Back to Journals » OncoTargets and Therapy » Volume 12
HOXC13-AS-miR-122-5p-SATB1-C-Myc feedback loop promotes migration, invasion and EMT process in glioma
Authors Liu N, Wang Z, Liu D, Xie P
Received 19 June 2019
Accepted for publication 16 August 2019
Published 2 September 2019 Volume 2019:12 Pages 7165—7173
DOI https://doi.org/10.2147/OTT.S220027
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr William C. Cho
Ning Liu,1,* Ziyu Wang,2,* Dachao Liu,3,* Peng Xie4
1Department of Neurosurgery, Huzhou Central Hospital, Huzhou, Zhejiang 313000, People’s Republic of China; 2Department of Emergency Intensive Care Unit, The Affiliated Huai’an Hospital of Xuzhou Medical University, The Second People’s Hospital of Huai’an, Huai’an 223002, People’s Republic of China; 3Department of Imaging, The Affiliated Huai’an Hospital of Xuzhou Medical University, The Second People’s Hospital of Huai’an, Huai’an 223002, People’s Republic of China; 4Department of Neurosurgery, The Affiliated Huai’an Hospital of Xuzhou Medical University, The Second People’s Hospital of Huai’an, Huai’an 223002, People’s Republic of China
Correspondence: Peng Xie
Department of Neurosurgery, The Affiliated Huai’an Hospital of Xuzhou Medical University, The Second People’s Hospital of Huai’an, No. 62, Huaihai Road (S.), Huai’an 223002, People’s Republic of China
Email [email protected]
*These authors contributed equally to this work
Purpose: Differentially expressed long non-coding ribonucleic acids (lncRNAs) have been reported as a key factor of glioma carcinogenesis, but the underlying mechanism involved is still unknown.
Materials and methods: In the present study, lncRNA HOXC13 antisense RNA (HOXC13-AS) was identified as a potential oncogene in glioma, and Western blotting, wound healing and Transwell assays were carried out to explore the effects of HOXC13-AS on the epithelial-mesenchymal transition (EMT) process as well as the migration and invasion of glioma cells.
Results: A further mechanistic study showed that HOXC13-AS sponged miR-122-5p to indirectly regulate SATB1 expression and affect the EMT process via the Wnt/β-catenin pathway. Meanwhile, the promoter activity was significantly increased via c-Myc, a key factor of the Wnt/β-catenin pathway, thus forming a positive HOXC13-AS-miR-122-5p-SATB1-c-Myc feedback loop to drive the malignant behavior in glioma.
Discussion: This study evidences the constitutive HOXC13-AS-miR-122-5p-SATB1-c-Myc feedback loop and provides a potential therapeutic target for glioma treatment.
Keywords: HOXC13-AS, epithelial-mesenchymal transition, competing endogenous RNA, miR-122-5p, glioma
Introduction
Glioma has been considered as the most common primary tumor in the brain of adults with the highest malignancy, in which glioblastoma is the most aggressive and lethal type.1,2 Despite that the multimodal therapeutic strategy has been improved over the past decades, the prognosis of glioma patients remains unoptimistic.3,4 Thus, it is urgent to explore the mechanism of strong invasiveness of glioma and develop new treatment strategies.
Long non-coding ribonucleic acids (lncRNAs) refer to a series of non-protein transcripts with more than 200 bp in length.5 In the past decade, multiple studies have focused on the biological processes of cancer including proliferation,6 migration,7 invasion,8 angiogenesis,9 and chemoresistance.10 Proposed by Salmena and colleagues in 2011, competing endogenous RNA (ceRNA) is a crucial mechanism of lncRNAs,11 based on which lncRNAs indirectly regulate target genes via forming RNA-induced silencing complex (RISC) with RNA-binding proteins,7,12 and the lncRNA-miRNA-mRNA network has been validated in several human cancers including glioma.8,13 Although multiple lncRNAs have been annotated, there remains a need to investigate the biological function and the potential mechanisms of lncRNAs in glioma. HOXC13 antisense RNA (HOXC13-AS), located on 12q13.13, is a gene accelerating tumor progression in breast cancer14 and nasopharyngeal carcinoma.15 However, the biological role and potential mechanism of HOXC13-AS in glioma remain unclear.
