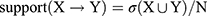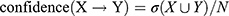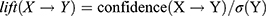Back to Journals » International Journal of General Medicine » Volume 16
Huangqin Qingre Chubi Capsule is Associated with Reduced Risk of Readmission in Patients with Rheumatoid Arthritis: A Real-World Retrospective Cohort Study
Authors Hu Y , Liu J , Xin L, Wan L, Qi Y, Li Y, Chen Y
Received 28 July 2023
Accepted for publication 13 October 2023
Published 26 October 2023 Volume 2023:16 Pages 4819—4834
DOI https://doi.org/10.2147/IJGM.S431124
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Luca Testarelli
Yuedi Hu,1,2 Jian Liu,1,3,4 Ling Xin,3,4 Lei Wan,1,3,4 Yajun Qi,1 Yang Li,1 Yiming Chen1
1Department of Rheumatology, The First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, Anhui, People’s Republic of China; 2College of Chinese Medicine, Anhui University of Chinese Medicine, Hefei, Anhui, People’s Republic of China; 3Institute of Rheumatology, Anhui University of Chinese Medicine, Hefei, Anhui, People’s Republic of China; 4Department of Internal Medicine Application Foundation Research and Development, Anhui Province—Key Laboratory of Modern Chinese Medicine, Hefei, Anhui, People’s Republic of China
Correspondence: Jian Liu, Department of Rheumatology, The First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, Anhui, 230031, People’s Republic of China, Tel +86 13955109537, Email [email protected]
Purpose: The therapeutic effects of Huangqin Qingre Chubi (HQC) in rheumatoid arthritis (RA) have been documented. However, there is a lack of real-world clinical evidence supporting its efficacy.
Methods: Patients diagnosed with RA were recruited from the First Affiliated Hospital of the Anhui University of Chinese Medicine. Patient information was obtained from the hospital’s database. Propensity score matching (PSM), Kaplan–Meier curve, and Cox proportional hazards model were used to control confounding factors and analyze the factors influencing readmission. Association rule analysis and random walk evaluation models were used to evaluate the correlations among HQC treatment, inflammation indicators, and self-perception of patients (SPP) scale.
Results: After PSM, 3423 patients were enrolled, with 1142 in the HQC group and 2281 in the non-HQC group. The readmission risk of the HQC group was significantly lower than that of the non-HQC group. Combined univariate and multivariate analysis results revealed that risk factors for readmission were age > 60 years, female sex, hypertension, chronic gastritis, and elevated levels of laboratory indices, including anticyclic citrullinated peptide and complement component 3 (C3) and C4. HQC, disease-modifying antirheumatic drugs, nonsteroidal anti-inflammatory drugs, and glucocorticoid therapy were protective factors for readmission. HQC treatment was closely associated with improvements in many factors, including erythrocyte sedimentation rate, C-reactive protein, C3, rheumatoid factor levels, visual analog scale, depression self-assessment scale, and patient-reported activity index scores with RA.
Conclusion: HQC treatment can reduce the risk of readmission and significantly improve immune inflammatory indicators and SPP in patients with RA, with no risk of hepatorenal toxicity.
Keywords: Huangqin Qingre Chubi capsule, traditional Chinese medicine, rheumatoid arthritis, readmission
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by hyperplasia and destruction of synovial joints.1,2 Severe and persistent inflammatory responses and extra-articular manifestations have led to a consistently higher RA mortality than that in the general population over the past 50 years.3–5 Chronic progression of RA is accompanied by continuous joint dysfunction or disability, which considerably affects the patient’s quality of life (QOL). Extra-articular complications further accelerate premature death.6,7 In the treatment of RA, the long-term use of conventional disease-modifying antirheumatic drugs (DMARDs), nonsteroidal anti-inflammatory drugs (NSAIDs), and glucocorticoids (GCs) inevitably cause side effects. Additionally, the use of biological agents is expensive and poses risks of infection and recurrence of tuberculosis,8 with several targeted drugs yet to be translated into clinical use.9
Traditional Chinese medicine (TCM) is a complementary and alternative therapy that plays a critical role in the treatment of RA by alleviating complications and adverse reactions. In a clinical retrospective cohort study of more than 10,000 patients, we found that TCM significantly reduced the risk of readmission, complications, surgical treatment, and all-cause mortality in patients with RA.10 Compared to individual components, TCM compound formulae allow for the combined advantage of individual components for treatment of the same disease. For example, Juanbi Decoction is a TCM compound that has been shown to relieve joint pain, swelling, and morning stiffness in patients with RA cold-dampness patterns and reduce the expression of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and rheumatoid factor (RF).11 Similarly, compared with Tripterygium wilfordii used individually, Xinfeng capsule (XFC) was strongly correlated with improvements of clinical inflammatory indices in patients with RA.12
In this study, we focused on the Huangqin Qingre Chubi capsule (HQC), which has been used clinically for more than 20 years at the First Affiliated Hospital of Anhui University of Chinese Medicine. HQC is a Chinese patent medicine for the treatment of RA Damp-Heat Patterns (RADHP) under the guidance of TCM pattern differentiation and treatment theory.13 HQC has a legal production license in China (Anhui Medicine System: Z20200001) and has obtained a national invention patent (Patent Number: ZL201110095718.X). HQC is composed of five traditional Chinese medicines, namely, Scutellaria baicalensis, Gardenia jasminoides, Semen coicis, Radix clematidis, and Prunus persica, in the ratio of 10:9:30:10:5.14 Previous pharmacokinetic studies have shown the high content and good stability of the five core chemical components of HQC, and the rigorous production process allows for controllable quality.15,16 We have explored the underlying mechanisms of HQC in RA. In vivo experiments showed that HQC inhibit secondary foot swelling in adjuvant-induced arthritis (AA) rats, reduce synovial tissue hyperemia, oedema and inflammatory cell infiltration, and reduce serum NO, Interleukin-1β, −6 levels. In addition, it can inhibit the pathogenesis of RA through CUL4B/Wnt-signalling pathways.17–19 In vitro experiments showed that HQC inhibit the proliferation of fibroblast-like synoviocytes in AA rats,14 and by increasing the total antioxidant capacity and the level of SOD in peripheral blood mononuclear cells of RA patients—reducing Malondialdehyde and Lipid peroxide levels. This activates AMP-activated protein kinase (AMPK) and forkhead box transcription factor family O subfamily 3a, playing an anti-inflammatory and anti-oxidative role.20 Through clinical observation, we have demonstrated the efficacy of HQC as combined with other drugs for the treatment of rheumatic diseases. HQC combined with XFC, or the external washing of TCM, can effectively improve patients’ mental health and reduce inflammation, oxidative damage, toxicity, and the side effects incurred by Western medicines.21,22 HQC combined with celecoxib can improve the Visual Analog Scales (VAS), the Western Ontario and McMaster Universities (WOMAC) score, ESR, and the CRP of patients with osteoarthritis than that of celecoxib alone.23 Although the results of small observational clinical studies provide evidence for the efficacy of HQC, we believe that, on a larger scale, high-level research evidence based on real-world cases is required to prove the auxiliary efficacy of HQC in patients with RADHP.
