Back to Journals » International Journal of Nanomedicine » Volume 17
How Nanotechniques Could Vitalize the O-GlcNAcylation-Targeting Approach for Cancer Therapy
Authors Yang R , Wang L, Wu Z, Yin Y, Jiang SW
Received 29 January 2022
Accepted for publication 11 April 2022
Published 24 April 2022 Volume 2022:17 Pages 1829—1841
DOI https://doi.org/10.2147/IJN.S360488
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Rui Yang,1,* Leilei Wang,2,* Zhifeng Wu,3 Yongxiang Yin,4 Shi-Wen Jiang1
1Center of Reproductive Medicine, State Key Laboratory of Reproductive Medicine, Research Institute for Reproductive Health and Genetic Diseases, The Affiliated Wuxi Maternity and Child Health Care Hospital of Nanjing Medical University, Wuxi, 214002, Jiangsu, People’s Republic of China; 2Department of Medical Genetics, Lianyungang Maternal and Child Health Hospital Affiliated to Yangzhou University, Lianyungang, 222000, Jiangsu, People’s Republic of China; 3Department of Ophthalmology, The Affiliated Wuxi Clinical College of Nantong University, Wuxi, 214002, Jiangsu, People’s Republic of China; 4Department of Pathology, The Affiliated Maternity and Child Health Hospital of Nanjing Medical University, Wuxi, 214002, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shi-Wen Jiang, Center of Reproductive Medicine, State Key Laboratory of Reproductive Medicine, Research Institute for Reproductive Health and Genetic Diseases, The Affiliated Wuxi Maternity and Child Health Care Hospital of Nanjing Medical University, Wuxi, 214002, Jiangsu, People’s Republic of China, Tel +86-13730975986, Fax +86-510-82725094, Email [email protected]
Abstract: Accumulated data indicated that many types of cancers have increased protein O-GlcNAcylation at cell surface and inside cells. The aberrant O-GlcNAcylation is considered a potential therapeutic target. Although several types of compounds capable of inhibiting O-GlcNAcylation have been developed, their low solubility, poor permeability and delivery efficiency have impeded the application for in vivo and pre-clinical studies. Nanocarriers have the advantages of controllable drug release and active cancer-targeting capability. Moreover, nanoparticles can improve drug delivery efficiency and reduce the non-specific distribution in normal tissues by the enhanced permeability and retention (EPR) effect in cancer. Taking the advantage of O-GlcNAc-specific antibodies or lectins, nanoparticles could further improve their cancer-targeting capability. Although nanocarriers targeting the canonical N- and O-linked glycosylation have been extensively investigated for cancer detection and therapy, application of nanotechniques for the specific targeting of O-GlcNAcylation has not been actively pursued. This review summarizes the general features of GlcNAcylation and its alterations in cancers. Analyses are focused on the following areas: How the nanocarriers may improve the solubility and/or cell permeability of O-GlcNAc transferase (OGT) inhibitors; The modification of nanocarriers with lectins or antibodies for active targeting of O-GlcNAc; The nanocarriers-mediated co-delivery of OGT inhibitors and conventional drugs, which may lead to synergistic effects. Unsolved issues impeding the research progression on O-GlcNAcylation-targeting scheme are also discussed.
Keywords: O-GlcNAcylation, nanocarriers, OGT inhibitor, targeted therapy, lectin, combined therapy
Graphical Abstract:
Introduction
Protein glycosylation refers to the transfer of a variety of sugar molecules to cellular proteins under the catalysis of various glycosyltransferases. The process involves the formation of covalent bonds between sugar molecules and the amino or hydroxyl groups of amino acid residues of proteins. Glycosylated proteins carry monosaccharide or polysaccharide chains that are either readily synthesized and added altogether, or sequentially added one sugar molecule by another. Based on the peptide motifs where the sugar chain is added to, glycosylation can be generally classified into the N-link glycosylation with the transfer of N-acetylglucosamine (GlcNAc), and the O-link glycosylation with the transfer of N-acetylgalactosamine (GalNAc). In mammalian cells, the extracellular and membrane proteins are frequently post-translationally modified by glycosylation. It is noteworthy that Torres and Hart reported the “non-canonical glycosylation” of O-linked N-acetylglucosaminylation (O-GlcNAcylation).1 Different to the glycons in the classical N- and O-link glycosylation, in this type of glycosylation, a monosaccharide GlcNAc group is specifically added by the OGT and removed by β-N-acetylglucosaminidase (OGA). The rapid turnover and the reversible nature of O-GlcNAcylation pointed to its tight regulation and dynamic bioactivity. While most O-GlcNAcylated proteins are detected in the cytoplasm, nuclei, and mitochondrions,2 several studies indicated that O-GlcNAcylation also exist in the extracellular domains of membrane proteins such as the Notch receptor and epidermal growth factor receptor (EGFR).3–5 Interestingly, the GlcNAc chain can be extended from the initial monosaccharide by sequential addition of a galactose and a terminal sialic acid (Figure 1). An increasing body of evidence supports that O-GlcNAcylation participates in the regulation of various cell functions including those for transcription, signal transduction, metabolism, proliferation and apoptosis.
Studies in recent years indicated that cancer cells often exhibit significant alterations in O-GlcNAcylation. The steady state level of O-GlcNAcylation is largely affected by two factors: the expression levels of O-GlcNAc-cycling enzymes of OGT and OGA, and the concentration of OGT substrate, uridine diphosphate GlcNAc (UDP-GlcNAc). While each type of cancer has a signature pattern of O-GlcNAcylation, the increased O-GlcNAcylation and OGT expression appear to be a common feature shared by cancers arising from the bladder, the column, and other tissues.6–8 UDP-GlcNAc is an end product of the hexosamine biosynthetic pathway (HBP). In order to support the fast proliferation, cancer cells deprive large amounts of nutrients including glucose and glutamine, and HBP is highly active, leading to an elevated level of UDP-GlcNAc.9,10 Indeed, an analysis of published data by Akella et al showed that in many cancer cell lines, the increased O-GlcNAcylation was accompanied by the elevation of UDP-GlcNAc level.11 In addition, as reviewed by Ferrer et al, OGT overexpression and the resultant hyper-O-GlcNAcylation may promote cancer development through their positive impacts on cancer cell glycolysis, growth, survival, invasion, metastasis, cancer angiogenesis, and reprograming of the epigenome.12 A study by Itkonen et al demonstrated that the blocking of OGT activity with an OGT inhibitor (OSMI-2) impaired the MYC O-GlcNAcylation, resulting in a slower androgen-independent proliferation of the PC3 prostate cancer cells.13 These findings strongly suggest that the cancer-related O-GlcNAcylation could be a promising target for cancer therapy.