Epithelial-mesenchymal transition (EMT) is a common mechanism in tumors. by which cells lose their epithelial characteristics and form highly invasive and migratory phenotypes.16,17 Therefore, EMT plays a pivotal role in tumor progression.18 Tumor cells and matrix components collaboratively participate in the malignant progression and recurrence of glioma.19 Hence, studies on the EMT process are essential to suppress the malignant progression of glioma.
In the present research, a novel lncRNA, HOXC13-AS, was reported as an oncogene in glioma. HOXC13-AS down-regulation repressed the migration, invasion and EMT process of glioma cells. Mechanistically, HOXC13-AS regulated the SATB1 expression via sponging miR-122-5p, and the Wnt/β-catenin pathway was involved in the biological role of HOXC13-AS. Interestingly, c-Myc, the target gene of the Wnt/β-catenin pathway could bind to the promoter region of HOXC13-AS and regulate the expression of HOXC13-AS at the transcription level, thereby forming a positive feedback loop.
Materials and methods
Tissues and cell lines
Twenty glioma tissue samples and seven cerebral trauma samples (non-neoplastic brain tissues, NBTs) were collected from the Department of Neurosurgery, Huzhou Central Hospital. The study was approved by the Ethics Committee of Huzhou Central Hospital and all patients were required to sign the informed consent. All patients clinical information were listed in Table S3. Normal human astrocytes were purchased from ScienCell Research Laboratories (Carlsbad, USA) and maintained using astrocyte medium (Carlsbad, USA) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics (penicillin and streptomycin). Glioma cell lines (LN229, U251, U87 and U118) were bought from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and N3 primary glioma cell was obtained from Tianjin Medical University. All glioma cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) and incubated with 5% CO2 at 37 °C. The use of N3 glioma cell line was approved by Ethics Committee of Huzhou Central Hospital.
Stable cell transfection and establishment
For stable transfection, lentiviruses carrying HOXC13-AS knockdown sequence (shHOXC13-AS) or control sequence (shCtrl) were packaged into LN229 and N3 cells via lentiviral packaging kit (Genechem Shanghai, China) according to manufacturer’s instructions and selected with puromycin at 2 days after transfection. For transient transfection, miR-122-5p mimics (miR-122-5p), control mimics, miR-122-5p inhibitor (anti-miR-122-5p) and inhibitor control were purchased from RiboBio (Guangzhou, China). SATB1 small interfering RNA (siSATB1), control siRNA (siCtrl), c-Myc siRNA (si-c-Myc) and control small interfering RNA (siRNA) (siCtrl) were bought from Genechem (Shanghai, China). RiboFECT CP Transfection Kit (Ribobio, Guangzhou, China) was employed for transient transfection according to manufacturer’s instructions. All sequences were listed in Table S1.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
The total RNA of glioma was extracted using Total RNA Kit I (Omega Bio-tek, GA, USA) based on the manufacturer’s instructions, followed by qRT-PCR analysis using SYBR Premix ExTaq (Takara). U6 was used as a negative control. Primers for HOXC13-AS, miR-122-5p and β-actin were purchased from RiboBio (Guangzhou, China). ABI 7500 RT-PCR system was utilized for qRT-PCR. The data were determined using 2−△△Ct method. All primer sequences were listed in Table S2.
The separation of nuclear and cytoplasmic fractions
The separation of nuclear and cytoplasmic fractions were performed using Ambion® PARIS™ system (Thermo Fisher, Shanghai, China) according to manufacturer’s instructions. After RNA extraction, qRT-PCR analysis were performed using SYBR Premix ExTaq (Takara). Primers for HOXC13-AS, β-actin and U6 were purchased from RiboBio (Guangzhou, China).
Western blotting assay
Western blotting assay was conducted as described previously.19 Antibodies against SATB1, Vimentin, N-cadherin, c-Myc and β-actin were purchased from Cell Signaling Technology (Danvers, MA, USA).