In this study, we collected information on HQC therapy by patients with RA and analyzed differences in demographics, laboratory indicators, and self-perception of patients(SPP) before and after medication, in combination with a follow-up survey, and determined the correlation between drug use and evaluation indicators. The evidence-based medicine findings provides a reference for evaluating the clinical efficacy of HQC.
Materials and Methods
Data Sources and Study Participants
In this telephone follow-up, we revisited 5576 patients with RA who were admitted to the Rheumatology Department of the First Affiliated Hospital of Anhui University of TCM between December 19, 2011 and June 22, 2021. Our follow-up process fully protected patient privacy and did not interfere with the treatment selection. This study was performed in accordance with the principles of the Declaration of Helsinki.
Inclusion and Exclusion Criteria
The included patients met the 2010 American College of Rheumatology/European Federation of Rheumatology Association RA criteria.24 Meanwhile, they need to have met the diagnostic criteria for RADHP, as per the 2018 Branch Committee of Rheumatology of China Association of Chinese Medicine’s “Rheumatoid Arthritis Disease Evidence Combined Diagnosis and Treatment Guidelines”25 which state that individuals: (1) Meet the 2010 American College of Rheumatology/European Federation of Rheumatology Associations RA classification criteria; and that a (2) diagnosis can be made by combining tongue and pulse readings with two of the following primary symptoms, or one of the primary symptoms plus two of the secondary symptoms. These symptoms include: primary symptoms include (A) joint swelling and heat pain; and (B) heat sensation of joint contact or self-consciousness; whereas secondary symptoms include (A) localized redness of the joints; (B) fever; (C) upset, (D) thirst or no desire to drink when thirsty; and (E) yellow urine. Tongue and pulse symptoms include: a red tongue, a thick yellowish coating on the tongue, and a smooth or slippery pulse.The exclusion criteria26 included (1) age <18 years; (2) incomplete clinical data, uncooperative follow-up, and missed follow-up; (3) serious cardiovascular, liver, renal, and hematopoietic diseases, and severe extra-articular lesions, such as hyperthermia, renal amyloidosis, and central nervous system vasculitis; (4) psychiatric disease; and (5) pregnant or lactating women.
Contents of Telephone Follow-Up and Data Processing
We retrieved patient data from the data processing system of First Affiliated Hospital of Anhui University of Chinese Medicine (Patent Number: 2017SR422234). Patient name, age, sex, telephone number, and diagnostic information that had been collected from the processing system were verified in the telephone follow-up. Other items queried in the follow-ups included HCQ use and duration and endpoint events.
The underlying medications included DMARDs (methotrexate, leflunomide, and sulfasalazine), NSAIDs (celecoxib, meloxicam, and lornoxicam), and GCs (methylprednisolone and betamethasone). The underlying diseases included hypertension, diabetes, and chronic gastritis. Primary endpoints were RA exacerbations leading to readmission, extra-articular RA lesions (interstitial lung disease, Sjogren’s syndrome), joint surgical treatment, and death. All follow-up examinations were conducted by physicians specializing in rheumatology. Each follow-up visit involved one medical doctor. Two medical doctors supervised the verification.
Observational research is prone to the presence of confounding variables which means that unadjusted analyses would lead to bias.27 Propensity score matching (PSM), as a statistical method proposed by Rosenbaum and Rubin in 1983, can effectively reduce the confounding bias in the design of observational studies.28 For patients with successful follow-up, propensity score matching (PSM) was performed at a 1:2 ratio with a 0.02 matching tolerance to balance the bias caused by the baseline data. The PSM generated HQC and non-HQC groups. In the HQC group, exposure was defined according to the length of time of continuous oral HQC therapy and was categorized as strongly exposed (≥12 months), exposed (≥1 month), weakly exposed (≥1 month and <12 months), and non-exposed (no HQC application or HQC tonics for <1 month) subgroups. Figure 1 provides a flowchart of the selection process for patients with RA.
 |
Figure 1 Flowchart of the study population. Abbreviations: RA, rheumatoid arthritis; HQC, Huangqin Qingre Chubi Capsule. |
Laboratory Examination and Self-Perception of Patients Scale
Laboratory examination indicators included ESR (reference value: male 0–15mm/h, female 0–20 mm/h), CRP (reference value:<2.87mg/L), RF (reference value:<20 U/mL), anticyclic citrullinated peptide antibody (anti-CCP)(reference value: negative), immunoglobulins A, G, and M (IgA, IgG, and IgM)(reference value:0.7–3.5 g/L;7.0–16.6 g/L;0.5–2.6 g/L), complement component 3 (C3) (reference value:0.8–0.1.5 g/L) and C4 (reference value:0.2–0.0.6 g/L), alanine aminotransferase (ALT)(reference value: 5–40 U/L), aspartate transferase (AST)(reference value: 8–40 U/L), serum creatinine (CREA)(reference value: male 53–106 μmol/L, female 44–97 μmol/L), blood urea nitrogen (BUN)(reference value: 3.2–7.1 mmol/L), and uric acid (UA)(reference value: male 150–416 μmol/L, female 89–357 μmol/L).29
To determine SPP, the medical outcomes study item short form-36 health survey (SF-36),30 visual analog scale (VAS), Chinese patient-reported activity index with rheumatoid arthritis (CPRI-RA),31 anxiety self-assessment scale (SAS), depression self-assessment scale (SDS), and TCM symptom score with RA Damp-Heat Patterns (CMSS-RADHP) were used. SF-36 measures nine domain scales and 36 items. The SF-36 measures nine domain, including physical function (PF), role limitations due to physical problems (RP), body pain (BP), general health (GH), vitality (VT), social function (SF), role limitations due to emotional problems (RE), mental health (MH), and reported health transition (HT). The VAS scores included joint pain (JP), patient assessment (PA), and doctor assessment (DA). All participants completed the paper version of the scale under the guidance of two medical doctors specializing in rheumatology. One doctor entered the data processing system, while the other supervised quality control.
