Back to Journals » Infection and Drug Resistance » Volume 16
How Long Will It Take to Launch an Effective Helicobacter pylori Vaccine for Humans?
Authors Li S , Zhao W , Xia L, Kong L, Yang L
Received 14 March 2023
Accepted for publication 2 June 2023
Published 15 June 2023 Volume 2023:16 Pages 3787—3805
DOI https://doi.org/10.2147/IDR.S412361
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Songhui Li,1,* Wenfeng Zhao,2,* Lei Xia,3 Lingyi Kong,1 Lei Yang1
1Jiangsu Key Laboratory of Bioactive Natural Product Research and State Key Laboratory of Natural Medicines, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing, 210009 People’s Republic of China; 2Department of Biochemistry, School of Life Science and Technology, China Pharmaceutical University, Nanjing, 210009 People’s Republic of China; 3Bloomage Biotechnology Corporation Limited, Jinan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lingyi Kong, Jiangning Campus, China Pharmaceutical University, No. 639 Longmian Avenue, Chunhua Street, Jiangning District, Nanjing City, Jiangsu Province, People’s Republic of China, Fax +86-25-8327-1405, Email [email protected] Lei Yang, Jiangning Campus, China Pharmaceutical University, No. 639 Longmian Avenue, Chunhua Street, Jiangning District, Nanjing City, Jiangsu Province, People’s Republic of China, Tel +86-13813925076, Email [email protected]
Abstract: Helicobacter pylori infection often occurs in early childhood, and can last a lifetime if not treated with medication. H. pylori infection can also cause a variety of stomach diseases, which can only be treated with a combination of antibiotics. Combinations of antibiotics can cure H. pylori infection, but it is easy to relapse and develop drug resistance. Therefore, a vaccine is a promising strategy for prevention and therapy for the infection of H. pylori. After decades of research and development, there has been no appearance of any H. pylori vaccine reaching the market, unfortunately. This review summarizes the aspects of candidate antigens, immunoadjuvants, and delivery systems in the long journey of H. pylori vaccine research, and also introduces some clinical trials that have displayed encouraging or depressing results. Possible reasons for the inability of an H. pylori vaccine to be available over the counter are cautiously discussed and some propositions for the future of H. pylori vaccines are outlined.
Keywords: Helicobacter pylori vaccines, antigen, adjuvant, delivery system, clinical trials
Graphical Abstract:
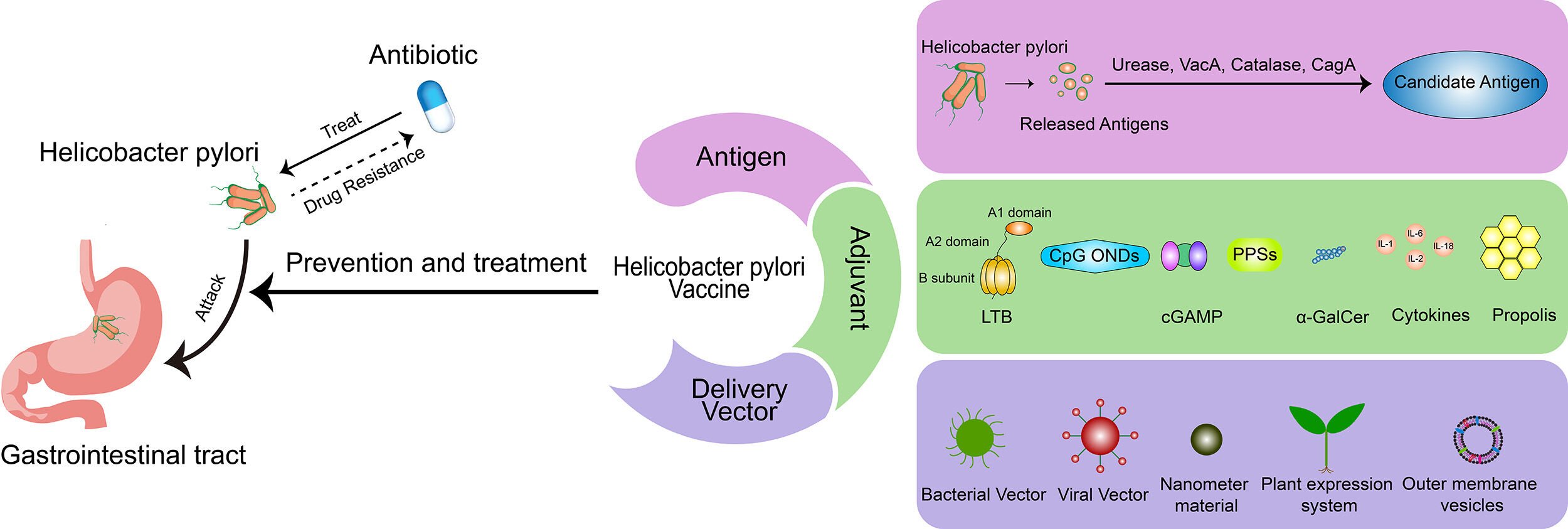
Introduction
Helicobacter pylori became a concern in 1979 when John Warren, a pathologist in Perth, Western Australia, discovered that the inflamed gastric mucosa was covered with bacteria that looked like Campylobacter.1 Warren and his colleague Barry Marshall cultured the first batch of these bacteria, newly named Helicobacter pylori, subsequently from 11 patients with gastritis in 1982.2 H. pylori is a spiral and slightly aerobic pathogenic bacteria that can survive in the stomach and colonize for life through contact transmission from the oral cavity or feces.3 It may lead to gastritis, digestive tract ulcers, lymphoproliferative gastric lymphoma, and even gastric cancer.4 Nearly 50% of the global population is infected, and the infection rate is even higher in developing countries.5,6 The pathogenic mechanism of H. pylori is a complex process that has been reviewed in detail by a considerable number of articles.
