Back to Journals » International Journal of Nanomedicine » Volume 18
How Nanotherapeutic Platforms Play a Key Role in Glioma? A Comprehensive Review of Literature
Authors Yang Y, Cheng N, Luo Q, Shao N, Ma X, Chen J , Luo L , Xiao Z
Received 29 March 2023
Accepted for publication 15 June 2023
Published 3 July 2023 Volume 2023:18 Pages 3663—3694
DOI https://doi.org/10.2147/IJN.S414736
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Yongqing Yang,* Nianlan Cheng,* Qiao Luo, Ni Shao, Xiaocong Ma, Jifeng Chen, Liangping Luo, Zeyu Xiao
The Guangzhou Key Laboratory of Molecular and Functional Imaging for Clinical Translation, The First Affiliated Hospital of Jinan University, Guangzhou, 510632, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zeyu Xiao; Liangping Luo, The Guangzhou Key Laboratory of Molecular and Functional Imaging for Clinical Translation, The First Affiliated Hospital of Jinan University, 613 Huangpu Avenue West, Guangzhou, Guangdong Province, 510632, People’s Republic of China, Tel +86 20-38688425 ; +86 20-85221507, Email [email protected]; [email protected]
Abstract: Glioblastoma (GBM), a highly aggressive form of brain cancer, is considered one of the deadliest cancers, and even with the most advanced medical treatments, most affected patients have a poor prognosis. However, recent advances in nanotechnology offer promising avenues for the development of versatile therapeutic and diagnostic nanoplatforms that can deliver drugs to brain tumor sites through the blood-brain barrier (BBB). Despite these breakthroughs, the use of nanoplatforms in GBM therapy has been a subject of great controversy due to concerns over the biosafety of these nanoplatforms. In recent years, biomimetic nanoplatforms have gained unprecedented attention in the biomedical field. With advantages such as extended circulation times, and improved immune evasion and active targeting compared to conventional nanosystems, bionanoparticles have shown great potential for use in biomedical applications. In this prospective article, we endeavor to comprehensively review the application of bionanomaterials in the treatment of glioma, focusing on the rational design of multifunctional nanoplatforms to facilitate BBB infiltration, promote efficient accumulation in the tumor, enable precise tumor imaging, and achieve remarkable tumor suppression. Furthermore, we discuss the challenges and future trends in this field. Through careful design and optimization of nanoplatforms, researchers are paving the way toward safer and more effective therapies for GBM patients. The development of biomimetic nanoplatform applications for glioma therapy is a promising avenue for precision medicine, which could ultimately improve patient outcomes and quality of life.
Keywords: nanomedicine, bionanotechnology, glioma therapy, drug delivery system
Graphical Abstract:
Introduction
Gliomas are the predominant primary malignant brain tumors, accounting for approximately 80% of primary brain malignancies. Gliomas are considered the most prevalent tumors affecting the central nervous system (CNS). Among them, glioblastomas (GBMs) are a highly aggressive and fatal tumors that are often associated with various clinical complications, and are characterized by a tendency to recur and an extremely poor prognosis.1–3 The conventional therapeutic approach for gliomas includes surgical resection, postoperative radiotherapy, and chemotherapy. Surgery can reduce tumor load, prolong survival and relieve symptoms caused by intracranial hypertension and tumor compression, but due to the infiltrative nature of gliomas, it is difficult to completely remove these tumors without damaging key structures near the tumor bed.4 In addition, moderately advanced gliomas and gliomas in some special sites cannot be treated surgically. The early postoperative application of radiotherapy has demonstrated efficacy in extending patient survival. However, postoperative radiotherapy is associated with a risk of inducing secondary malignancy in the irradiated area and causing radiation damage. Chemotherapy is an indispensable therapeutic approach for glioma that can increase progression-free and overall survival in affected patients. However, this treatment modality is associated with several systemic side effects, including liver dysfunction, cardiotoxicity, and bone marrow suppression.5 In recent years, photodynamic therapy (PDT),6 photothermal therapy (PTT),7 sonodynamic therapy (SDT),8 chemodynamic therapy (CDT),9 gene therapy10 and immunotherapy11 have been used to treat glioma in place of or in combination with conventional therapies, and have received widespread attention because they have shown unparalleled advantages.
Nonetheless, a noteworthy challenge in the effective treatment of glioma is posed by the blood-brain barrier (BBB), which is a multifaceted composition of brain microvascular endothelial cells (ECs), pericytes, astrocytes, tight junctions (TJs), neurons, and basement membranes (Figure 1).12 The BBB is a protective physical barrier that provides a defense mechanism for the brain to maintain CNS homeostasis, but the BBB also prevents large molecules such as drugs from entering the brain.13 In addition, factors such as the complexity of the tumor microenvironment, the heterogeneity of tumor tissues and drug tolerance often lead to unsatisfactory efficacy.14
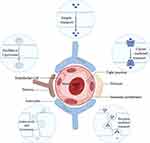 |
Figure 1 Composition of neurovascular structural units and the different mechanisms by which molecules cross the BBB. Created with Biorender.com. |
Nanoparticles (NPs) have been skillfully designed to extend the systemic circulation of drugs. The selective targeting of the surface fraction of NPs and the enhanced permeability and retention (EPR) effect render them highly effective as delivery vehicles for transporting drugs across the BBB to the tumor site and improving drug therapeutic efficacy. Multiple drugs can be linked into individual NPs through encapsulation, adsorption or covalent linkage to build a versatile therapeutic system, which is of exceptional significance in the treatment of intricate and multifaceted diseases such as cancer.15,16 Moreover, NPs can reduce immunogenicity and side effects, and biocompatible nanoparticle matrices can prevent drug enzymatic or environmental degradation, while reducing exposure to drug toxic effects and increasing the maximum tolerated dose of a drug.5,15,17
Traditional NPs are primarily synthesized from polymers, lipids, and metals, and are capable of accommodating an assortment of active ingredients such as drugs, contrast agents, proteins, and nucleic acids, among others, for various biomedical applications. However, when NPs are injected into the circulation, the liver and spleen remove most of the nanomaterials and thus prevent nanomaterial delivery to tumor tissue.18,19 Safety remains a major concern in the development/utilization of NP-based therapeutics.20 For example, metals and metal oxides can cause neurotoxicity by inducing apoptosis and elevating oxidative stress.21 Specific antibodies are produced after PEGylation administration, leading to rapid clearance after repeated dosing.22 In addition, most NP preparations accumulate significantly in the spleen, liver, and kidneys, leading to systemic toxicity.23 Another legitimate concern is the potential neurotoxicity of untargeted NPs to healthy brain tissue.24 Therefore, scientists have been trying to optimize nanomaterials to minimize side effects.
In recent years, biomimetic NPs have become a crucial carrier of drugs to enhance their bioavailability, achieve targeted delivery of anticancer drugs in solid tumors, and mitigate drug side effects. Biomimetic NPs can be broadly categorized into two types: cell membrane (CM)-based biomimetic NPs and protein/peptide template-based biomimetic NPs.25,26 Biomimetic NPs, which consist of a synthetic core with multiple functionalities and are encapsulated with different types of CMs, such as those of cancer cells, red blood cells (RBCs), leukocytes, and platelets, have been developed to enhance drug bioavailability and achieve selective delivery, penetration and accumulation of anticancer drugs in solid tumors.27 These NPs utilize the complex biological functions of CMs and the versatility of NPs, allowing them to achieve immune escape, prolonged circulation in the bloodstream, and targeting capability toward tumors.20,27,28 Peptides or proteins as endogenous carriers are another branch of bionanomaterials that are equally nonimmunogenic and biocompatible.29 Commonly used carriers are albumin, transferrin (TF) and lipoproteins.30 Cancer cell surface proteins can also be used to enhance the targeting of NPs.31
A number of reviews have summarized the notable achievements in the development of glioma-targeting materials. Recent advances in magnetic resonance contrast agents and multimodal imaging of GBM were summarized by Zhuang et al.32 Tang et al reviewed important recent advances in targeting nanotechnology via the BBB, focusing on the design of multifunctional nanoplatforms.23 In recent years, biomimetic NPs have rapidly evolved and shown great advantages over conventional nanoplatforms, and a few summaries and in-depth discussions addressing the application of biomimetic NPs in glioma have been reported. In this paper, we aim to explore the structure and advantages of the latest bionano platforms. We classify and discuss various biological nanoplatforms for glioma diagnosis and treatment based on different materials. Finally, we conclude the article by discussing the challenges and prospects for future applications of glioma-targeted nanotherapies.
What are Membrane-Modified Biomimetic Nanoparticles?
NPs modified by CMs have gradually become the focus of research since 2011, when the team of Hu et al first synthesized RBC membrane-camouflaged NPs through a top-down method.33 Commonly used biological membranes include RBC membranes, tumor CMs, immune CMs, vesicles, etc. Different types of CMs exhibit varying properties. CM-encapsulated NPs retain membrane lipids, surface proteins, and glycans, thereby inheriting the inherent biological properties of the source cells. Compared with synthetic NPs, these biomimetic NPs showed better biosafety, significantly improved the ability of NPs to cross the BBB, increased drug delivery efficiency, and revealed superior efficacy for cancer treatment. Table 1 lists the applications of biofilm-modified biomimetic nanoplatforms in the management of glioma in recent years.
 |
Table 1 Application of Biomimetic Biofilm Nanoplatform in Glioma |
Tumour Membranes
Despite their infamy, cancer cells possess some distinctive and wondrous characteristics. For example, during the development of cancer, tumor cells develop complex mechanisms to counteract and avoid immune surveillance. Tumor-targeting agents mainly recognize overexpressed cancer cell surface antigens, such as CD133 and CD44,76 while cancer CM (CCM)-encapsulated NPs (CCM-NPs) can achieve homologous targeting.77 Nanoparticle surface functionalization using natural CMs is a novel approach. NPs encapsulated by CMs derived from cancer cells can exhibit excellent immune escape and homologous targeting of genetically autologous cancer cells.78,79
Red Blood Cell Membranes
The concept of employing RBCs as a vehicle for drug delivery was initially investigated by Dale et al during the 1970s.80 As the most common cells in blood, the specific properties of RBCs make them inherently suitable for intravascular transport. First, because RBCs lack a nucleus and organelles, most of their volume can be used for drug encapsulation. Then, as RBCs possess a lengthy lifespan, the duration of drug circulation can be extended, thereby sustaining the drug concentration at a secure and efficient level for an extended period.81 In addition, RBCs are plastic and deformable and can pass through capillaries 2–3 μm in diameter without damaging cells. In addition, RBCs are safe and nontoxic and show a high degree of biocompatibility. In short, the unique shape, size, structure and mechanical flexibility of RBCs can provide NPs with extraordinary biological properties. Combined with suitable targeting substances on the surface of RBCs, engineered bionanodrugs with BBB crossing ability can be constructed to effectively target glioma tissues and release drugs efficiently.81–83
Leukocyte Membranes
Leukocytes are immune cells that act as a defense system and include macrophages, neutrophils (NE), and natural killer (NK) cells, etc.84 There are approximately 4.5–12 billion leukocytes per liter of blood. By expressing cell adhesion molecules (CAMs) that bind to inflamed ECs, leukocytes can achieve targeted migration.85 Virchow first noted the presence of leukocytes in tumors in 1863, and suggested that there may be some connection between cancer and inflammation. The findings of subsequent studies confirmed that inflammation is present throughout tumor growth, including tumorigenesis, growth, invasion, and metastasis. During the development of cancer, a large number of immune cells are recruited into the tumor microenvironment (TME).86 The encapsulation of NPs with CMs retains the properties of the original biofilm. NPs encapsulated by immune CMs have specific immune functions that enable active immune responses, targeted migration to tumor sites, and the inhibition of tumor growth and metastasis, and thus hold great promise for targeted drug delivery.20
Macrophages
The cells of the mononuclear phagocyte system have intrinsic homing properties, responding to cytokine/chemokine excretion from diseased tissues, migrating across the endothelium to tumor sites, and protecting NPs from hepatic metabolism.55,87,88 Macrophages are considered as potential carriers of anticancer drugs because of their long in vivo circulation time and their ability to mediate immune evasion.27 Pang et al demonstrated that macrophages, in the presence of CAMs, can carry NPs across the BBB, overcome high interstitial fluid pressure to enter deeper parts of gliomas and better kill tumor cells. In addition, because M1 macrophages are in a proinflammatory state, they are better able to internalize particles than normal macrophages.55,56
Microglia
Microglia originate from primitive yolk sac myeloid precursors that enter the CNS during embryonic development and become the key immune effector cells of the CNS89–91. Gliomas can attract microglia by secreting a variety of cytokines that cause them to migrate toward the tumor site in a targeted manner.92 Therefore, the establishment of a microglial CM-based GBM-targeted drug delivery system has become a possibility.
Neutrophils
Inflammation is an important process in tumorigenesis.86 NEs adhere to brain ECs activated by inflammation and enter brain tissue via the BBB. Superoxide production during the NE response to inflammatory factors can also cause an increase in BBB permeability.93 Thus, coating the surface of NPs with NE CMs helps to target inflammatory brain ECs, thereby allowing NPs to enter the damaged brain.94
Natural Killer Cells
NK cells are important immune cells in vivo that play a key role in cancer immune surveillance, transplant rejection and early viral immunity.95 Since their discovery in the 1970s, numerous studies have illustrated that NK cells can elicit cytotoxicity against tumor cells and kill them spontaneously. Moreover, NK cells secrete a variety of cytokines and chemokines that initiate and modulate a multifaceted immune response, culminating in durable and protective immunity against tumors.96 Integrins on lymphocytes binding to CAMs on ECs can trigger intracellular signaling cascades that lead to TJ disruption and actin backbone recombination, creating a gap in the BBB that allows the penetration of NK cells. In addition, NK cells have potential tumor targeting ability. NPs wrapped by NK CMs can mimic the properties of NK cells.
Platelets
Platelets are one of the three main components of blood. Platelets have the advantages of wide sourcing, easy separation and fast renewal.65 Platelets reduce the uptake of drugs by macrophages and prolong the circulation of drugs while avoiding their undesirable accumulation in the body.97 NPs wrapped by platelet membranes can bind specifically to cancer cells through their surface proteins.98 In addition, platelets, as circulating sentinels of vascular injury, have an inherent adhesive effect on the injured vascular system, and thus the exposed collagen fibers of damaged vessels in tumors can attract platelets for targeted adhesion.99 Thus, the use of platelet bionanotechnology strategies will confer tumor-targeting properties to nanoprobes.