Zhu and Harrt summarized the abnormal O-GlcNAcylation in cancers, diabetes and neurodegenerative diseases, and discussed the novel approaches for therapeutic application of O-GlcNAcylation.14 Although several types of compounds capable of inhibiting O-GlcNAcylation have been developed,15 technical barriers such as the low solubility, the poor permeability and delivery efficiency need to be cleared for in vivo and pre-clinical studies. Nanocarriers have the advantages of controllable drug release and active cancer-targeting capability. For example, compared to free doxorubicin, the pegylated liposomal doxorubicin (PLD) preferentially concentrates in cancer tissues. Its clinical application showed an improved efficacy for breast and ovarian cancers, with a significantly reduced cardiotoxicity.16 BIND-014 is a polymeric nanoparticle that loaded with docetaxel in the core and modified with the ligand for prostate-specific membrane antigen (PSMA) at the edge. Preclinical studies indicated that its selective cancer accumulation led to a 10-fold increase of efficacy as well as an 80% reduction of adverse effects.17 The properties of glycan-binding antibodies and lectins, and the potential applications of nanoparticles conjugated with glycan-binding molecules for cancer detection and treatment have been previously reviewed.18 However, O-GlcNAcylation inhibitors and their application with the aid of nanotechnique have not been analyzed in detail. The current review focuses on how nanotechnology can be applied for passive and active targeting of O-GlcNAcylation. The issues concerning the design, technical approach and possible side effects of nanoparticles are also raised and discussed.
Compounds Inhibiting O-GlcNAcylation
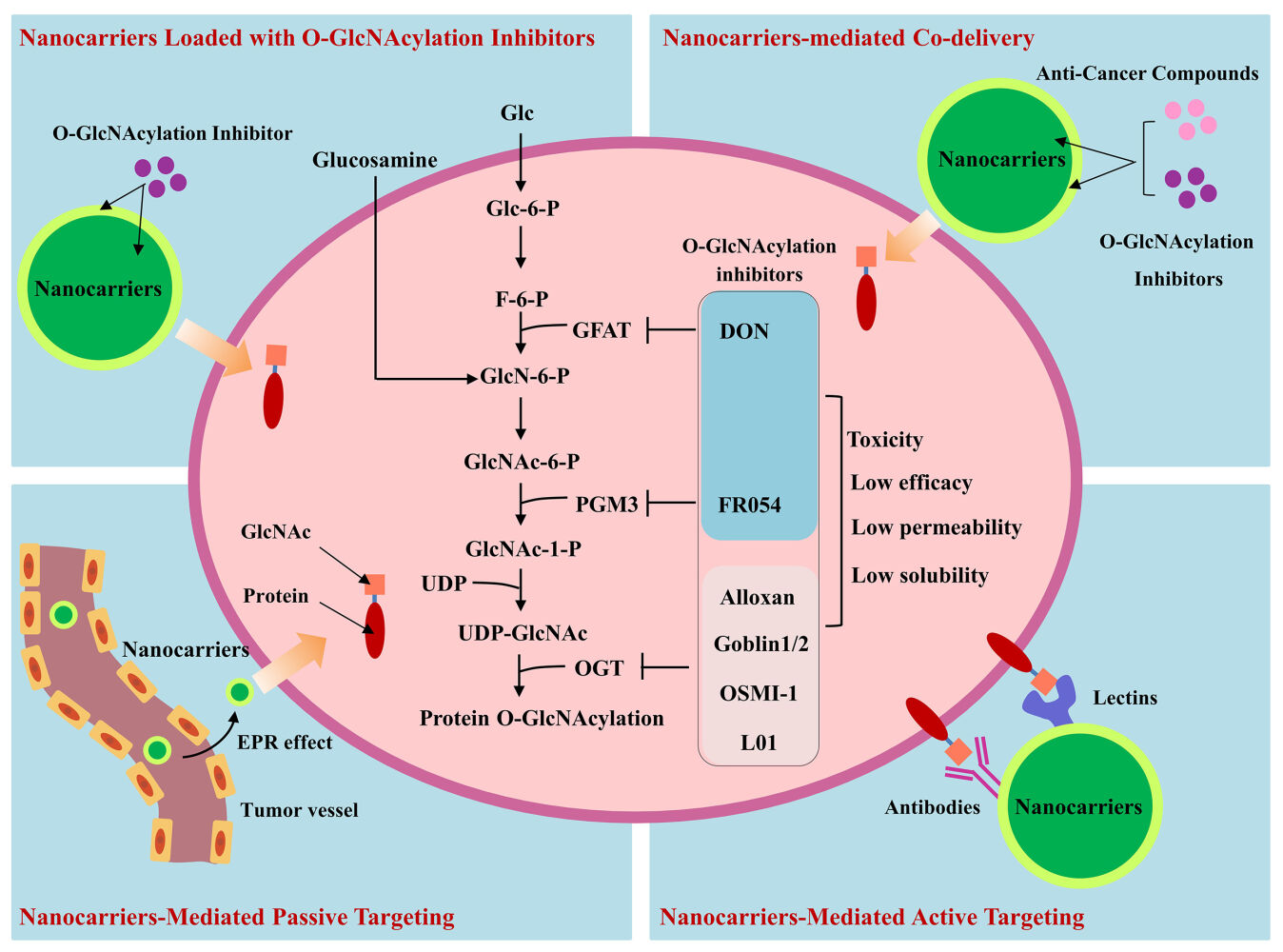
As illustrated in Figure 2, in terms of the mechanisms for the reduction of O-GlcNAcylation, there are two categories of compounds: 1) Those to limit the intracellular levels of UDP-GlcNAc, the substrate providing the GlcNAc group for O-GlcNAcylation; 2) The OGT inhibitors, including the UDP-GlcNAc analogues or non-UDP-GlcNAc analogous, small molecules identified by high throughput screening. These compounds may have therapeutic potentials by disruption of the aberrant glycosylation in cancer cells.