Wound healing and Transwell assays
Wound healing and Transwell assays were performed as described above.19
In vitro three-dimensional (3D) invasion assay
Collagen I gels, purchased from Inamed (Fremont, CA, USA), were pre-treated with NaOH and mixed liquor containing DMEM and 10% FBS. LN229 and N3 glioma cells aggregated and were implanted into 3D collagen I gels.20 Finally, Nikon ECLIPSE E800 fluorescence microscope system was used to observe the results.
Dual-luciferase assay
Luciferase report plasmid carrying wild type (WT) or mutant (MUT) HOXC13-AS or SATB1 sequences were compound by Genechem (Shanghai, China). Control mimics or miR-122-5p mimics were co-transfected with related luciferase report plasmids into LN229 and N3 glioma cells. The luciferase assay was implemented using Dual-Luciferase Kit (Promega, Wisconsin, USA) and luciferase activities were analyzed by Dual Luciferase Reporter Assay System (Promega, Wisconsin, USA).
RNA immunoprecipitation (RIP) assay
LN229 and N3 glioma cells were treated with magnetic beads pre-conjugated with Argonaute2 (Ago2) antibody. Normal mouse IgG functioned as a negative control. RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) was utilized for RIP assay in accordance with the manufacturer’s protocol.
Chromatin immunoprecipitation (CHIP) assay
CHIP Kit (Millipore, Massachusetts, USA) was employed for CHIP assay in line with the manufacturer’s protocol. The chromatins of N3 and LN229 were conjugated with 3 μg c-Myc antibodies, followed by detection of immunoprecipitated DNA via RT-PCR.
Statistical analysis
All results were analyzed by Graphpad Prism 7 (La Jolla, CA, USA) and SPSS 22.0 software (IBM, Corp., Armonk, New York, USA). Data were evaluated by Student’s t-test or ANOVA and expressed as mean ± standard deviation (SD). p<0.05 represented that the difference was statistically significant.
Results
HOXC13-AS was highly expressed in glioma and affects the malignant phenotype of glioma
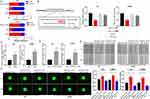
It was found from TCGA database that there was a high HOXC13-AS level in high-grade glioma (HGG) compared with low-grade glioma (LGG) (Figure 1A) and high HOXC13-AS level represented poor prognosis (Figure 1B), indicating that HOXC13-AS may function as an oncogene in glioma, which was confirmed by HOXC13-AS expression level detected via qRT-PCR analysis. To confirm the results of bioinformatics analysis, 20 glioma tissues and 7 NBTs obtained from 7 cerebral trauma patients were selected, and the HOXC13-AS level was analyzed by qRT-PCR. The results showed that glioma tissues had a higher HOXC13-AS level (Figure 1C), and the same results were also obtained in glioma cells (Figure 1D). The above data indicate that HOXC13-AS may act as a tumor stimulator in glioma.
To further investigate the biological function of HOXC13-AS, its expression was knocked down by lentiviruses in LN229 and N3 cells (with a high HOXC13-AS level, Figure 1D), and qRT-PCR analysis confirmed the knockdown efficiency (Figure S1A). On account of the reduction of epithelial cells, E-cadherin was poorly expressed in glioma,21 so the changes in N-cadherin and Vimentin expressions were tested to analyze the EMT process in glioma. As expected, the protein levels of N-cadherin and Vimentin were markedly down-regulated after the reduction of HOXC13-AS in N3 and LN229 cells (Figure 1E). As the EMT process is always accompanied by the changes in cell migration and invasion, wound healing and Transwell assays were conducted in both N3 and LN229 cells. It was discovered that knockdown of HOXC13-AS delayed wound healing and the decrease of invasive cell number, which indicated the reduction of mobility and invasiveness of glioma cells (Figure 1F and G). Briefly, the above findings denote that HOXC13-AS promotes migration, invasion and EMT process in glioma.