SF-36 uses 0–100 general health rating index as a “criterion”.32 The VAS scale criterion is as follows: 0 for no pain, 1–3 for mild pain, 4–6 for moderate pain, and 7–10 for severe pain.33 CPRI-RA was positively correlated with the degree of disease activity.31 SDS and SAS were developed by Zung in 1965 and 1971, respectively, and were subsequently popularized worldwide.34,35 The scoring standard of this study was as follows: the cut-off value of the SAS standard score was 50 points, in which 50–59 points indicated mild anxiety, 60–69 points indicated moderate anxiety, and more than 70 points indicated severe anxiety; an SDS score of less than 50 was defined as no depression present. A score of 50–59 indicated mild depression, whereas a; score of 60–69 indicated moderate-to-severe depression. A score above 70 is considered to indicate severe depression’s presence. CMSS-RADHP is positively correlated with the severity of damp-heat syndrome in RA.36
Assessment of Readmission Risk Factors
Survival analysis is a commonly-used method in biomedical research. A Kaplan-Meier (K-M) curve is often used to visually summarize time-to-event data from treatment to relapse in cohort studies.37 According to whether the patients used HQC, readmission, and readmission time, online bioinformatics tools 38 were used to draw K–M curves and analyze the risk factors for readmission.38
The Cox proportional-hazards model is a statistical method widely used in medical research to analyze the effect of one or more predetermined variables on the survival time of patients.39 Different to the K-M curve commonly used in univariate analysis, the Cox model can use both univariate and multivariate survival analysis methods, which can extend the survival analysis method to evaluate the impact of several risk factors on survival time at the same time.40 Cox proportional hazards model analysis took the readmission of patients with RA as the dependent variable and the use of HQC, age, sex, basic diseases and drugs, and RA-specific clinical laboratory indicators as the covariables. Combined with readmission and follow-up times, the Cox proportional risk model analysis was performed. Univariate and multivariate analyses were performed to obtain preliminary and multivariate data, respectively.
Association Rule Analysis and Random Walk Evaluation Model
Association rule analysis is a data mining technique that identifies frequently-occurring patterns, associations or correlations between item sets or object sets in transaction data, relational data, or other information carriers by searching for frequent attribute value conditions within data sets.41 The Apriori module in International Business Machines Corporation (IBM) Statistical Product and Service Solutions (SPSS) modeler software (version 18.0) was used to perform the association rule analysis. In the analysis evaluating HQC treatment and the improvement among indicators, HQC treatment was defined as “T” and non-HQC treatment as “F”. After HQC treatment, ESR, CRP, RF, anti-CCP, IgA, IgG, IgM, C3, and C4 decreased to T and stabilized or increased to F. Readmission was defined as “F” and no readmission was defined as “T”. In the analysis of association rules that evaluated the association between HQC treatment and changes in the SPP scale, HQC treatment was defined as “T” and non-HQC treatment as “F”. After the HQC treatment, VAS(JP), VAS(DA), SDS, VAS(PA), CPRI-RA, CMSS-RADHP, and SAS decreased to T and stabilized or increased to F. MH, VT, PF, GH, SF, RE,RP, HT, and BP increased to T and stabilized or decreased to F. Readmission was defined as “F” and no readmission was defined as “T”.
The specific calculation formula for the association rule analysis described by Huang et al42 was used:
where X and Y are item sets, σ is the minimum support threshold, and N is total number of item sets.
The random walk model takes the single treatment effect of each patient as a point. When the size of the patient reaches a certain level, a random walk route is formed, which is called the long-term association, and can be used to judge the improvement of the overall efficacy of the patient.The ORACLE 10g tool was used to realize a random walk model43 of immune inflammatory indicators to detect the improvement in laboratory indicators by HQC.
Statistical Analysis
All data were analyzed using SPSS v.26.0. Counting data are expressed as frequency and percentage. Measurement data are expressed as median [interquartile range]. Chi-square test was used for counting data, t-test was used for data conforming to normal distribution and homogeneity of variance, and non-parametric test was used for data not conforming to normal distribution. Statistical significance was set at P<0.05.
Results
Baseline Characteristics of Patients Before and After Propensity Score Matching
The clinical data of 5576 patients with RA were returned, and 5262 cases were screened for inclusion in the successful follow-up, with a shedding rate of 6%. Of the 5262 patients, 1142 were treated with HQC and 4120 were not. The two groups differed significantly in age, sex, combined chronic gastritis, DMARDs, NSAIDs, and readmission between the two groups (all P<0.05). To control for confounding factor bias, PSM was performed. The 3423 enrolled patients included 1142 patients in the HQC group and 2281 patients in the non-HQC group. The differences in the various influencing factors between the two groups were better balanced after PSM (Table 1).
 |
Table 1 Comparison of Demographic Characteristics Between Patients with RA with and without HQC |
Influencing Factors for the Readmission of Patients
K–M curve analysis was used to compare the risk of readmission between the HQC and non-HQC groups and to further assess the impact of HQC intervention time on the risk of readmission. Between 15–90 months, the proportion of non-readmissions was higher in HQC than in the non-HQC group, indicating that the HQC group had a significantly lower risk of readmission than the non-HQC group (hazard ratio [HR] = 0.85, P = 0.011; Figure 2A). From 30 months, the proportion of non-readmission was higher in the high-exposure group than in the low-exposure group, indicating that the risk of readmission was significantly lower in the high-exposure group than in the low-exposure group (HR=0.26, P<0.001; Figure 2B). Therefore, long-term use of HQC will reduce the risk of patient readmission.
