Back to Journals » OncoTargets and Therapy » Volume 16
HOXC Cluster Antisense RNA 3, a Novel Long Non-Coding RNA as an Oncological Biomarker and Therapeutic Target in Human Malignancies
Received 15 June 2023
Accepted for publication 10 October 2023
Published 24 October 2023 Volume 2023:16 Pages 849—865
DOI https://doi.org/10.2147/OTT.S425523
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Nagashree Seetharamu
Yunhe Xie,1,2 Jiarong Ye,3 Hongliang Luo1
1Department of Gastrointestinal Surgery, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, 330008, People’s Republic of China; 2Department of General Surgery, Jiujiang Hospital of Traditional Chinese Medicine, Jiujiang, Jiangxi, 332007, People’s Republic of China; 3Queen Mary School, Nanchang University, Nanchang, Jiangxi, 330038, People’s Republic of China
Correspondence: Hongliang Luo, Department of Gastrointestinal Surgery, the Second Affiliated Hospital of Nanchang University, No. 1 Minde Road, Nanchang, Jiangxi Province, 330008, People’s Republic of China, Tel +86 13097280001, Email [email protected]
Abstract: HOXC cluster antisense RNA 3 (HOXC-AS3) is a novel long noncoding RNA (lncRNA) that exhibits aberrant expression patterns in various cancer types. Its expression is closely related to clinicopathological features, demonstrating significant clinical relevance across multiple tumors. And HOXC-AS3 plays multifaceted roles in tumor progression, impacting cell proliferation, apoptosis, migration, invasion, epithelial-mesenchymal transition (EMT), autophagy, senescence, tumor growth, and metastasis. In this review, we summarized and comprehensively analyzed the expression and clinical significance of HOXC-AS3 as a diagnostic and prognostic biomarker for malignancies. Additionally, we presented an in-depth update on HOXC-AS3’s functions and regulatory mechanisms in cancer pathogenesis. This narrative review underscores the importance of HOXC-AS3 as a promising lncRNA candidate in cancer research and its potential as a predictive biomarker and therapeutic target in clinical applications.
Keywords: long non-coding RNA, HOXC-AS3, malignant neoplasms, neoplastic processes, biological marker
Introduction
In recent years, the discovery and characterization of long non-coding RNAs (lncRNAs) have greatly advanced our understanding of the non-coding transcriptome and its relevance to human diseases, particularly cancer.1–4 Dysregulation of lncRNAs has been implicated in disease onset and progression, and their functional investigations have unveiled their essential role in various molecular processes, including cell growth, cell cycle, autophagy,5–7 and metabolism.8–10 Consequently, lncRNAs have emerged as novel disease targets for clinical applications, driven by an increased in-depth understanding of their functions and molecular mechanisms.
Among these lncRNAs, HOXC cluster antisense RNA 3 (HOXC-AS3) has garnered significant attention due to its dysregulated expression patterns observed in multiple malignancies.11–17 A number of studies have revealed the implication of HOXC-AS3 expression in a range of human cancers, and elevated HOXC-AS3 expression has been correlated with clinicopathologic characteristics such as tumor grade, size, metastasis, and TNM stage and prognosis.17–22 Research has further unveiled the regulatory effects of HOXC-AS3 on the initiation and progression of diverse human tumors, involving modulation of cellular processes such as cell proliferation, apoptosis, invasion, metastasis, and metabolic reprogramming.11–15 HOXC-AS3 exhibits promising potential for diagnostic, prognostic, and therapeutic applications.11–15,17,21,22
To gather relevant literature for the comprehensive synthesis of HOXC-AS3’s role in cancer biology and its potential clinical relevance. We systematically searched databases, including PubMed, Web of Science, and Google Scholar, using keywords “HOXC-AS3” and “HOXC cluster antisense RNA 3”. Articles published in English up to August 1, 2023, were considered, and sources were included based on predefined criteria, focusing on studies investigating the expression, clinicopathological correlations, biological functions, and clinical implications of HOXC-AS3 in various human tumor types.
This review aims to provide a thorough overview of HOXC-AS3’s expression profiles, clinicopathologic features, biological roles, molecular mechanisms, and clinical implications across different tumor types. By consolidating existing knowledge, it aims to contribute to our integrated understanding of HOXC-AS3’s significance as a compelling lncRNA candidate in cancer research, paving the way for its potential clinical applications in cancer management.
Characteristics of HOXC-AS3
Homo sapiens HOXC-AS3 is classified as a non-coding RNA (ncRNA) gene type. It is located on the reverse strand of Chromosome 12 at coordinates 53,983,951 to 53,985,519, spans three exons, and has a length of 1569 nucleotides (nt) [source: https://www.ncbi.nlm.nih.gov/gene/100874365]. HOXC-AS3 is a natural antisense transcript of HOXC10.22 LncRNAs generated within HOX genes have been documented to play pivotal roles in tumorigenesis. For example, HOXA11-AS,23–25 an antisense transcript of HOXA11, has been identified as an oncogenic lncRNA in various cancer types. Another well-known oncogenic lncRNA, HOTAIR,26–29 originates from the antisense strand of HOXC11. Notably, HOXC-AS3 is situated within the HOX gene cluster, sharing the genomic location 12q13.13 with HOTAIR. This association suggests a potential role for HOXC-AS3 in tumor formation and progression.
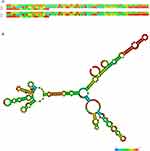
HOXC-AS3 has been detected in both the cytoplasm and nucleus in most reported tumor cells,11,13–15,22 with more prominent expression in the cytoplasm of glioma cells.16 Experimental and bioinformatic analyses have confirmed HOXC-AS3 as a classic lncRNA with no coding potential,22 emphasizing its role solely as a lncRNA. Consequently, the observed functions are attributed exclusively to the lncRNA itself. Furthermore, the Minimum Free Energy (MFE) secondary structure, a widely recognized model commonly used for understanding RNA stability and potential functional roles,30,31 was predicted for HOXC-AS3 using the RNAfold web server (http://rna.tbi.univie.ac.at//cgi-bin/RNAWebSuite/RNAfold.cgi?PAGE=3&ID=zB2AoN9m89), as illustrated in Figure 1.