HOXC13-AS acts as a molecular sponge for miR-122-5p to regulate SATB1 expression
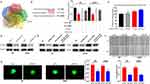
Based on the ceRNA hypothesis, the function of lncRNAs is related to their intracellular localization. This study revealed that HOXC13-AS was mainly located in the cytoplasm in glioma cells (Figure 2A), which suggested that HOXC13-AS may function as a ceRNA to regulate the malignant phenotype of glioma. Besides, bioinformatics analysis (DIANA tools, http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=site/index and miRcode, http://www.mircode.org/) demonstrated that miR-122-5p was present in both two databases (Figure S2A), indicating that miR-122-5p is a predict target for HOXC13-AS. Meanwhile, miR-122-5p was down-regulated in HGG compared with LGG in CGGA database (Figure S2B). To clarify whether miR-122-5p binds directly to HOXC13-AS, dual-luciferase report gene assay was performed, which showed that miR-122-5p-mediated suppression of luciferase activity was inhibited by HOXC13-AS mutation (Figure 2B). Moreover, RIP was conducted using antibody against Ago2, the key factor of RISC. As expected, HOXC13-AS and miR-122-5p were present in Ago2 precipitates (Figure 2C) and knockdown of HOXC13-AS up-regulated miR-122-5p (Figure 2D). All in all, the above findings imply that HOXC13-AS suppresses the miR-122-5p expression through RISC-dependent manner.
In order to verify whether miR-122-5p participates in HOXC13-AS-mediated migration and invasion of glioma cells, miR-122-5p was overexpressed or knocked down in glioma cells with reduced HOXC13-AS. After that, wound healing and 3D-spheroid invasion assays were employed to analyze the mobility and invasiveness of glioma cells. The results manifested that the knockdown of HOXC13-AS regulated the migration and invasion of glioma cells, which could be suppressed by miR-122-5p overexpression but facilitated by miR-122-5p inhibition (Figures 2E, F and S2C).
HOXC13-AS indirectly regulated the SATB1 expression
To further analyze the ceRNA network between HOXC13-AS, miR-122-5p and its targets gene in glioma, 5 bioinformatics websites (DIANA, miRanda, miRDB, miRwalk and Targetscan) were used to predict the potential target genes of miR-122-5p. According to the results, SATB1, a well-defined oncogene, is involved in all these databases (Figure 3A). In addition, SATB1 was identified to be a directly target of miR-122-5p via dual-luciferase report gene assay (Figure 3B). Subsequently, pGL3-basic-SATB1, miR-122-5p and HOXC13-AS overexpression plasmids were co-transfected into HEK293T cells. The enhanced luciferase activity has a relationship with HOXC13-AS in a dose-dependent manner, indicating that HOXC13-AS sequester miR-122-5p and stabilize SATB1-mediated luciferase activity. Moreover, it was discovered that knockdown of HOXC13-AS decreased the SATB1 expression, and the SATB1 protein level suppressed by shHOXC13-AS was rescued by miR-122-5p inhibitors (Figure 3D and E). Wound healing and 3D-spheroid invasion assays were conducted to detect the biological role of SATB1 in glioma cells. N3 and LN229 cells were transfected with SATB1 siRNA (Figure S3A), and the results showed that the migration and invasion abilities of glioma cells were significantly reduced after SATB1 depletion (Figure 3F and G). The above results evidence the presence of the HOXC13-AS/miR-122-5p/SATB1 axis in glioma.