Hybrid Membranes
CM coating technology is a method to enable NPs to acquire the inherent properties of the source CM. NPs coated with CMs have better biocompatibility, enhanced pharmacokinetic characteristics and specific targeting ability.100 Different types of CMs have different characteristics. As mentioned above, CCM-NPs have antigens and receptors for cancer cells and have specific tumor targeting capabilities.101 RBCm-encapsulated NPs can achieve immune escape and prolong circulation time in vivo.102 Leukocyte membrane-encapsulated NPs have high affinity for inflammatory regions in vivo and easily cross the biological barrier.84 Platelet membrane-encapsulated NPs have strong immunocompatibility and high affinity for pathogens and injured vascular systems.103 The fusion of membranes from different cell types allows the acquisition of properties from both membranes, and the use of hybrid membranes to camouflage NPs can effectively address the functional limitations of individual CMs. This approach has been shown to enhance the properties of nanomaterials, as the resulting hybrid membrane can incorporate the desirable features of multiple CMs.104
Extracellular Vesicles
Extracellular vesicles (EVs), which are small vesicles consisting of CMs and associated membrane proteins, offer great promise as drug delivery vehicles for the treatment of gliomas. EVs possess a relatively stable structure that protects their contents from degradation, and they have the ability to target specific cells, including the ability to pass the BBB.105–108 Exosomes (Exos) are EVs that have shown potential for the treatment of GBM.
The Fabrication of Biomimetic Nanoparticles
The CM camouflage technique is a straightforward and practical top-down method to enhance the safety and efficiency of nanoparticle delivery without specific restrictions on the core nanomaterial. The production of CM-derived NPs generally involves three stages: the extraction of membranes, the preparation of nanoparticle cores, and the encapsulation of the cores with CMs (as shown in Figure 2). In addition, biofilms can be modified to obtain additional functions. The method of NP core preparation often depends on the core material and personalization and has been reported in several papers109,110, therefore, it is not reviewed here.
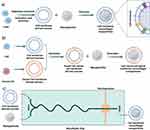 |
Figure 2 Different methods of CM nanoparticle camouflaging. (a) Single CM (b) Hybrid CMs and (c) Electroporation to collect NPs. Note: Reprinted from International Journal of Pharmaceutics, 621, Imran M, Jha L A, Hasan N, et al “Nanodecoys”-Future of drug delivery by encapsulating nanoparticles in natural cell membranes. 121,790, Copyright 2022, with permission from Elsevier.111 |
The Methods of Biofilm Extraction
CMs are phospholipid-rich bilayers, and the proteins, peptides and sugars on their surface determine their function. Therefore, it is essential to maintain the surface properties of CMs during the isolation and extraction process. The cell structure of nonnucleated cells (RBCs, platelets, etc.) is simple and the extraction of membranes is also simple. Repeated freeze‒thaw cycles or hypotonic incubation can be used to perform cell lysis, followed by the isolation of the cytoplasmic membrane by centrifugation.33,99,112 The isolation and extraction of CMs from nucleated cells is a more complex process. First, cells need to be screened based on markers on the CM surface. Then the cells are induced to rupture and the cell structure is further disrupted using ultrasound (US). Finally, the CMs are separated using differential centrifugation.77,113–116 CM hybridization involves fusing CMs from different cells together by US and extrusion, and then coating the fused membranes on individual NPs to give the NPs the functionality of membranes from both cells.117,118
Ultracentrifugation, including differential centrifugation, is the most commonly used method to isolate Exos because it is simple and inexpensive.119 Immunoaffinity capture is the most efficient method for isolating Exos with desirable structures.120 In addition, microfluidic techniques have recently been developed for Exo isolation.121,122 Passive microfluidic techniques utilize elastic lifting forces on particles in a viscoelastic medium to achieve separation.123 Active microfluidics can separate Exos using inhomogeneous electric fields or acoustic radiation forces depending on the size and dielectric properties of the Exos.124 Compared to Exos generated by ultracentrifugation, Exos generated by microfluidic methods have higher purity and recovery rates,125 making them potentially suitable for scaled-up production. In addition, Silva et al cultured human umbilical vascular ECs with gold NPs, iron oxide NPs or graphene quantum dots (GQDs) and observed that the cells released vesicles containing these exogenous NPs.126
The Methods of Biofilm Encapsulation
Currently, there are three main techniques for fusing biofilm-derived NPs: extrusion, ultrasonic fusion, and electroporation. Among these methods, physical extrusion uses mechanical forces to disrupt the membrane structure. CMs and NPs are first mixed well, and then fused by repeated extrusion using polycarbonate membranes with different pore sizes. Although this technology is convenient, the purity and integrity of the produced bionanoparticles are limited and challenges are encountered in large-scale preparation.127,128 The US method uses high-intensity US to assemble CMs and NPs into core-shell structures via electrostatic interactions. This method is associated with less material loss than extrusion, but may disrupt the nanoparticle core.129,130 In addition, microfluidic electroporation is a new method to facilitate the coating of nanocores with biofilm vesicles. Microfluidic electroporation uses electrical pulses between two electrodes to induce electroporation, resulting in the formation of many short-lived micropores in the CM, and these tiny pores allow the entry and exit of NPs into and out of the cell, allowing the efficient encapsulation of NPs within the CM.111 Microfluidic-based techniques for the preparation of biofilm-derived NPs offer better colloidal stability and higher encapsulation efficiency than conventional extrusion-based methods.130
The Methods of Biofilm Modification
The activity of NPs depends to a large extent on the surface functional proteins of the CM. Therefore, CM modification and functionalization can alter the characteristics of CM-encapsulated NPs.131,132 There are three main approaches for CM modification: physical, chemical and genetic engineering approaches. The spontaneous functionalization of specific ligands onto the CMs through the interaction of lipid membranes and their mobility is referred to as physical engineering of CMs. For example, lipid vesicles holding the target molecule are fused to the CMs.133 Physical engineering is the simplest method for modifying proteins and customizing delivery as a carrier. Chemical engineering techniques focus on membrane-associated proteins, peptides and polysaccharides.134 Chemical techniques can endow cells with the ability to perform new activities while maintaining their biological function. However, the lack of selectivity of chemical techniques may reduce the biological activity of natural proteins and disrupt their original function. Genetic engineering techniques involving viral and nonviral vectors allow the highly selective embedding of desired peptides or proteins on the cell surface. This approach is frequently utilized to incorporate protein into CMs, resulting in targeted delivery and enhanced therapeutic responses.135,136 Further exploration of genetically engineered CMs is needed, because although they are highly specific and improve the precision of CM engineering, they are associated with potential toxicity or immunogenicity when used in combination with chemical or physical modification strategies.111
The Applications of Membrane Modified NPs in Glioma
Imaging
Nanoparticle-based contrast agents have been widely used for optical image-guided procedures and disease monitoring, and their performance depends heavily on their molecular structure and physical, chemical, and optical properties. However, the intrinsic properties of NPs make them poorly biocompatible.137 To address this problem, Santos-Coquillat et al have developed a dual-labeled nanoprobe by radiolabeling and fluorescently tagging small EVs. This nanoprobe exhibits strong in vivo uptake in tumor tissue, making it a valuable tool for diagnostic assessment in animal models of GBM.74 Kong et al synthesized NIR-PLNPs with dual imaging and therapeutic functions based on mesoporous silica loaded with doxorubicin (DOX), ZnGa2O4:Cr3+, and Sn4+, wrapping RBC membranes (RBCm) for prolonged drug circulation time and further coupling T7 peptide on the RBC surface for blood-brain tumor barrier (BBTB) penetration and glioma targeting. The NPs showed good tissue penetration and exhibited in vivo NIR luminescence for more than 30 hours, allowing long-term autofluorescence imaging. This long-lived rechargeable nanoprobe has high imaging sensitivity for enhanced antitumor efficacy.45
Magnetic Particle Imaging (MPI) is an emerging non-invasive imaging technique based on tracer agents, which offers unique advantages such as background-free tissue signals and excellent imaging contrast. The MPI signal is generated by superparamagnetic particles, such as superparamagnetic iron oxide (SPIO) NPs. To enhance the performance of MPI for precise diagnosis of brain tumors, Huang et al developed a nanosensor called CM-Coated SPIO (CCM-SPIO) by coating the membrane of GBM cells onto the surface of SPIO. This nanosensor can penetrate the BBB and subsequently target brain GBMs, demonstrating excellent magnetic and photothermal effects. It can be used for early and accurate detection and intervention of GBM.43
Although the gliomas of some patients are inoperable, surgical treatment is still a key measure to reduce or delay the recurrence of most glioma. Developing diagnostic techniques to localize GBM tumors, visualize their borders during surgery, and remove as much of the tumor as possible while preserving normal brain tissue can improve patient prognosis and reduce tumor recurrence.138,139 Near-infrared (NIR) optical imaging is a new imaging method. Combined with sensitive detection devices and versatile fluorescent probes, this technique can be used for real-time imaging of a wide range of molecules in vivo, clearly defining and characterizing the physical edges of tumor tissue.137,140 Intraoperatively use of NIR imaging allows more precise identification of the boundaries of GBM tumors during surgery, enabling neurosurgeons to remove tumors with high sensitivity and precision.141 Men et al designed a CCM-encapsulated conjugated polymer dot nanoplatform for glioma-targeted detection. This biomimetic nanoprobe retains the complex biological functions of CCM-NPs while having the ability to be used for NIR-II fluorescence imaging.34 Wang et al designed lanthanum-doped elemental NPs encapsulated in brain tumor CMs for brain tumor imaging and surgical navigation with high stability, high spatiotemporal resolution and a low background signal, making brain tumor boundaries clearly visible.35
Chemotherapy
In addition to surgery, which is a crucial initial therapeutic intervention for malignant gliomas, chemotherapy serves as an additional treatment option to improve prognosis. However, the majority of cytotoxic agents have the limitation of not being able to effectively penetrate the BBB, which hampers their efficacy. The appropriate approach for penetrating the CNS tissue and cerebrospinal fluid (CSF) plays a crucial role in the delivery and effectiveness of chemotherapy for malignant gliomas. Nanomaterials that are coated with tumor CMs have high targeting ability, are capable of penetrating the BBB, and have demonstrated good antitumor effects both in vitro and in vivo.142
The GBM-CM camouflaged mimetic NPs (MNPs) developed by Zou et al could effectively be co-loaded with temozolomide (TMZ) and cisplatin Cisplatin (CDDP) (Figure 3), target GBMs through the BBB, and control drug release, achieving a strong anti-GBM effect and greatly prolonging survival. Histological analysis and routine blood tests showed no significant side effects.36 Fan et al successfully employed CCMs from C6 cells to camouflage a 10-hydroxycamptothecin nanosuspension (NS), achieving effective antitumor outcomes. NS is a method to stabilize the particle size of a drug at the nanoscale while increasing the surface area of the drug by using a small amount of surfactant or polymeric material as a stabilizer. This method is simple to perform; improves the dissolution rate, solubility and bioavailability of the drug and mitigates the drug side effects. In addition, CCC-encapsulated NSs are immune to clearance and can cross the BBB for selective targeting of tumor tissues.37
 |
Figure 3 TMZ and CDDP codelivery of bionanodrugs (a) Construction of NPs. (b) Size distribution of NPs. (c) Submicrostructure of NPs. (d) Flow cytometric analysis. (e) DNA damage induction description. (f and g) Cell proliferation of different MNPs after 48 h incubation. (h) Mechanistic description of the synergistic effect of TMZ and CDDP. (i and j) ELISA measurement of the MGMT concentration (*p < 0.05, **p < 0.01, ***p < 0.001). Note: Reprinted from Advanced Materials, 34(33), Zou Y, Wang Y, Xu S, et al. Brain Co-Delivery of Temozolomide and Cisplatin for Combinatorial Glioblastoma Chemotherapy. e2203958, Copyright 2022, with permission from Wiley-VCHGmbH.36 |
The safe and effective transport of chemotherapeutic drugs remains the key to improving the effectiveness of cancer treatment. RCBm-modified nanodrugs bound to targeting ligands show highly prolonged drug circulation times and high BBB penetration. Chai et al introduced tumor-targeting ligand c (RGDyK) to the RBCm surface via multivalent affinity-biotin interactions and encapsulated drug NCs (RBC-NCs) with it.46 Fu et al designed a T7 and NGR peptide dual-modified RBCm-encapsulated lipid nanoparticle that could be used for crossing the BBB and BBTB and targeting gliomas to improve the therapeutic efficacy of gliomas (Figure 4).47 Zou et al used vascular endothelin-2 to functionalize the RBCm surface and loaded PH-sensitive NPs (polymer, DOX) and lexiscan (Lex) to generate Ang-RBCm@NM-(DOX/Lex).48 Chai et al incorporated neurotoxin-derived targeting ligands bound to RBCCM-encapsulated NPs that could target nicotinic acetylcholine receptors (nAChRs) expressed by brain ECs.49 RBCms with attached targeting ligands conferred nanocarrier targeting ability along with prolonged blood circulation time, thus improving drug delivery efficiency and enhancing therapeutic efficacy.