The Cox proportional hazards model was used to compare the risk factors affecting readmission in patients with RA. Univariate analysis (Table 2) revealed significant associations with increased risk of readmission for those aged >60 years (HR=1.012, 95% confidence interval [CI]=1.003–1.012, P=0), hypertension (HR=1.285, 95% CI=1.089–1.516, P=0), and chronic gastritis (HR=1.223, 95% CI=1.007–1.485, P=0.04). Significantly reduced risk of admission was evident for oral HQC (HR=0.85, 95% CI=0.752–0.961, P=0), DMARDs (HR=0.84, 95% CI=0.734–0.957, P=0.01), NSAIDs (HR=0.597, 95% CI=0.450–0.792, P=0), and GC therapy (HR=0.805, 95% CI=0.712–0.911, P=0). Significant associations with a higher risk of readmission were evident for laboratory indicators of anti-CCP (HR=1, 95% CI=1, P=0.01), C3 expression (HR=1.002, 95% CI=1.001–1.003, P=0), and C4 expression (HR=1.006, 95% CI=1.002–1.01, P=0). Multifactorial analysis (Table 2) showed that HQC (HR=0.42, 95% CI=0.29–0.72, P=0.04) and GC therapy (HR=0.669, 95% CI=0.489–0.914, P=0.01) were protective factors against patient readmission. Age >60 years (HR=1.007, 95% CI=1.002–1.012, P=0), female gender (HR=1.196, 95% CI=1.031–1.388, P=0.02), and hypertension (HR=1.13, 95% CI=0.947–1.348, P=0.01) were risk factors for readmission.
 |
Table 2 Cox Proportional Hazards Model of Univariate and Multivariate Analyses for Readmission of Patients with RA Treated with HQC |
Figure 3 displays a forest map based on multivariate analysis visualizing the protective and risk factors for patient readmission. Forest maps are graphs based on statistical indexes and statistical analysis methods, drawn by numerical operation results. Centered on a vertical null line (the horizontal scale ranges between 0 and 1), it describes the effect size and confidence interval of each study included with multiple line segments parallel to the horizontal axis, which can describe the statistical results in a simple and intuitive manner.44
 |
Figure 3 Forest map of risk factors for readmission. Abbreviation: HQC, Huangqin Qingre Chubi Capsule. Notes: The blue icon: protective factor;The rde icon: risk factor. |
Effects of Huangqin Qingre Chubi Capsule on Immune Inflammatory Indices, and Liver and Kidney Function in Patients with RA
We assessed changes in the levels of immune inflammatory indicators and liver and kidney function indicators in the HQC and non-HQC groups before and after treatment (Tables 3 and 4). In the non-HQC group, ESR, CRP, anti-CCP, IgG, C3, and C4 levels were significantly decreased after treatment compared with those before treatment (P<0.05). In the HQC group, the ESR, CRP, RF, anti-CCP, IgA, IgG, C3, and C4 levels decreased after treatment (P<0.05). In the non-HQC group, ALT and BUN levels increased (P<0.05), and CREA and UA levels decreased (P<0.05) after treatment compared with those before treatment. In the HQC group after treatment, ALT and BUN levels increased (P < 0.05), and CREA and UA levels decreased (P<0.05). There was no significant difference in the AST levels (P>0.05).
 |
Table 3 Effects of HQC on Immune Inflammatory Indices in Patients with RA |
 |
Table 4 Effects of HQC on Liver and Kidney Function in Patients with RA |
Analysis of Association Rules and Establishment of Random Walk Modeling Between Huangqin Qingre Chubi Capsule Treatment and Laboratory Indicators
Association rule analysis was performed with the HQC used as the antecedent and improved laboratory indicators as the consequent. HQC was strongly correlated with improvements in ESR, CRP, C3, and RF. In these results, support was >30%, confidence was >50%, and lift was >1, except for CRP (Table 5).
 |
Table 5 Analysis of Association Rules Between HQC Treatment and Laboratory Indicators in Patients with RA |
We evaluated ESR, CRP, RF, IgA, IgG, IgM, C3, and C4 as indicators in the random walk model and obtained the maximum stochastic volatility, walking steps, positive growth rate of walking, stochastic fluctuation power law value, rate of increase, and ratio for each indicator (Table 6). In the model evaluation, the ESR, CRP, RF, IgA, IgG, and C4 indicators were clinically significant; for each step of improvement in ESR, CRP, RF, IgA, IgG, and C4, 7.8, 3.6, 11.99, 23.04, 21.65, and 5.59 steps were required to walk, respectively. In addition, we plotted a model of walking steps versus index improvement (Figure 4). ESR, CRP, RF, IgA, IgG, IgM, and C4 levels improved as the number of steps increased.
 |
Table 6 Evaluation of Immunoinflammatory Indicators Random Walk Model in the HQC Group |
Effects of Huangqin Qingre Chubi Capsule on Self-Perception of Patient in Patients with RA
Of the 3423 patients included, 789 completed the SPP scale on admission and discharge, with 587 and 202 in the HQC and non-HQC groups, respectively. SPP scale changes were consistent between the groups. In the HQC and non-HQC groups, PF, RP, BP, GH, VT, SF, RE, and MH levels significantly improved (P<0.05) after treatment, whereas the HT levels showed no significant changes (P<0.05). In addition, for both groups VAS(JP), VAS(PA), VAS(DA), CPRI-RA, SAS, SDS, and TCMSS-RADHP levels significantly decreased after treatment (P<0.05; Table 7).
 |
Table 7 Effects of HQC on SPP in Patients with RA |
An association rule analysis was performed to explore the relationship between HQC and SPP. HQC was strongly correlated with improvements in VAS(JP), VAS(DA), SDS, VAS(PA), CPRI-RA, TCMSS-RADHP, MH, VT, PF, RP, GH, SF, and RE scores. Support was >70%, confidence was >60%, and the lift was >1 (Table 8).
 |
Table 8 Analysis of Association Rules Between HQC Treatment and SPP Indicators in Patients with RA |
Discussion
The conventional clinical drugs used for RA treatment delay disease progression to a large extent. However, all these drugs are associated with adverse events.45 Adverse reactions caused by drugs also greatly reduce patients’ QOL. In a systematic study of 88 articles, Salliot et al found that nearly 13% of patients had elevated liver enzyme levels as a result of methotrexate monotherapy, and 3.7% of patients permanently discontinued the drug due to liver toxicity.46 In a retrospective study of 1114 patients, Jayachandran et al reported that 10.1% of patients experienced adverse reactions, such as gastritis, liver enzyme disturbance, and hepatitis, when using sulfasalazine.47 In addition, the use of GCs and emerging biologics is associated with a high risk of severe infection.48,49 Therefore, we focused on designing alternative or auxiliary drug therapies that are robust and can effectively alleviate the toxicity and side effects associated with RA drugs used in Western medicine.