HOXC13-AS and c-Myc formed a positive feedback loop
Several studies have reported that SATB1 can regulate tumor progression via the Wnt/β-catenin pathway.22 In order to investigate the involvement of Wnt/β-catenin pathway in the HOXC13-AS-induced tumorigenic effects, the protein levels of N-cadherin, Vimentin, β-catenin as well as c-Myc, a target gene of Wnt/β-catenin pathway were examined. It was found that HOXC13-AS knockdown suppressed N-cadherin, Vimentin, β-catenin and c-Myc expression levels, implying that HOXC13-AS may regulate EMT process via the Wnt/β-catenin pathway (Figure 4A). What’s interesting was that JASPAR database (http://jaspar.genereg.net/) denoted that c-Myc had the potential to bind to the promoter area of HOXC13-AS. To confirm this conjecture, c-Myc was knocked down by siRNA (Figure 4B), and the expression level of HOXC13-AS was analyzed. The results manifested that the depletion of c-Myc caused the decrease of HOXC13-AS (Figure 4C). Then we scanned the promoter region of HOXC13-AS and found that there were three potential binding sites of c-Myc (Figure 4D). To better elucidate the binding of c-Myc and HOXC13-AS promoter region, CHIP assay was conducted, and it was observed that only putative site 1 had PCR production, indicating that c-Myc can bind to HOXC13-AS promoter at −809 to −798 region (Figure 4E). To better investigate the expression of HOXC13-AS-miR-122-5p-SATB1-c-Myc feedback loop, we performed linear regression analyses by using 20 glioma tissues. The results showed that HOXC13-AS is positively correlated with SATB1 and c-Myc, while SATB1 is negatively correlated with miR-122-5p (Figure S3B–D). In summary, the above findings suggest that c-Myc can regulate HOXC13-AS at the transcription level and form a positive feedback loop.
Discussion
Increasing evidences have confirmed that ncRNAs, including circRNAs and lncRNAs, can interact with mRNAs if they contain same miRNA binding sites which reveal the ceRNA theory.11,23 Based on the results in this study, it was proposed that the present of HOXC13-AS-miR-122-5p-SATB1-c-Myc positive feedback loop which is essential for the regulation of malignant behavior of glioma provides a potential therapeutic target for glioma.
Only three percent of transcribed human gene can encode protein, which makes the studies on ncRNAs become important. Over the past decade, there have been more studies on lncRNAs in cancer.24 By reason of the biological function on malignant behavior of cancers, including proliferation,12 migration,25 invasion26 and chemoresistance,10 lncRNAs have been considered as an essential regulator in cancer. EMT plays the key role in tumor invasiveness enhancement and is involved in malignant progression of glioma.19,27 In the current study, a novel lncRNA which hasn’t been identified in glioma, HOXC13-AS (ENSG00000249641), was reported to be upregulated in both glioma cells and tissues. Knockdown of HOXC13-AS represented the suppression of migration, invasion and EMT process of glioma cells. Thus, our results shed light on the oncogenic function of HOXC13-AS to promote malignant behaviors of glioma cells.
CeRNA is a basic mechanism of lncRNAs which is widely present in tumors. LncRNAs can “talk” with target mRNAs via binding and titrating them off their binding sites on protein coding messengers.11,28 To further explore the molecular mechanism of the biological function of HOXC13-AS, its intracellular localization was assessed. Bioinformatics analysis, RIP, dual-luciferase report and Western blot assays demonstrated that miR-122-5p was a target of HOXC13-AS and SATB1, and further in vitro studies confirmed the effect of the HOXC13-AS/miR-122-5p/SATB1 axis on tumor progression. In brief, the above findings prove the existence of the HOXC13-AS/miR-122-5p/SATB1 axis, which can accelerate the migration and invasion of glioma.
SATB1 tethers multiple genomic loci and recruit multiple enzymes to regulate gene expression.29 As a genome organizer, SATB1 has been verified to promote tumor growth and metastasis.30,31 In glioma, SATB1 has been reported as a activator of tumor development and progression,32 and Wnt/β-catenin has been considered as an important pathway of SATB1-induced tumor progression.22,33 In the current study, SATB1 was discovered to accelerate the migration and invasion of glioma cells, and the Wnt/β-catenin pathway was involved in HOXC13-AS-induced EMT process. c-Myc is the target gene and the key factor of Wnt/β-catenin pathway,34 whose expression is positively correlated with HOXC13-AS level. Interestingly, c-Myc could bind to the promoter region of HOXC13-AS to regulate its expression at the transcription level. As previously reported, transcription factors exert their biological functions at the transcriptional level to regulate genes’ expression, such as ZEB1,35 STAT19 and NFAT5.36 Thus our results support the notion that lncRNAs and their target genes can form a feedback regulatory loop which may play a crucial role in glioma development and progression.