 |
Figure 4 Dual targeted, RBCm-camouflaged vincristine nanodrugs for T7 and NGR peptides enable effective targeted drug delivery to GBM in situ (*P< 0.05). Note: Reprinted from ACS Appl Mater Interfaces, 11(2), Fu S, Liang M, Wang Y, et al. Dual-Modified Novel Biomimetic Nanocarriers Improve Targeting and Therapeutic Efficacy in Glioma. 1841–1854, Copyright 2018, with permission from American Chemical Society.47 |
Xue et al constructed paclitaxel containing (PTX) liposomes with NE coatings. Inflammatory factors released after tumor removal, such as TNF-α and CXCL1/KC, guided NPs into the brain and triggered the release of liposomal PTX to kill residual tumor cells, effectively slowing tumor recurrence in rodents and improving survival rates.62
Niu et al have devised a novel drug delivery system whereby natural grapefruit EVs were layered onto the surface of heparin-loaded NPs (DNs) containing DOX. This biomimetic EV-DN hybrid construct exhibited the remarkable ability to traverse the BBB/BBTB through transcytosis and membrane fusion, thereby penetrating the glioma tissues. This not only considerably enhanced the cellular internalization and antiproliferative potency of the therapeutic agent but also extended its circulation time, culminating in an improved therapeutic outcome for glioma.73
Wu et al developed NPs with excellent targeting and immune escape capabilities by hybridizing platelet and glioma CCMs.66 Hao et al developed a chemotherapeutic delivery vehicle based on docetaxel (DTX) NS with mixed glioma CCMs and a dendritic cell (DC) membrane envelope. The homologous targeting ability of CCMs provided a driver for precise drug delivery. At the same time, the platform achieved efficient synergistic effects of chemotherapy and immunotherapy due to the specialized antigen presentation properties of the DC membrane.67 Jiao et al used RBCms and CCMs to generate a hybrid membrane, and used this membrane to coat Gboxin-loaded mesoporous silica NPs for GBM chemotherapy. The coating of the RBCm/CCM composite membrane extended the cycle time of the NPs, allowing them to target gliomas.68 Targeting the TME is an important area of research in tumor targeting. Ma et al developed a novel approach to target the TME by fusing glioma-associated stromal cells (GASC) with GBM CMs and encapsulating them with polylactic-co-glycolic acid (PLGA) NPs. The resulting NPs inherit membrane proteins from both the GBM membrane and GASC membrane, significantly enhancing tumor targeting efficiency compared to NPs solely coated with CCMs.143
Photothermal Therapy
PTT is an approach that utilizes phototherapeutic agents distributed within diseased tissues to absorb electromagnetic radiation and convert it into heat. This process is aimed at killing cells through local heating. Under NIR light irradiation, the photothermal agents generate heat, which leads to the destruction of CMs and protein denaturation. PTT has the advantage of avoiding the nonselective destruction of cells that can occur with chemotherapy and radiotherapy, while also avoiding damage to nontargeted tissues as much as possible.144,145 Therefore, PTT may be the ideal method to remove residual tumor cells after surgery. Ren et al encapsulated GQDs and DOX in a homotypic CCM to construct a multifunctional bionanoplasmic nanoplatform for synergistic chemotherapeutic PTT targeting gliomas. Graphene GQD nanomaterials have unique physicochemical properties, and CCMs can actively target tumor cells, leading to significantly enhanced cellular uptake. Under external laser stimulation, the membranes may be disrupted, leading to rapid DOX release. This study provided an effective combination strategy for precision tumor therapy.38
Maintaining effective drug concentrations deep in the tumor remains one of the challenges of the photothermal treatment of gliomas. Zhang et al established a nanogel system by cross-linking prululam with a degradable conjugated polymer and loaded indocyanine green (ICG) and TMZ. The modification of the RBCm with an Apoe peptide further extended the circulation time of the nanosystem, enhancing its ability to cross the BBB and reach gliomas. Upon tumor irradiation with NIR, ICG generated ROS to deform the nanogel, resulting in the localized release of the drug and consequent eradication of tumor cells, leading to a substantial increase in survival rates.50
Wang et al successfully designed a macrophage membrane-encapsulated photothermal nanoprobe, Fe3O4-Cy5.5, that exhibited optimal detection depth and a high signal-to-noise ratio in fluorescence, photoacoustic and magnetic resonance imaging. This nanoprobe could distinguish gliomas from adjacent normal tissue and effectively guide surgery. In addition, Fe3O4-Cy5.5 induced PTT and effectively prevented glioma recurrence after surgical intervention.57
NPs wrapped by NK CMs can mimic the properties of NK cells. Chai et al developed NK cell-mimicking nanorobots (NK@AIEdots) with aggregation-induced emission (AIE) characteristics by mimicking the viral budding process and covering an AIE-active polymer endoskeleton with an NK CM. The prepared NK@AIEdots showed excellent NIR-II fluorescence. The NK@AIEdots could cross the BBB by pulling apart the TJs and accumulated specifically in brain tumors for in situ penetrating fluorescence imaging and PTT.63
Geng et al demonstrated an IR-II light therapy nanoprobe with platelet membrane camouflaging NPs that could target multiple cancer cells, such as pancreatic cancer, breast cancer, and glioma cells. The nanoprobe showed good biocompatibility, avoided macrophage clearance, and improved the treatment of glioma.65
Wang et al demonstrated that targeted EVs loaded with ICG and PTX could induce glioma cell apoptosis and inhibit tumor growth through combined chemotherapy-thermal therapy.69 However, the low yield of natural Exos limits their application in nanomedicine. To overcome this limitation, Wu et al compared the delivery of Exos with bioinspired nanovesicles (BNVs) in brain tumor drug delivery. They found that BNVs derived from brain-derived ECs had similar pharmacokinetic profiles and prolonged DOX cycle times, suggesting that autologous BNVs are an effective alternative to natural Exos for brain tumor nanodrug delivery.70 The formation of a protein corona (PC) on nanocarriers may also affect their behavior. Wu et al developed a multifunctional Exo mimic (EM) modified with Ang to enhance GBM drug delivery by manipulating the PC. The EM could evade macrophage phagocytosis, and the targeting ligand Ang retained its BBB penetration capability, providing a promising platform for targeted brain drug delivery and GBM therapy.71
Sonodynamic Therapy
SDT is a noninvasive treatment modality that kills cancer cells by generating reactive oxygen species (ROS) through US excitation of acoustic sensitizers. As a mechanical wave with a frequency greater than 20 kHz, US has the unique advantage of low tissue scattering and can penetrate deep soft tissues and activate acoustic sensitizers. In addition, by controlling the frequency, US can be accurately targeted to the tumor site, avoiding damage to the surrounding normal tissues. Nanoparticle-based acoustic sensitizers exhibit obvious advantages due to their properties, such as improved SDT efficacy, binding affinity, target specificity and synergistic treatment.146–148 For example, Zhu et al constructed multifunctional tumor homology-targeting MNPs (PIOC@CM NPs) encapsulating Fe3O4 and Ce6 as glioma acoustic sensitizers. US combined with circulating microbubbles for safe and instantaneous BBB opening and simulated nanosensitizer-mediated SDT combined with iron therapy for targeted synergistic treatment of gliomas.39
Chemodynamic Therapy
CDT is a rising anticancer strategy that involves inducing apoptosis by converting endogenous hydrogen peroxide (H2O2) into toxic hydroxyl radicals through Fenton and Fenton-like reactions. To improve the efficacy of CDT, NPs are used due to their good biocompatibility and stability. CM coating can further improve the stability of NPs and enable them to cross the BBB and traverse and target glioma.40,149 This approach was demonstrated in the experiments of Du et al, where a DOX-coupled manganese dioxide bionanomedical system (MnO2-DOX-C6) encapsulated by C6 CMs was prepared. MnO2 can promote the decomposition of H2O2 to alleviate the hypoxic TME; in addition, MnO2 can generate ROS through Fenton-like reactions to further induce oxidative stress to kill cancer cells. The hydrazone bond linking DOX and MnO2 allows DOX to be released exclusively in the acidic environment of the tumor, thereby reducing its toxicity and side effects. MnO2-DOX-C6, which wraps around the membrane of glioma C6 cancer cells, has homologous targeting ability and can regulate the rate of drug release.41
Selective targeting of copper (Cu) in tumor cells using chelating agents to induce elevated ROS may be an effective strategy for the treatment of GBM. The Cu chelator di-2-pyridone-4,4-dimethyl-3-thiosemicarbazone (Dp44mT) can form Cu-Dp44mT complexes with high redox activity, resulting in potent antitumor activity. Nevertheless, the high toxicity of this approach has hindered its practical application. To address this limitation, Muhammad Ismail et al utilized vasopeptide-2 functionalized RBCm (Ang-M) camouflaged f Cu-loaded NPs. These NPs can penetrate the BBB, target delivery of Dp44mTb to GBM cells and increase tumor Cu loading, resulting in Cu-dependent antitumor activity.52
The polymeric nanogels developed by Xiao et al have multifunctional properties and contain both manganese dioxide (MnO2) and cisplatin, which are camouflaged with macrophage membranes. These nanogels are intended for use in MRI-guided in situ glioma chemotherapy/CDT. The Mn ions present in the nanogels are capable of enhancing CDT through Fenton-like reactions. Furthermore, cisplatin loaded into nanogels not only has chemotherapeutic effects but also enhances CDT efficacy by promoting the production of ROS (Figure 5).58
 |
Figure 5 (a) Preparation of macrophage membrane-encapsulated bionanoparticles and (b) crossing the BBB for MRI-guided in situ glioma chemotherapy/CDT combination therapy. Note: Reprinted from ACS Nano, 15(12), Xiao T, He M, Xu F, et al. Macrophage Membrane-Camouflaged Responsive Polymer Nanogels Enable Magnetic Resonance Imaging-Guided Chemotherapy/CDT of Orthotopic Glioma. 20377–20390, Copyright 2021, with permission from American Chemical Society.58 |
Immunotherapy
The immune system plays a critical role in immune surveillance, identifying cancerous cells and triggering cellular immune responses.150 However, cancer cells have developed multiple mechanisms to evade immune surveillance, including heightened expression of negative regulatory pathways, antigen delivery mechanism impairment, and immunosuppressive cell recruitment.151 Immunotherapy, which aims to augment autoimmunity to eradicate tumor cells, represents a significant breakthrough in cancer treatment and has emerged as an effective clinical tool.152 Nevertheless, the downside of immunotherapy is that it can cause excessive activation of the patient’s autoimmunity, resulting in an attack on healthy tissues.153 Nanoparticle delivery strategies can target tumor and/or immune cells more effectively and reduce the adverse effects triggered by off-targeting.152
He et al found that the synergistic action of an Mcl −1 specific inhibitor (A12) with Bcl-2 and Bcl-xl inhibitors (ABT) induced strong apoptosis of GBM cancer stem cells. They designed apolipoprotein E (ApoE) peptide-modified RBCm-encapsulated pH-sensitive dextran NPs for targeted delivery of ABT and A12. After nanodrug encapsulation, their antiglioma synergistic effects were preserved and they were biocompatible, effectively penetrating the BBB and inhibiting tumor growth with no significant side effects.53
Zhao et al have utilized carbonated calcium carbonate NPs coated with CCM modified with cRGD to deliver interleukin-12 messenger RNA (IL-12 mRNA@cRGD-CM-CaCO3NPs). The calcium carbonate NPs (CaCO3NPs) generate bubbles under the acidic conditions of tumor lysosomes and induce immunogenic cell death under US exposure, resulting in a synergistic immune response when combined with IL-12 mRNA. These biomimetic NPs exhibit excellent targeting capabilities and immune therapeutic effects.44
In addition, GBM is an immuno-cooled tumor type, and the use of immune CM modified NPs has the additional effect of improving the TME and helps to improve the efficacy of immunotherapy. Ma et al utilized activated mature DC membrane-coated rapamycin (RAPA)-loaded PLGA to construct a nanoplatform. DCs, serving as antigen-presenting cells, can stimulate and regulate innate and adaptive immune responses, promoting the activation and proliferation of T cells and NK cells, and significantly inhibiting GBM growth. PD-L1 is overexpressed in tumor cells and tumor-associated macrophages, and it binds to PD-1 expressed on tumor-infiltrating lymphocytes, thereby inducing T-cell apoptosis and exhaustion, inhibiting the activation of CD8 cytotoxic T lymphocytes, and triggering immune escape of tumor cells. Local chemotherapy combined with anti-PD-1 helps enhance the anti-tumor immune response in GBM and exhibits significant synergistic effects.64 Yin et al utilized macrophage membrane-coated poly(lactic-co-glycolic acid) NPs loaded with RAPA, which overexpress PD-1, to construct NPs that enhance PD-1 expression (PD-1-MM@PLGA/RAPAs). These NPs can penetrate the BBB, respond to the TME, and demonstrate remarkable anti-tumor efficacy.59
Liu et al connected siPD-L1 with Fe3O4 NPs using disulfide bonds, and further packaged Microglia them in a microglia membrane (M-BV2) to form the biomimetic brain-targeting NPs Fe3O4-siPD-L1@M-BV2. The microglial membrane interacts with GBM cells to actively deliver NPs to GBM cells. Thus, ferroptosis and immunotherapy exert synergistic effects.60 Qiao et al prepared Zoledronate (ZOL) loaded NPs with REDOX properties and encapsulated them in a microglial membrane to form NPs ZOL@CNPs. ZOL@CNPs can induce the apoptosis and inhibit the migration and invasion of GBM cells, significantly inhibiting the growth of GBMs.61
Gene Therapy
The utilization of gene therapy for treating gliomas has gained significant attention in recent years. Gene therapy involves the targeted delivery of genetic material into tumor cells with the aim of triggering apoptosis or programmed cell death. Although RNA interference therapy may specifically inhibit the proliferation of GBMs via the gene expression silencing of candidate GBM oncogenes with relatively low-dose requirements and negligible toxicity, its practical application has been severely hindered by the extremely short half-life of small molecule interfering RNA (siRNA) in the blood and the presence of the BBB. However, with the advancement of nanotechnology, innovative solutions have emerged for siRNA treatment of glioma. Researchers have explored various strategies for bypassing the BBB and delivering nucleic acids to gliomas through the use of NPs.154 Tumor CM-encapsulated nanomaterials are a good choice. Han et al used NPs encapsulated in CCMs for gene delivery. Under CCM coverage, polyethyleneimine/plasmid DNA complexes form stable NPs with negatively charged surfaces that can deliver genes to the brain, improving delivery efficiency and reducing side effects.155
Liu et al developed a charge transfer bio-nano platform with a three-layer core-shell structure that overcomes the barrier to siRNA delivery to GBM. This platform uses RBCm as the surface structure to shield siRNA from non-specific clearance and immune response, while the low-density lipoprotein receptor-related protein receptor Angiopep-2 (Ang) modification enhances the penetration of the BBB and targeting of GBMs. The charge conversion strategy combined with a biomimetic membrane in an “all-in-one” platform facilitates efficient siRNA release triggered by negative to positive charge conversion in cancer cell lysosomes (pH 5.0–6.5), leading to successful target gene silencing. This nanoplatform improves the efficiency of siRNA delivery and enhances therapeutic efficacy.51
Liang et al have designed an Exo-based delivery system loaded with signal transducers and activators of transcription 3 siRNA, functionalized with Ang, for the treatment of GBM. This system demonstrates high blood stability and cellular uptake capability, as well as excellent BBB penetration and remarkable in vitro anti-GBM efficacy75. To achieve signal transducer and activator of transcription 3 inhibition and the delivery of TMZ to GBM, Fawad et al modified bone marrow mesenchymal stem cell (BMSC) isolated Exos (BMSCExos) with heme oxygenase 1-specific peptide as a targeted and multifunctional nanocarrier for TMZ-resistant GBM treatment (Figure 6). Mesenchymal stem cells can migrate to damaged tissues and have the ability to differentiate to multiple cell lines, which can promote tissue regeneration. In addition, they are able to regulate the TME through a complex secretion mechanism. This BMSCExo has great TMZ and siRNA loading capacity and can cross the BBB and accumulate specifically in brain tumors (Figure 6).72
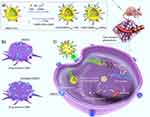 |
Figure 6 Specifically targeted Exos for glioma delivery. (a) Description of Exo surface modifications. (b) GBM surface targeting markers. (c) Exos are capable of penetrating the BBB. Note: Reprinted from Journal of Controlled Release, 345, Rehman FU, Liu Y, Yang Q, et al. Heme Oxygenase-1 targeting exosomes for TMZ resistant glioblastoma synergistic therapy. 696–708, Copyright 2021, with permission from Elsevier.72 |
Metabolic Therapy
Altered metabolism is a hallmark of cellular carcinogenesis, and glycolysis is a vital pathway for metabolism in cancer cells. Through glycolysis, cancer cells consume glucose, convert it to lactate (LA), and produce ATP, which supports their rapid proliferation in the hypoxic TME. While LA was previously viewed as a waste product in the TME, recent evidence indicates that it can promote invasive metastasis of cancer.156 Consequently, targeting LA metabolism has become an emerging strategy for cancer treatment. Lu et al developed CCM-encapsulated self-assembled NPs that provide drugs for photothermal synergistic metabolic therapy These NPs can cross the BBB and target GBM through isotype recognition. The lactate oxidase in the NPs converts LA to pyruvate (PA) and H2O2. PA blocks histone expression and induces cell cycle arrest, thus inhibiting cancer cell growth. The photosensitizer chlorin e6 is codelivered and generates cytotoxic singlet oxygen using the energy released from the reaction of H2O2 with bisoxalate to kill glioma cells.42 Such NPs with self-aggregation and self-encapsulation properties offer a simple and effective approach for creating multifunctional nanocarrier platforms.