Currently, there are four main first-line treatment regimens for H. pylori: clarithromycin-containing triple therapy, sequential therapy, concomitant therapy, and bismuth quadruple therapy. Quadruple therapy is the recommended first-line treatment. In areas where the incidence of clarithromycin resistance is low and in patients who have not previously used macrolides, a 14-day triple therapy containing clarithromycin is recommended.7 The anti-inflammatory and antioxidant mechanisms of probiotics can improve intestinal microecology and general health, but cannot increase the eradication rate of H. pylori infection.8 Therefore, probiotic therapy can only be used as an adjunctive therapy to reduce antibiotic-related adverse events. Although clarithromycin-containing triple therapies initially achieve a radical cure rate of >90%,9 due to the increase in macrolide drug resistance, mainly clarithromycin, the efficacy of these therapies has been reduced to unacceptably low levels (≤80%) in most parts of the world.10 Vaccination is effective in the prophylaxis and therapy of infectious diseases and has been verified after hundreds of years of application practice. The focus has shifted to H. pylori infection, even though there is no available vaccine as yet. In 1990, Pallen et al thought that an anti-urease vaccine was promising,11 and now urease is one of the most important vaccine antigens. Czinn et al proposed in 1991 that oral H. pylori antigen plus cholera toxin could increase the level of antibodies in serum and intestinal tract.12 Then, in 1993 Czinn et al proved that the resistance to H. pylori comes from IgG and IgA produced by oral immunization. Active oral or passive immunization of IgA can provide resistance to H. pylori in mice.13 Since then, the search for an H. pylori vaccine has entered a period of rapid development. In the process, there have been >10 kinds of candidate antigen identified to use alone or in combination, nearly 10 kinds of adjuvant that have been tested to enhance immunoresponse, and various delivery systems introduced to help antigen presentation. Several H. pylori vaccines have been undergoing or completed clinical trials.14–26 This review focuses on an H. pylori vaccine, provides references to counterparts, summarizes the progress of candidate antigens, immunoadjuvants, delivery systems, and clinical trials, analyzes the current situation cautiously, and looks forward to the future confidently.