Globally, a significant number of patients with arthritis are treated with TCM in addition to conventional Western medicine.50–52 Over the past few decades, several in-depth studies have investigated the role of TCM in the treatment of RA. TCM is effective in reducing disease activity, protecting joints,53 and ameliorating the adverse effects of conventional Western medicine.54 Fang et al demonstrated that TCM with anti-inflammatory effects is no less effective than methotrexate in the treatment of RA and that the combination of TCM and DMARDs can significantly reduce the occurrence of adverse events.55
HQC is effective in treating joint swelling, heat and pain, aggravation of clinical indicators, red tongue, and yellow greasy fur in the acute or active stage of RA. In China, HQC is a Chinese patent medicine created under the guidance of the basic theories of TCM combined with the experience of clinical diagnosis and treatment of rheumatism. The prescription comprises five traditional Chinese medicines: S. baicalensis, G. jasminoides, S. coicis, R. clematidis, and P. persica. In TCM, S. baicalensis and G. jasminoides clear heat and dampness, purify fire, and detoxify poison; S. coicis strengthens the spleen and removes dampness; R. clematidis alleviates wind and dampness and removes pain; and P. persica promotes blood circulation and removes blood stasis, which has a significant effect on the clinical treatment of damp-heat pattern rheumatism.21,56,57 In our previous studies encompassing 20 years of clinical observations and data mining involving patients with RA, the evidence indicates that HQC can effectively improve immune inflammatory indicators, red blood cell parameters, and patient perception.58,59 We identified the main chemical components of HQC as baicalin, geniposide, luteolin, oleanolic acid, coixol, and amygdalin.16 Identification of these chemical components may provide reasonable explanations for the anti-inflammatory, antioxidant, and immunoregulatory effects of HQC.60,61
In this study, long-term use of HQC prolonged the readmission time of patients with chronic RA. Univariate analysis revealed that hypertension, chronic gastritis, and elevated anti-CCP, C3, and C4 levels increased the risk of readmission, while DMARDs, NSAIDs, and GCs therapy decreased this risk. These findings are consistent with clinical practice. To better screen all factors, we performed a multivariate analysis, which provided results consistent with the univariate analysis and also showed that age <60 years and female sex were both risk factors for the readmission of patients. These findings are consistent with epidemiological results.60,61
We also observed that ESR, CRP, C3, and C4 levels were reduced after oral HQC treatment, presumably because we did not restrict patients to other clinical medications. Nevertheless, association rule analysis results demonstrated that HQC was closely related to improvements in the ESR, CRP, C3, and RF levels. Additionally, the HQC group performed significantly better than the non-HQC group in reducing the sensitivity index of RF level.
The random walk model is an evaluation system that was established to further evaluate the correlation between drug use and laboratory indicators. The long-range correlation and mathematical probability theory of the random walk model were similar to the disease development law. The long-range correlation of the model determines the effectiveness of the index system.62,63 The randomized walk model of this study considers the effect of a single treatment in each patient as an individual point. The 1142 patients treated with HQC in this study reached the size of dots to form a random walk route. The relationship between the number of steps walked and the improvement of indicators was used to determine the improvement of the overall efficacy of patients. In this study, random walk model analysis suggest that the changes in ESR, CRP, RF, IgA, IgG, IgM, and C4 were associated with HQC treatment in the long-term and that HQC treatment affected the changes in immune inflammatory indicators in patients.
Hepatorenal toxicity caused by TCM have been the focus of research worldwide. In this study, we evaluated the changes in the liver and kidney function of patients and found an insignificant difference between the HQC and non-HQC groups. CREA and UA levels in the HQC group were decreased, AST levels were not significantly different, and ALT and BUN levels were slightly increased. All were within the normal range and no liver or kidney injury caused by TCM was observed.
The evaluation of patients’ self-perception and QOL is an important reference criterion for evaluating the effectiveness of treatment. This evaluation has attracted extensive attention in clinical research. In the past, researchers have used the SF-36, study item short form-12 health survey, EuroQol 5-dimension 3-level version, and RA-specific QOL to evaluate patients’ QOL;64–67 SAS and SDS to evaluate patients’ anxiety and depression;68 disease activity score and VAS to evaluate JP and disease activity.64,69 The SPP scale used in this study has been promoted worldwide by the International Alliance for the Measurement of Health Outcomes.70 Based on this, we created an original production and added scoring of TCM syndromes. We believe that the QOL of patients should be based on comprehensive judgment of their physical, mental, and disease states. In previous studies, we found that changes in SAS, SDS, and SF-36 in SPP were closely related to the dysregulation of disease activity and immunoinflammatory markers.71,72 In the present study there were insignificant differences in the overall improvement of the SF-36, VAS, SAS, SDS, and TCMSS-RADHP scores between the HQC and non-HQC groups. This may be because we did not restrict patients to other conventional treatments (such as Western medicine, Chinese herbal decoction pieces, and acupuncture) besides HQC treatment. Therefore, all patients received routine personalized treatment and their diseases were effectively controlled after treatment. After drug treatment, the levels of PF, RP, BP, GH, VT, SF, RE, and MH increased and the levels of VAS, SAS, SDS, and TCMSS-RADHP decreased, indicating that drug treatment improved the physical function and mental outlook of the patients. Association rule analysis results strongly correlated HQC with improvements in VAS(JP), VAS(DA), SDS, VAS(PA), PRI-RA, TCMSS-RADHP, MH, VT, PF, RP, GH, SF, and RE, confirming the improvements of SPP associated with HQC as an adjuvant therapeutic drug.