Compounds Reducing the UDP-GlcNAc Level
The intracellular UDP-GlcNAc level can be reduced by inhibiting the key enzymes of HBP. Glutamine fructose-6-phosphate amidotransferase (GFAT) is the initial and rate-limiting enzyme in HBP. The GFAT inhibitor 6-diazo-5-oxo-norleucine (DON) can effectively reduce O-GlcNAcylation, and display cytotoxic effects in cell lines established from bladder cancer and acute myeloid leukemia.19,20 However, in the Phase I and II clinical trials, this compound elicited adverse gastric-intestinal reactions, which limited its potentials for clinical application.21 The N-acetylglucosamine-phosphate mutase (PGM3) inhibitor FR054 is a small molecule compound.22 In vitro experiments using the MDA-MB-231 breast cancer cell line showed that FR054 downregulated both N-link glycosylation and O-GlcNAcylation, and caused cell apoptosis at a high concentration of 1 mmol/L. In the mouse xenograft model, an anti-cancer activity was observed when FR054 was administered by intraperitoneal injection (500 mg/kg) twice a day for 5 consecutive days. This effect was accompanied by the downregulation of N-link glycosylation and O-GlcNAcylation in cancer tissues. Although no severe side effect was observed in mice, this high dosage is equivalent to approximately 60 g/day for an adult human, an enormous amount to be administered. Thus, at this point, the OGT inhibitors’ potentials for clinical application are restricted by their high toxicity and/or low efficacy.
OGT Inhibitors
UDP-GlcNAc analogues inhibit OGT activity by competing with UDP-GlcNAc for binding to OGT. Alloxan, a uracil mimic, has been shown to bind to various enzymes that uses UDP-sugars as substrate, and to inhibit a broad-spectrum of enzymes including OGT and other glycosylation enzymes.15 This low-specificity not only affected its OGT-inhibitory efficiency, but may also cause side effects. The later developed bisubstrate-linked inhibitor goblin1/2 contains an UDP group covalently connected to the serine of a synthetic peptide (VTPVSTA).23 In biochemical experiments, these compounds inhibited human OGT at the micromolar range, and may have improved specificity to OGT. But they could not perpetrate the cell membrane due to the large molecular size and ionic nature.
Another type of OGT inhibitors comes from the high-throughput screening of compound libraries. These small molecules have been extensively investigated, with many showing some decent inhibitory efficiencies. Jaskiewicz reported that OSMI-1 (MW 563.64) was able to inhibit protein O-GlcNAcylation in the endometrial cancer Ishikawa cell line. OSMI-1 at a concentration of 25 μM significantly inhibited the cell proliferation following 72 hours of treatment. Unfortunately, OSMI-1 has a low aqueous solubility and a poor cell permeability,24 and could not be conveniently applied to in vivo study.
Overall, although several prototypes of O-GlcNAcylation inhibitors have been developed, their high toxicity, low efficiency, low water solubility and cell permeability make the in vivo studies difficult. As discussed later, properly engineered nanocarriers may help to overcome these problems.
Potential Applications of the Nanotechnique for Targeting O-GlcNAcylation
The intrinsic advantages of nanoparticles can be taken for O-GlcNAcylation-targeting in 4 respects: 1) Physical encapsulation or chemical coupling of OGT inhibitors to nanocarriers will facilitate the inhibitor’s solubility and permeability; 2) Making a use of the EPR effect of solid tumors, nanocarriers can help to passively increase the local concentration of a therapeutic compound, and reduce the collateral toxicity; 3) Antibody- or lectin-conjugated nanocarriers can specifically deliver the compounds to cancer cells through active targeting of O-GlcNAcylation. 4) Nanocarriers-mediated co-delivery of OGT inhibitor with other compounds may generate synergistic effects. These aspects are discussed separately in the following 4 sections.
Nanocarrier-Aided Solubility and Permeability of OGT Inhibitors
Nanocarriers can significantly improve the solubility and cell permeability of many compounds by encapsulating them to the core or loading them to the external layer. The US Food and Drug Administration (FDA) has approved the medical applications of liposomal, polymeric, micellar and proteinaceous nanocarriers.25 Take an example of liposomal nanocarriers, in the mode of physical encapsulation, a hydrophilic drug can be carried in the aqueous core of spherical liposomal particles, and a hydrophobic drug can be loaded between the two phospholipid layers. Alternatively, a drug can be chemically coupled to either side of the phospholipid layers of liposomal nanocarriers.26 Previously, Cremophor EL, a compatible excipient type of solvent vehicles, has been used to facilitate the clinical application of low-solubility drugs such as paclitaxel. However, Cremophor EL was found to cause severe allergy by itself in many individuals, and the prophylactic use of glucocorticoids and anti-histamines could not fully prevent the occurrence of allergic reactions.27 Through liposomal encapsulation of paclitaxel, the commercialized formula of Paclitaxel Liposome for Injection has largely overcome the drawback. It is noteworthy that recent studies indicated that the peptide-based nanoparticles exhibited some beneficial features such as the low immunogenicity, unique antitumor activities, and the easiness for functionalization.28 Moreover, the carrier-free type of nanoparticles formed by self-assembly of one or multiple drugs can avoid the adverse effects caused by nanomaterials, which provides an obvious advantage.29
The currently available UDP-analogous and OGT-catalytic inhibitors including Ac-5S-GlcNAc, L01, and OSMI-1 have a common shortage of low solubility.15 It is noted that these compounds carry the hydroxyl and other ionizable groups that potentiate the chemical coupling to the phospholipid bilayers of a liposomal nanocarrier. However, the multiple labile groups tend to form diversified by-products during modification, which may complicate the downstream purification process. Moreover, in compounds like L01, the hydroxyl groups for modification are closely located to the OGT-binding sites, and chemical modifications could affect their bioactivity and therapeutic efficacy. On the other hand, the hydrophilic bisubstrate mimic OGT inhibitors such as goblin1/2 usually have satisfactory solubility, but their large molecule sizes limit the cell-permeability. Liposomal particles can be used to carry large molecules into target cells via the fusion and endocytosis mechanisms. While nanotechnology provides a possible approach to improve the solubility and/or permeability of these inhibitors, nanomaterials themselves may introduce side effects. In vitro studies using cell culture models have clearly demonstrated various cytotoxicities of nanomaterials, including the damage of cell membranes, interference of cell metabolism, induction of ROS production, and ultimately cell death.30 In vivo, deposition of nanomaterials in the lungs, intestines, liver, spleen, and kidney can lead to inflammation, damage of structural integrity, and organ dysfunctions.31
Through the Cancer EPR Effects, Nanocarriers Can Passively Increase Drug Concentrations in Cancer Tissues and Reduce the Side Effects
The upregulated angiogenesis and high exchange rate due to the fast expansion of cancer renders an increased blood vessel-tissue permeability of cancer tissues. At the same time, the impaired lymphatic drainage favors the retention of large molecules in cancer tissues. These events converge to an EPR (enhanced permeability and retention) effect in cancer tissues. It is believed that nanoparticles with sizes approximately ≥100 nm often display a strong EPR effect. Moreover, some nanocarriers with neutral charge can partially avoid the destruction by the mononuclear phagocyte system (MPS), which extends their survival. In addition to the EPR effects, tumor cells exhibit an increase capability to take up glucose and other nutrients. Glycoconjugated drugs are being experimented for improved selectivity in cancer targeting.32 These “passive” targeting features of nanocarriers have been successfully used to enhance the drug accumulation in cancer tissues and alleviate the side effects. For example, cisplatin is frequently used for the treatment of ovarian cancers, non-small-cell lung cancers (NSCLC), cervical cancers and head and neck cancers. But the severe side effects of nephrotoxicity, neurotoxicity, nausea-vomiting, asthenia, and hematological toxicity have limited its clinical applications. A meta-analysis of the NSCLC patients by Xu et al indicated that a phospholipid-loaded formulation of cisplatin (Lipoplatin) had an improved efficacy as well as a reduced side effect in patients.33 It is expected that the nanocarrier-loading of O-GlcNAcylation inhibitors could facilitate the in vitro as well as translational studies.