Altering the availability and metabolism of glucose may be an interesting strategy for tumor treatment. To this end, Ke et al created a multifunctional bioreactor for synergistic chemotherapy and starvation therapy. The bioreactor was created by encapsulating glucose oxidase (GOX) and DOX in a metal-organic framework (MOF) that was camouflaged by tumor-targeting ligand (RGD)-modified RBCm (called RGD-mgzd). The long blood circulation time and RGD peptide modification inherited from RBCs allow the bioreactor to preferentially target the tumor. Upon reaching the target area, GOX immediately consumes intratumoral glucose and oxygen. In addition, the acidic microenvironment in the tumor region induces the release of DOX from MOF catabolism, enhancing chemotherapy. The development of this bioreactor has paved the way for a synergistic model that promotes cancer therapy in a spatiotemporally controlled manner.54
What are Protein/Peptide-Modified Biomimetic Nanoparticles?
Peptide and protein based methods are another approach to developing bionanoplasmic nanoplatforms. The approach is mainly based on two strategies: nanoplatforms modified by targeting ligands and nanoplatforms based on protein modifications. For the first strategy, ligands are modified on NPs, giving the NPs cell-like long circulation times and specific targeting capabilities for glioma diagnosis, therapy and therapeutic diagnostic applications. The second strategy involves the design of biologically active proteins as carriers for nanoplatforms with good biocompatibility for drug delivery.
Nanoparticles Modified with Targeting Ligands
Bioactive ligands with different physiological functions can be covalently linked to nanoplatforms and specifically direct their behavior according to actual clinical needs. Nanoplatforms modified with ligands can exhibit the properties of these peptides or proteins while increasing local drug concentrations and minimizing drug toxicity. In addition, functional nanoplatforms can be developed by designing and modulating peptide modal sequences that can achieve good responsiveness and target recognition to external stimuli (pH, temperature, pressure, etc.).
Liposomal Nanoparticles Modified with Individual Ligands
Liposomes, which consist of phospholipids that can form closed lipid bilayers in aqueous solvents, can readily form nanoscale particles. As a result, liposomal NPs have been extensively employed for drug delivery to encapsulate drugs and improve their water solubility.157,158 Liposomes are also biocompatible and nonimmunogenic,159,160 and can cross biological barriers such as CMs and the BBB. However, unmodified liposomes have significant limitations, such as a lack of target selectivity, short circulation time and poor in vivo stability.161 To overcome these limitations, liposomes are modified with ligands to have the ability to recognize specific receptors on the cell to form targeted liposomes. Ligand modification of liposomes can be achieved through three main strategies. The first approach is the membrane anchoring method, also known as the pre-conjugation strategy, where the ligand-lipid conjugate is prepared beforehand and then added to the lipid mixture during liposome preparation. This method is relatively straightforward but may result in ligand modification primarily on the inner layer of the liposome. Furthermore, certain ligands may be unstable in the presence of organic solvents. In the second approach, known as the post-suffix or post-insertion method, the targeting ligand is attached to functional groups on the liposome surface after liposome formulation. Compared to the first method, this approach avoids drug-ligand contact during encapsulation, preventing drug degradation or conformational changes of proteins and peptides, and ensures that the targeting ligand is located on the liposome surface.162
For example, liposome nanoplatforms can be constructed using proteins derived from CCMs. These bionanosomes are highly stable, exhibit excellent homologous targeting capabilities and immune escape characteristics, and can cross the BBB and target gliomas. These bionanosomes can enhance chemotherapeutic efficacy by carrying chemotherapeutic drugs31,163, and can carry ICG for infrared imaging to guide surgery as well as for PTT, efficiently suppressing glioma cell proliferation.164 In addition, Lin et al developed a targeted siRNA delivery system by assembling protamine/chondroitin sulfate/siRNA/cationic liposomes and modifying them with Seven-peptide (T7). T7 is a ligand that binds to the TF receptor (TfR), which is highly expressed in brain ECs. T7-mediated nucleoshell NPs can cross the BBB and serve as a ligand for a drug delivery system targeting gliomas.165
Furthermore, liposomes have been modified with peptides derived from the transmembrane protein transduction structural domain of HIV-1 virus (PTDHIV-1 peptide)166 and L1 papillomavirus type 16 capsid-derived lipopeptide (L1 motif sequence)167 to facilitate transmembrane transport. The PTDHIV-1 peptide can mediate the inward transport of various macromolecules and nanocarriers through the CM, and it has a high affinity for brain ECs at the BBB due to electrostatic attraction.166 The L1 motif sequence from the type 16 papillomavirus capsid protein can trigger heparan sulfate recognition and effective gene transfer to target cells via endocytosis.167 These liposomes can cross the BBB in the circulating blood system and accumulate in the glioma region.
Ismail et al proposed a unique ApoE-functionalized liposomal nanoplatform based on artesunate (ART)-phosphatidylcholine (ARTPC) encapsulated with temozolomide (ApoE-ARTPC@TMZ). ART can inhibit DNA repair through the Wnt/β-catenin signaling cascade, thereby enhancing the sensitivity of GBM to TMZ and synergistically inducing DNA damage and cell apoptosis.168
Wang et al have developed a biomimetic nanoplatform by co-modifying liposomes with apolipoprotein apoA-I biomimetic peptide (D4F) and α-Melittin (the main component of bee venom with immunomodulatory effects that can reshape the immune microenvironment and activate DCs. This nanoplatform is capable of carrying arsenic trioxide and manganese chloride (MnCl2) for achieving multimodal therapeutic and diagnostic capabilities with real-time guidance. The NPs effectively remodel the immune microenvironment, inhibit the recurrence and invasion of GBM. When used in combination with TMZ, the survival rate of GBM mice significantly improves.169
Liposomal Nanoparticles Modified with Multiple Ligands
To further improve transport efficiency, the construction of dual-targeted ligand-modified liposomes has been explored. The most common modifications thus far have been the binding of TF or TF-binding peptides to another ligand. TF is a plasma protein used for iron ion delivery. When the TfR is encountered, the iron-containing TF protein binds to the TfR and is endocytosed into cells. TfR is overexpressed on glioma cells. Therefore, coupling TF to drug-loaded liposomes can give them the ability to target gliomas. For instance, Tian et al created an epirubicin liposomes modified with tamoxifen (TAM) and TF for crossing the BBB and targeting gliomas. TAM, an antagonist of estrogen receptors, inhibits drug efflux from brain tumor cells and improves therapeutic efficacy.170
Li et al used TF and CCM proteins to modify liposomes coated with elemene and carbamazel, and excellent tumor targeting and immune escape ability was shown.171 Xue et al created a dual-targeting liposome, erythromycin liposomes modified with p-aminophenyl-alpha-D-mano-pyranoside and TF, for crossing the BBB and targeting gliomas.172 Mu et al obtained the TfR binding peptide TfR-T12 by phage display. This peptide was able to bind different sites of TfR. TfR-T12 and octa-arginine conjugated stearoyl-R8 were loaded onto liposomes. Stearoyl- R8 is a conjugate of octa-arginine and stearyl groups. Due to its multicationic center, stearoyl- r8 is also used as a functional material to allow lipid vesicles to cross the BBB and to mediate the endocytosis of vesicles. These liposomes can pass through the BBB and kill cancer cells by inducing necrosis, apoptosis and autophagy.173
In addition to using TF, Yang et al utilized Ang and neuropilin-1 receptor (tLyP-1) for brain tumor targeting and tumor infiltration. The core of the delivery system consisted of vascular endothelial growth factor (VEGF), siRNA and the chemotherapeutic agent DTX. The bipeptide-modified liposomes exhibited an ability to target glioma cells and augmented drug internalization via endocytosis and enhanced tissue penetration. The dipeptide-modified liposomes demonstrated superior gene silencing and antiproliferative activity, with minimal toxicity and no activation of the innate immune response.174
Other Nanoparticles Modified with Ligands
Besides the most common liposomes, there are also other NPs endowed with different functions by peptides. Wu et al have devised small-sized Zeolitic imidazolate framework 8 (ZIF-8) NPs modified with rabies virus glycoprotein 15 (RVG15) peptide (RVG15-PEG@DTX@ZIF-8) for targeted delivery of DTX to mouse GBM. These NPs have demonstrated exceptional BBB penetration rate and safety, effectively suppressing the growth and metastasis of GBM, thereby enhancing the survival rate of the mice175. Dube et al have developed a novel nanoparticle by combining anisotropic gold nanoroses (AuNs) with the chemotherapy drug DOX and the NIR responsive dye ICG. This nanoparticle is designed to facilitate combination chemotherapy and optical cell imaging, enabling enhanced therapeutic effects. Additionally, the nanoparticle incorporates a naive peptide drug with dual functionality, targeting the BBB and GBM specifically. This synergistic approach offers improved treatment outcomes for GBM and helps mitigate chemotherapy-related adverse events.176
The use of micellar polymers for drug targeting was first proposed by Yokoyama et al in 1992.177 The micelles have a multifunctional drug-carrying hydrophobic core and a biocompatible hydrophilic shell with dimensions of tens to a hundred nanometer. This size allows micelles to bypass the renal clearance and reticuloendothelial system and thus makes them good vehicles for drug transport through EPR within the tumor tissue. Moreover, the modification of peptides on polymer micelles can improve their biological properties.178,179 For example, Ran et al developed a variety of peptide modification micelles such as the quorum-sensing, d-peptide RI-VAP and d-vap (Figure 7), GRP78 protein and “y-type” quorum-sensing receptor “well-designed ligands”. After these well-designed peptide modifications, the accumulation of micelles in gliomas was significantly enhanced and showed no immunogenicity or cytotoxicity, ensuring safety for use in vivo.180–182 Ruan et al reduced receptor-associated protein (RAP) to a short RAP12 helper peptide modified on the surface of PEG-PLA micelles. RAP is a chaperone protein that binds tightly to low-density lipoprotein receptor-associated protein-1, which is overexpressed in gliomas, the BBB/BBTB; participates in tumor neovascularization; serves as an angiogenesis mimic; and can effectively facilitate micelle penetration into BBB/BBTB.183
 |
Figure 7 Targeting ability of different conformations of tumor homing peptide (l-VAP) to gliomas and their antitumor mechanisms. Note: Reprinted from Journal of Controlled Release, 255, Ran D, Mao J, Shen Q, et al. GRP78 enabled micelle-based glioma targeted drug delivery. 120–131, Copyright 2017, with permission from Elsevier.180 |
Nanoparticles Based on Proteins
Proteins have gained significant attention as highly effective biological carriers in the synthesis of NPs due to their distinctive three-dimensional structures, unique sequences, and self-assembly properties. In addition, the abundance of chemical groups and binding sites present in proteins facilitates further modifications, thus enhancing their versatility in drug delivery applications to glioma sites. Furthermore, proteins possess superior biocompatibility, biodegradability, low antigenicity, and high stability, all of which are crucial characteristics that make them exceptional carriers for drug delivery to glioma sites. Consequently, the use of proteins as carriers for drug delivery has become increasingly popular in recent years.
Albumin
One of the most common proteins in plasma is albumin, which has multiple ligand binding sites and can bind ligands through covalent, noncovalent or fusion expression, making it an ideal carrier for hydrophobic substance delivery. Yang et al created a biomimetic peroxidase-albumin phototherapeutic nanoprobe that enables precision imaging to guide surgery. Through albumin-binding protein-mediated transport, this nanoprobe can penetrate the BBB and accumulate to deep gliomas. In addition, the nanoprobe can induce hyperthermia and decompose H2O2 into oxygen, further amplifying the effect of phototherapy (Figure 8).184
 |
Figure 8 Schematic design of a peroxidase-albumin integrated phototherapeutic nanoprobe for precise imaging-guided surgery and assisted phototherapy for gliomas. Note: Reprinted from ACS Nano, 14(5), Yang Z, Du Y, Sun Q, et al. Albumin-Based Nanotheranostic Probe with Hypoxia Alleviating Potentiates Synchronous Multimodal Imaging and Phototherapy for Glioma. 6191–6212, Copyright 2020, with permission from American Chemical Society.184 |
Furthermore, the tumor-targeting ability of albumin can be further enhanced by ligand modification. Lin et al employed a cell-penetrating peptide, LMWP, to modify albumin NPs to improve BBB permeability and tumor targeting, and promote the uptake of NPs by tumor cells.185 Zhu et al designed an albumin nanosystem modified with brain-targeting peptides for the delivery of TGF-β receptor I inhibitors and mTOR inhibitors. These NPs can cross the BBB and target nAChRs overexpressed on tumor cells, thus facilitating drug delivery to gliomas.186 In addition, Zhao et al demonstrated that albumin NPs modified with dual ligands (TfR-binding peptide T12 and mannose) can efficiently pass through the BBB and target nutrient transporters overexpressed in glioma cells, thus enabling biomimetic delivery to gliomas.187
Ferritin
In addition to albumin, ferritin is also commonly used as a nanoplatform carrier. Mo et al developed cell-penetrating peptide lactoferrin NPs. They coencapsulated simvastatin and fenpropidin in D-α-tocopherol polyethylene glycol succinate to form an amphiphilic structure. Targeted biodelivery is achieved through low-density lipoprotein receptor-related protein 1. This lactoferrin nanoparticle enhances glioma treatment through tumour associated macrophages repolarization and ROS modulation mechanisms.188 Wang et al constructed a fusion protein loaded with interferon gene stimulating factor by genetically engineering different glioma-targeting molecules to fuse to a self-assembled ferritin. These NPs were able to penetrate deep tissues and target gliomas after crossing the BBB. These bioengineered ferritin NPs, as a drug delivery carrier, can enable the targeted delivery of immunomodulators into glioma and improve the efficacy of immunotherapy.189
Jia et al190 have developed a biomimetic nanoplatform, termed HFn-Cu-REGO NPs, by utilizing human heavy chain ferritin (HFn) loaded with Cu ions and the chemotherapeutic drug regorafenib. Within this nanoplatform, HFn imparts the ability for BBB penetration, tumor targeting, and pH responsiveness. Regorafenib effectively inhibits autophagosome-lysosome fusion and induces autophagy arrest, while Cu2+ disrupts Cu homeostasis in GBM cells, leading to Cu-induced cell death and achieving significant GBM suppression.