Expression of HOXC-AS3 and Its Clinical Value as a Novel Biomarker for Human Cancer
Numerous studies have demonstrated the dysregulation of HOXC-AS3 in different human tumors, along with its association with clinicopathological characteristics and patients’ clinical outcomes (Table 1). This section provides an overview and investigates the alterations in HOXC-AS3 expression, its correlation with clinicopathologic features, and its clinical potential as a promising biomarker in various tumors.
 |
Table 1 Expression of lncRNA HOXC-AS3 in Tissue Samples and Its Relationship with Clinical Characteristics and Survival in Human Tumors |
Transcriptomic Patterns of HOXC-AS3 in Bulk Tissues
HOXC-AS3 has emerged as a promising candidate involved in many biological processes, such as development, differentiation, and tumorigenesis. In order to gain a deeper understanding of its potential roles in physiological contexts, an integrated assessment of its expression across diverse normal human tissues was performed with the extensive transcriptomic data available in the Genotype-Tissue Expression (GTEx) project (https://www.gtexportal.org/) (Figure 2A and B). The expression of HOXC-AS3 varies greatly in different tissues, with relatively high expression in the kidney, skin, and testis, and relatively low expression in most human tissues (Figure 2A and B). The higher expression in kidney and skin tissues may indicate an important role of HOXC-AS3 in the development and homeostasis of these organs. Likewise, the relatively higher expression in the testis may imply its contribution to spermatogenesis or reproductive processes. In short, the observed tissue-specific expression patterns of HOXC-AS3 may provide clues to its potential biological regulation of tissue development and function relevant to cancer research.
 |
Figure 2 Expression of HOXC-AS3 across diverse normal human tissues was shown in violin plots (A) and cluster heatmaps (B) from GTEx. Abbreviation: TPM, Transcripts Per Million. |
Expression Profile of HOXC-AS3 in Malignancies
Abnormal HOXC-AS3 expression has been reported in various human malignant tissues, including gastric cancer, nasopharyngeal carcinoma, glioma, breast cancer, ovarian cancer, cervical cancer, hepatocellular carcinoma, colorectal cancer, lung cancer and mesenchymal stromal cells (MSCs) derived from multiple myeloma (MM) patients (Table 1). To comprehensively explore the expression profile of HOXC-AS3 in human pan-cancer, we assessed HOXC-AS3 expression levels in 33 cancer types using UCSC XENA (https://xenabrowser.net/datapages/) (Figure 3A–C).
The results showed that HOXC-AS3 was significantly up-regulated in several malignancies of the digestive and respiratory systems (Figure 3A), including esophageal carcinoma (ESCA), liver hepatocellular carcinoma (LIHC), pancreatic adenocarcinoma (PAAD), lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), and genitourinary and gynecologic cancers (Figure 3B), such as adrenocortical carcinoma (ACC), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), and uterine corpus endometrial carcinoma (UCEC). Conversely, HOXC-AS3 was notably down-regulated in kidney chromophobe (KICH), kidney renal clear cell carcinoma (KIRC), and testicular germ cell tumors (TGCT) (Figure 3B).
These differential expression patterns across various cancer types suggest that HOXC-AS3 expression levels may have significant clinical relevance in predicting disease onset and progression.
Clinical Significance of HOXC-AS3 in Tumors
HOXC-AS3 as a Prognostic Marker
HOXC-AS3 expression levels have been determined to correlate with clinical features of various cancers, see Table 1 for details. High expression of HOXC-AS3 in tumor samples showed a significant positive correlation with clinicopathological features, including tumor grade, metastasis, and TNM stage.11,13,19,20 Furthermore, the relationship between HOXC-AS3 expression and patient prognosis was investigated in several studies (Table 1). HOXC-AS3 displayed different prognostic significance in different cancer types. In most of the reported tumors, including gastric cancer,17 glioma,16 breast cancer,11,19,20 cervical cancer,36 hepatocellular carcinoma,15 HOXC-AS3 overexpression tends to predict poor prognosis, whereas in colorectal cancer (CRC),12 high expression of HOXC-AS3 indicates good prognosis.
Moreover, apart from the aforementioned cancer types, we also evaluated the correlation between HOXC-AS3 and the prognosis of various other forms of cancer, based on the TCGA dataset available at (https://portal.gdc.cancer.gov/).
Our investigation encompassed overall survival (OS), disease-specific survival (DSS), and disease-free interval (DFI) for these additional cancer types (Figure 4A). Kaplan–Meier (KM) plots analysis results further showed that HOXC-AS3 expression level was significantly correlated with the prognosis of patients with ACC, Glioblastoma multiforme (GBM), and Skin cutaneous melanoma (SKCM) (Figure 4B). High expression of HOXC-AS3 indicated worse OS, DSS, and DFI in ACC, shorter OS, and DSS in GBM, while better OS, DSS, and DFI in SKCM (Figure 4B). These findings indicate that HOXC-AS3 holds distinct prognostic implications across various cancer types, making it a potentially valuable prognostic indicator for a range of malignancies.
HOXC-AS3 as a Cancer Diagnostic Biomarker
Moreover, receiver operating characteristic (ROC) curve analysis revealed that HOXC-AS3 could be used as a potent diagnostic biomarker in multiple cancer types (Figure 5A), particularly in Pheochromocytoma and paraganglioma (PCPG), ESCA, SKCM, and TGCT, where the area under the curve (AUC) exceeded 0.9 (Figure 5B). These results indicate that HOXC-AS3 has the potential to act as a valuable diagnostic marker in a wide range of tumors.