In conclusion, the data in this study highlight the importance of correlations of HOXC13-AS with miR-122-5p, miR-122-5p’s target SATB1 and Wnt/β-catenin pathway in regulation of glioma cell migration, invasion and EMT process. HOXC13-AS can sponge miR-122-5p to indirectly regulate SATB1 and Wnt/β-catenin pathway. The key factor of Wnt/β-catenin pathway, c-Myc, can then in turn regulate HOXC13-AS expression, thereby forming a positive feedback loop. Therefore, the results of this study confirmed the presence of HOXC13-AS-miR-122-5p-SATB1-c-Myc feedback loop in glioma which may be a potential therapeutic target for glioma treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pekmezci M, Rice T, Molinaro AM, et al. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: additional prognostic roles of ATRX and TERT. Acta Neuropathol. 2017;133:1001–1016. doi:10.1007/s00401-017-1690-1
2. Kotliarova S, Fine A. SnapShot: glioblastoma multiforme. Cancer Cell. 2012;21:710–710.e1. doi:10.1016/j.ccr.2012.04.031
3. Stupp R, Hegi M, Mason W, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi:10.1016/S1470-2045(09)70025-7
4. Reardon DA, Rich JN, Friedman HS, Bigner DD. Recent advances in the treatment of malignant astrocytoma. J Clin Oncol. 2006;24:1253–1265. doi:10.1200/JCO.2005.04.5302
5. Tuck AC, Tollervey D. A transcriptome-wide atlas of RNP composition reveals diverse classes of mRNAs and lncRNAs. Cell. 2013;154:996–1009.
6. Lv J, Qiu M, Xia W, et al. High expression of long non-coding RNA SBF2-AS1 promotes proliferation in non-small cell lung cancer. J Exp Clin Cancer Res. 2016;35:75. doi:10.1186/s13046-016-0444-6
7. Zhang E, Han L, Yin D, et al. H3K27 acetylation activated-long non-coding RNA CCAT1 affects cell proliferation and migration by regulating SPRY4 and HOXB13 expression in esophageal squamous cell carcinoma. Nucleic Acids Res. 2017;45:3086–3101. doi:10.1093/nar/gkw1247
8. Zhou K, Zhang C, Yao H, et al. Knockdown of long non-coding RNA NEAT1 inhibits glioma cell migration and invasion via modulation of SOX2 targeted by miR-132. Mol Cancer. 2018;17:105. doi:10.1186/s12943-018-0849-2
9. Yu H, Zheng J, Liu X, et al. Transcription factor NFAT5 promotes glioblastoma cell-driven angiogenesis via SBF2-AS1/miR-338-3p-mediated EGFL7 expression change. Front Mol Neurosci. 2017;10:301. doi:10.3389/fnmol.2017.00301
10. Fan Y, Shen B, Tan M, et al. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. FEBS J. 2014;281:1750–1758. doi:10.1111/febs.12737
11. Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi:10.1016/j.cell.2011.07.014
12. Sun M, Nie F, Wang Y, et al. LncRNA HOXA11-AS promotes proliferation and invasion of gastric cancer by scaffolding the chromatin modification factors PRC2, LSD1, and DNMT1. Cancer Res. 2016;76:6299–6310. doi:10.1158/0008-5472.CAN-16-0356
13. Zhu Y, Zhang X, Qi L, et al. HULC long noncoding RNA silencing suppresses angiogenesis by regulating ESM-1 via the PI3K/Akt/mTOR signaling pathway in human gliomas. Oncotarget. 2016;7:14429–14440. doi:10.18632/oncotarget.7418
14. Li X, Wang Q, Rui Y, et al. HOXC13-AS promotes breast cancer cell growth through regulating miR-497-5p/PTEN axis. J Cell Physiol. 2019. undefined.
