence requirements for the WHO model list of essential medicines immediate release, solid oral dosage forms. WHO (2005). Available from: http://www.who.int/medicines/services/expertcommittees/pharmprep/QAS04_109Rev1_Waive_invivo_bioequiv.pdf [online]. Accessed December 15, 2017. | ||
Lea L. Sublingual absorption (1996). Available from: http://www.positivehealth.com/article/colonhealth/sublingual-absorption [online]. Accessed December 18, 2017. | ||
Makino I, Shibata K, Makino Y, Kangawa K, Kawarabayashi T. Adrenomedullin attenuates the hypertension in hypertensive pregnant rats induced by N(G)-nitro-L-arginine methyl ester. Eur J Pharmacol. 1999;371(2–3):159–167. | ||
Michael J. Rathbone, Bernadette K. Drummond, Ian G. Tucker. The oral cavity as a site for systemic drug delivery. Adv Drug Deliver Rev. 1994;13(1–2):1–22. | ||
Bartlett JA, van der Voort Maarschalk K, Maarschalk Kvanderv. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS Pharm Sci Tech. 2012;13(4):1110–1115. | ||
Narang N, Sharma J. Sublingual mucosa as a route for systemic drug delivery. Int J Pharm Sci. 2011;3(12):65–73. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