The Role of HOXC‑AS3 and Its Regulatory Mechanisms in Various Tumors
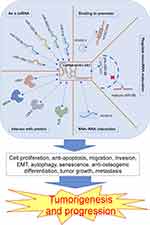
HOXC-AS3 is involved in regulating biological functions and cancer progression through a series of mechanisms, including cell proliferation, apoptosis, migration, invasion, epithelial-mesenchymal transition (EMT), autophagy, senescence, tumor growth, metastasis, and osteogenic differentiation (Table 2 and Figure 6). It has demonstrated significant regulatory roles in multiple cancers, such as breast cancer and non-small cell lung cancer. HOXC‑AS3 holds promise as a novel therapeutic target for human cancers.
 |
Table 2 The Role and Regulatory Mechanism of lncRNA HOXC-AS3 in Multiple Human Malignancies |
HOXC‑AS3 is distributed in both cytoplasm and nucleus, exerting crucial modulatory roles at transcriptional and post-transcriptional levels. LncRNAs can act as negative regulators of miRNA biogenesis, revealing a novel mechanism of lncRNA-miRNA crosstalk.38–41 HOXC‑AS3 has been reported to suppress the maturation of miR-96 by inhibiting the transportation of premature miR-96 from the nucleus to the cytoplasm in ovarian and lung cancer cells.13,35
One crucial lncRNA mechanism is acting as a competing endogenous RNA (ceRNA),42–44 sequestering miRNAs from their target mRNAs and forming a lncRNA-miRNA-mRNA network. Several studies have shown HOXC‑AS3 could functions as a ceRNA by interacting with miRNAs, including miR-216 in glioma,16 miR-3922-5p20 and miR-1224-5p in breast cancer,11 miR-105-5p in cervical cancer,36 miR-1269 in colorectal cancer.12 Additionally, several assays, including luciferase reporter, RNA immunoprecipitation (RIP), and RNA pull-down, have identified miRNA binding sites on HOXC-AS3. Functional assays demonstrated that miRNAs and their target mRNAs regulate HOXC-AS3’s effects.
Moreover, a growing body of evidence reveals the regulatory mechanisms of lncRNAs include regulating transcriptional or splicing regulation and interaction with RNA-binding proteins.45–47 Here, HOXC-AS3 can interact with proteins, including YBX1,14,19 SIRT6,11 CDK2,15 and FUS,18 further influencing tumorigenesis. HOXB13 can upregulate HOXC-AS3 expression by binding to its promoter region, exerting malignant potential in glioblastoma.21 RNA-RNA interactions can alter RNA’s secondary or tertiary structure, shielding it from RNase degradation and enhancing its stability.48,49 LncRNA HOXC-AS3 interacts with HOXC10 to form an RNA-RNA structure, reducing HOXC10 RNA decay, and subsequently up-regulating the expression of HOXC10.22 For different malignant tumors, the detailed molecular mechanisms underlying HOXC-AS3’s regulatory effects are discussed in the following sections.
Gastrointestinal Tract System Tumors
Gastric Cancer
HOXC-AS3 exhibits high expression in gastric cancer tissues, and its overexpression is associated with a dismal prognosis.17 In addition, HOXC-AS3 expression serves as an independent predictor of OS and TNM stage in gastric cancer patients.17 Elevated HOXC-AS3 expression levels are also observed in gastric cancer cell lines, including BGC823, SGC-7901, MGC-803, and SNU-601.17 Functional studies involving ASO-mediated knockdown and plasmid-mediated overexpression reveal that HOXC-AS3 regulates gastric cancer cell proliferation and migration in vitro, while also influencing tumor growth and lung metastasis in mouse xenograft models.17 Mechanistically, lncRNA HOXC-AS3 participates in the tumorigenesis of gastric cancer by binding to YBX1, thereby modulating the transcriptional regulation of several target genes, including MMP7, WNT10B, and HDAC5.17
Colorectal Cancer
Enteghami et al37 first detected HOXC‑AS3 expression in CRC and found that it was higher in CRC tumor tissue compared to adjacent normal tissues, although not significantly altered. Interestingly, HOXC‑AS3 was significantly positively correlated with the well-known oncogenic lncRNA HOTAIR, but negatively correlated with HOXC10.37 In a subsequent study by Zhang et al,12 HOXC-AS3 was reported to be significantly downregulated in CRC tissues and CRC cell lines. The observed differences in HOXC-AS3 expression in CRC tissues may be attributed to the ethnicity of the individuals in these two studies. Zhang et al12 also found that low HOXC-AS3 levels were indicative of poor survival in CRC patients. Notably, HOXC-AS3 overexpression effectively impedes TGF-β2-induced migration and invasion in CR4 and RKO colorectal cancer cells by acting as a sponge for miR-1269.12 This is of significance as a positive feedback loop between TGF-β signaling and miR-1269 has been reported to promote metastasis in colorectal cancer.50
Hepatocellular Carcinoma
In hepatocellular carcinoma, HOXC-AS3 is highly expressed in cancer tissues and is observed in five cancer cell lines (97H, HLF, HepG2, Hep3B and PLC/PRF/5), and it has been associated with poor overall survival.15 In terms of biological functions, HOXC-AS3 has been demonstrated, through in vitro and in vitro experiments, to promote the proliferation in cells and facilitate tumor proliferation and growth in tumor xenograft models.15 The interaction of HOXC-AS3 with CDK2 reduces CDK2’s binding to p21, thereby enhancing CDK2 activity. This results in increased RB phosphorylation, promoting cell cycle progression from G1 to S-phase, ultimately enhancing cell proliferation, and advancing the progression of hepatocellular carcinoma.15
Gynecologic System Tumors
Ovarian Cancer
HOXC-AS3 has been found to be upregulated in ovarian cancer compared to paired non-neoplastic tissues.35 To assess its effects, a CCK-8 assay and apoptosis assay were conducted, revealing that HOXC-AS3 overexpression led to increased cell proliferation while reducing cell apoptosis in Caov3 and OVCAR3 cells.35 Additionally, changes in the expression of apoptosis, senescence, and autophagy-related proteins upon the addition of HOXC-AS3 indicated its involvement in these cellular behaviors of ovarian cancer cells.35 A study by Vera et al51 showed that HOXC-AS3 was significantly upregulated in cisplatin-resistant ovarian cancer cells, suggesting its potential biological significance in cisplatin resistance in ovarian cancer. Furthermore, HOXC-AS3 may be implicated in the ivermectin-mediated lncRNA-EIF4A3-mRNA pathways in ovarian cancer.52