This study has some limitations. First, we obtained and verified important data, such as medication use, time, underlying diseases, and endpoint events of patients, after treatment through telephone follow-up. Limited by patients’ subjective initiatives, there may be a risk of response and recall bias. Second, we did not exclude the influence of Western medicine and other Chinese medicines on the efficacy of HQC, nor did we evaluate the combined efficacy of Western medicine, other Chinese medicines, and HQC. In addition, this study was conducted at a single institution and thus the results may be applicable to only a narrow range of beneficiaries. Therefore, further multicenter prospective studies are required to evaluate the exact role of the HQC. Finally, owing to patient compliance, we did not include the SPP data of all matched patients in the analysis. Thus, the SPP results were not verified in a large sample of patients. We believe that the SPP is a comprehensive assessment of patients’ QOL by which we can grasp the comprehensive assessment of patients’ improvement in physical symptoms, mental outlook, and TCM symptoms after drug treatment. This is consistent with the treatment concept of comprehensive conditioning and holistic treatment of TCM. The purpose of patient treatment was to improve the indicators and also to improve QOL. In future studies, we will refine the SPP rules and explore the correlation between the improvement in rules and indicators.
Conclusion
Based on the clinical practice of our institution, our study retrospectively analyzed the therapeutic effect of Chinese patent medicine HQC in RA. The findings demonstrate that HQC treatment, as a protective factor, is associated with the reduction of the risk of hospital readmission in patients with RA. Furthermore, HQC can significantly improve the immunoinflammatory indicators of patients without the risk of hepatorenal toxicity. Importantly, HQC treatment can improve SPP and improve QOL and satisfaction of patients with RA.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This research was reviewed and approved and exempted patients from the right of informed consent by the Ethics Committee of the First Affiliated Hospital of Anhui University of Chinese Medicine (registration number: 2022MCZQ01).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or across all these areas; all authors took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Nature Fund Program [No. 82274490, No. 82074373, No.81973655], Anhui Provincial Laboratory of Applied Basis and Development of Internal Medicine of Modern Traditional Chinese Medicine [No. 2016080503B041], Anhui Famous Traditional Chinese Medicine Liu Jian Studio Construction Project [Traditional Chinese Medicine Development Secret [2018] No. 11], Key Laboratory of Xin’an Ministry of Medical Education [No. 2020xayx10], and 2021 Anhui Province Major Difficult Diseases Collaborative Project of Traditional Chinese and Western Medicine [Anhui Traditional Chinese Medicine Development Secret [2021] No. 70].
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Deane KD, Holers VM. The natural history of rheumatoid arthritis. Clin Ther. 2019;41(7):1256–1269. doi:10.1016/j.clinthera
2. Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46(2):183–196. doi:10.1016/j.immuni
3. Dadoun S, Zeboulon-Ktorza N, Combescure C, et al. Mortality in rheumatoid arthritis over the last fifty years: systematic review and meta-analysis. Jt Bone Spine. 2013;80(1):29–33. doi:10.1016/j.jbspin.2012.02.005
4. Lee Y, Ahn GY, Lee J, et al. Excess mortality persists in patients with rheumatoid arthritis. Int J Rheum Dis. 2021;24(3):364–372. doi:10.1111/1756-185X.14058
5. Videm V, Houge IS, Liff MH, Hoff M. Inflammation mediates approximately one quarter of excess relative all-cause mortality in persons with rheumatoid arthritis: the Trøndelag Health Study. Sci Rep. 2022;12(1):18599. doi:10.1038/s41598-022-21977-9
6. Ometto F, Fedeli U, Schievano E, Botsios C, Punzi L, Corti MC. Cause-specific mortality in a large population-based cohort of patients with rheumatoid arthritis in Italy. Clin Exp Rheumatol. 2018;36(4):636–642.
7. Scott DL, Coulton BL, Symmons DPM, Popert AJ. Long-term outcome of treating rheumatoid arthritis: results aft er 20 years. Lancet. 1987;329(8542):1108–1111. doi:10.1016/S0140-6736(87)91672-2
8. Shen Q, Du Y. A comprehensive review of advanced drug delivery systems for the treatment of rheumatoid arthritis. Int J Pharm. 2023;635:122698. doi:10.1016/j.ijpharm.2023.122698
9. Feng X, Chen Y. Drug delivery targets and systems for targeted treatment of rheumatoid arthritis. J Drug Target. 2018;26(10):845–857. doi:10.1080/1061186X.2018.1433680
10. Fang Y, Liu J, Xin L, et al. Traditional Chinese medicine compound preparations are Associated with low disease-related complication rates in patients with rheumatoid arthritis: a retrospective cohort study of 11,074 patients. BioMed Res Int. 2023;2023:1019290. doi:10.1155/2023/1019290
11. Gu XH, Chen P, Zhu L, et al. Efficacy observation of juanbi decoction adjuvant therapy for active stage rheumatoid arthritis. Chin J Integr Tradit West Med. 2021;41(7):812–816.
12. Dong WZ, Liu J, Ling X, Fang YY, Wen JT. Data mining research on different formulations of tripterygium wilfordii ameliorating immune inflammation in patients with rheumatoid arthritis. J IMMUNOL. 2018;34(10):894–899+905. doi:10.13431/j.cnki.immunol.j.20180138
13. Huang D, Liu J, Wan L, Jiang H, Sun Y, Long Y. Study on treating “Bi” from spleen. J Rheum Arthritis. 2021;10(1):46–50.
14. Zhou W, Wang Y, Huang Y, et al. Huangqin qingre qubi capsule inhibits RA pathology by binding FZD8 and further inhibiting the activity of Wnt/β-catenin signaling pathway. J Ethnopharmacol. 2023;302:115886. doi:10.1016/j.jep.2022.115886
15. Liu JQ, Liu XC, Liu J, Zhang YY, Wang TJ, Zhou A. HPLC fingerprint of huangqin qingre chubi capsule preliminary study and determination of three components. Chin Med Biotechnol. 2022;17(1):56–58.
16. Dong XT, Gan PR, Ke JT, et al. Simultaneous determination of six active components in huangqin qingre chure chubi capsule by ultra-high-performance liquid chromatography-tandem mass spectrometry. J Anhui Univ Chinese Med. 2021;40(6):97–102.
17. Ge P, Zhang H, Sun XC, et al. Effect of huangqin qingre chubi capsule on serum IL-1β and IL-6 of adjuvant arthritis rats. Trad Chin Drug Res Clin Pharmacol. 2014;25(1):8–10. doi:10.3969/j.issn.1003-9783.2014.01.003
18. Jiang Y, Zhang J, Meng M, et al. Experimental study of huangqin qingre chubi capsule on anti-inflammatory action of adjuvant arthritis in rats. West China Med J. 2015;30(2):178–180.