Nanocarriers Modified for Active O-GlcNAc-Targeting
O-GlcNAc at the surface of cancer cells can be used as biomarkers or “receptors” for active targeting. The antibodies raised against O-GlcNAc or the O-GlcNAc-binding lectins can be linked to nanocarriers for selective or a “relatively specific” delivery of either O-GlcNAcylation inhibitors or conventional drugs to cancer cells.
O-GlcNAc Antibody-Mediated Targeting of O-GlcNAc
The antibody molecules contain multiple COOH- and -NH2 groups that can be used for covalent modification in the manufacturing of nanocarriers capable of O-GlcNAc targeting. Multiple monoclonal antibodies raised against O-GlcNAcylated proteins, including CTD110.6, HGAC85, RL2, 10D8, 18B10.C7(#3), 9D1.E4(#10) and 1F5.D6(#14) have been used for detecting O-GlcNAcylation. Tashima and Stanley reported that among these frequently used O-GlcNAc antibodies, while CTD110.6 equivalently reacted with not only O-GlcNAcylated proteins, but also with the terminal β-GlcNAc on complex N-glycans, CTD110.6/18B10.C7(#3)/9D1.E4 can be used to specifically detect the extracellular domains of O-GlcNAcylated proteins when N-Glycans were removed.34 The specificity of these monoclonal antibodies for cancer-related O-GlcNAc requires further investigation. Notch receptor and EGFR are known transmembrane proteins with hyper-O-GlcNAcylation in cancer cells. Barua et al reported that the O-GlcNAcylation of Notch receptor participated in the regulation of proliferation and migration of Panc-1 pancreatic cancer cells.35 Stateva and Villalobo reported the presence of O-GlcNAcylated EGFR in the A431 skin squamous and the A549 lung cancer cell lines.5 Moreover, Wang et al observed that knockdown of OGT in the 786-O kidney cancer cell line led to a downregulation of EGFR O-GlcNAcylation.36 Unlike successful generation of a variety of “double-specificity” antibodies against phosphorylated proteins, in which a given antibody specifically recognizes both the unique -PO4 group and its amino acid sequences, possibly due to the lower immunogenicity of O-GlcNAc group, no double-specificity antibody against O-GlcNAcylated Notch receptor/EGFR is currently available.37 These “pan-antibodies” react with the O-GlcNAc group, but could not distinguish different proteins. This situation constitutes a technical hurdle for specific targeting of O-GlcNAcylated Notch receptor/EGFR or other proteins. Nevertheless, nanocarriers conjugated by the “pan-antibodies” against the O-GlcNAc group may still benefit the drug deliver to cancer tissues to a certain extent.
Lectin-Mediated Targeting of O-GlcNAc
Lectins are carbohydrate-binding proteins extensively exist in diversified organisms. Since lectins are able to recognize the sugar groups with an appreciable specificity, they have been used for the detection of glycoproteins. Application of lectins for the construction of glycosylation-targeting nanocarriers are being actively pursued. Wheat germ agglutinin (WGA) represents a type of phytolectins conferring a relatively high affinity to the GlcNAc and sialic acid groups. WGA has almost no immunogenicity, which could be an advantage for in vivo applications. As illustrated in Figure 1, sialic acids are often found at the termini of N-link glycans, canonical O-link glycans and O-GlcNAc chains. Lochner et al explored the mechanism of physical interactions between WGA and the Caco-2 colon cancer cells. It was found that the WGA-binding to terminal sialic acids accounted for at least 85% of its interaction with all types of glycosylation.38 Patrícia et al reported that succinylation of WGA (succinyl WGA, sWGA) improved the affinity and specificity of WGA for the recognition of GlcNAc.39 Machon et al reported that the recombinant Psathyrella velutina (rPVL) produced by E. coli could bind to GlcNAc with a 10-fold higher affinity than to sialic acidic monosaccharide, suggesting that it can be used to substitute WGA for more specific detection of O-GlcNAcylated proteins.40 Indeed, the same study demonstrated that 0.2 µg/mL of rPVL had an equivalent power to 0.66 µg/mL of the RL2 monoclonal antibody against O-GlcNAc for detecting GlcNAc in the Western blotting assay. Importantly, Audfray et al reported that the majority of breast cancer specimens were positively stained with biotinylated rPVL, while in the adjacent normal tissues only a few canular epithelial cells showed a weak staining.41 Liu et al reported that the Agrocybe aegerita lectin 2 (AANL) bound to the terminal GlcNAc of the sugar chain with a high specificity.42 Moreover, Su et al reported that mutagenesis of the 6 carbohydrate-binding sites in AANL led to identification of one recombinant AANL with an increased specificity to O-GlcNAc over the wild type AANL.43 Thus, the variety of natural lectins, with most of them so far uninvestigated, appear to provide some opportunities for identifying certain lectins that may be suitable for efficient O-GlcNAc-targeting. Chemical modification as well as recombinant manipulation of lectins are practical approaches to further enhance their O-GlcNAc-targeting efficiency.