Cervical Cancer
HOXC-AS3 is up-regulated in cervical cancer tissues and cancer cell lines (CaSki, SiHa, HeLa, ME180, and C33A).36 Overexpression of HOXC-AS3 has been associated with higher FIGO stage, larger tumor size, and poorer prognosis in cervical cancer.36 HOXC-AS3 exerts carcinogenic effects by enhancing proliferation, anti-apoptosis, migration, invasion, tumor growth, and metastasis.36 LncRNA HOXC-AS3 exacerbates cervical cancer progression and metastasis by up-regulating SOS1 expression level through miR-105-5p sequestration, further activating the ErbB signaling pathway.36
Other Tumors
Glioma
Glioma is the most prevalent primary brain tumor, with glioblastomas as grade IV astrocytomas exhibiting the highest aggressiveness and mortality in adults.53–56 HOXC-AS3 has shown a significant increase in glioma tissues compared to normal brain tissues and is highly expressed in glioma cell lines (U87, U25, T98G, LN229, and A172) compared to normal human astrocytes (NHAs).16 Additionally, the expression level of HOXC-AS3 was found to be inversely correlated with the prognosis of glioma patients.16 Furthermore, HOXC-AS3 could facilitate glioma cell proliferation, migration, invasion, and tumor growth by upregulating F11R expression through sponging miR-21616 or participate in HOXB13-induced glioblastomas cell proliferation, migration, and invasion.21
Breast Cancer
It was found that the expression of HOXC-AS3 in breast cancer tissue was significantly higher than in normal breast tissue.11,19,20 Similarly, the expression of HOXC-AS3 in breast cancer cells (SK-BR-3, MDA-MB-231, HCC-1954, T47D, MDA-MB-468) was also markedly elevated than that in normal mammary epithelial cell line (MCF-10A).20 In addition, HOXC-AS3 has demonstrated potential as a promising prognostic marker in breast cancer.11,19,20 Both in vivo and in vitro studies11,19,20 have provided evidence that HOXC-AS3 contributes the breast cancer progression by regulating cell proliferation, migration, apoptosis, epithelial-mesenchymal transition, tumor growth, metastasis, and metabolic reprogramming. Mechanistically (Figure 7), HOXC-AS3 regulates breast cancer progression by directly transcriptionally activating TK1 by binding to YBX119 or promoting breast cancer metastasis by upregulating PPP1R1A expression as a miR-3922-5p sponge.20 Furthermore, it has been reported that overexpression of HOXC-AS3 induced by low glucose levels promotes metabolic reprogramming in breast cancer. The HOXC-AS3/SP1/miR-1224–5p axis forms a positive feedback loop that enhances its cancer-promoting roles11 (Figure 7).
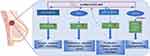 |
Figure 7 Regulatory mechanisms of lncRNA HOXC‑AS3 in breast cancer. |
Non-Small Cell Lung Cancer
Non-small cell lung cancer (NSCLC), accounting for about 80–90% of all lung cancer cases, mainly includes squamous cell carcinoma and adenocarcinoma.57–59 Within adenocarcinoma, invasive mucinous adenocarcinoma (IMA) is classified as a unique histologic subtype of lung adenocarcinoma.60–62
The role of HOXC-AS3 in lung cancers has been reported. In a study by Yang et al,18 HOXC-AS3 was found to be approximately two- to-eight-fold up-regulated in IMA cell lines (A549, SPC-A1, H441, H1299) compared with the non-cancer cell line HBE. Knock-down of HOXC-AS3 inhibited the proliferation and migration of IMA cells and promoted apoptosis. Mechanistically (Figure 8), HOXC-AS3 enhances the stability of FOXM1 mRNA and promotes the expression of FOXM1 by recruiting FUS (Figure 8), thereby accelerating cell proliferation, migration and inhibiting cell apoptosis in IMA. FUS is a well-known RNA binding protein (RBP)63–65 that acts as an mRNA stabilizer recruited by lncRNAs.66,67 In NSCLC, HOXC-AS3 was significantly elevated in NSCLC tissues13,14 and also remarkably upregulated in NSCLC cells (A549, H522, H460, and H1299).14 The subcellular location of HOXC-AS3 was detected in the cytoplasm and nuclear.13,14 HOXC-AS3 over-expression in NSCLC tissues was closely correlated with unfavorable clinical features.13 In vitro functional assays showed that HOXC-AS3 could promote invasion and migration,13,14 and enhance cell proliferation.14 A nude mouse xenograft model demonstrated that knockdown of HOXC-AS3 inhibited NSCLC tumor growth and metastasis in vivo.14 The oncogenic roles of HOXC-AS3 were achieved by sponging premature miR-9613 or by stabilizing YBX1 and thereby increasing HOXC8 transcription14 (Figure 8).
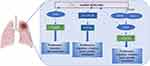 |
Figure 8 Regulatory mechanisms of lncRNA HOXC-AS3 in non-small cell lung cancer. |
Multiple Myeloma
Multiple myeloma (MM) is a hematologic malignancy known to causes severe imbalances in bone remodeling, resulting in serious skeletal lesions.68–70 The level of HOXC-AS3 was found to be upregulated in mesenchymal stromal cells (MSCs) derived from MM relative to those from healthy volunteer donors.22 RNA FISH further showed the presence of HOXC-A3 in the cytoplasm and nucleus of MSCs.22 Moreover, HOXC-AS3 transcripts were found to positively regulate the expression of HOXC10. Specifically, lncRNA HOXC-AS3 interacts with HOXC10, forming an RNA-RNA structure that protects HOXC10 RNA from degradation by RNase enzymes. This interaction leads to the upregulation of HOXC10 expression, ultimately repressing the osteogenic differentiation of MSCs.22 This regulatory mechanism has been substantiated through in vivo and in vitro experimental data.22
Conclusions and Perspectives
HOXC-AS3 is an emerging lncRNA that exhibits dysregulated expression in various human malignancies.11,14–16,19,20,35 Extensive research has unveiled its valuable clinical significance,11,19,20 owing to its tissue-specific expression patterns and significant clinical relevance in tumors.