Lipoprotein
Lipoproteins are also commonly used as nanoplatform carriers. For example, Geng et al modified low-density lipoproteins (LDL) with Ang to deliver targeted drugs to the brain. Synergistic targeting of Ang peptide and lipoprotein B increases the ability to cross the BBB, further enhancing the permeability of the nanocarrier to tumor tissue and improving the GBM therapeutic efficacy.191 The E3-reconfigured high-density lipoprotein (HDL) created by Huang et al was used to wrap calcium phosphate loaded with siRNA, facilitating its penetration into the BBB and targeting GBM cells. The nanostructures exerted significant RNA interference efficiency, increased apoptosis and inhibited tumor cell growth in GBM cells.192 Taking inspiration from the natural structure and properties of HDL, Wang et al developed a lipoprotein with tumor-penetrating abilities by fusing tLyP-1 with apolipoprotein A-I-mimicking peptides, incorporating ICG, and anchoring a surface of lipophilic siRNA targeted hypoxia-inducible factor-1 for photogene therapy at specific sites. The tLyP-1 peptide was incorporated onto the surface of HDL to facilitate BBB permeability, tumor-homing capacity, and the accumulation of photosensitizers and siRNA at the tumor site.193
Protein Hybrid Nanoparticles
Wu et al designed a novel multifunctional protein hybrid nanoplatform consisting of TF and oxygen-carrying hemoglobin (ODP-TH). TRF is an iron carrier protein that can recognize TfR on the surface of tumor cells, enabling tumor targeting. Hemoglobin (Hb) has the ability to carry oxygen and autonomously release it in hypoxic regions deep within tumors, significantly alleviating TME hypoxia and helping overcome tumor drug resistance for better anti-tumor effects. This protein hybrid framework is further engineered to encapsulate the photosensitizer protoporphyrin IX (PpIX) and DOX, allowing penetration through the BBB and selective accumulation in hypoxic tumors through protein homology recognition. It downregulates the expression of multidrug resistance gene 1 (MDR1) and hypoxia-inducible factor-1α (HIF-1α), resulting in improved therapeutic outcomes.194
Hydrogel
Hydrogels, which are water-soluble, three-dimensional polymer networks, have garnered significant interest in recent years due to their tunable physicochemical properties.195 The hydrophilic functional groups attached to the polymer backbone facilitate water absorption and retention, while cross-linking between the polymer chains ensures their stability in an aqueous environment. In addition, hydrogels are responsive to various external stimuli, such as changes in pH or temperature.196 Biodegradable hydrogels that can be injected have emerged as a promising option for intratumoral drug delivery, as they allow continuous and controlled drug release at the tumor site while minimizing systemic drug exposure and associated adverse effects.197 Moreover, they can promote tissue repair in the surrounding area postinjury.198 As an illustration, PTT may cause harm to adjacent tissue by means of photoabsorber leakage. To overcome this challenge, Yao et al created a green hydrogel that utilizes a protein-based biomaterial, silk fibroin, to incorporate biliverdin. The enhanced biliverdin concentration accelerates the heating process and raises the final temperature. This biomimetic hydrogel not only enables photothermal-mediated ablation of glioma cells but also facilitates tissue reconstruction and wound healing.199
Challenges and Solutions
The treatment of glioma has always been a pressing clinical problem, and the BBB poses a challenge for glioma treatment. The application of NPs has led to a new possibilities in drug delivery for glioma treatment, and the therapeutic effect is significantly enhanced. However, conventional nanomaterials have certain limitations. Biological NPs have garnered significant attention in recent years. Biological nanomaterials have good biocompatibility and can precisely target tumors and control drug release, greatly improving biosafety and reducing side effects. Despite significant progress being made with bionanoparticle platforms, there is still a considerable distance to cover before their clinical application can be realized.
First, the safety of bionanomaterials needs to be further explored. There are thousands of proteins present on the surface of CMs, but only a limited number of them have been identified as tumor-specific antigens that can be targeted. Moreover, immune-related biofilm and protein-like modifications can induce an in vivo immune response and induce or exacerbate inflammation. Therefore, further studies on immunogenicity and potential cytotoxicity are required before clinical use. Topical intratumoural administration via hydrogels may allow intratumoural drug aggregation and to some extent ameliorate the systemic tissue and organ toxicity that results from systemic administration.200 The hydrogel also has a slow-release feature that allows timed and dosed drug release, reducing the overactivation of the immune system triggered by uncontrolled drug release.
Another important aspect to take into consideration is the formation of PC, as this can potentially impact the release profile, targeting ability, and therapeutic efficacy of the encapsulated drugs, while also decreasing the half-life of NPs.201,202 The modification of nanomaterials (eg polyethylene glycol) can effectively reduce the adsorption of proteins on their surface, thus maintaining their targeting properties. The presence of protein crowns also has several benefits, such as effectively mediating the phagocytosis of nanomaterials by cells and alleviating the acute cytotoxicity of positively charged nanomaterials. Studying PCs can provide valuable insights into the mechanisms and processes by which nanomaterials function in biological applications. By regulating PCs, it is possible to mitigate the negative effects of nanomaterials and enhance their efficacy in biomedical applications. Advanced techniques such as synchrotron radiation, cryo-electron microscopy, and 4D ultrafast electron diffraction can be employed to analyze the structure of protein/peptide-related NPs, leading to a better understanding of their interactions with the biological environment and potential for targeted drug delivery.203
Furthermore, the biological applications of NPs can be extended by building modular peptides or novel multifunctional proteins. This process involves designing nanoplatforms with multiple structural domains, one of which is explicitly designed for a particular function, while the others remain available for other purposes, such as drug delivery, and targeting. Such advanced functional integration can enhance the efficiency of nanomaterial development and facilitate nanomaterial management.203
In addition, although some bionanostructural modifications have enabled nanomaterials to have active targeting capabilities, most of these drug carriers currently lack propulsion and navigation capabilities and suffer from low drug delivery efficiency, short cycle times and poor permeability.204 Nanomotors are nanosystems capable of converting chemical or other forms of energy from the surrounding environment into their own mechanical motion.205 With the outstanding advantages of small size, autonomous motion and strong drug loading capacity, nanomotors can achieve intelligent propulsion and spatiotemporal control,206–208 and have excellent application prospects in improving drug delivery efficiency in the treatment of gliomas.
Finally, the clinical translation of NPs is challenging. First, the complex and inefficient process of preparing membrane-encapsulated NPs, coupled with the difficulty of generating bionanoparticles with high purity and integrity for large-scale production, restricts their further application. The advent of microfluidics in recent years has enabled the collection of Exos with higher purity and recovery rates,125 offering the possibility of large-scale production. Furthermore, in vitro models may not fully simulate the complex physiological effects in vivo, including interactions between nanomedicines and the immune system or other cells in the TME. Therefore, it is crucial to use in vivo models and clinical trials to evaluate the biosafety and efficacy of cancer nanodrugs. The nanoplatform in question is now in clinical trials for application in glioma. Kumthekar et al used brain-permeable RNA interference-based spherical nucleic acid (NU-0129) to treat patients with postoperative recurrent GBM or gliosarcoma. siRNA-based SNA can be safely administered by microdose intravenous administration, crosses the BBB/BBTB, and accumulates in GBM tumor cells with reduced target protein levels without acute or long-term toxicity.209 In addition, there are selected clinical trials underway, including a Phase I trial of the nanoliposome CPT-11 in patients with recurrent high-grade glioma. Polysiloxane Gd-Chelates based NPs (AGuIX) in combination with radiotherapy plus temozolomide for newly diagnosed GBM. Panobinostat Nanoparticle Formulation MTX110 for the treatment of newly diagnosed diffuse endogenous pontine glioma by convection-enhanced delivery. Convection-enhanced, image-assisted delivery of liposomal irinotecan in recurrent high-grade glioma. Water-soluble Panobinostat nanoparticle formulation MTX110 and gadolinium is safe for use in children with newly diagnosed diffuse midline glioma. Phase II study of temozolomide in combination with the cationic liposome complex SGT-53 for recurrent GBM. However, there are fewer clinical trials related to bionanomaterials, and further studies are needed in the future. Additionally, biomarkers and imaging tools can provide valuable information about the distribution, metabolism, and elimination of nanodrugs in vivo, which can aid in optimizing the design and dosing of nanodrugs for clinical translation.
Conclusion and Perspectives
In conclusion, as research in the medical field continues to evolve, bio-NPs are a promising multifunctional method due to their natural advantages. The unique properties of natural CMs and their derivatives make them an ideal choice for promoting prolonged cycle times, immune escape and homologous targeting. Ligand modifications can also provide appropriate biological functions to nanoplatforms, while proteins have a high degree of specificity and modification flexibility, making them excellent carriers. Moreover, bio-NPs are highly customizable and can be personalized by adjusting their size, shape, surface charge, hydrophilic/hydrophobic properties, chemical composition and structure, as well as their surface reactivity and chemistry. These properties of bio-NPs indicate their great potential for use in precision medicine. Notably, bionanoparticles show promise in the treatment of glioma, a type of brain tumor that poses significant challenges due to its location and invasiveness. The ability to target glioma cells specifically with bionanoparticles can significantly improve treatment outcomes, while the biocompatible, biodegradable, and bio-eliminable nature of these materials provides an added layer of safety for patients. With their unique properties and potential for personalized medicine, bionanoparticles offer hope for more effective, targeted, and less invasive treatments for patients with glioma and other diseases. In short, the potential of bio-nanoparticles in the field of medicine is immense and constantly evolving. Although there are still limitations, we believe that the ongoing development and optimization of bionanoplasmic nanoplatforms will lead to more groundbreaking advancements and provide better treatment options for patients with glioma in the years ahead.
Abbreviations
CNS, central nervous system; GBMs, glioblastomas; PDT, photodynamic therapy; PTT, photothermal therapy; SDT, sonodynamic therapy; CDT, chemodynamic therapy; BBB, blood-brain barrier; ECs, endothelial cells; TJs, tight junctions; NPs, nanoparticles; EPR, enhanced permeability and retention; CM, cell membrane; RBCs, red blood cells; TF, transferrin; CCM, cancer CM; CCM-NPs, cancer CM-encapsulated NPs; NE, neutrophils; NK, natural killer; CAMs, cell adhesion molecules; TME, tumor microenvironment; EVs, Extracellular vesicles; Exos, Exosomes; US, ultrasound; GQDs, graphene quantum dots; DOX, doxorubicin; RBCm, RBC membranes; BBTB, blood-brain tumor barrier; MPI, Magnetic Particle Imaging; SPIO, superparamagnetic iron oxide; NIR, Near-infrared; CSF, cerebrospinal fluid; MNPs, mimetic NPs; TMZ, temozolomide; CDDP, Cisplatin; NS, Nanosuspension; LEX, lexiscan; nAChRs, nicotinic acetylcholine receptors; PTX, paclitaxel containing; DNs, heparin-loaded NPs; DTX, docetaxel; DC, dendritic cell; GASC, glioma-associated stromal cells; PLGA, polylactic-co-glycolic acid; AIE, aggregation-induced emission; PC, protein corona; EM, Exo mimic; ROS, reactive oxygen species; Dp44mT, di-2-pyridone-4,4-dimethyl-3-thiosemicarbazone; ApoE, apolipoprotein E; RAPA, rapamycin; ZOL, Zoledronate; siRNA, small molecule interfering RNA; Ang, Angiopep-2; BMSC, bone marrow mesenchymal stem cell; LA, lactate; PA, pyruvate; GOX, glucose oxidase; MOF, metal-organic framework; ICG, indocyanine green; T7, Seven-peptide; TfR, transferrin receptor; PTDHIV-1 peptide, protein transduction structural domain of HIV-1 virus; ART, artesunate; ARTPC, artesunate-phosphatidylcholine; TAM, tamoxifen; tLyP-1, neuropilin-1 receptor; VEGF, vascular endothelial growth factor; ZIF-8, Zeolitic imidazolate framework 8; RVG15, rabies virus glycoprotein 15; AuNs, gold nanoroses; RAP12, receptor-associated protein; HFn, human heavy chain ferritin; LDL, low-density lipoproteins; HDL, high-density lipoprotein; ODP-TH, oxygen-carrying hemoglobin; Hb, Hemoglobin; PpIX, protoporphyrin IX; MDR1, multidrug resistance gene 1; HIF-1α, hypoxia-inducible factor-1α.
