Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Hyaluronic Acid Fillers and ASIA Syndrome: Case Studies
Authors Owczarczyk-Saczonek A , De Boulle K
Received 3 July 2023
Accepted for publication 29 August 2023
Published 5 October 2023 Volume 2023:16 Pages 2763—2771
DOI https://doi.org/10.2147/CCID.S419716
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Agnieszka Owczarczyk-Saczonek,1 Koenraad De Boulle2
1Department of Dermatology, Sexually Transmitted Diseases and Clinical Immunology, Collegium Medicum, University of Warmia and Mazury, Olsztyn, Poland; 2Aalst Dermatology Clinic, Aalst Belgium and University College London, London, UK
Correspondence: Agnieszka Owczarczyk-Saczonek, Email [email protected]
Abstract: A continuous increase in the popularity of esthetic procedures with the use of substances as HA has been observed for many years, which might be contributing to an increase in the number of adverse events. The autoimmune/inflammatory syndrome induced by adjuvants (ASIA) can be provoked by hyaluronic acid (HA), which belongs to substances meeting the criteria of adjuvants. Mechanisms of the innate and acquired immune response are activated, leading to the dysregulation of T and B lymphocytes, inability to recognize one’s own antigens, inflammation, damage to one’s own tissues, and ultimately to autoimmunity. The objective of this article is to present a case-series study of patients who developed ASIA syndrome following HA injection after delayed inflammatory reaction (DIR) and emphasize the importance of the need for long-term monitoring after such the reaction. Lack of knowledge about ASIA can lead to delayed diagnosis and serious consequences for the patients. People with a history of immunization reactions, severe allergic reactions, individual predisposition to autoimmunity or family predisposition to autoimmunity and previous exposure to adjuvants require special attention and long-term follow-up. This applies primarily to cases of DIR after the using of bioimplants, especially with treatment resistance, as in our reported cases.
Keywords: hyaluronic acid, bioimplants, ASIA syndrome, immunogenicity
Erratum for this paper has been published.
Introduction
The autoimmune/inflammatory syndrome induced by adjuvants (ASIA) was first defined by Shoenfeld et al in 2011, explaining the mechanism of adverse immune reactions after vaccination using aluminum compounds as an adjuvant.1,2 The role of the adjuvant is to protect antigens from degradation, enabling prolonged exposure to the cells presenting the antigen, which results in greater immunogenicity and, thus, the effectiveness of vaccination. Adjuvants may also include infectious agents, bioimplants (silicone, acrylamides, hyaluronic acid (HA) and methacrylate compounds).2–4
Immediately after the implantation of the biomaterial, the host protein layer settles on its surface, causing the attraction of phagocytes (mainly pro-inflammatory M1 macrophages).5 Additionally, if a biofilm is formed after implantation, the microorganisms in this biofilm may also act as adjuvants.5 Exposure to an adjuvant leads to activation of the innate and adaptive immune systems by mimicking damage-associated molecular patterns, pathogen-associated molecular patterns and alarmins, which can activate Toll-like receptors and the inflammasome system (IL-1β overproduction), causing the release of Th1 inflammatory cytokines and increasing the activity of dendritic cells, lymphocytes and tissue macrophages.3,6 It is followed by macrophage apoptosis, which results in the release of adjuvant-containing particles that may be reabsorbed by other macrophages, further stimulating the inflammatory response. Additionally, the production of IL-17 and the influx of neutrophils are stimulated. It results in the production of reactive oxygen species (ROS) and the release of enzymes such as myeloperoxidase, thereby intensifying inflammation.5 Consequently, the innate and acquired immune response is activated, which then intensifies the local and systemic response to antigens that are presented in regional lymph nodes, leading to the dysregulation of T and B lymphocytes, inability to recognize one’s own antigens, inflammation, damage to one’s own tissues, and ultimately to autoimmunity.2,4,5,7 The destruction of one’s own tissues is facilitated by an imbalance between effector T cells and regulatory Treg cells, which additionally stimulates the production of autoantibodies.7
Predisposing genetic factors play an important role in this reaction. It is mediated by specific HLA antigens predisposing to the development of autoimmune diseases, especially the simultaneous presence of the HLA-DRB1 and PTPN22 gene.8
Our aim is to present three cases of ASIA development after HA procedure to draw attention to a rarely recognized problem leading to serious consequences.
Case 1
A 64-year-old woman underwent partial (left-sided) mastectomy followed by chemotherapy 15 years earlier. She had no history of drug hypersensitivity.