Antigens
Urease
H. pylori urease is a polyenzyme composed of 12 UreA and UreB heterodimers, accounting for 10%–15% of the total protein content of bacteria.27 Urease can catalyze the hydrolysis of urea to CO2 and NH3. NH3 can neutralize excess gastric acid, inhibit the function of neutrophils,28 promote the formation of cytotoxic NH3-derived compounds,29 and destroy the integrity of gastric epithelial cell connections,30,31 thus providing conditions for the colonization of pathogens in the stomach. CO2 can protect bacteria from ONOO– cytotoxicity, thereupon then promoting the colonization of pathogens.32 The discrete surface of urease complex can directly interact with host components involved in immunomodulatory activities, thereby ensuring the continuous colonization of H. pylori.33 Inhibition of urease activity will impair the ability of H. pylori to colonize in the stomach, and plays a role in the prevention and treatment of H. pylori.34 Most studies have used urease as a candidate antigen, such as Nasr-Esfahani et al, who showed that injecting the recombinant plasmid pcDNA3.1 (+)-ureA into mice can stimulate an immunoresponse in an animal model infected with H. pylori.35 Intranasal immunization with recombinant UreB vaccine with plant polysaccharides as adjuvants protected mice from H. pylori infection, which may be related to increased gastrointestinal specific SIgA and Th1/Th17 CD4+ T-cell response.36 Most of the vaccines that have entered the clinical research stage contain urease antigen.14–17,19–23,25 It is worth mentioning that the H. pylori vaccine with urease and heat-labile enterotoxin subunit B (LTB) developed by Zeng et al in 2015 has achieved gratifying results in phase III clinical trials.25
VacA
Vacuolating cytotoxin A (VacA) is a protein component that causes vacuolization of eukaryotic cells.37 All H. pylori strains contain the vacA gene, while only about 50% of H. pylori strains produce cytotoxin encoded by the vacA gene.37,38 VacA is a kind of secretory pore-forming toxin, which can form anion-selective channels in planar lipid bilayers and have extensive effects on host cells,38 such as leading to epithelial vacuolization, enhancing H. pylori colonization in the stomach, and interfering with MHC II antigen presentation.39 Recently, using a polysaccharide adjuvant (PA) containing Lycium barbarum polysaccharides (LBPs) and chitosan as adjuvant, Guo et al designed a vaccine FVpE containing Th1 immunoadjuvant NAP, functional fragments of CagA and VacA, and urease multiepitope peptides. Compared with natural urease vaccine, FVpE can induce higher levels of antigen-specific antibodies and significantly reduce the number of H. pylori in infected mice.40 Phase II clinical trials have been carried out on the multiepitope vaccine containing vacuolating toxin. Compared with the placebo group, this vaccine can enhance the systemic humoral response to key H. pylori antigens, which have fully demonstrated the potential of vacuolating toxins as vaccine candidates.24,41
cagPAI
The cag pathogenicity island (cagPAI) is a chromosome region of 40 kb encoding a functional type IV secretory system, which plays an important role in the pathogenesis of H. pylori.41 The three genes cagA, cagL, and cagW encoded by this region can be used as candidate antigens.
CagA
Cytotoxin-associated gene A (cagA) is located at one end of cagPAI, which is closely related to the production of VacA.41,42 The protein encoded by cagA is a bacterial surface protein with a relative molecular weight of 120–128 kDa containing a carboxyl terminal variable region.43 The expression rate of CagA in strains found in Western countries is about 60%, while almost all East Asian strains express CagA.44 It has been indicated that the fragments of CagA can produce immunogenicity in human models.45 Shapouri et al successfully expressed the 38 kD recombinant protein CagA (rCagA) in Escherichia coli BL21, which was able to bind to human antiserum.46 After mice were immunized with rCagA and lipopolysaccharide, compared with the control group, the combined-immunization group produced a strong Th1 immunoresponse. Importantly, the number of H. pylori cells decreased sixfold.47 Similarly, Paydarnia et al proposed that a CpG adjuvant carrying H. pylori lipopolysaccharide and rCagA protein not only maintained the antigenicity of the recombinant protein throughout the experiment but also induced a strong Th1-biased immunoresponse.48 CagA has also been used as a candidate antigen in clinical experiments and has been proved to be effective in stimulating immunoresponses.24,26
CagW
CagW is the key subunit of the protein transport type IV secretory system, which is the VirB6-like protein of the Cag system.49,50 The interaction between CagW and CagA provides conditions for CagA to pass through the bacterial membrane barrier.49,50 Chehelger et al successfully prepared high-quality chitosan nanoparticle (NP)-encapsulated cagW gene DNA vaccine-pcDNA3.1 (+)-cagW-CS-NPs, which induced high mucosal and humoral immunoresponses in mice.51
CagL
CagL is a highly conserved protein encoded by cagPAI located on the surface of bacterial type IV secretory pili.52,53 It has been shown to bind integrin receptor α5β1, activate EGFR, and mediate virulence-factor infusion into host target cells, so it has the potential to be used as a candidate antigen.45 Aliramaei et al cloned the cagL gene in a pAMJ2008 vector and transferred the recombinant plasmid into Lactococcus lactis MG1363. Mice immunized with recombinant L. lactis MG1363/pAMJ2008 cagL can produce specific anti-cagL IgA.54