Acknowledgments
We acknowledge funding by the National Natural Science Foundation of China (82102005 and 82271943), the Guangdong Basic and Applied Basic Research Foundation (2023A1515012660), the Fundamental Research Funds for the Central Universities (21622102), and the Medical Joint Fund of Jinan University (YXJC2022008).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ostrom QT, Price M, Neff C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro Oncol. 2022;24(Suppl 5). doi:10.1093/neuonc/noac202
2. Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. doi:10.1001/jama.2013.280319
3. Paw I, Carpenter RC, Watabe K, Debinski W, Lo H-W. Mechanisms regulating glioma invasion. Cancer Lett. 2015;362(1):1–7. doi:10.1016/j.canlet.2015.03.015
4. Albert FK, Forsting M, Sartor K, Adams HP, Kunze S. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery. 1994;34(1):45–60; discussion 60–1. doi:10.1097/00006123-199401000-00008
5. Koo Y-EL, Reddy GR, Bhojani M, et al. Brain cancer diagnosis and therapy with nanoplatforms. Adv Drug Deliv Rev. 2006;58(14):1556–1577. doi:10.1016/j.addr.2006.09.012
6. Ji B, Wei M, Yang B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics. 2022;12(1):434–458. doi:10.7150/thno.67300
7. Lu L, Wang K, Lin C, et al. Constructing nanocomplexes by multicomponent self-assembly for curing orthotopic glioblastoma with synergistic chemo-photothermal therapy. Biomaterials. 2021;279:121193. doi:10.1016/j.biomaterials.2021.121193
8. Bunevicius A, Pikis S, Padilla F, Prada F, Sheehan J. Sonodynamic therapy for gliomas. J Neurooncol. 2022;156(1):1–10. doi:10.1007/s11060-021-03807-6
9. Li C, Wan Y, Zhang Y, et al. In situ sprayed starvation/chemodynamic therapeutic gel for post-surgical treatment of IDH1 (R132H) glioma. Adv Mater. 2022;34(5):e2103980. doi:10.1002/adma.202103980
10. Tamura R, Miyoshi H, Yoshida K, Okano H, Toda M. Recent progress in the research of suicide gene therapy for malignant glioma. Neurosurg Rev. 2021;44(1):29–49. doi:10.1007/s10143-019-01203-3
11. Hosseini R, Sarvnaz H, Arabpour M, et al. Cancer exosomes and natural killer cells dysfunction: biological roles, clinical significance and implications for immunotherapy. Mol Cancer. 2022;21(1):15. doi:10.1186/s12943-021-01492-7
12. Dong X. Current strategies for brain drug delivery. Theranostics. 2018;8(6):1481–1493. doi:10.7150/thno.21254
13. Terstappen GC, Meyer AH, Bell RD, Zhang W. Strategies for delivering therapeutics across the blood-brain barrier. Nat Rev Drug Discov. 2021;20(5):362–383. doi:10.1038/s41573-021-00139-y
14. Yang K, Wu Z, Zhang H, et al. Glioma targeted therapy: insight into future of molecular approaches. Mol Cancer. 2022;21(1):39. doi:10.1186/s12943-022-01513-z
15. Maeda H. SMANCS and polymer-conjugated macromolecular drugs: advantages in cancer chemotherapy. Adv Drug Deliv Rev. 2001;46(1–3):169–185. doi:10.1016/S0169-409X(00)00134-4
16. Wang J, Li Y, Nie G. Multifunctional biomolecule nanostructures for cancer therapy. Nat Rev Mater. 2021;6(9):766–783. doi:10.1038/s41578-021-00315-x
17. Quader S, Kataoka K. Nanomaterial-enabled cancer therapy. Mol Ther. 2017;25(7):1501–1513. doi:10.1016/j.ymthe.2017.04.026
18. Tsoi KM, MacParland SA, X-z M, et al. Mechanism of hard-nanomaterial clearance by the liver. Nat Mater. 2016;15(11):1212–1221. doi:10.1038/nmat4718
19. Fan Z, Zhu P, Zhu Y, Wu K, Li CY, Cheng H. Engineering long-circulating nanomaterial delivery systems. Curr Opin Biotechnol. 2020;66:131–139. doi:10.1016/j.copbio.2020.07.006
20. Chen L, Hong W, Ren W, Xu T, Qian Z, He Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct Target Ther. 2021;6(1):225. doi:10.1038/s41392-021-00631-2
21. Attarilar S, Yang J, Ebrahimi M, et al. The toxicity phenomenon and the related occurrence in metal and metal oxide nanoparticles: a brief review from the biomedical perspective. Front Bioeng Biotechnol. 2020;8:822. doi:10.3389/fbioe.2020.00822
22. Yang Q, Lai SK. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(5):655–677. doi:10.1002/wnan.1339
23. Tang W, Fan W, Lau J, Deng L, Shen Z, Chen X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem Soc Rev. 2019;48(11):2967–3014. doi:10.1039/c8cs00805a
24. Pehlivan SB. Nanotechnology-based drug delivery systems for targeting, imaging and diagnosis of neurodegenerative diseases. Pharm Res. 2013;30(10):2499–2511. doi:10.1007/s11095-013-1156-7
25. Li B, Wang F, Gui L, He Q, Yao Y, Chen H. The potential of biomimetic nanoparticles for tumor-targeted drug delivery. Nanomedicine. 2018;13(16):2099–2118. doi:10.2217/nnm-2018-0017
26. Menilli L, Milani C, Reddi E, Moret F. Overview of nanoparticle-based approaches for the combination of Photodynamic Therapy (PDT) and chemotherapy at the preclinical stage. Cancers. 2022;14(18):4462. doi:10.3390/cancers14184462
27. Liu Y, Luo J, Chen X, Liu W, Chen T. Cell membrane coating technology: a promising strategy for biomedical applications. Nanomicro Lett. 2019;11(1). doi:10.1007/s40820-019-0330-9
28. Luk BT, Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release. 2015;220(Pt B):600–607. doi:10.1016/j.jconrel.2015.07.019
29. Parayath NN, Amiji MM. Therapeutic targeting strategies using endogenous cells and proteins. J Control Release. 2017;258:81–94. doi:10.1016/j.jconrel.2017.05.004
30. Neumann E, Frei E, Funk D, et al. Native albumin for targeted drug delivery. Expert Opin Drug Deliv. 2010;7(8):915–925. doi:10.1517/17425247.2010.498474
31. Jia Y, Sheng Z, Hu D, et al. Highly penetrative liposome nanomedicine generated by a biomimetic strategy for enhanced cancer chemotherapy. Biomater Sci. 2018;6(6):1546–1555. doi:10.1039/c8bm00256h
32. Zhuang D, Zhang H, Hu G, Guo B. Recent development of contrast agents for magnetic resonance and multimodal imaging of glioblastoma. J Nanobiotechnology. 2022;20(1):284. doi:10.1186/s12951-022-01479-6
33. Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108(27):10980–10985. doi:10.1073/pnas.1106634108
34. Men X, Geng X, Zhang Z, et al. Biomimetic semiconducting polymer dots for highly specific NIR-II fluorescence imaging of glioma. Mater Today Bio. 2022;16:100383. doi:10.1016/j.mtbio.2022.100383
35. Wang Z, Zhang M, Chi S, Zhu M, Wang C, Liu Z. Brain tumor cell membrane-coated lanthanide-doped nanoparticles for NIR-IIb luminescence imaging and surgical navigation of glioma. Adv Healthc Mater. 2022;11(16):e2200521. doi:10.1002/adhm.202200521
36. Zou Y, Wang Y, Xu S, et al. Brain co-delivery of temozolomide and cisplatin for combinatorial glioblastoma chemotherapy. Adv Mater. 2022;34(33):e2203958. doi:10.1002/adma.202203958
37. Fan Y, Hao W, Cui Y, et al. Cancer cell membrane-coated nanosuspensions for enhanced chemotherapeutic treatment of glioma. Molecules. 2021;26(16):5103.
38. Ren Y, Miao C, Tang L, et al. Homotypic cancer cell membranes camouflaged nanoparticles for targeting drug delivery and enhanced chemo-photothermal therapy of glioma. Pharmaceuticals. 2022;15(2):157. doi:10.3390/ph15020157
39. Zhu M, Wu P, Li Y, Zhang L, Zong Y, Wan M. Synergistic therapy for orthotopic gliomas biomimetic nanosonosensitizer-mediated sonodynamic therapy and ferroptosis. Biomater Sci. 2022;10(14):3911–3923. doi:10.1039/d2bm00562j
40. Liu G, Zhu J, Guo H, et al. Mo2C-derived polyoxometalate for NIR-II photoacoustic imaging-guided chemodynamic/photothermal synergistic therapy. Angew Chem Int Ed. 2019;58(51):18641–18646. doi:10.1002/anie.201910815
41. Du J, Sun J, Liu X, et al. Preparation of C6 cell membrane-coated doxorubicin conjugated manganese dioxide nanoparticles and its targeted therapy application in glioma. Eur J Pharm Sci. 2022;180:106338. doi:10.1016/j.ejps.2022.106338
42. Lu G, Wang X, Li F, et al. Engineered biomimetic nanoparticles achieve targeted delivery and efficient metabolism-based synergistic therapy against glioblastoma. Nat Commun. 2022;13(1):4214. doi:10.1038/s41467-022-31799-y
43. Huang X, Hui H, Shang W, et al. Deep penetrating and sensitive targeted magnetic particle imaging and photothermal therapy of early-stage glioblastoma based on a biomimetic nanoplatform. Adv Sci. 2023:e2300854. doi:10.1002/advs.202300854
44. Zhao P, Tian Y, Lu Y, et al. Biomimetic calcium carbonate nanoparticles delivered IL-12 mRNA for targeted glioblastoma sono-immunotherapy by ultrasound-induced necroptosis. J Nanobiotechnology. 2022;20(1):525. doi:10.1186/s12951-022-01731-z
45. Kong J, Zou R, Law G-L, Wang Y. Biomimetic multifunctional persistent luminescence nanoprobes for long-term near-infrared imaging and therapy of cerebral and cerebellar gliomas. Sci Adv. 2022;8(10):eabm7077. doi:10.1126/sciadv.abm7077
46. Chai Z, Ran D, Lu L, et al. Ligand-modified cell membrane enables the targeted delivery of drug nanocrystals to glioma. ACS Nano. 2019;13(5):5591–5601. doi:10.1021/acsnano.9b00661
47. Fu S, Liang M, Wang Y, et al. Dual-modified novel biomimetic nanocarriers improve targeting and therapeutic efficacy in glioma. ACS Appl Mater Interfaces. 2019;11(2):1841–1854. doi:10.1021/acsami.8b18664
48. Zou Y, Liu Y, Yang Z, et al. Effective and targeted human orthotopic glioblastoma xenograft therapy via a multifunctional biomimetic nanomedicine. Adv Mater. 2018;30(51):e1803717. doi:10.1002/adma.201803717
49. Chai Z, Hu X, Wei X, et al. A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. J Control Release. 2017;264:102–111. doi:10.1016/j.jconrel.2017.08.027
50. Zhang D, Tian S, Liu Y, et al. Near infrared-activatable biomimetic nanogels enabling deep tumor drug penetration inhibit orthotopic glioblastoma. Nat Commun. 2022;13(1):6835. doi:10.1038/s41467-022-34462-8
51. Liu Y, Zou Y, Feng C, et al. Charge conversional biomimetic nanocomplexes as a multifunctional platform for boosting orthotopic glioblastoma RNAi therapy. Nano Lett. 2020;20(3):1637–1646. doi:10.1021/acs.nanolett.9b04683
52. Ismail M, Yang W, Li Y, et al. Biomimetic Dp44mT-nanoparticles selectively induce apoptosis in Cu-loaded glioblastoma resulting in potent growth inhibition. Biomaterials. 2022;289:121760. doi:10.1016/j.biomaterials.2022.121760
53. He W, Li X, Morsch M, et al. Brain-targeted codelivery of Bcl-2/Bcl-xl and Mcl-1 inhibitors by biomimetic nanoparticles for orthotopic glioblastoma therapy. ACS Nano. 2022;16(4):6293–6308. doi:10.1021/acsnano.2c00320
54. Ke R, Zhen X, Wang H-S, et al. Surface functionalized biomimetic bioreactors enable the targeted starvation-chemotherapy to glioma. J Colloid Interface Sci. 2022;609:307–319. doi:10.1016/j.jcis.2021.12.009
55. Pang L, Qin J, Han L, et al. Exploiting macrophages as targeted carrier to guide nanoparticles into glioma. Oncotarget. 2016;7(24):37081–37091. doi:10.18632/oncotarget.9464
56. Pang L, Zhu Y, Qin J, Zhao W, Wang J. Primary M1 macrophages as multifunctional carrier combined with PLGA nanoparticle delivering anticancer drug for efficient glioma therapy. Drug Deliv. 2018;25(1):1922–1931. doi:10.1080/10717544.2018.1502839
57. Wang S, Shen H, Mao Q, et al. Macrophage-mediated porous magnetic nanoparticles for multimodal imaging and postoperative photothermal therapy of gliomas. ACS Appl Mater Interfaces. 2021;13(48):56825–56837. doi:10.1021/acsami.1c12406
58. Xiao T, He M, Xu F, et al. Macrophage membrane-camouflaged responsive polymer nanogels enable magnetic resonance imaging-guided chemotherapy/chemodynamic therapy of orthotopic glioma. ACS Nano. 2021;15(12):20377–20390. doi:10.1021/acsnano.1c08689
59. Yin T, Fan Q, Hu F, et al. Engineered macrophage-membrane-coated nanoparticles with enhanced PD-1 expression induce immunomodulation for a synergistic and targeted antiglioblastoma activity. Nano Lett. 2022;22(16):6606–6614. doi:10.1021/acs.nanolett.2c01863
60. Liu B, Ji Q, Cheng Y, et al. Biomimetic GBM-targeted drug delivery system boosting ferroptosis for immunotherapy of orthotopic drug-resistant GBM. J Nanobiotechnology. 2022;20(1):161. doi:10.1186/s12951-022-01360-6
61. Qiao S, Cheng Y, Liu M, et al. Chemoattractants driven and microglia based biomimetic nanoparticle treating TMZ-resistant glioblastoma multiforme. J Control Release. 2021;336:54–70. doi:10.1016/j.jconrel.2021.06.015
62. Xue J, Zhao Z, Zhang L, et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12(7):692–700. doi:10.1038/nnano.2017.54
63. Deng G, Peng X, Sun Z, et al. Natural-killer-cell-inspired nanorobots with aggregation-induced emission characteristics for near-infrared-II fluorescence-guided glioma theranostics. ACS Nano. 2020;14(9):11452–11462. doi:10.1021/acsnano.0c03824
64. Ma X, Kuang L, Yin Y, et al. Tumor-antigen activated dendritic cell membrane-coated biomimetic nanoparticles with orchestrating immune responses promote therapeutic efficacy against glioma. ACS Nano. 2023;17(3):2341–2355. doi:10.1021/acsnano.2c09033
65. Geng X, Gao D, Hu D, et al. Active-targeting NIR-II phototheranostics in multiple tumor models using platelet-camouflaged nanoprobes. ACS Appl Mater Interfaces. 2020;12(50):55624–55637. doi:10.1021/acsami.0c16872
66. Wu L, Li Q, Deng J, et al. Platelet-tumor cell hybrid membrane-camouflaged nanoparticles for enhancing therapy efficacy in glioma. Int J Nanomedicine. 2021;16:8433–8446. doi:10.2147/IJN.S333279
67. Hao W, Cui Y, Fan Y, et al. Hybrid membrane-coated nanosuspensions for multi-modal anti-glioma therapy via drug and antigen delivery. J Nanobiotechnology. 2021;19(1):378. doi:10.1186/s12951-021-01110-0