19. Wang X, Chang J, Zhou G, et al. The traditional Chinese medicine compound huangqin qingre chubi capsule inhibits the pathogenesis of rheumatoid arthritis through the CUL4B/Wnt pathway. Front Pharmacol. 2021;12:750233. doi:10.3389/fphar.2021.750233
20. Guo JC, Liu J, Zhang XJ, Zhou Q, Huang D, Song Q. Effect of huangqin qingre chubi capsules containing serum on oxidative stress and protein expression of AMPK and FoxO3a in rheumatoid arthritis patients. China J Chin Mater Med. 2020;45(13):3228–3232. doi:10.19540/j.cnki.cjcmm.20200427.502
21. Ding BJ, Huang CB, Fu P, Chen LL. Clinical observation on 30 cases of rheumatoid arthritis with damp-heat bi-obstructing syndrome treated by Huangqin Qingre Chubi Jiaonang combined with shire bizu waixi fang. J Gansu Univ Chin Med. 2022;39(4):53–57. doi:10.16841/j.issn1003-8450.2022.04.09
22. Cao YH, Liu J. Clinical efficacy of heat-clearing and diuresis-promoting prescription combined with western medicine treatment for damp-heat rheumatoid arthritis. J Anhui Univ Chinese Med. 2014;33(6):19–22.
23. Cheng LL, Huang CB, Fu P, Ding BJ. Clinical observation of huangqin qingre chubi capsule combined with celecoxib in the treatment of knee osteoarthritis. Clin J Trad Chin Med. 2021;33(7):1375–1378. doi:10.16448/j.cjtcm.2021.0739
24. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–1588. doi:10.1136/ard.2010.138461
25. Jiang Q, Wang HL, Gong X, Luo CG. The diagnosis and treatment guidelines of rheumatoid arthritis combined with disease and pattern. J Tradit Chin Med. 2018;59(20):1794–1800. doi:10.13288/j.11-2166/r.2018.20.018
26. Wen JT, Liu J, Wan L, Xin L, Dong WZ, Fang YY. Outcome events of 1468 patients with rheumatoid arthritis: a cohort study. J Anhui Univ Chinese Med. 2017;36(5):13–17.
27. Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359(9302):248–252. doi:10.1016/S0140-6736(02)07451-2
28. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi:10.1093/biomet/70.1.41
29. Wan XH, Lu XF. Diagnostics.
30. Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–164. doi:10.1136/bmj.305.6846.160
31. Han M, Liu HX, Tang XP, et al. Expert consensus on clinical outcome scales reported by patients with rheumatoid arthritis in China. J Tradit Chin Med. 2018;59(10):897–900. doi:10.13288/j.11-2166/r.2018.10.020
32. Davies AR, Ware JE. Measuring Health Perceptions in the Health Insurance Experiment. Santa Monica, CA: Rand Corporation; 1981.
33. Burckhardt SC, Jones DK. Adult measures of pain: the McGill Pain Questionnaire (MPQ), Rheumatoid Arthritis Pain Scale (RAPS), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Verbal Descriptive Scale (VDS), Visual Analog Scale (VAS), and West Haven‐Yale Multidisciplinary Pain Inventory (WHYMPI)[J]. Arthritis Care Res. 2003;49(S5). doi:10.1002/art.11440
34. Zung WWK. A self-rating depression scale. Arch Gen Psychiatry. 1965;12(1):63. doi:10.1001/archpsyc.1965.01720310065008
35. Goyer PF, Brown GL, Minichiello MD, Major LF. Mood-altering effects of disulfiram in alcoholics. J Stud Alcohol. 1984;45(3):209–213. doi:10.15288/jsa.1984.45.209
36. Zheng XY. Guiding Principles for Clinical Research of New Traditional Chinese Medicine. Beijing: China Medical Science and Technology Press; 2002.
37. Ranstam J, Cook JA, Ranstam J, Cook JA. Kaplan–Meier curve. Br J Surg. 2017;104(4):442. doi:10.1002/bjs.10238
38. The xiantao academic. Available from: https://www.xiantaozi.com/products/apply/1150d1f4-ccad-4cec-a19d-7826235c52e5/analyse/2057dbc9-c8aa-4b3f-88c8-206cf336bb68.
39. Fisher LD, Lin DY. Time-dependent covariates in the cox proportional-hazards regression model. Annu Rev Public Health. 1999;20(1):145–157. doi:10.1146/annurev.publhealth.20.1.145
40. Austin PC, Goel V, Van Walraven C. An introduction to multilevel regression models. Can J Public Health. 2001;92(2):150–154. doi:10.1007/BF03404950
41. Han J, Pei J, Kamber M. Data Mining: Concepts and Techniques.
42. Huang D, Liu J, Xin L, et al. Data mining study on prescription patterns of different dosage forms of Chinese herbal medicines for treating and improving immune-inflammatory indices in patients with rheumatoid arthritis. Chin J Integr Med. 2022;28(3):215–222. doi:10.1007/s11655-020-3480-1
43. Fang Y, Liu J, Xin L, et al. Identifying compound effect of drugs on rheumatoid arthritis treatment based on the association rule and a random walking-based model. BioMed Res Int. 2020;2020:1–10. doi:10.1155/2020/4031015
44. Liu GJ, Wu T. Clinical implemation of forest plots in meta-analysis. Clin Implem Forest Plots Meta Anal. 2004;4(3):198–201.