Three years earlier, the patient was administered HA preparation (Perfectha®) into her nasolabial folds, chin and glabellar area. In April 2019, after pharyngitis treated with amoxicillin and clavulanic acid, inflammatory lumps appeared at the sites of preparation application (the zygomatic area, nasolabial folds, marionette lines, glabella, upper lip). The lesions were tender on palpation (Figure 1). The patient complained of general malaise and feeling uneasy. She had sore muscles and low-grade fever. The surgeon diagnosed abscesses, incised the lumps in the nasolabial folds and applied a surgical drain. Transparent crystal-like secretion, without purulent discharge was obtained. After the treatment, both local and general clinical symptoms persisted.
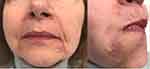 |
Figure 1 The patient after three weeks of treatment: the inflammation has subsided. However, the lesions are still visible. |
The patient was admitted to our clinic, where she was diagnosed with DIR (delayed inflammatory reaction). Laboratory tests did not show any abnormalities. The ultrasound examination revealed HA deposits appeared as a dark hypoechoic region with well-defined contours in the subcutaneous tissue of the left zygomatic region (Figure 2). The following drugs were administered: cloxacillin 3×1 g iv + metronidazole 2×0.5 g iv 7 days, followed by clindamycin 2×300 mg orally and prednisone 40 mg. Intralesional hyaluronidase 1500 U injections were administered for 3 days. Although the patient’s clinical condition had improved (the inflammation had subsided), solid lumps were still palpable and they tended to get periodically swollen. The dose of prednisone was gradually lowered to 20 mg/d for 10 months. The symptoms recurred when the oral steroid dose was drastically lowered.
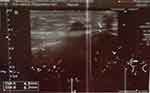 |
Figure 2 HA deposit appeared as a dark hypoechoic region with well-defined contours in the subcutaneous tissue of the left zygomatic region. |
The administration of hyaluronidase was continued: 3000 U every 2 to 4 weeks for 6 months and 1500 U every 4 weeks for another 6 months. After one year of treatment with prednisone at a dose of 2.5 mg applied every 4 weeks, it was decided to terminate the treatment. Regrettably, the inflammation recurred on the third day at the sites of previous preparation application. After administering 2.5 mg of prednisone once again, the lesions subsided (Figure 3). The treatment was ended after 1.5 years.
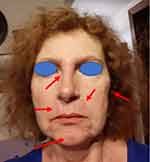 |
Figure 3 The patient after one year of treatment, the third day after the withdrawal of prednisone 2.5 mg. (red arrows – sites of oedema). |
During this observation period, the patient developed symptoms that enabled the diagnosis of the ASIA syndrome (Tab. I): muscle weakness and pain, joint pain, feeling of chronic fatigue with sleep and memory disorders, positive antinuclear antibody titer 1:160 (immunoblotting test negative), elevated antithyroid antibodies (anti-TPO, anti-TG). Moreover, autoimmune thyroiditis was diagnosed.
Case 2
A 60-year-old female without noteworthy preexisting medical history receives cosmetic treatment with botulinum toxin and fillers for 14 years. In the 3 first years, she has been treated with a collagen-based product (Evolence and Evolence Breeze) (Figure 4A and B). From the last 11 years, she has been treated with hyaluronic acid (Juvederm Ultra 3, Voluma, Volift and Volbella).
 |
Figure 4 (A) After 3 y of BTX /collagen fillers. (B) After 10 years of BTX and fillers. |
The last injections in midface, mandibular angle and marionette lines with HA dated back 2.5 years. Shortly after this treatment vague complaints of intractable itch in the sacroilical area are noted. Extensive investigations are performed, but no specific reason could be retrieved, except eventual diagnosis of notalgia paresthetica. Some discrete swelling in the periorbital area is noted at that time. One year ago, a remaining vertical glabellar line was treated with a minute quantity of HA.
Last year all of a sudden, she develops fatigue, joint and muscle pain. Hospitalisation is needed. Cutaneous vasculitis signs on the lower limbs are seen, and a skin biopsy shows necrotizing arteritis in small and medium-sized arteries with granulocytes and mononuclear cells in the vessel wall. Extensive lab work up is done with raised inflammatory parameters, but no hepatitis; ANCA is also negative. Arteritis in one salpinx needed a unilateral salpingectomy. The diagnosis of polyarteritis nodosa and vasculitis was made. A relation with previous COVID vaccinations could not be ruled out. The areas where the HA fillers have been injected all became very edematous, but no erythema, no infiltration, no nodules. This edematous swelling shows a more or less cyclic course (Figure 5).