WGA is the best-studied lectin for nanocarrier-aided detection and targeting of glycosylation (Table 1). Recently, Mukwaya et al covalently linked the Alexa Fluor 647-labeled WGA to polystyrene-co-polyacrylic acid, obtaining the 120 nm-diameter spherical nanoparticles.44 Their experiments demonstrated that WGA recognized a GlcNAc-containing cell membrane mimic formed by semipermeable polysaccharide-polymer microcapsules. Duan et al engineered a WGA-conjugated, matrine-loaded nanoparticle that was able to target the HT-29 colon cancer cells in vitro.45 This nanocarrier displayed an increased inhibition of HT-29 cells compared to the plain matrine-loaded nanoparticles without WGA-conjugation. Sialic acids are often present at the termini of O-GlcNAc chain. The phytolectin concanavalin A-functionalized nanocarriers showed a selective binding to sialic acids as well as an enhanced internalization in the mouse and human osteosarcoma cell cultures.46 It should be borne in mind that besides the diverse features of lectins, even a given type of lectins may display divergent affinities to different types of cancers. For example, biotinylated rPVL effectively stained the lung squamous carcinomas and adenocarcinomas, but reacted poorly with the bronchioalveolar, mucoepidermoid, large cell, and small cell, types of lung cancers.41 To design a meaningful O-GlcNAc-targeting scheme, a pair-wise determination of the interactions between a lectin and a type of cancer cells is required. It should be noted that many studies have indicated that protein corona is formed on the surface of virtually all types of nanoparticles in vivo, which may exert positive and/or negative impacts on the targeting efficiency of nanoparticles.47–49 The protein corona could compromise the ligand-receptor interaction, or increase the nonspecific endocytosis of nanoparticles by the MPS, both reducing the targeting efficiency of nanoparticles.50,51 On the other side, the corona containing apolipoprotein can bind to the low-density lipoprotein (LDL) receptors that are overexpressed in tumors, and facilitate nanoparticles to enter tumor cells.52 Javid et al demonstrated that the graphene oxide (GO) sheets can form coronas with varied compositions to elicit differential biological responses in patients suffering different diseases,53 pointing to the patient- and disease-specific design and application of nanocarriers. How the corona formation would affect the lectin- or O-GlcNAc antibody-mediated cancer targeting deserves further investigation.
 |
Table 1 Nanoparticles Modified with WGA for Glycosylation Detection or Targeting |
TME-Responsive Nanocarriers Improve the Drug Delivery Efficiency
The “off-target” problem will compromise the overall efficiency of nanocarriers that are conjugated with either lectins or antibodies. O-GlcNAcylated proteins broadly exist in normal tissues and circulation.60 Albeit at lower levels relative to malignant tissues, the O-GlcNAcylated proteins of non-cancerous sources can divert the O-GlcNAc-targeting nanocarriers, and causes the off-target effects. It is recognized that malignant tissues possess characteristic tumor microenvironment (TME), a concept broader than EPR to include tumorigenic factors, inflammation, hypoxia, and angiogenesis. The nanocarriers designed based on the TME features can partially alleviate the off-target problem. As illustrated in Figure 3, EPR effects promote the penetration of nanocarriers from the vessel compartment to tumor tissues and the retention of nanocarriers in tumor tissues. In addition, the cancer tissues often have an acidic (pH 6.5–6.9) and reducing TME. In a pH-responsive nanocarrier, the targeting ligand is covered by the functional group outside the TME. But in TME, the low-pH triggers the protonation/ionization of the functional group to expose the targeting ligand.61 Alternatively, the low-pH can trigger the cleavage of the acid-labile bonds or degradation of the shield moisty, resulting in an exposure of the ligand. Subsequently, the ligand can bind to its receptor or marker on the surface of tumor cells. Through the receptor-mediated endocytosis, the nanocarriers will enter tumor cells and release the drug molecules to a certain cellular compartment depending on the features of nanocarriers and cell responses.
The short arginine-glycine-aspartic acid (RGD) peptide can specifically bind to integrin receptors that are rich on the membranes of cancer cells. The RGD-modified graphene oxide nanoparticles exhibit an appreciable in vivo efficacy for enhanced cancer detection and therapy.62,63 Poly(2-(hexamethyleneimino)ethyl methacrylate)) (PC7A) represents a pH-hypersensitive functional group that is protonized at pH 6.9. A RGD-modified PC7A nanocarrier, namely the hierarchical responsive nanomedicine (HRNM), was constructed to facilitate the targeting of integrin-overexpressing cancer cells.64 The HRNM nanocarrier is composed of RGD, PC7A, the POEG (poly(oligo-(ethylene glycol) monomethyl ether methacrylate) matrix, and the chemotherapy drug pssCPT (poly reduction-responsive camptothecin prodrug). Under a physiological condition, the RGD-targeting ligand is shielded by PC7A. In an acidic TME, the RGD peptide is exposed, and binds to integrin to realize a specific targeting. Upon internalization, the bisulfide bond between CPT and POEG will be broken under the high-GSH (glutathione), reducing condition, leading to a selective drug releasing in cancer cells. Compared to the particle without PC7A-mediated pH response, the complete HRNM particle showed a 1.32-fold increase of uptake by tumor tissues in a mouse xenograft model. Thus, the hierarchical design significantly enhances the drug delivery efficiency and treatment safety.
The amide, ester, imine, oxime, acetal, and ketal bonds can be incorporated into the design of a nanocarrier structure, and their breakage by low-pH TME will cause a shedding of the coating moisty to expose the targeting ligands.61 For example, 2-propionic-3-methylmaleic anhydride, a low-pH TME degradable compound, was used to construct the PEG micelle nanocarrier. 2-propionic-3-methylmaleic anhydride was linked to the PEG micelle at one end, and to the nona-arginine (R9) peptide at the other end. At physiological pH, the PEG shell protects the nanocarrier against the clearance by MPS. The shedding of PEG shell at low-pH TME exposes R9 that helps the remaining part of nanocarrier to penetrate through, and accumulate in, cancer cells.65 This nanocarrier was successfully used to deliver a siRNA to knockdown the CDK4 expression of A549 lung cancer cells in a mouse xenograft model.65 Besides concerns on the connection between the covering groups and ligands, how to link the ligand to the nanomaterials also needs to be considered. The multiple -SH, -COOH and -NH2 groups of antibodies make it possible to covalently link an antibody to the matrix of nanocarriers. Similarly, lectins contain -NH2 groups that can be used for nanocarrier construction. Conceivably, nanocarriers equipped with the TME-responsive mechanisms may enhance the efficiency of antibodies- or lectins-mediated O-GlcNAc targeting.