45. Costello R, David T, Jani M. Impact of adverse events associated with medications in the treatment and prevention of rheumatoid arthritis. Clin Ther. 2019;41(7):1376–1396. doi:10.1016/j.clinthera.2019.04.030
46. Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009;68(7):1100–1104. doi:10.1136/ard.2008.093690
47. Jayachandran M, Koshy V, David R, et al. Adverse reaction profile of sulfasalazine and its persistence in chronic therapy of rheumatoid arthritis and spondyloarthritis: a multicentric observational study. Int J Clin Pharmacol Ther. 2022;60(08):327–335. doi:10.5414/CP204120
48. Askling J, Dixon W. The safety of anti-tumour necrosis factor therapy in rheumatoid arthritis. Curr Opin Rheumatol. 2008;2(20):138–144. doi:10.1097/BOR.0b013e3282f4b392
49. George MD, Baker JF, Winthrop K, et al. Risk for serious infection with low-dose glucocorticoids in patients with rheumatoid arthritis: a cohort study. Ann Intern Med. 2020;173(11):870–878. doi:10.7326/M20-1594
50. Xue CC, Zhang AL, Lin V, Myers R, Polus B, Story DF. Acupuncture, chiropractic and osteopathy use in Australia: a national population survey. BMC Public Health. 2008;8(1):105. doi:10.1186/1471-2458-8-105
51. Yang L, Adams J. The role of traditional Chinese medicine in arthritis management: why we need public health and health services research. Front Public Health. 2020;8:597917. doi:10.3389/fpubh.2020.597917
52. Yang L, Sibbritt D. Factors associated with Chinese herbal medicine use among middle-aged and older women with arthritis: evidence from China. Sci Rep. 2022;12(1):12566. doi:10.1038/s41598-022-16927-4
53. Wang YR, Liu L, Wang XY, et al. The efficacy of the traditional Chinese medicine Jia Wei Niu Bang Zi granule combined with methotrexate in treating active rheumatoid arthritis: a multicenter, randomized, double-blinded controlled clinical trial. Medicine. 2019;98(6):14424. doi:10.1097/MD.0000000000014424
54. Xing Q, Fu L, Yu Z, Zhou X. Efficacy and safety of integrated traditional Chinese medicine and western medicine on the treatment of rheumatoid arthritis: a meta-analysis. Evid Based Complement Alternat Med. 2020;2020:1–15. doi:10.1155/2020/4348709
55. Fang J, Liu M, Huang Z, et al. Efficacy and safety of TCMs with anti-inflammatory effect in patients with rheumatoid arthritis: a network meta-analysis. Front Immunol. 2023;14:1114930. doi:10.3389/fimmu.2023.1114930
56. Fan HX, Liu J, Huang CB, et al. Clinical effects of huangqin qingre chubi capsules combined with western medicine in the treatment of acute gouty arthritis. WJTCM. 2021;16(17):2600–2604+2610.
57. Huang D, Liu J, Wan L, Song Q, Guo JC, Zhou Q. Effects of huangqin qingre chubi capsule on efficacy and oxidative stress of ankylosing spondylitis. Chin J Immunol. 2019;35(12):1448–1452.
58. Sun Y, Liu J, Xin L, et al. Traditional Chinese medicine is associated with reduced risk of readmission in rheumatoid arthritis patients with anemia: a retrospective cohort study. Evid Based Complement Alternat Med. 2022;2022:1–10. doi:10.1155/2022/4553985
59. Wang J, Liu J, Wen JT, et al. Effect of xinfeng capsule combined with huangqin qingre chubi capsule on rheumatic arthritis association rules analysis of self-perception of patient. Chin J Clin Health. 2021;24(5):639–645.
60. Eriksson JK, Neovius M, Ernestam S, Lindblad S, Simard JF, Askling J. Incidence of rheumatoid arthritis in Sweden: a nationwide population-based assessment of incidence, its determinants, and treatment penetration: assessment of RA incidence in Sweden. Arthritis Care Res. 2013;65(6):870–878. doi:10.1002/acr.21900
61. Intriago M, Maldonado G, Cárdenas J, Ríos C. Clinical characteristics in patients with rheumatoid arthritis: differences between genders. Sci World J. 2019;2019:1–6. doi:10.1155/2019/8103812c
62. Guo JC, Liu J, Xin L, Huang D, Zhou Q, Song Q. Effects of furong ointment external application combined with Chinese medicine oral administration on immune inflammation in rheumatoid arthritis patients explored by HIS data analysis. J IMMUNOL. 2018;34(3):230–238. doi:10.13431/j.cnki.immunol.j.20180035
63. Guo JC, Liu J, Xin L, Huang D, Zhou Q, Song Q. Evaluation of effect of hibiscus paste external application combined with traditional Chinese medicine on inflammatory indices of patients with active rheumatoid arthritis based on random walk model. Chin J Immunol. 2018;34(6):854–860.
64. Deniz O, Cavusoglu C, Satis H, et al. Sleep quality and its associations with disease activity and quality of life in older patients with rheumatoid arthritis. Eur Geriatr Med. 2023;14(2):317–324. doi:10.1007/s41999-022-00739-w
65. Hsieh SC, Tsai PH, Kuo CF, et al. Health-related quality of life improvement by Adalimumab therapy in patients with rheumatoid arthritis in Taiwan: a nationwide prospective study. J Chin Med Assoc. 2023;86(4):366–374. doi:10.1097/JCMA.0000000000000889
66. Matcham F, Scott IC, Rayner L, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):123–130. doi:10.1016/j.semarthrit.2014.05.001
67. Qian J, Li M, Zhang X, et al. Long-term prognosis of patients with systemic lupus erythematosus-associated pulmonary arterial hypertension: CSTAR-PAH cohort study. Eur Respir J. 2019;53(2):1800081. doi:10.1183/13993003.00081-2018
68. Zhang C, Wu X, Yuan Y, et al. Effect of solution-focused approach on anxiety and depression in patients with rheumatoid arthritis: a quasi-experimental study. Front Psychol. 2022;13:939586. doi:10.3389/fpsyg.2022.939586
69. Yoshii I, Sawada N, Chijiwa T. Associations between clinical metrics of joint deformity, disease duration, disease activity, functional capacity, quality of life, pain, and fatigue in patients with rheumatoid arthritis. Clin Rheumatol. 2023;42(4):1027–1038. doi:10.1007/s10067-022-06432-4
70. Oude Voshaar MAH, Das Gupta Z, Bijlsma JWJ, et al. International consortium for health outcome measurement set of outcomes that matter to people living with inflammatory arthritis: consensus from an international working group. Arthritis Care Res. 2019;71(12):1556–1565. doi:10.1002/acr.23799
71. Sun Y, Liu J, Fang L, Zhu FB, Tan B, Zhang PH. Study on anxiety and depression of 604 patients with rheumatoid arthritis. J Rheum Arthritis. 2016;5(9):9–15.
72. Zhang Y, Liu J, Huang D, et al. Feeling changes of 135 patients with rheumatoid arthritis and related analyses. J Rheum Arthritis. 2019;8(11):15–19.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.