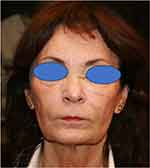 |
Figure 5 Signs of cyclic massive swelling over all injected areas. |
The patient left the hospital on oral steroids (methylprednisone) in high dose, which gradually were tapered to 8 mg methylprednisone daily. This treatment is ongoing.
Criteria for ASIA syndrome is definitely met for this patient. Dissolving the HA is put on hold as can be expected that now, almost 2 years after the last implant, gradually all HA will be biodegraded.
Case 3
A 59-year-old patient with almost 30-year history of psoriasis, first vulgaris, afterwards evolving into psoriasis arthropathica (HLA B27 positive) has been treated for this condition with UV, methotrexate, cyclosporin and then consecutive different biologicals. She also suffers from a chronic nephritis needing sometimes antibiotic treatment.
She got a cosmetic treatment since 2014 with hyaluronic acid for a full-face treatment (total of 5 cc HA). (Figure 6A) Her main medication at that time consisted of adalimumab. She left the office uneventful, but a couple of days complained of a flu-like condition (possibly already on the day of treatment) and a little over 1 week later she presented with massive swelling, periorbital edema and hard infiltrate of all HA injected areas (Figure 6B). The patient was put on oral steroids (methylprednisone 32 mg daily) for 1 week and then first tapered in a fast mode with multiple relapses, afterwards gradually tapered down over 2 months, as there were relapses, notwithstanding dissolving of the products with hyaluronidase. Fatigue and malaise were present as well for some months.
 |
Figure 6 (A) Before treatment with HA. (B) 1 week after injection. |
As she was actually very happy with the result of the fillers right after the treatment, and before the adverse event appeared she asked 6 months later for a test to see if she could have any further treatment in the future. The products used for the facial treatment have been injected in the ventral side of the forearm: 2 weeks later a serious local reaction could be noted, suggesting a real hypersensitivity for one compound and a lesser reaction to the other product used (Figure 7). At that time, the patient also felt some reactive symptoms: swelling and itching, in the formerly injected areas.
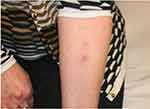 |
Figure 7 Positive intradermal testing. |
This case fuels the discussion about the role or the nonexistence of type I or IV hypersensitivity in reactions on HA (pure hypersensitivity, ASIA syndrome) even more. Up to now no fillers of any kind were injected anymore and the only treatment she got on regular base has been botulinum toxin injections with a totally uneventful course.
The patient’s sequence of events will also fit into the diagnosis of ASIA syndrome: preexisting and predisposing condition (psoriasis arthritis and chronic nephritis) – flu-like episode – HA injection as adjuvant – cutaneous facial reactions – only disappearing after dissolving the product – positive intradermal test with (discrete) reactivation of the facial reaction.
Discussion
A continuous increase in the popularity of esthetic procedures with the use of substances as HA has been observed for many years. The American Society for Dermatologic Surgery (ASDS) reported that dermatologic surgeons performed almost 1.6 million soft-tissue filler injections in 2019. The number of filler procedures had increased by 78% since 2012.9 Those data concerned only the legal market and did not include procedures performed by unauthorized persons and using unregistered products, which might be contributing to an increase in the number of adverse events. While these procedures are nowadays often performed by individuals, lacking necessary knowledge and often working outside a medically registered and insured frame, the costs of eventual treatments and/or even hospitalization will be passed onto the state health care system. There exists a wild growth in new and hardly or even not at all pretested injectable compounds of sometimes questionable quality. Neither the FDA nor EMA approved these products: some are fake copies of existing regular brand, or are not intended for injection, hence posing a risk factor for new types of adverse events.