HOXC-AS3 expression levels were associated with tumor clinical features. For example, in gastric cancer,17 HOXC-AS3 levels are significantly linked to histological grade, tumor invasion depth, lymph node metastasis, and TNM stage, high HOXC-AS3 levels indicated a more aggressive and advanced disease state. Similarly, in breast cancer,11,19 HOXC-AS3 expression levels were positively correlated with lymph node metastasis, clinical stage, and different breast cancer subtypes, highlighting its influence on cancer characteristics and behavior. In NSCLC,13 high HOXC-AS3 expression levels indicated deeper T stages, tumor metastasis, and advanced clinical stages, suggesting its involvement in disease progression and metastasis. Since HOXC-AS3 has been implicated in multiple biological processes,11,14–16,18–20,35,36 HOXC-AS3 overexpression promotes cell proliferation, migration, invasion, and metastasis, thereby influencing tumor progression and development. The function of HOXC-AS3 on tumors may contribute to understanding the impact of HOXC-AS3 levels on key clinical parameters in various cancers.
Furthermore, HOXC-AS3 holds potential as a dual-purpose biomarker, serving as both a prognostic predictor and a diagnostic marker in various tumors. It exhibits robust prognostic value across different cancer types, offering valuable insights for prognostic prediction and personalized treatment strategies. According to reported studies and analysis of TCGA database data, high expression of HOXC-AS3 in tumor samples generally indicates an unfavorable prognosis, except for CRC and SKCM, where it is associated with a more favorable prognosis. This may involve multiple factors, including the molecular characteristics of cancer, the biology of the disease, and the interactions of HOXC-AS3 with other genes or pathways. Further research is needed to understand these differences. And HOXC-AS3 expression could be also used to distinguish patients with or without lymph node metastases and different tumor stages in breast cancer,11 and HoxC-AS3’s diagnostic potential for distinguishing between tumor and normal tissue was evaluated using large online datasets from UCSC XENA. It demonstrated strong diagnostic value across various cancers, with notable effectiveness in PCPG, ESCA, SKCM, and TGCT.
HOXC-AS3 exerts its regulatory effects through molecular mechanisms that involve interactions with microRNAs, leading to the formation of ceRNA networks and modulation of target gene expression. Additionally, HOXC-AS3 has demonstrated interactions with proteins and other non-coding RNAs, as well as binding to the promoters of target genes, thereby exerting regulatory influence at both the transcriptional and post-transcriptional levels. A comprehensive understanding of these regulatory mechanisms is crucial in elucidating the precise roles of HOXC-AS3 and identifying potential therapeutic targets. Moreover, HOXC-AS3 has been implicated in tumor progression and metastasis through several signaling pathways, including the ErbB signaling pathway36 and metabolic pathways,11 which have been identified as promising targets for therapeutic interventions in the context of tumor treatment.71–77
In addition, the presence of chemoresistance poses a major obstacle in cancer therapy, hindering the effectiveness of chemotherapy.78,79 Recent studies suggest that HOXC-AS3 expression may be associated with chemotherapy response in certain cancers.51,52 For example, in ovarian cancer, the inclusion of ivermectin as a treatment option may prove beneficial, as HOXC-AS3 potentially influences the lncRNA-EIF4A3-mRNA pathways regulated by ivermectin.52 And the significant upregulation of HOXC-AS3 in cisplatin-resistant ovarian cancer cells suggests its potential biological significance in conferring resistance to cisplatin treatment.51 These studies indicate that targeting HOXC-AS3 could serve as a viable therapeutic strategy to enhance chemosensitivity in cancer.
Despite the progress made in understanding the implications of HOXC-AS3 in human malignancies, several areas require further special attentions. First, in-depth investigations into the underlying molecular mechanisms and signaling pathways regulated by HOXC-AS3 are needed in different cancer types for the development of novel therapeutic strategies. Future studies should focus on identifying small molecules, nucleic acid-based therapeutics, or gene editing approaches that can selectively modulate HOXC-AS3 expression or disrupt its interactions with regulatory molecules. Second, due to the inconvenience and limited accessibility of obtaining HOXC-AS3 from tumor tissue biopsies, it is necessary to further explore the specificity and stability of HOXC-AS3 expression in minimally invasive body fluids, such as blood and urine. Establishing a reliable and non-invasive method to detect and quantify HOXC-AS3 expression levels in the clinical setting will expedite its translation into clinical practice. Third, additional studies are warranted to explore the diagnostic and prognostic value of HOXC-AS3 in larger patient cohorts and across diverse populations. Validation of its potential as a biomarker will provide valuable insights into its utility for personalized cancer management.