15. Gao C, Lu W, Lou W, Wang L, Xu Q. Long noncoding RNA HOXC13-AS positively affects cell proliferation and invasion in nasopharyngeal carcinoma via modulating miR-383-3p/HMGA2 axis. J Cell Physiol. 2019;234:12809–12820. doi:10.1002/jcp.27915
16. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi:10.1172/JCI39104
17. Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi:10.1038/cr.2009.5
18. Ye X, Weinberg RA. Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol. 2015;25:675–686. doi:10.1016/j.tcb.2015.07.012
19. Karsy M, Gelbman M, Shah P, Balumbu O, Moy F, Arslan E. Established and emerging variants of glioblastoma multiforme: review of morphological and molecular features. Folia Neuropathol. 2012;50:301–321.
20. Zeng A, Yin J, Li Y, et al. miR-129-5p targets Wnt5a to block PKC/ERK/NF-κB and JNK pathways in glioblastoma. Cell Death Dis. 2018;9:394. doi:10.1038/s41419-018-0343-1
21. Lewis-Tuffin LJ, Rodriguez F, Giannini C, et al. Misregulated E-cadherin expression associated with an aggressive brain tumor phenotype. PLoS One. 2010;5:e13665. doi:10.1371/journal.pone.0013665
22. Mir R, Pradhan SJ, Patil P, Mulherkar R, Galande S. Wnt/β-catenin signaling regulated SATB1 promotes colorectal cancer tumorigenesis and progression. Oncogene. 2016;35:1679–1691. doi:10.1038/onc.2015.232
23. Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi:10.1038/nature12986
24. Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi:10.1016/j.tcb.2011.04.001
25. Liu L, Meng T, Yang X-H, et al. Prognostic and predictive value of long non-coding RNA GAS5 and mircoRNA-221 in colorectal cancer and their effects on colorectal cancer cell proliferation, migration and invasion. Cancer Biomarkers. 2018;22:283–299. doi:10.3233/CBM-171011
26. Liang H, Yu T, Han Y, et al. LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol Cancer. 2018;17:119. doi:10.1186/s12943-018-0870-5
27. Siebzehnrubl FA, Silver DJ, Tugertimur B, et al. The ZEB1 pathway links glioblastoma initiation, invasion and chemoresistance. EMBO Mol Med. 2013;5:1196–1212. doi:10.1002/emmm.201302827
28. Jalali S, Bhartiya D, Lalwani M, Sivasubbu S, Scaria V. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS One. 2013;8:e53823. doi:10.1371/journal.pone.0053823
29. Han H-J, Russo J, Kohwi Y, Kohwi-Shigematsu T. SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature. 2008;452:187–193. doi:10.1038/nature06781
30. Kohwi-Shigematsu T, Poterlowicz K, Ordinario E, Han H-J, Botchkarev VA, Kohwi Y. Genome organizing function of SATB1 in tumor progression. Semin Cancer Biol. 2013;23:72–79. doi:10.1016/j.semcancer.2012.06.009
31. Tu W, Luo M, Wang Z, et al. Upregulation of SATB1 promotes tumor growth and metastasis in liver cancer. Liver Int. 2012;32:1064–1078. doi:10.1111/j.1478-3231.2012.02815.x
32. Chu SH, Ma YB, Feng DF, Zhang H, Zhu ZA, Li ZQ. Upregulation of SATB1 is associated with the development and progression of glioma. J Transl Med. 2012;10(undefined):149. doi:10.1186/1479-5876-10-233
33. Meng W-J, Yan H, Li Y, Zhou Z-G. SATB1 and colorectal cancer in Wnt/β-catenin signaling: is there a functional link? Med Hypotheses. 2011;76:277–279. doi:10.1016/j.mehy.2010.10.022
34. Ji Q, Liu X, Fu X, et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. PLoS One. 2013;8(11):e78700. doi:10.1371/journal.pone.0078700
35. Krebs AM, Mitschke J, Lasierra Losada M, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19(5):518–529. doi:10.1038/ncb3513
36. Kang M, Ren M, Li Y, Fu Y, Deng M, Li C. Exosome-mediated transfer of lncRNA PART1 induces gefitinib resistance in esophageal squamous cell carcinoma via functioning as a competing endogenous RNA. J Exp Clin Cancer Res. 2018;37(1):171. doi:10.1186/s13046-018-0845-9
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.