Hyaluronic acid (HA) is a glycosaminoglycan, a natural component of the extracellular matrix. The identical structure of the molecule in all living organisms causes a minimal likelihood of immunogenicity, which is why it is conveniently used as a material for filling and revitalization.10–13 Moreover, being a highly hydrophilic substance, it has the properties of binding large amounts of water molecules in relation to the mass of HA causing formation of bulky conglomerates, enabling rapid hydration of tissues, an increase in skin tone and the effect of filling tissues.10–12
Hyaluronic acid is not organ- nor species-specific, so it might be assumed that it would not cause hypersensitivity. However, in certain situations delayed reactions may occur after weeks, months, or even years. The risk of such reactions is increased by preparations of unknown origin, poor purification containing DNA, proteins or bacterial toxins or the presence of biofilm.14–18 Moreover, it is believed that LMW-HA (low molecular weight HA) may act as danger-associated molecular patterns (DAMPs) initiating the mechanisms of innate immunity, similarly to bacterial proteins or heat-shock proteins, while HMW-HA (high-molecular HA) has primarily an anti-inflammatory effect.18,19 However, a recent study suggests that HA products alone do not stimulate an inflammatory/immune response, regardless of molecular weight or cross-linking. On the other hand, the immune response after stimulation of heat-inactivated Cutibacterium acnes bacteria confirm a potential initiating role for contaminants such as bacteria and a modulating role for HA.20 The technique of the procedure and the individual immune sensitivity of the patient are also important.14–18 The next trigger factor is stress. Recent studies have identified a population of proinflammatory monocytes/macrophages that are released from the bone marrow in response to the sympathetic nervous system which release proinflammatory cytokines into circulation but also have the ability to traffic to stress- and anxiety-related brain regions.21 However, it should be remembered that HA belongs to substances meeting the criteria of adjuvants.
The ASIA syndrome following the implantation of bioimplants is still recognized too rarely. It is probably due to the lack of knowledge or diagnostic difficulties, because the symptoms are non-specific and there is no immune marker of this syndrome. Furthermore, it may develop several years after the procedure and it is difficult for both the patient and the physician to associate this relationship. With regards the presented case, the symptoms of the ASIA syndrome were detected early enough due to the ongoing DIR, as in our cases (Tab. I). Macrophages play a decisive role in both reactions. Like in ASIA syndrome, presumably neither type I nor type IV hypersensitivity plays a role in DIR to hyaluronic acid (HA) fillers, although intradermal skintesting with different brands of HA might result in positive skin tests, further obscuring the precise mechanism of these reactions.6,17,22 Potentially a similar pathophysiological mechanism of the reaction may explain the initiating role of DIR in the development of ASIA.
Montealegre et al described a series of 13 cases of patients who developed ASIA syndrome after the injection of biopolymers into the buttocks. Initially, symptoms corresponding to the local inflammatory process characterized by continuous or intermittent pain, and hyperemia occurred at the implantation site. They developed even several months or years after the application of the modelling substance.4 Our patients were initially diagnosed with DIR, although the resistance of this inflammatory reaction to antibiotic therapy, steroids and hyaluronidase were observed, which was suggestive of the development of ASIA. The authors evaluated patients according to the ASIA score, which predicts the probability of the occurrence of this disorder, comprising age, sex (more common in middle-aged women), individual or family coexistence of autoimmune diseases, exposure to other treatments with adjuvant substances (including bioimplants, tattoos, hair dyes, piercings and earrings, smoking). The following questionnaires were used in case 1: Functional Assessment of Chronic Illness Therapy (FACIT), whose higher score indicates a better quality of life, the Fatigue Severity Scale (FSS), and the Medical Research Council (MRC) Scale for Muscle Strength and the Unstimulated Whole Salivary Flow Rate (UWSF) (positive if ≤1.5 mL/15 min).4 It is worth using the above analysis in clinical practice when assessing patients.