In conclusion, the comprehensive understanding of the expression and implications of HOXC-AS3 in human malignancies contributes to our understanding of the non-coding transcriptome and its role in cancer biology. The clinical significance of HOXC-AS3 as a diagnostic and prognostic biomarker, as well as its involvement in diverse cellular processes, underscores its potential as a therapeutic target for cancer management. Further efforts in in vivo studies and clinical trials are necessary to elucidate the detailed mechanisms of HOXC-AS3 and explore its clinical applications. Moreover, future experiments should focus on testing the efficacy and safety of targeted HOXC-AS3 drugs, with the ultimate goal of improving cancer management.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgment
We thank Xiantao Tools (https://www.xiantaozi.com/) for helping to obtain analysis data datasets from TCGA, Genotype-Tissue Expression (GTEx), and UCSC Xena to evaluate the expression of HOXC-AS3 and its clinical outcome in tumors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Natural Science Foundation of Jiangxi Province, China (S2020ZRMSB0672).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Mattick JS, Amaral PP, Carninci P, et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol. 2023;24(6):430–447. doi:10.1038/s41580-022-00566-8
2. Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genomics Proteomics Bioinformatics. 2016;14(1):42–54. doi:10.1016/j.gpb.2015.09.006
3. Bartonicek N, Maag JL, Dinger ME. Long noncoding RNAs in cancer: mechanisms of action and technological advancements. Mol Cancer. 2016;15(1):43. doi:10.1186/s12943-016-0530-6
4. Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193(3):651–669. doi:10.1534/genetics.112.146704
5. Li J, Tian H, Yang J, Gong Z. Long noncoding RNAs regulate cell growth, proliferation, and apoptosis. DNA Cell Biol. 2016;35(9):459–470. doi:10.1089/dna.2015.3187
6. Ou C, He X, Liu Y, Zhang X. lncRNA cytoskeleton regulator RNA (CYTOR): diverse functions in metabolism, inflammation and tumorigenesis, and potential applications in precision oncology. Genes Dis. 2023;10(2):415–429. doi:10.1016/j.gendis.2021.08.012
7. Wu J, Zhu Y, Cong Q, Xu Q. Non‑coding RNAs: role of miRNAs and lncRNAs in the regulation of autophagy in hepatocellular carcinoma (Review). Oncol Rep. 2023;49(6). doi:10.3892/or.2023.8550
8. Kudriashov V, Sufianov A, Mashkin A, et al. The role of long non-coding RNAs in carbohydrate and fat metabolism in the liver. Non-Coding RNA Res. 2023;8(3):294–301. doi:10.1016/j.ncrna.2023.03.003
9. Sanya DRA, Onésime D. Roles of non-coding RNAs in the metabolism and pathogenesis of bladder cancer. Hum Cell. 2023:1–30. doi:10.1007/s13577-023-00915-5
10. Hu Q, Li Y, Li D, et al. Amino acid metabolism regulated by lncRNAs: the propellant behind cancer metabolic reprogramming. Cell Commun Signal. 2023;21(1):87.
11. Zhu W, Chen X, Guo X, et al. Low glucose-induced overexpression of HOXC-AS3 promotes metabolic reprogramming of breast cancer. Cancer Res. 2022;82(5):805–818. doi:10.1158/0008-5472.CAN-21-1179
12. Zhang TT, Chen HP, Yu SY, Zhao SP. LncRNA HOXC-AS3 overexpression inhibits TGF-β2-induced colorectal cancer cell migration and invasion by sponging miR-1269. Hum Exp Toxicol. 2022;41:9603271221093630. doi:10.1177/09603271221093630
13. Wan L, Cheng Z, Sun Q, Jiang K. LncRNA HOXC-AS3 increases non-small cell lung cancer cell migration and invasion by sponging premature miR-96. Expert Rev Respir Med. 2022;16(5):587–593.
14. Su H, Fan G, Huang J, Qiu X. LncRNA HOXC-AS3 promotes non-small-cell lung cancer growth and metastasis through upregulation of YBX1. Cell Death Dis. 2022;13(4):307. doi:10.1038/s41419-022-04723-x
15. Su C, Wang W, Mo J, et al. Long noncoding RNA HOXC-AS3 interacts with CDK2 to promote proliferation in hepatocellular carcinoma. Biomarker Res. 2022;10(1):65. doi:10.1186/s40364-022-00411-2
16. Li Y, Peng L, Cao X, et al. The long non-coding RNA HOXC-AS3 promotes glioma progression by sponging miR-216 to regulate F11R expression. Front Oncol. 2022;12:845009. doi:10.3389/fonc.2022.845009
17. Zhang E, He X, Zhang C, et al. A novel long noncoding RNA HOXC-AS3 mediates tumorigenesis of gastric cancer by binding to YBX1. Genome Biol. 2018;19(1):154. doi:10.1186/s13059-018-1523-0
18. Yang Z, Hu T. Long noncoding RNA HOXC-AS3 facilitates the progression of invasive mucinous adenocarcinomas of the lung via modulating FUS/FOXM1. In Vitro Cell Dev Biol Anim. 2020;56(1):15–23. doi:10.1007/s11626-019-00414-8
19. Su J, Yu B, Zhang C, et al. Long noncoding RNA HOXC-AS3 indicates a poor prognosis and regulates tumorigenesis by binding to YBX1 in breast cancer. Am J Transl Res. 2020;12(10):6335–6350.