68. Jiao X, Yu X, Gong C, et al. Erythrocyte-cancer hybrid membrane-camouflaged mesoporous silica nanoparticles loaded with gboxin for glioma-targeting therapy. Curr Pharm Biotechnol. 2022;23(6):835–846. doi:10.2174/1389201022666210719164538
69. Wang M, C-y L, S-A L, et al. Near infrared light fluorescence imaging-guided biomimetic nanoparticles of extracellular vesicles deliver indocyanine green and paclitaxel for hyperthermia combined with chemotherapy against glioma. J Nanobiotechnology. 2021;19(1):210. doi:10.1186/s12951-021-00907-3
70. J-Y W, Y-J L, X-b H, et al. Exosomes and biomimetic nanovesicles-mediated anti-glioblastoma therapy: a head-to-head comparison. J Control Release. 2021;336:510–521. doi:10.1016/j.jconrel.2021.07.004
71. J-y W, Y-j L, Wang J, et al. Multifunctional exosome-mimetics for targeted anti-glioblastoma therapy by manipulating protein Corona. J Nanobiotechnology. 2021;19(1):405. doi:10.1186/s12951-021-01153-3
72. Rehman FU, Liu Y, Yang Q, et al. Heme oxygenase-1 targeting exosomes for temozolomide resistant glioblastoma synergistic therapy. J Control Release. 2022;345:696–708. doi:10.1016/j.jconrel.2022.03.036
73. Niu W, Xiao Q, Wang X, et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21(3):1484–1492. doi:10.1021/acs.nanolett.0c04753
74. Santos-Coquillat A, Herreros-Pérez D, Samaniego R, et al. Dual-labeled nanoparticles based on small extracellular vesicles for tumor detection. Biol Direct. 2022;17(1):31. doi:10.1186/s13062-022-00345-7
75. Liang S-F, Zuo -F-F, Yin B-C, Ye B-C. Delivery of siRNA based on engineered exosomes for glioblastoma therapy by targeting STAT3. Biomater Sci. 2022;10(6):1582–1590. doi:10.1039/d1bm01723c
76. Pietras A, Katz AM, Ekström EJ, et al. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014;14(3):357–369. doi:10.1016/j.stem.2014.01.005
77. Chen Z, Zhao P, Luo Z, et al. Cancer cell membrane–biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10(11):10049–10057. doi:10.1021/acsnano.6b04695
78. Wang H, Liu Y, He R, et al. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater Sci. 2020;8(2):552–568. doi:10.1039/c9bm01392j
79. Rao L, L-L B, Cai B, et al. Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv Mater. 2016;28(18):3460–3466. doi:10.1002/adma.201506086
80. Dale GL, Kuhl W, Beutler E. Incorporation of glucocerebrosidase into gaucher’s disease monocytes in vitro. Proc Natl Acad Sci. 1979;76(1):473–475. doi:10.1073/pnas.76.1.473
81. Sun Y, Su J, Liu G, et al. Advances of blood cell-based drug delivery systems. Eur J Pharm Sci. 2017;96:115–128. doi:10.1016/j.ejps.2016.07.021
82. Pierigè F, Serafini S, Rossi L, Magnani M. Cell-based drug delivery. Adv Drug Deliv Rev. 2008;60(2):286–295. doi:10.1016/j.addr.2007.08.029
83. Javed S, Alshehri S, Shoaib A, et al. Chronicles of nanoerythrosomes: an erythrocyte-based biomimetic smart drug delivery system as a therapeutic and diagnostic tool in cancer therapy. Pharmaceutics. 2021;13(3):368. doi:10.3390/pharmaceutics13030368
84. Mohale S, Kunde SS, Wairkar S. Biomimetic fabrication of nanotherapeutics by leukocyte membrane cloaking for targeted therapy. Colloids Surf B Biointerfaces. 2022;219:112803. doi:10.1016/j.colsurfb.2022.112803
85. Fukuta T, Yoshimi S, Tanaka T, Kogure K. Leukocyte-mimetic liposomes possessing leukocyte membrane proteins pass through inflamed endothelial cell layer by regulating intercellular junctions. Int J Pharm. 2019;563:314–323. doi:10.1016/j.ijpharm.2019.04.027
86. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi:10.1016/S0140-6736(00)04046-0
87. Brynskikh AM, Zhao Y, Mosley RL, et al. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson’s disease. Nanomedicine. 2010;5(3):379–396. doi:10.2217/nnm.10.7
88. Jinushi M, Komohara Y. Tumor-associated macrophages as an emerging target against tumors: creating a new path from bench to bedside. Biochim Biophys Acta. 2015;1855(2):123–130. doi:10.1016/j.bbcan.2015.01.002
89. Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–27. doi:10.1038/nn.4185
90. Gutmann DH, Kettenmann H. Microglia/brain macrophages as central drivers of brain tumor pathobiology. Neuron. 2019;104(3):442–449. doi:10.1016/j.neuron.2019.08.028
91. Green LA, Nebiolo JC, Smith CJ, Eroglu C. Microglia exit the CNS in spinal root avulsion. PLoS Biol. 2019;17(2):e3000159. doi:10.1371/journal.pbio.3000159
92. Guo X, Pan Y, Gutmann DH. Genetic and genomic alterations differentially dictate low-grade glioma growth through cancer stem cell-specific chemokine recruitment of T cells and microglia. Neuro Oncol. 2019;21(10):1250–1262. doi:10.1093/neuonc/noz080
93. Feng L, Dou C, Xia Y, et al. Neutrophil-like cell-membrane-coated nanozyme therapy for ischemic brain damage and long-term neurological functional recovery. ACS Nano. 2021;15(2):2263–2280. doi:10.1021/acsnano.0c07973
94. Joice SL, Mydeen F, Couraud P-O, et al. Modulation of blood-brain barrier permeability by neutrophils: in vitro and in vivo studies. Brain Res. 2009;1298:13–23. doi:10.1016/j.brainres.2009.08.076
95. Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17(9):1025–1036. doi:10.1038/ni.3518
96. Chiossone L, Dumas P-Y, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18(11):671–688. doi:10.1038/s41577-018-0061-z
97. Lu Y, Hu Q, Jiang C, Gu Z. Platelet for drug delivery. Curr Opin Biotechnol. 2019;58:81–91. doi:10.1016/j.copbio.2018.11.010
98. Chen Z, Hu Q, Gu Z. Leveraging engineering of cells for drug delivery. Acc Chem Res. 2018;51(3):668–677. doi:10.1021/acs.accounts.7b00526
99. C-mj H, Fang RH, Wang K-C, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–121. doi:10.1038/nature15373
100. Zeng Z, Pu K. Improving cancer immunotherapy by cell membrane‐camouflaged nanoparticles. Adv Funct Mater. 2020;30(43):2004397. doi:10.1002/adfm.202004397
101. Jin J, Bhujwalla ZM. Biomimetic nanoparticles camouflaged in cancer cell membranes and their applications in cancer theranostics. Perspective. Front Oncol. 2020;9. doi:10.3389/fonc.2019.01560
102. Xia Q, Zhang Y, Li Z, Hou X, Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm Sin B. 2019;9(4):675–689. doi:10.1016/j.apsb.2019.01.011
103. Kunde SS, Wairkar S. Platelet membrane camouflaged nanoparticles: biomimetic architecture for targeted therapy. Int J Pharm. 2021;598:120395. doi:10.1016/j.ijpharm.2021.120395
104. Wang Y, Luan Z, Zhao C, Bai C, Yang K. Target delivery selective CSF-1R inhibitor to tumor-associated macrophages via erythrocyte-cancer cell hybrid membrane camouflaged pH-responsive copolymer micelle for cancer immunotherapy. Eur J Pharm Sci. 2020;142:105136. doi:10.1016/j.ejps.2019.105136
105. de Jong OG, Kooijmans SAA, Murphy DE, et al. Drug delivery with extracellular vesicles: from imagination to innovation. Acc Chem Res. 2019;52(7):1761–1770. doi:10.1021/acs.accounts.9b00109
106. Aryani A, Denecke B. Exosomes as a nanodelivery system: a key to the future of neuromedicine? Mol Neurobiol. 2016;53(2):818–834. doi:10.1007/s12035-014-9054-5
107. Tran HLP, Duan W, Lee B-J, Tran TDT. Current perspectives on delivery systems using extracellular vesicles in neurological disease. Curr Pharm Des. 2020;26(7):764–771. doi:10.2174/1381612826666200102125847
108. Hallal S, Ebrahimkhani S, Shivalingam B, Graeber MB, Kaufman KL, Buckland ME. The emerging clinical potential of circulating extracellular vesicles for non-invasive glioma diagnosis and disease monitoring. Brain Tumor Pathol. 2019;36(2):29–39. doi:10.1007/s10014-019-00335-0
109. Amaral M, Cruz N, Rosa A, et al. An update of advanced nanoplatforms for glioblastoma multiforme management. EXCLI J. 2021;20:1544–1570. doi:10.17179/excli2021-4393
110. d’Angelo M, Castelli V, Benedetti E, et al. Theranostic nanomedicine for malignant gliomas. Front Bioeng Biotechnol. 2019;7:325. doi:10.3389/fbioe.2019.00325
111. Imran M, Jha LA, Hasan N, et al. “Nanodecoys” - future of drug delivery by encapsulating nanoparticles in natural cell membranes. Int J Pharm. 2022;621:121790. doi:10.1016/j.ijpharm.2022.121790
112. Qiao B, Song X, Zhang N, et al. Artificial nano-red blood cells nanoplatform with lysosomal escape capability for ultrasound imaging-guided on-demand pain management. Acta Biomater. 2023;158:798–810. doi:10.1016/j.actbio.2023.01.004
113. Lopes J, Lopes D, Pereira-Silva M, et al. Macrophage cell membrane-cloaked nanoplatforms for biomedical applications. Small Methods. 2022;6(8):e2200289. doi:10.1002/smtd.202200289
114. Pan W-L, Tan Y, Meng W, et al. Microenvironment-driven sequential ferroptosis, photodynamic therapy, and chemotherapy for targeted breast cancer therapy by a cancer-cell-membrane-coated nanoscale metal-organic framework. Biomaterials. 2022;283:121449. doi:10.1016/j.biomaterials.2022.121449
115. Khosravi N, Pishavar E, Baradaran B, Oroojalian F, Mokhtarzadeh A. Stem cell membrane, stem cell-derived exosomes and hybrid stem cell camouflaged nanoparticles: a promising biomimetic nanoplatforms for cancer theranostics. J Control Release. 2022;348:706–722. doi:10.1016/j.jconrel.2022.06.026
116. Wei X, Zhang G, Ran D, et al. T-cell-mimicking nanoparticles can neutralize HIV infectivity. Adv Mater. 2018;30(45):e1802233. doi:10.1002/adma.201802233
117. Jiang Q, Liu Y, Guo R, et al. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials. 2019;192:292–308. doi:10.1016/j.biomaterials.2018.11.021
118. Wang D, Dong H, Li M, et al. Erythrocyte-cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. ACS Nano. 2018;12(6):5241–5252. doi:10.1021/acsnano.7b08355
119. Dad HA, Gu T-W, Zhu A-Q, Huang L-Q, Peng L-H. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29(1):13–31. doi:10.1016/j.ymthe.2020.11.030
120. Tauro BJ, Greening DW, Mathias RA, et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56(2):293–304. doi:10.1016/j.ymeth.2012.01.002
121. Zhang L, Feng Q, Wang J, Sun J, Shi X, Jiang X. Microfluidic synthesis of rigid nanovesicles for hydrophilic reagents delivery. Angew Chem Int Ed Engl. 2015;54(13):3952–3956. doi:10.1002/anie.201500096
122. Yang S, Guo F, Kiraly B, et al. Microfluidic synthesis of multifunctional janus particles for biomedical applications. Lab Chip. 2012;12(12):2097–2102. doi:10.1039/c2lc90046g
123. Liu C, Guo J, Tian F, et al. Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano. 2017;11(7):6968–6976. doi:10.1021/acsnano.7b02277
124. Lee K, Shao H, Weissleder R, Lee H. Acoustic purification of extracellular microvesicles. ACS Nano. 2015;9(3):2321–2327. doi:10.1021/nn506538f
125. Wu Z, Zhang H, Yan J, Wei Y, Su J. Engineered biomembrane-derived nanoparticles for nanoscale theranostics. Theranostics. 2023;13(1):20–39. doi:10.7150/thno.76894
126. Silva AKA, Di Corato R, Pellegrino T, et al. Cell-derived vesicles as a bioplatform for the encapsulation of theranostic nanomaterials. Nanoscale. 2013;5(23):11374–11384. doi:10.1039/c3nr01541f
127. Ye S, Wang F, Fan Z, et al. Light/pH-triggered biomimetic red blood cell membranes camouflaged small molecular drug assemblies for imaging-guided combinational chemo-photothermal therapy. ACS Appl Mater Interfaces. 2019;11(17):15262–15275. doi:10.1021/acsami.9b00897
128. Zhu L, Zhong Y, Wu S, et al. Cell membrane camouflaged biomimetic nanoparticles: focusing on tumor theranostics. Mater Today Bio. 2022;14:100228. doi:10.1016/j.mtbio.2022.100228
129. Wu T, Liu Y, Cao Y, Liu Z. Engineering macrophage exosome disguised biodegradable nanoplatform for enhanced sonodynamic therapy of glioblastoma. Adv Mater. 2022;34(15):e2110364. doi:10.1002/adma.202110364
130. Rao L, Cai B, L-L B, et al. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano. 2017;11(4):3496–3505. doi:10.1021/acsnano.7b00133
131. Zalba S, Ten Hagen TLM. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat Rev. 2017;52:48–57. doi:10.1016/j.ctrv.2016.10.008
132. Kainuma R, Motohashi Y, Nishihara T, Kurihara R, Tanabe K. Modulation of cell membrane functionalization with aggregates of oligodeoxynucleotides containing alkyl chain-modified uridines. Org Biomol Chem. 2020;18(28):5406–5413. doi:10.1039/d0ob00943a
133. Escribá PV, Busquets X, Inokuchi J-I, et al. Membrane lipid therapy: modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog Lipid Res. 2015;59:38–53. doi:10.1016/j.plipres.2015.04.003
134. Sletten EM, Bertozzi CR. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew Chem Int Ed Engl. 2009;48(38):6974–6998. doi:10.1002/anie.200900942
135. Levy O, Zhao W, Mortensen LJ, et al. mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood. 2013;122(14):e23–e32. doi:10.1182/blood-2013-04-495119
136. Lao Y-H, Li M, Gao MA, et al. HPV oncogene manipulation using nonvirally delivered CRISPR/Cas9 or Natronobacterium gregoryi Argonaute. Adv Sci. 2018;5(7):1700540. doi:10.1002/advs.201700540
137. Vats M, Mishra SK, Baghini MS, Chauhan DS, Srivastava R, De A. Near infrared fluorescence imaging in nano-therapeutics and photo-thermal evaluation. Int J Mol Sci. 2017;18(5):924. doi:10.3390/ijms18050924
138. Young RM, Jamshidi A, Davis G, Sherman JH. Current trends in the surgical management and treatment of adult glioblastoma. Ann Transl Med. 2015;3(9):121. doi:10.3978/j.issn.2305-5839.2015.05.10
139. Reichel D, Sagong B, Teh J, et al. Near infrared fluorescent nanoplatform for targeted intraoperative resection and chemotherapeutic treatment of glioblastoma. ACS Nano. 2020;14(7):8392–8408. doi:10.1021/acsnano.0c02509