Hyaluronic acid, the most commonly used bioimplant in esthetic medicine, may cause late immune-mediated adverse reactions, some of which meet the ASIA criteria. Alijotas-Reig et al described a series of 25 patients who developed delayed immune reactions after HA injection. As many as 5 of them developed clinical symptoms corresponding to the ASIA syndrome.3 Watad et al analyzed 500 cases and found that HA caused the syndrome in as many as 29.2% of them.2
The autoimmune/inflammatory syndrome induced by adjuvants (ASIA) was first defined by Shoenfeld et al in 2011, explaining the mechanism of adverse immune reactions after vaccination using aluminum compounds as an adjuvant.1,2 The role of the adjuvant is to protect antigens from degradation, enabling prolonged exposure to the cells presenting the antigen, which results in greater immunogenicity and, thus, the effectiveness of vaccination. Adjuvants may also include infectious agents, bioimplants (silicone, acrylamides, hyaluronic acid (HA) and methacrylate compounds).2–4
Immediately after the implantation of the biomaterial, the host protein layer settles on its surface, causing the attraction of phagocytes (mainly pro-inflammatory M1 macrophages).5 Additionally, if a biofilm is formed after implantation, the microorganisms in this biofilm may also act as adjuvants.5 Exposure to an adjuvant leads to activation of the innate and adaptive immune systems by mimicking damage-associated molecular patterns, pathogen-associated molecular patterns and alarmins, which can activate Toll-like receptors and the inflammasome system (IL-1β overproduction), causing the release of Th1 inflammatory cytokines and increasing the activity of dendritic cells, lymphocytes and tissue macrophages.3,6 It is followed by macrophage apoptosis, which results in the release of adjuvant-containing particles that may be reabsorbed by other macrophages, further stimulating the inflammatory response. Additionally, the production of IL-17 and the influx of neutrophils are stimulated. It results in the production of reactive oxygen species (ROS) and the release of enzymes such as myeloperoxidase, thereby intensifying inflammation.5 Consequently, the innate and acquired immune response is activated, which then intensifies the local and systemic response to antigens that are presented in regional lymph nodes, leading to the dysregulation of T and B lymphocytes, inability to recognize one’s own antigens, inflammation, damage to one’s own tissues, and ultimately to autoimmunity.2,4,5,7 The destruction of one’s own tissues is facilitated by an imbalance between effector T cells and regulatory Treg cells, which additionally stimulates the production of autoantibodies.7
Predisposing genetic factors play an important role in this reaction. It is mediated by specific HLA antigens predisposing to the development of autoimmune diseases, especially the simultaneous presence of the HLA-DRB1 and PTPN22 gene.8
The analysis of 300 patients with the ASIA syndrome revealed that the group included mostly women at the average age of 38 years, and the median time between exposure to adjuvant stimuli and the development of autoimmune disorders ranged from 2 weeks to 5 years. Moreover, up to 45% of patients were at risk of undifferentiated connective tissue disease (UCTD), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis (SS), Sjögren’s syndrome (SjS), eosinophilic fasciitis (EF), polymyositis, silicone implant incompatibility syndrome (SIIS) and chronic fatigue syndrome (CFS) which were the most commonly reported diseases.2,4,5 Cohen Tervaert et al showed that the above mentioned percentage increased to 50% in patients with a reaction provoked by silicone implants. In addition, up to a quarter of patients developed IgG immunoglobulin deficiency.5
When qualifying patients for treatment with bioimplants, including HA, it is worth considering the group which is at a particular risk of complications, both early and delayed. This group includes people with a history of immunization reactions, severe allergic reactions, individual predisposition to autoimmunity or family predisposition to autoimmunity and previous exposure to adjuvants.3,18,23 Regarding our first case, the patient’s father suffered from systemic lupus erythematosus (SLE), which indicates a strong genetic predisposition to autoimmunity.24 For the second and third case, the intractable itch diagnosed as notalgia paresthetica and the psoriasis arthropathica and chronic nephritis might also point in the same direction.
Conclusion
The possibility of provoking the ASIA syndrome by bio-implants, including HA, means that every patient with a history of adverse reactions (eg, DIR) should be monitored for many months.
Ethics and Consent Statements
The authors obtained the informed consents from all of the patients. The study participants gave consent to publish. All three patients provided informed consent to publish their case details and any accompanying images.
The Department of Dermatology, Sexually Transmitted Diseases and Clinical Immunology, The University of Warmia and Mazury approved to publish the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shoenfeld Y, Agmon-Levin N. ‘ASIA’ - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36(1):4–8. doi:10.1016/j.jaut.2010.07.003
2. Watad A, Bragazzi NL, McGonagle D, et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) demonstrates distinct autoimmune and autoinflammatory disease associations according to the adjuvant subtype: insights from an analysis of 500 cases. Clin Immunol. 2019;203:1–8. doi:10.1016/j.clim.2019.03.007
3. Alijotas-Reig J, Esteve-Valverde E, Gil-Aliberas N, et al. Autoimmune/inflammatory syndrome induced by adjuvants-ASIA-related to biomaterials: analysis of 45 cases and comprehensive review of the literature. Immunol Res. 2018;66(1):120–140. doi:10.1007/s12026-017-8980-5
4. Montealegre G, Uribe R, Martínez-Ceballos MA, et al. ASIA syndrome symptoms induced by gluteal biopolymer injections: case-series and narrative review. Toxicol Rep. 2021;8:303–314. doi:10.1016/j.toxrep.2021.01.011
5. Cohen Tervaert JW. Autoinflammatory/autoimmunity syndrome induced by adjuvants (ASIA; Shoenfeld’s syndrome): a new flame. Autoimmun Rev. 2018;17(12):1259–1264. doi:10.1016/j.autrev.2018.07.003
6. Decates T, Kadouch J, Velthuis P, et al. Immediate nor delayed type hypersensitivity plays a role in late inflammatory reactions after hyaluronic acid filler injections. Clin Cosmet Investig Dermatol. 2021;14:581–589. doi:10.2147/CCID.S312198
7. Jara LJ, Garcia-Collinot G, Medina G, et al. Severe manifestations of autoimmune syndrome induced by adjuvants (Shoenfeld’s syndrome). Immunol Res. 2017;65(1):8–16. doi:10.1007/s12026-016-8811-0
8. Facciolà A, Visalli G, Laganà A, et al. An overview of vaccine adjuvants: current evidence and future perspectives. Vaccines. 2022;10(5):819. doi:10.3390/vaccines10050819
9. ASDS report. Available from: https://www.asds.net/skin-experts/news-room/press-releases/asds-members-performed-nearly-14-million-treatments-in-2019.