20. Shi SH, Jiang J, Zhang W, et al. A novel lncRNA HOXC-AS3 acts as a miR-3922-5p sponge to promote breast cancer metastasis. Cancer Invest. 2020;38(1):1–12. doi:10.1080/07357907.2019.1695816
21. Wang X, Sun Y, Xu T, et al. HOXB13 promotes proliferation, migration, and invasion of glioblastoma through transcriptional upregulation of lncRNA HOXC-AS3. J Cell Biochem. 2019;120(9):15527–15537. doi:10.1002/jcb.28819
22. Li B, Han H, Song S, et al. HOXC10 regulates osteogenesis of mesenchymal stromal cells through interaction with its natural antisense transcript lncHOXC-AS3. Stem Cells. 2019;37(2):247–256. doi:10.1002/stem.2925
23. Xu Y, Ren Z, Wang X, Ren M. The lncRNA HOXA11-AS acts as a tumor promoter in breast cancer through regulation of the miR-125a-5p/TMPRSS4 axis. J Gene Med. 2022;24(5):e3413. doi:10.1002/jgm.3413
24. Wei C, Zhang X, Peng D, et al. LncRNA HOXA11-AS promotes glioma malignant phenotypes and reduces its sensitivity to ROS via Tpl2-MEK1/2-ERK1/2 pathway. Cell Death Dis. 2022;13(11):942. doi:10.1038/s41419-022-05393-5
25. Liu Y, Yan W, Zhou D, Jin G, Cheng X. Long non‑coding RNA HOXA11‑AS accelerates cell proliferation and epithelial‑mesenchymal transition in hepatocellular carcinoma by modulating the miR‑506‑3p/Slug axis. Int J Mol Med. 2020;46(5):1805–1815. doi:10.3892/ijmm.2020.4715
26. Xu HW, Chen YR, Ouyang SS, Li P, Wang MQ, Zhu SL. HOTAIR plays an oncogenic role in gastric cancer through microRNA and SNP. Neoplasma. 2021;68(3):465–471. doi:10.4149/neo_2021_210127N138
27. Ghafouri-Fard S, Dashti S, Farsi M, Taheri M. HOX transcript antisense RNA: an oncogenic lncRNA in diverse malignancies. Exp Mol Pathol. 2021;118:104578. doi:10.1016/j.yexmp.2020.104578
28. Rajagopal T, Talluri S, Akshaya RL, Dunna NR. HOTAIR LncRNA: a novel oncogenic propellant in human cancer. Clin Chim Acta. 2020;503:1–18. doi:10.1016/j.cca.2019.12.028
29. Liu FT, Qiu C, Luo HL, et al. The association of HOTAIR expression with clinicopathological features and prognosis in gastric cancer patients. Panminerva Med. 2016;58(2):167–174.
30. Martens L, Rühle F, Witten A, et al. A genetic variant alters the secondary structure of the lncRNA H19 and is associated with dilated cardiomyopathy. RNA Biol. 2021;18(sup1):409–415. doi:10.1080/15476286.2021.1952756
31. Lorenz R, Bernhart SH, Höner Zu Siederdissen C, et al. ViennaRNA Package 2.0. Algorithms Mol Biol. 2011;6(1):26. doi:10.1186/1748-7188-6-26
32. Dong Y, Li X, Lin Z, et al. HOXC-AS1-MYC regulatory loop contributes to the growth and metastasis in gastric cancer. J Exper Clin Cancer Res. 2019;38(1):502. doi:10.1186/s13046-019-1482-7
33. Zhou LL, Jiao Y, Chen HM, et al. Differentially expressed long noncoding RNAs and regulatory mechanism of LINC02407 in human gastric adenocarcinoma. World J Gastroenterol. 2019;25(39):5973–5990. doi:10.3748/wjg.v25.i39.5973
34. Xu YZ, Chen FF, Zhang Y, Liang H, Li XJ, He C. Identification of potential long noncoding RNA associated with nasopharyngeal carcinoma using deep sequencing. J Int Med Res. 2019;47(7):3271–3281. doi:10.1177/0300060519845973
35. Yang B, Sun L, Liang L. LncRNA HOXC-AS3 suppresses the formation of mature miR-96 in ovarian cancer cells to promote cell proliferation. Reprod Sci. 2021;28(8):2342–2349. doi:10.1007/s43032-021-00500-x
36. Zhao R, Song J, Jin Y, Liu Y. Long noncoding RNA HOXC-AS3 enhances the progression of cervical cancer via activating ErbB signaling pathway. J Mol Histol. 2021;52(5):991–1006. doi:10.1007/s10735-021-10007-z
37. Enteghami M, Ghorbani M, Zamani M, Galehdari H. HOXC10 is significantly overexpressed in colorectal cancer. Biomed Rep. 2020;13(3):18. doi:10.3892/br.2020.1325
38. Yu Y, Nangia-Makker P, Farhana L, Majumdar APN. A novel mechanism of lncRNA and miRNA interaction: CCAT2 regulates miR-145 expression by suppressing its maturation process in colon cancer cells. Mol Cancer. 2017;16(1):155. doi:10.1186/s12943-017-0725-5
39. Jin Z, Li H, Long Y, Liu R, Ni X. MicroRNA-1269 is downregulated in glioblastoma and its maturation is regulated by long non-coding RNA SLC16A1 antisense RNA 1. Bioengineered. 2022;13(5):12749–12759. doi:10.1080/21655979.2022.2070581
40. Li X, Zhu L, Luo Y. Long non-coding RNA HLA-F antisense RNA 1 inhibits the maturation of microRNA-613 in polycystic ovary syndrome to promote ovarian granulosa cell proliferation and inhibit cell apoptosis. Bioengineered. 2022;13(5):12289–12297. doi:10.1080/21655979.2022.2070965
41. Zhang H, Hao Y, Yang A, et al. TGFB3-AS1 promotes Hcy-induced inflammation of macrophages via inhibiting the maturity of miR-144 and upregulating Rap1a. Mol Ther Nucleic Acids. 2021;26:1318–1335. doi:10.1016/j.omtn.2021.10.031
42. Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi:10.1016/j.cell.2011.07.014
43. Karreth FA, Pandolfi PP. ceRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov. 2013;3(10):1113–1121. doi:10.1158/2159-8290.CD-13-0202
44. Dhawan A. Mathematical modeling of ceRNA-based interactions. Methods Mol Biol. 2021;2324:105–114.
45. Sideris N, Dama P, Bayraktar S, Stiff T, Castellano L. LncRNAs in breast cancer: a link to future approaches. Cancer Gene Ther. 2022;29(12):1866–1877. doi:10.1038/s41417-022-00487-w
46. Alsayed R, Sheikhan K, Alam MA, et al. Epigenetic programing of cancer stemness by transcription factors-non-coding RNAs interactions. Semin Cancer Biol. 2023;92:74–83. doi:10.1016/j.semcancer.2023.04.005
47. Khan M, Hou S, Chen M, Lei H. Mechanisms of RNA export and nuclear retention. Wiley Interdiscip Rev RNA. 2023;14(3):e1755. doi:10.1002/wrna.1755
48. Su WY, Li JT, Cui Y, et al. Bidirectional regulation between WDR83 and its natural antisense transcript DHPS in gastric cancer. Cell Res. 2012;22(9):1374–1389. doi:10.1038/cr.2012.57
49. Faghihi MA, Modarresi F, Khalil AM, et al. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14(7):723–730. doi:10.1038/nm1784
50. Bu P, Wang L, Chen KY, et al. miR-1269 promotes metastasis and forms a positive feedback loop with TGF-β. Nat Commun. 2015;6:6879. doi:10.1038/ncomms7879
51. Vera O, Rodriguez-Antolin C, de Castro J, Karreth FA, Sellers TA, Ibanez de Caceres I. An epigenomic approach to identifying differential overlapping and cis-acting lncRNAs in cisplatin-resistant cancer cells. Epigenetics. 2018;13(3):251–263.