Nanocarriers for Co-Delivery of OGT Inhibitor and Other Therapeutic Compounds to Achieve Synergistic Effect
Since simultaneous targeting of different pathways/mechanisms tend to result in synergistic effects, the combined usage of OGT inhibitor with other drugs offers an opportunity to achieve an enhanced efficacy. Lee and Kwon demonstrated that treatment of the HepG2 hepatocarcinoma cells with doxorubicin (1 μM) alone for 15 hours led to a 20% inhibition of cell viability, and treatment with the OGT inhibitor OSMI-1 (20 μM) did not have a significant effect. However, a combined treatment with the same concentrations of the two compounds accomplished a much increased inhibition rate of 70%.66 OSMI-2, generated by optimization of the OSMI-1 structure, was shown to reduce the O-GlcNAcylation in various cell lines. Itkonen et al screened 5000 bioactive compounds for their synergism with OSMI-2 and identified one compound AT7519 (a pan-cyclin-dependent kinase inhibitor) capable of achieving an enhanced efficacy with OSMI-2 in experiments with the use of a prostate cancer organoid.67 Although the investigators were seeking an OSMI-2 partner for an efficient combined therapy in a general sense, a nanocarrier capable of co-delivering the two drugs simultaneously to cancer tissues could further improve the synergy. The US FDA has approved the clinical application of Vyxeos® for treating the acute myelocytic leukemia in 2017. This double-delivery liposomal nanocarrier encapsulates cytarabine and daunorubicin in a 5:1 molar ratio. A post hoc analysis on the results of a Phase 3 trial by Lin et al covering 309 newly diagnosed, high-risk/secondary patients indicated that Vyxeos® chemotherapy accomplished a deeper remission as well as a longer median overall survival compared to the conventional chemotherapy of plain cytarabine and daunorubicin not co-delivered by the nanocarrier.68
Concluding Remarks
Sufficient data has proven a key role(s) of O-GlcNAcylation for the tumorigenesis, cancer invasion and metastasis. Interference of aberrant O-GlcNAcylation in cancer cells either by reduction of UDP-substrates or direct inhibition of OGT activity provides a new treatment modality for cancer therapy. Nanotechnique can be used to improve the efficiency of O-GlcNAcylation targeting in several ways. As an immediate benefit, a properly designed nanocarrier will enable the in vivo studies, eg, in animal models, by improving the solubility and/or cell permeability of these compounds. Through the EPR effects nanocarriers can passively increase the local concentration of these compounds and reduce the side effects. Nanocarriers can be conjugated with lectins or antibodies for a selective delivery of OGT inhibitors or other compounds to cancer tissues. Also, the manipulation of the structure of lectins by mutagenesis should be able to optimize their binding affinity and specificity with O-GlcNAc. Nanocarriers equipped with the TME-responsive mechanism would enhance the active cancer targeting. A combined use of OGT inhibitor and other drugs, or co-delivery of multiple compounds by a single nanocarrier may produce a synergistic effect, with the latter to be a more potent approach.
Current studies on the O-GlcNAc interfering compounds for cancer therapy are limited to experimental stage. Although lectin functionalized nanocarriers capable of targeting N- and O-linked glycosylation in cancer cells are extensively investigated, selective targeting of O-GlcNAcylation has not been actively pursued for therapeutic purpose. In addition to the off-targeting and other challenges discussed above, several issues impede the development of useful O-GlcNAc-targeting nanocarriers. The regulation of O-GlcNAcylation as well as its cognate enzymes OGT and OGA are poorly understood; there is a lack of data regarding the turnover rates of different types of glycosylation in cancer cells, and the time-dependent responses of cancer cells to OGT inhibitors have not been characterized; finally, the adverse effects by O-GlcNAc-targeting nanoparticles remain undetermined. Such investigation and acknowledgement should cover the whole body as well as the organ, tissue and cellular levels before attempt of clinical trials. Future studies focusing on these issues are required to take a full advantage of nanotechniques for the targeting of cancer-related O-GlcNAcylation.
Acknowledgments
This research work was funded by Grants LJ20A and WX201918 from the Leading Talents in Medical and Health Profession and Fenghuanchao Program of Wuxi Taihu Talent Plan (SWJ); Grant JS201920 from Jiangsu Shuangchuang Talent Program (SWJ); Grant 2018M630605 from the China Postdoctoral Science Foundation (RY); Grant BK20171148 from the Natural Science Foundation of Jiangsu Province (RY); and Grants BJ2020074 (RY) and BJ2020072 (YY) from the Wuxi Taihu Talent Plan.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259(5):3308–3317. doi:10.1016/S0021-9258(17)43295-9
2. Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol. 2017;18(7):452–465. doi:10.1038/nrm.2017.22
3. Matsuura A, Ito M, Sakaidani Y, et al. O-Linked N-Acetylglucosamine is present on the extracellular domain of Notch receptors. J Biol Chem. 2008;283(51):35486–35495. doi:10.1074/jbc.M806202200
4. Ogawa M, Senoo Y, Ikeda K, Takeuchi H, Okajima T. Structural divergence in O-GlcNAc Glycans displayed on epidermal growth factor-like repeats of mammalian Notch1. Molecules. 2018;23(7):1745. doi:10.3390/molecules23071745
5. Stateva SR, Villalobo A. O-GlcNAcylation of the human epidermal growth factor receptor. Org Biomol Chem. 2015;13(30):8196–8204. doi:10.1039/C5OB00443H
6. Wang L, Chen S, Zhang Z, et al. Suppressed OGT expression inhibits cell proliferation while inducing cell apoptosis in bladder cancer. BMC Cancer. 2018;18(1):1141. doi:10.1186/s12885-018-5033-y
7. Jang TJ. Differential membranous E-cadherin expression, cell proliferation and O-GlcNAcylation between primary and metastatic nodal lesion in colorectal cancer. Pathol Res Pract. 2016;212(2):113–119. doi:10.1016/j.prp.2015.12.003
8. Qian K, Wang S, Fu M, et al. Transcriptional regulation of O-GlcNAc homeostasis is disrupted in pancreatic cancer. J Biol Chem. 2018;293(36):13989–14000. doi:10.1074/jbc.RA118.004709
9. Chiaradonna F, Ricciardiello F, Palorini R. The nutrient-sensing hexosamine biosynthetic pathway as the Hub of cancer metabolic rewiring. Cells. 2018;7(6):53. doi:10.3390/cells7060053
10. Decourcelle A, Very N, Djouina M, et al. O-GlcNAcylation links nutrition to the epigenetic downregulation of UNC5A during colon carcinogenesis. Cancers. 2020;12(11):3168. doi:10.3390/cancers12113168
11. Akella NM, Ciraku L, Reginato MJ. Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 2019;17(1):52. doi:10.1186/s12915-019-0671-3
12. Ferrer CM, Sodi VL, Reginato MJ. O-GlcNAcylation in cancer biology: linking metabolism and signaling. J Mol Biol. 2016;428(16):3282–3294. doi:10.1016/j.jmb.2016.05.028
13. Itkonen HM, Urbanucci A, Martin SE, et al. High OGT activity is essential for MYC-driven proliferation of prostate cancer cells. Theranostics. 2019;9(8):2183–2197. doi:10.7150/thno.30834
14. Zhu Y, Hart GW. Targeting O-GlcNAcylation to develop novel therapeutics. Mol Aspects Med. 2021;79:100885. doi:10.1016/j.mam.2020.100885
15. Ju Kim E. O-GlcNAc transferase: structural characteristics, catalytic mechanism and small-molecule inhibitors. ChemBioChem. 2020;21(21):3026–3035. doi:10.1002/cbic.202000194
16. Franco Y, Vaidya T, Ait-Oudhia S. Anticancer and cardio-protective effects of liposomal doxorubicin in the treatment of breast cancer. Breast Cancer Targets Ther. 2018;10:131–141.