10. Bukhari SNA, Roswandi NL, Waqas M, et al. Hyaluronic acid, a promising skin rejuvenating biomedicine: a review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J Biol Macromol. 2018;120(Pt B):1682–1695. doi:10.1016/j.ijbiomac.2018.09.188
11. Rohrich RJ, Bartlett EL, Dayan E. Practical approach and safety of hyaluronic acid fillers. Plast Reconstr Surg Glob Open. 2019;7(6):e2172. doi:10.1097/GOX.000000000000217
12. Viscusi KS, Hanke CW. Soft tissue augmentation. In: Baran R, Maybach HI, editors. Textbook of Cosmetic Dermatology,
13. Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143(2):155–163. doi:10.1001/archderm.143.2.155
14. Chung KL, Convery C, Ejikeme I, et al. A systematic review of the literature of delayed inflammatory reactions after hyaluronic acid filler injection to estimate the incidence of delayed type hypersensitivity reaction. Aesthet Surg J. 2020;40(5):NP286–NP300. doi:10.1093/asj/sjz222
15. Heydenrych I, De Boulle K, Kapoor KM, et al. The 10-Point Plan 2021: updated Concepts for Improved Procedural Safety During Facial Filler Treatments. Clin Cosmet Investig Dermatol. 2021;14:779–814. doi:10.2147/CCID.S315711
16. Bitterman-Deutsch O, Kogan L, Nasser F. Delayed immune mediated adverse effects to hyaluronic acid fillers: report of five cases and review of the literature. Dermatol Reports. 2015;7(1):5851. doi:10.4081/dr.2015.5851
17. Decates TS, Velthuis PJ, Jhingoerie R, et al. No association found between late-onset inflammatory adverse events after soft tissue filler injections and the adaptive immune system. J Cosmet Dermatol. 2023;22(2):458–463. doi:10.1111/jocd.15098
18. Owczarczyk-Saczonek A, Zdanowska N, Wygonowska E, et al. The immunogenicity of hyaluronic fillers and its consequences. Clin Cosmet Investig Dermatol. 2021;14:921–934. doi:10.2147/CCID.S316352
19. Sadeghpour M, Quatrano NA, Bonati LM, et al. Delayed-Onset Nodules to differentially crosslinked hyaluronic acids: comparative incidence and risk assessment. Dermatol Surg. 2019;45(8):1085–1094. doi:10.1097/DSS.0000000000001814
20. Hee CK, Messina DJ. In vitro inflammatory and immune response to uncrosslinked hyaluronic acid (HA) and HA fillers. J Immunol Regen Med. 2022;17. doi:10.1016/j.regen.2022.100065
21. Flux MC, Lowry CA. Inflammation as a mediator of stress-related psychiatric disorders. In: Zigmond MJ, Wiley CA, Chesselet MF, editors. Neurobiology of Brain Disorders.
22. Shalmon D, Cohen JL, Landau M, et al. Management Patterns of Delayed Inflammatory Reactions to Hyaluronic Acid Dermal Fillers: an Online Survey in Israel. Clin Cosmet Investig Dermatol. 2020;13:
23. Kaczor T. Recognizing Autoimmune/Autoinflammatory Syndrome Induced by Adjuvants (ASIA). Useful knowledge for identifying patients with underlying immune dysregulation. Nat Med J. 2017;9:676.
24. Aggarwal R, Namjou B, Li S, et al. Male-only systemic lupus. J Rheumatol. 2010;37(7):1480–1487. doi:10.3899/jrheum.090726
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