52. Li N, Zhan X. Anti-parasite drug ivermectin can suppress ovarian cancer by regulating lncRNA-EIF4A3-mRNA axes. EPMA J. 2020;11(2):289–309. doi:10.1007/s13167-020-00209-y
53. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi:10.1007/s00401-007-0243-4
54. Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee SU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prevent. 2017;18(1):3–9. doi:10.22034/APJCP.2017.18.1.3
55. Kumari S, Gupta R, Ambasta RK, Kumar P. Multiple therapeutic approaches of glioblastoma multiforme: from terminal to therapy. Biochimica Et Biophysica Acta Rev Cancer. 2023;1878(4):188913. doi:10.1016/j.bbcan.2023.188913
56. POLIVKA JR J, Polivka J, Holubec L, et al. Advances in experimental targeted therapy and immunotherapy for patients with glioblastoma multiforme. Anticancer Res. 2017;37(1):21–33. doi:10.21873/anticanres.11285
57. Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–1640. doi:10.1016/j.mayocp.2019.01.013
58. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. doi:10.1016/S0025-6196(11)60735-0
59. Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5(3):288–300. doi:10.21037/tlcr.2016.06.07
60. Cui D, Xie S, Liu Q. Postoperative survival of pulmonary invasive mucinous adenocarcinoma versus non-mucinous invasive adenocarcinoma. BMC Pulm Med. 2023;23(1):9. doi:10.1186/s12890-023-02305-x
61. Xu L, Li C, Lu H. Invasive mucinous adenocarcinoma of the lung. Transl Cancer Res. 2019;8(8):2924–2932. doi:10.21037/tcr.2019.11.02
62. Cha YJ, Shim HS. Biology of invasive mucinous adenocarcinoma of the lung. Transl Lung Cancer Res. 2017;6(5):508–512. doi:10.21037/tlcr.2017.06.10
63. Ishigaki S, Masuda A, Fujioka Y, et al. Position-dependent FUS-RNA interactions regulate alternative splicing events and transcriptions. Sci Rep. 2012;2(1):529. doi:10.1038/srep00529
64. Colombrita C, Onesto E, Megiorni F, et al. TDP-43 and FUS RNA-binding proteins bind distinct sets of cytoplasmic messenger RNAs and differently regulate their post-transcriptional fate in motoneuron-like cells. J Biol Chem. 2012;287(19):15635–15647. doi:10.1074/jbc.M111.333450
65. Nakaya T, Alexiou P, Maragkakis M, Chang A, Mourelatos Z. FUS regulates genes coding for RNA-binding proteins in neurons by binding to their highly conserved introns. RNA. 2013;19(4):498–509. doi:10.1261/rna.037804.112
66. Ge Z, Cheng Z, Yang X, et al. Long noncoding RNA SchLAH suppresses metastasis of hepatocellular carcinoma through interacting with fused in sarcoma. Cancer Sci. 2017;108(4):653–662. doi:10.1111/cas.13200
67. Xin Y, Shang X, Sun X, Xu G, Liu Y, Liu Y. SLC8A1 antisense RNA 1 suppresses papillary thyroid cancer malignant progression via the FUS RNA binding protein (FUS)/NUMB like endocytic adaptor protein (Numbl) axis. Bioengineered. 2022;13(5):12572–12582. doi:10.1080/21655979.2022.2073125
68. Mukkamalla SKR, Malipeddi D. Myeloma bone disease: a comprehensive review. Int J Mol Sci. 2021;22(12):6208. doi:10.3390/ijms22126208
69. Terpos E, Christoulas D, Gavriatopoulou M, Dimopoulos MA. Mechanisms of bone destruction in multiple myeloma. Eur J Cancer Care. 2017;26(6):e12761. doi:10.1111/ecc.12761
70. Giuliani N, Rizzoli V, Roodman GD. Multiple myeloma bone disease: pathophysiology of osteoblast inhibition. Blood. 2006;108(13):3992–3996. doi:10.1182/blood-2006-05-026112
71. Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21(2):177–184. doi:10.1016/j.ceb.2008.12.010
72. Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19(13):3159–3167. doi:10.1093/emboj/19.13.3159
73. Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25(3):282–303. doi:10.1016/j.ccr.2014.02.025
74. Dillon M, Lopez A, Lin E, Sales D, Perets R, Jain P. Progress on Ras/MAPK signaling research and targeting in blood and solid cancers. Cancers. 2021;13(20):5059. doi:10.3390/cancers13205059
75. Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17(4):351–359. doi:10.1038/ncb3124
76. Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10(9):671–684. doi:10.1038/nrd3504
77. Gong Y, Ji P, Yang YS, et al. Metabolic-pathway-based subtyping of triple-negative breast cancer reveals potential therapeutic targets. Cell Metab. 2021;33(1):51–64.e59. doi:10.1016/j.cmet.2020.10.012
78. Ramos A, Sadeghi S, Tabatabaeian H. Battling chemoresistance in cancer: root causes and strategies to uproot them. Int J Mol Sci. 2021;22(17):9451. doi:10.3390/ijms22179451
79. Emran TB, Shahriar A, Mahmud AR, et al. Multidrug resistance in cancer: understanding molecular mechanisms, immunoprevention and therapeutic approaches. Front Oncol. 2022;12:891652.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.