17. Hrkach J, Von Hoff D, Mukkaram Ali M, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4(128):128ra139. doi:10.1126/scitranslmed.3003651
18. Bloise N, Okkeh M, Restivo E, Della Pina C, Visai L. Targeting the “sweet side” of tumor with Glycan-binding molecules conjugated-nanoparticles: implications in cancer therapy and diagnosis. Nanomaterials. 2021;11(2):289. doi:10.3390/nano11020289
19. Jin L, Yuan F, Dai G, et al. Blockage of O-linked GlcNAcylation induces AMPK-dependent autophagy in bladder cancer cells. Cell Mol Biol Lett. 2020;25:17. doi:10.1186/s11658-020-00208-x
20. Asthana A, Ramakrishnan P, Vicioso Y, Zhang K, Parameswaran R. Hexosamine biosynthetic pathway inhibition leads to AML cell differentiation and cell death. Mol Cancer Ther. 2018;17(10):2226–2237. doi:10.1158/1535-7163.MCT-18-0426
21. Lemberg KM, Vornov JJ, Rais R, Slusher BS. We’re not “DON” yet: optimal dosing and prodrug delivery of 6-Diazo-5-oxo-L-norleucine. Mol Cancer Ther. 2018;17(9):1824–1832. doi:10.1158/1535-7163.MCT-17-1148
22. Ricciardiello F, Votta G, Palorini R, et al. Inhibition of the hexosamine biosynthetic pathway by targeting PGM3 causes breast cancer growth arrest and apoptosis. Cell Death Dis. 2018;9(3):377. doi:10.1038/s41419-018-0405-4
23. Borodkin VS, Schimpl M, Gundogdu M, et al. Bisubstrate UDP-peptide conjugates as human O-GlcNAc transferase inhibitors. Biochem J. 2014;457(3):497–502. doi:10.1042/BJ20131272
24. Jaskiewicz NM, Townson DH. Hyper-O-GlcNAcylation promotes epithelial-mesenchymal transition in endometrial cancer cells. Oncotarget. 2019;10(30):2899–2910. doi:10.18632/oncotarget.26884
25. Chariou PL, Ortega-Rivera OA, Steinmetz NF. Nanocarriers for the delivery of medical, veterinary, and agricultural active ingredients. ACS Nano. 2020;14(3):2678–2701. doi:10.1021/acsnano.0c00173
26. Almeida B, Nag OK, Rogers KE, Delehanty JB. Recent progress in bioconjugation strategies for liposome-mediated drug delivery. Molecules. 2020;25(23):5672. doi:10.3390/molecules25235672
27. Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590–1598. doi:10.1016/S0959-8049(01)00171-X
28. An H-W, Mamuti M, Wang X, et al. Rationally designed modular drug delivery platform based on intracellular peptide self-assembly. Exploration. 2021;1(2):20210153. doi:10.1002/EXP.20210153
29. Mei H, Cai S, Huang D, Gao H, Cao J, He B. Carrier-free nanodrugs with efficient drug delivery and release for cancer therapy: from intrinsic physicochemical properties to external modification. Bioact Mater. 2021;8:220–240. doi:10.1016/j.bioactmat.2021.06.035
30. Liu Y, Zhu S, Gu Z, Chen C, Zhao Y. Toxicity of manufactured nanomaterials. Particuology. 2022;69:31–48. doi:10.1016/j.partic.2021.11.007
31. Wang X, Cui X, Zhao Y, Chen C. Nano-bio interactions: the implication of size-dependent biological effects of nanomaterials. Sci China Life Sci. 2020;63(8):1168–1182. doi:10.1007/s11427-020-1725-0
32. Calvaresi EC, Hergenrother PJ. Glucose conjugation for the specific targeting and treatment of cancer. Chem Sci. 2013;4(6):2319–2333. doi:10.1039/c3sc22205e
33. Xu B, Zeng M, Zeng J, Feng J, Yu L. Meta-analysis of clinical trials comparing the efficacy and safety of liposomal cisplatin versus conventional nonliposomal cisplatin in nonsmall cell lung cancer (NSCLC) and squamous cell carcinoma of the head and neck (SCCHN). Medicine. 2018;97(46):e13169–e13169. doi:10.1097/MD.0000000000013169
34. Tashima Y, Stanley P. Antibodies that detect O-linked β-D-N-acetylglucosamine on the extracellular domain of cell surface glycoproteins. J Biol Chem. 2014;289(16):11132–11142. doi:10.1074/jbc.M113.492512
35. Barua R, Mizuno K, Tashima Y, et al. Bioinformatics and functional analyses implicate potential roles for EOGT and L-fringe in pancreatic cancers. Molecules. 2021;26(4):882. doi:10.3390/molecules26040882
36. Wang L, Chen S, Zhang J, et al. Suppressed OGT expression inhibits cell proliferation and modulates EGFR expression in renal cell carcinoma. Cancer Manag Res. 2019;11:2215–2223. doi:10.2147/CMAR.S190642
37. Zhu Q, Yi W. Chemistry-assisted proteomic profiling of O-GlcNAcylation. Front Chem. 2021;9:702260. doi:10.3389/fchem.2021.702260
38. Lochner N, Pittner F, Wirth M, Gabor F. Wheat germ agglutinin binds to the epidermal growth factor receptor of artificial Caco-2 membranes as detected by silver nanoparticle enhanced fluorescence. Pharm Res. 2003;20(5):833–839. doi:10.1023/A:1023406224028
39. de Fátima Menegoci Eugênio P, Assunção NA, Sciandra F, Aquino A, Brancaccio A, Carrilho E. Quantification, 2DE analysis and identification of enriched glycosylated proteins from mouse muscles: difficulties and alternatives. Electrophoresis. 2016;37:321–334. doi:10.1002/elps.201500362
40. Machon O, Baldini SF, Ribeiro JP, et al. Recombinant fungal lectin as a new tool to investigate O-GlcNAcylation processes. Glycobiology. 2017;27(2):123–128. doi:10.1093/glycob/cww105
41. Audfray A, Beldjoudi M, Breiman A, et al. A recombinant fungal lectin for labeling truncated Glycans on human cancer cells. PLoS One. 2015;10(6):22. doi:10.1371/journal.pone.0128190
42. Liu W, Han G, Yin Y, et al. AANL (Agrocybe aegerita lectin 2) is a new facile tool to probe for O-GlcNAcylation. Glycobiology. 2018;28(6):363–373. doi:10.1093/glycob/cwy029