140. Mahmood U, Weissleder R. Near-infrared optical imaging of proteases in cancer. Mol Cancer Ther. 2003;2(5):489–496.
141. Ferraro N, Barbarite E, Albert TR, et al. The role of 5-aminolevulinic acid in brain tumor surgery: a systematic review. Neurosurg Rev. 2016;39(4):545–555. doi:10.1007/s10143-015-0695-2
142. Kortmann R-D, Jeremic B, Weller M, Plasswilm L, Bamberg M. Radiochemotherapy of malignant glioma in adults. Clinical experiences. Strahlenther Onkol. 2003;179(4):219–232. doi:10.1007/s00066-003-1027-y
143. Ma J, Dai L, Yu J, et al. Tumor microenvironment targeting system for glioma treatment via fusion cell membrane coating nanotechnology. Biomaterials. 2023;295:122026. doi:10.1016/j.biomaterials.2023.122026
144. Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657–674. doi:10.1038/s41571-020-0410-2
145. Jaque D, Martínez Maestro L, Del Rosal B, et al. Nanoparticles for photothermal therapies. Nanoscale. 2014;6(16):9494–9530. doi:10.1039/c4nr00708e
146. Son S, Kim JH, Wang X, et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem Soc Rev. 2020;49(11):3244–3261. doi:10.1039/c9cs00648f
147. Pan X, Wang H, Wang S, et al. Sonodynamic therapy (SDT): a novel strategy for cancer nanotheranostics. Sci China Life Sci. 2018;61(4):415–426. doi:10.1007/s11427-017-9262-x
148. Zhang Y, Zhang X, Yang H, et al. Advanced biotechnology-assisted precise sonodynamic therapy. Chem Soc Rev. 2021;50(20):11227–11248. doi:10.1039/d1cs00403d
149. Dong -Z-Z, Yang C, Wang Z, Zhong Z, Wong M-S, H-w L. Tumor microenvironment-responsive Zn/Cu nanoparticles for enhanced chemodynamic therapy. Smart Mater Struct. 2023;4:286–293. doi:10.1016/j.smaim.2022.11.002
150. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–821. doi:10.1038/s41423-020-0488-6
151. Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi:10.1146/annurev.immunol.25.022106.141609
152. Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18(3):175–196. doi:10.1038/s41573-018-0006-z
153. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86–104. doi:10.3322/caac.21596
154. Tobias A, Ahmed A, Moon K-S, Lesniak MS. The art of gene therapy for glioma: a review of the challenging road to the bedside. J Neurol Neurosurg Psychiatry. 2013;84(2):213–222. doi:10.1136/jnnp-2012-302946
155. Han S, Lee Y, Lee M. Biomimetic cell membrane-coated DNA nanoparticles for gene delivery to glioblastoma. J Control Release. 2021;338:22–32. doi:10.1016/j.jconrel.2021.08.021
156. Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123(9):3685–3692. doi:10.1172/JCI69741
157. Dymek M, Sikora E. Liposomes as biocompatible and smart delivery systems - the current state. Adv Colloid Interface Sci. 2022;309:102757. doi:10.1016/j.cis.2022.102757
158. Guimarães D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571. doi:10.1016/j.ijpharm.2021.120571
159. Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;975. doi:10.2147/ijn.s68861
160. Pattni BS, Chupin VV, Torchilin VP. New developments in liposomal drug delivery. Chem Rev. 2015;115(19):10938–10966. doi:10.1021/acs.chemrev.5b00046
161. Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1(3):297–315.
162. Mojarad-Jabali S, Farshbaf M, Walker PR, et al. An update on actively targeted liposomes in advanced drug delivery to glioma. Int J Pharm. 2021;602:120645. doi:10.1016/j.ijpharm.2021.120645
163. Zhao Y, Ren W, Zhong T, et al. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J Control Release. 2016;222:56–66. doi:10.1016/j.jconrel.2015.12.006
164. Jia Y, Wang X, Hu D, et al. Phototheranostics: active targeting of orthotopic glioma using biomimetic proteolipid nanoparticles. ACS Nano. 2019;13(1):386–398. doi:10.1021/acsnano.8b06556
165. Wei L, Guo X-Y, Yang T, Yu M-Z, Chen D-W, Wang J-C. Brain tumor-targeted therapy by systemic delivery of siRNA with transferrin receptor-mediated core-shell nanoparticles. Int J Pharm. 2016;510(1):394–405. doi:10.1016/j.ijpharm.2016.06.127
166. R-j J, Zeng F, Liu L, et al. Destruction of vasculogenic mimicry channels by targeting epirubicin plus celecoxib liposomes in treatment of brain glioma. Int J Nanomedicine. 2016;11:1131–1146. doi:10.2147/IJN.S94467
167. Weyland M, Griveau A, Bejaud J, Benoit JP, Coursaget P, Garcion E. Lipid nanocapsule functionalization by lipopeptides derived from human papillomavirus type-16 capsid for nucleic acid delivery into cancer cells. Int J Pharm. 2013;454(2):756–764. doi:10.1016/j.ijpharm.2013.06.013
168. Ismail M, Yang W, Li Y, et al. Targeted liposomes for combined delivery of artesunate and temozolomide to resistant glioblastoma. Biomaterials. 2022;287:121608. doi:10.1016/j.biomaterials.2022.121608
169. Wang R, Zhang X, Huang J, et al. Bio-fabricated nanodrugs with chemo-immunotherapy to inhibit glioma proliferation and recurrence. J Control Release. 2023;354:572–587. doi:10.1016/j.jconrel.2023.01.023
170. Tian W, Ying X, Du J, et al. Enhanced efficacy of functionalized epirubicin liposomes in treating brain glioma-bearing rats. Eur J Pharm Sci. 2010;41(2):232–243. doi:10.1016/j.ejps.2010.06.008
171. Li J, Zeng H, You Y, et al. Active targeting of orthotopic glioma using biomimetic liposomes co-loaded elemene and cabazitaxel modified by transferritin. J Nanobiotechnology. 2021;19(1):289. doi:10.1186/s12951-021-01048-3
172. Ying X, Wen H, Lu W-L, et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 2010;141(2):183–192. doi:10.1016/j.jconrel.2009.09.020
173. Mu L-M, Bu Y-Z, Liu L, et al. Lipid vesicles containing transferrin receptor binding peptide TfR-T and octa-arginine conjugate stearyl-R efficiently treat brain glioma along with glioma stem cells. Sci Rep. 2017;7(1):3487. doi:10.1038/s41598-017-03805-7
174. Yang -Z-Z, J-q L, Wang -Z-Z, Dong D-W, X-r Q. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials. 2014;35(19):5226–5239. doi:10.1016/j.biomaterials.2014.03.017
175. Wu H, Liu Y, Chen L, et al. Combined biomimetic MOF-RVG15 nanoformulation efficient over BBB for effective anti-glioblastoma in mice model. Int J Nanomedicine. 2022;17:6377–6398. doi:10.2147/IJN.S387715
176. Dube T, Kompella UB, Panda JJ. Near infrared triggered chemo-PTT-PDT effect mediated by glioma directed twin functional-chimeric peptide-decorated gold nanoroses. J Photochem Photobiol B. 2022;228:112407. doi:10.1016/j.jphotobiol.2022.112407
177. Yokoyama M, Kwon GS, Okano T, Sakurai Y, Seto T, Kataoka K. Preparation of micelle-forming polymer-drug conjugates. Bioconjug Chem. 1992;3(4):295–301. doi:10.1021/bc00016a007
178. Miyata K, Christie RJ, Kataoka K. Polymeric micelles for nano-scale drug delivery. React Funct Polym. 2011;71(3):227–234. doi:10.1016/j.reactfunctpolym.2010.10.009
179. Wanjale MV, Kumar GSV. Peptides as a therapeutic avenue for nanocarrier-aided targeting of glioma. Expert Opin Drug Deliv. 2017;14(6):811–824. doi:10.1080/17425247.2017.1242574
180. Ran D, Mao J, Shen Q, et al. GRP78 enabled micelle-based glioma targeted drug delivery. J Control Release. 2017;255:120–131. doi:10.1016/j.jconrel.2017.03.037
181. Ran D, Zhou J, Chai Z, et al. All-stage precisional glioma targeted therapy enabled by a well-designed D-peptide. Theranostics. 2020;10(9):4073–4087. doi:10.7150/thno.41382
182. Ran D, Mao J, Zhan C, et al. d-retroenantiomer of quorum-sensing peptide-modified polymeric micelles for brain tumor-targeted drug delivery. ACS Appl Mater Interfaces. 2017;9(31):25672–25682. doi:10.1021/acsami.7b03518
183. Ruan H, Chai Z, Shen Q, et al. A novel peptide ligand RAP12 of LRP1 for glioma targeted drug delivery. J Control Release. 2018;279:306–315. doi:10.1016/j.jconrel.2018.04.035
184. Yang Z, Du Y, Sun Q, et al. Albumin-based nanotheranostic probe with hypoxia alleviating potentiates synchronous multimodal imaging and phototherapy for glioma. ACS Nano. 2020;14(5):6191–6212. doi:10.1021/acsnano.0c02249
185. Lin T, Zhao P, Jiang Y, et al. Blood-brain-barrier-penetrating albumin nanoparticles for biomimetic drug delivery via albumin-binding protein pathways for antiglioma therapy. ACS Nano. 2016;10(11). doi:10.1021/acsnano.6b04268
186. Zhu S, Sun F, Zhao P, et al. Brain-targeting biomimetic nanoparticles for codelivery of celastrol and LY2157299 for reversing glioma immunosuppression. Int J Pharm. 2022;619:121709. doi:10.1016/j.ijpharm.2022.121709
187. Zhao P, Wang Y, Kang X, et al. Dual-targeting biomimetic delivery for anti-glioma activity remodeling the tumor microenvironment and directing macrophage-mediated immunotherapy. Chem Sci. 2018;9(10):2674–2689. doi:10.1039/c7sc04853j
188. Mo X, Zheng Z, He Y, et al. Antiglioma via regulating oxidative stress and remodeling tumor-associated macrophage using lactoferrin-mediated biomimetic codelivery of simvastatin/fenretinide. J Control Release. 2018;287:12–23. doi:10.1016/j.jconrel.2018.08.012
189. Wang B, Tang M, Yuan Z, et al. Targeted delivery of a STING agonist to brain tumors using bioengineered protein nanoparticles for enhanced immunotherapy. Bioact Mater. 2022;16:232–248. doi:10.1016/j.bioactmat.2022.02.026
190. Jia W, Tian H, Jiang J, et al. Brain-targeted HFn-Cu-REGO nanoplatform for site-specific delivery and manipulation of autophagy and cuproptosis in glioblastoma. Small. 2023;19(2):e2205354. doi:10.1002/smll.202205354
191. Geng W, Zou H, Wang H, et al. Dual-triggered biomimetic vehicles enable treatment of glioblastoma through a cancer stem cell therapeutic strategy. Nanoscale. 2021;13(15):7202–7219. doi:10.1039/d0nr08899d
192. Huang J-L, Jiang G, Song Q-X, et al. Lipoprotein-biomimetic nanostructure enables efficient targeting delivery of siRNA to ras-activated glioblastoma cells via macropinocytosis. Nat Commun. 2017;8:15144. doi:10.1038/ncomms15144
193. Wang R, Wang X, Li J, Di L, Zhou J, Ding Y. Lipoprotein-biomimetic nanostructure enables tumor-targeted penetration delivery for enhanced photo-gene therapy towards glioma. Bioact Mater. 2022;13:286–299. doi:10.1016/j.bioactmat.2021.10.039
194. S-y W, Y-x Y, Zhang Q, et al. Multifunctional protein hybrid nanoplatform for synergetic photodynamic-chemotherapy of malignant carcinoma by homologous targeting combined with oxygen transport. Adv Sci. 2023;10(5):e2203742. doi:10.1002/advs.202203742
195. Cao H, Duan L, Zhang Y, Cao J, Zhang K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct Target Ther. 2021;6(1):426. doi:10.1038/s41392-021-00830-x
196. Gutierrez AM, Frazar EM, Klaus MV X, Paul P, Hilt JZ. Hydrogels and hydrogel nanocomposites: enhancing healthcare through human and environmental treatment. Adv Healthc Mater. 2022;11(7):e2101820. doi:10.1002/adhm.202101820
197. Norouzi M, Nazari B, Miller DW. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov Today. 2016;21(11):1835–1849. doi:10.1016/j.drudis.2016.07.006
198. Zhang A, Liu Y, Qin D, Sun M, Wang T, Chen X. Research status of self-healing hydrogel for wound management: a review. Int J Biol Macromol. 2020;164:2108–2123. doi:10.1016/j.ijbiomac.2020.08.109
199. Yao Q, Lan Q-H, Jiang X, et al. Bioinspired biliverdin/silk fibroin hydrogel for antiglioma photothermal therapy and wound healing. Theranostics. 2020;10(25):11719–11736. doi:10.7150/thno.47682
200. Melero I, Castanon E, Alvarez M, Champiat S, Marabelle A. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat Rev Clin Oncol. 2021;18(9):558–576. doi:10.1038/s41571-021-00507-y
201. Mirshafiee V, Mahmoudi M, Lou K, Cheng J, Kraft ML. Protein corona significantly reduces active targeting yield. Chem Comm. 2013;49(25):2557. doi:10.1039/c3cc37307j
202. Salvati A, Pitek AS, Monopoli MP, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8(2):137–143. doi:10.1038/nnano.2012.237
203. Yang W, Guo W, Chang J, Zhang B. Protein/peptide-templated biomimetic synthesis of inorganic nanoparticles for biomedical applications. J Mater Chem B. 2017;5(3):401–417. doi:10.1039/c6tb02308h
204. Gao C, Lin Z, Lin X, He Q. Cell membrane-camouflaged colloid motors for biomedical applications. Adv Ther. 2018;1(5):1800056. doi:10.1002/adtp.201800056
205. Ou J, Liu K, Jiang J, et al. Micro-/nanomotors toward biomedical applications: the recent progress in biocompatibility. Small. 2020;16(27):e1906184. doi:10.1002/smll.201906184
206. Gao W, Wang J. Synthetic micro/nanomotors in drug delivery. Nanoscale. 2014;6(18):10486–10494. doi:10.1039/c4nr03124e
207. Wang J, Dong R, Wu H, Cai Y, Ren B. A review on artificial micro/nanomotors for cancer-targeted delivery, diagnosis, and therapy. Nanomicro Lett. 2020;12(1):1–9.
208. Zhang X, Yang T, Wu Y, He Q. Research progress in the application of colloidal motors for precision medicine. Nanoscale. 2022;14(35):12547–12559. doi:10.1039/d2nr03963j
209. Kumthekar P, Ko CH, Paunesku T, et al. A first-in-human Phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci Transl Med. 2021;13(584). doi:10.1126/scitranslmed.abb3945
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