43. Su Y, Ye X, Xu B, et al. CBS homogenization mutation strategy narrows the glycan binding profile of a GlcNAc-specific lectin AANL. Glycobiology. 2020;30(3):159–173. doi:10.1093/glycob/cwz089
44. Mukwaya V, Zhang PP, Guo HZ, et al. Lectin-Glycan-mediated nanoparticle docking as a step toward programmable membrane catalysis and adhesion in synthetic protocells. ACS Nano. 2020;14(7):7899–7910. doi:10.1021/acsnano.0c02127
45. Duan XY, Cheng YF, Sang F, et al. Enhanced targeting function and anti-colon cancer efficacy by wheat germ agglutinin-modified nanoparticles for matrine delivery. Int J Pharmacol. 2020;16(6):470–478. doi:10.3923/ijp.2020.470.478
46. Martínez-Carmona M, Lozano D, Colilla M, Vallet-Regí M. Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2018;65:393–404. doi:10.1016/j.actbio.2017.11.007
47. Corbo C, Molinaro R, Tabatabaei M, Farokhzad OC, Mahmoudi M. Personalized protein Corona on nanoparticles and its clinical implications. Biomater Sci. 2017;5(3):378–387. doi:10.1039/C6BM00921B
48. Pinals RL, Chio L, Ledesma F, Landry MP. Engineering at the nano-bio interface: harnessing the protein Corona towards nanoparticle design and function. Analyst. 2020;145(15):5090–5112. doi:10.1039/D0AN00633E
49. Chen D, Ganesh S, Wang W, Amiji M. Protein Corona-enabled systemic delivery and targeting of nanoparticles. AAPS J. 2020;22(4):83. doi:10.1208/s12248-020-00464-x
50. Fasoli E. Protein Corona: Dr. Jekyll and Mr. Hyde of nanomedicine. Biotechnol Appl Biochem. 2021;68(6):1139–1152. doi:10.1002/bab.2035
51. Farshbaf M, Valizadeh H, Panahi Y, et al. The impact of protein Corona on the biological behavior of targeting nanomedicines. Int J Pharm. 2022;614:121458. doi:10.1016/j.ijpharm.2022.121458
52. Francia V, Yang K, Deville S, Reker-Smit C, Nelissen I, Salvati A. Corona composition can affect the mechanisms cells use to internalize nanoparticles. ACS Nano. 2019;13(10):11107–11121. doi:10.1021/acsnano.9b03824
53. Hajipour MJ, Raheb J, Akhavan O, et al. Personalized disease-specific protein Corona influences the therapeutic impact of graphene oxide. Nanoscale. 2015;7(19):8978–8994. doi:10.1039/C5NR00520E
54. Chio L, Del Bonis-O’Donnell JT, Kline MA, et al. Electrostatic assemblies of single-walled carbon nanotubes and sequence-tunable peptoid polymers detect a lectin protein and its target sugars. Nano Lett. 2019;19(11):7563–7572. doi:10.1021/acs.nanolett.8b04955
55. Li X, Yang G, Guan F. Preparation and application of lectin modified magnetosomes. J Biol. 2015;32(6):96–99.
56. Ferreira JA, Daniel-da-silva AL, Alves RMP, et al. Synthesis and optimization of tectin functionalized nanoprobes for the selective recovery of Glycoproteins from human body fluids. Anal Chem. 2011;83(18):7035–7043. doi:10.1021/ac200916j
57. Parker LM, Reineck P, Ghanii-Fard M, et al. Utilising glycobiology for fluorescent nanodiamond uptake and imaging in the central nervous system.
58. Pooja D, Kulhari H, Kuncha M, et al. Improving efficacy, oral bioavailability, and delivery of paclitaxel using protein-grafted solid lipid nanoparticles. Mol Pharm. 2016;13(11):3903–3912. doi:10.1021/acs.molpharmaceut.6b00691
59. Upadhyay A, Kandi R, Rao CP. Wheat germ agglutinin modified magnetic iron oxide nanocomplex as a cell membrane specific receptor target material for killing breast cancer cells. J Mat Chem B. 2018;6(36):5729–5737. doi:10.1039/C8TB01170B
60. Verathamjamras C, Sriwitool T-E, Netsirisawan P, et al. Aberrant RL2 O-GlcNAc antibody reactivity against serum-IgA1 of patients with colorectal cancer. Glycoconj J. 2021;38(1):55–65. doi:10.1007/s10719-021-09978-8
61. Yan Y, Ding H. pH-responsive nanoparticles for cancer immunotherapy: a brief review. Nanomaterials. 2020;10(8):1613. doi:10.3390/nano10081613
62. Akhavan O, Ghaderi E. Graphene nanomesh promises extremely efficient in vivo photothermal therapy. Small. 2013;9(21):3593–3601. doi:10.1002/smll.201203106
63. Ouyang A, Zhao D, Wang X, Zhang W, Jiang T. Covalent RGD-graphene-phthalocyanine nanocomposite for fluorescence imaging-guided dual active/passive tumor-targeted combinatorial phototherapy. J Mater Chem B. 2022;10(2):306–320. doi:10.1039/D1TB02254G
64. Wang S, Yu G, Wang Z, et al. Hierarchical tumor microenvironment-responsive nanomedicine for programmed delivery of chemotherapeutics. Adv Mater. 2018;30:e1803926–e1803926. doi:10.1002/adma.201803926
65. Sun CY, Shen S, Xu CF, et al. Tumor acidity-sensitive polymeric vector for active targeted siRNA delivery. J Am Chem Soc. 2015;137(48):15217–15224. doi:10.1021/jacs.5b09602
66. Lee SJ, Kwon O-S. O-GlcNAc transferase inhibitor synergistically enhances doxorubicin-induced apoptosis in HepG2 cells. Cancers. 2020;12(11):3154. doi:10.3390/cancers12113154
67. Itkonen HM, Poulose N, Steele RE, et al. Inhibition of O-GlcNAc transferase renders prostate cancer cells dependent on CDK9. Mol Cancer Res. 2020;18(10):1512–1521. doi:10.1158/1541-7786.MCR-20-0339
68. Lin TL, Rizzieri DA, Ryan DH, et al. Older adults with newly diagnosed high-risk/secondary AML who achieved remission with CPX-351: phase 3 post hoc analyses. Blood Adv. 2021;5(6):1719–1728. doi:10.1182/bloodadvances.2020003510
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.