Catalase
Catalase (CAT) is a ubiquitous enzyme in eukaryotes and most prokaryotes to protect bacteria from hydrogen peroxide.55 H. pylori CAT is a homologous tetramer protein with an isoelectric point of 9.0–9.3, which accounts for about 1% of the total protein of H. pylori.55–57 CAT protects bacteria from reactive oxygen species produced by the host and helps bacteria escape the phagocytosis of macrophages.57,58 Recently, the immunodominant Th1 epitopes of CAT have been fully identified. Seven new epitopes of CAT can produce significant Th1 response by expressing IFNγ.59 Three Th-cell epitopes and five B-cell epitopes from H. pylori antigen (HpaA, UreB and CAT), adjuvant LTB, and an appropriate linker were selected to form the multivalent epitope vaccine LHUC. After oral administration of fusion peptide LHUC, antigen-specific antibodies were detected in mouse serum, and the secretion of IFNγ, IL4, and IL17 by lymphocytes increased significantly. LHUC can significantly prevent C57BL/6 mice from being infected with H. pylori.60
HpaA Adhesion
H. pylori adhesion A (HpaA), which is described as hemagglutinin, belongs to the H. pylori outer-membrane proteins and the adhesin family. It is a highly conserved H. pylori-specific lipoprotein, which can bind to neuraminyllactose and various glycosylated components on the surface of gastric epithelial cells to ensure H. pylori attachment to the surface of gastric mucosa.61,62 Additionally, HpaA is highly conserved and is expressed on the surface and intracellular regions of bacteria.63,64 Also, it mediates binding with sialic acid in vitro, participates in the maturation of dendritic cells in vivo, and may affect antigen presentation.65,66 HpaA with deletion of the amino-terminal region may lose immunogenicity, and consequently the ability of lipoprotein to activate TLR2 depends on its N-terminal lipid part.67 Xue et al synthesized two new lipopeptides (LP1 and LP2) by predicting the lipid-modification sites of natural HpaA and simulating the terminal structure of natural HpaA. The results showed that LP2 could enhance the protective effect of rHpaA against H. pylori infection, which might be closely related to its ability to simulate the terminal structure of natural HpaA to activate TLR2.68
NAP
H. pylori neutrophil activating protein is a highly conserved spherical dodecameric protein with molecular weight of 17 kDa.69 It is a member of the adhesion family and exists in almost all H. pylori isolates.70,71 NAP has the ability to specifically bind to high-molecular-weight mucins and mediate the adhesion of H. pylori to host cells.70,72 NAP can also protect bacteria from DNA oxidative damage by inducing the host to produce oxidative stress.73 NAP has proinflammatory and immunomodulatory effects, which play an important role in the pathogenesis of H. pylori.74,75 Recently, new advances have been made in the study of NAP as a candidate antigen of H. pylori. After oral administration of H. pylori multicomponent subunit vaccine composed of NAP, UreA, UreB, and double-mutant heat-labile toxin (dmLT) from E. coli, mice produced significant Th1/Th17 immunoresponse and antigen-specific antibodies.76,77 Jafari et al screened three main CD4+T cell epitopes of H. pylori antigens UreB, HpaA, and NapA by immunoinformatics. After ligation with a suitable linker, the fusion protein was successfully expressed in E. coli BL21(DE3), but the effectiveness of the vaccine needs to be further studied.78 Clinical studies have shown that NAP combined with other antigens can enhance the systemic humoral immunoresponse.24,26
OipA
H. pylori outer-membrane inflammatory protein A (OipA) is one of the outer-membrane proteins with relative molecular weight of 34 kDa, which is one of the important virulence factors of H. pylori.79 A functional OipA should have the ability to promote the production of proinflammatory cytokines, participate in the attachment of bacteria to host cells, and help the host adapt to the environment.80,81 Soudi et al proved that both oral and injection of recombinant OipA can induce mice to produce IFNγ and promote Th1 immunoresponse.82
HcpD
Cysteine-rich H. pylori protein (Hcp) is a unique protein of H. pylori expressed in the natural environment.83 HcpD (HP0160) is also a member of the Hcp family, which can covalently bind to penicillin derivatives.84 Nasr et al prepared recombinant plasmid pCDNA3.1 (-)-hcpD and synthesized chitosan NPs by ion gelation. The results showed that pCDNA3.1 (-)-hcpD vaccine stimulated the immune system of vaccinated mice, either alone or in combination with chitosan NPs.85
Flagellin
FlaA
Flagella play a major driving role in the movement of bacteria, which means that flagellin is essential for H. pylori infection and continuous colonization.86,87 H. pylori flagellin consists of two subunits, FlaA, the main flagellin of 53 kDa, and FlaB, the secondary flagellin of 54 kDa.86,87 In view of the important role of the two flagellins in gastric mucosal injury, FlaA and FlaB may be used as candidate antigens against H. pylori infection.88,89 Recombinant expression vector pBudCE4.1-flaA was successfully expressed in human dermal fibroblasts, and immunoresponse was activated by intramuscular injection of the vaccine in mice.90 Hamzehloo et al constructed the recombinant plasmid pET32a-flaA-ureB. The recombinant protein can be recognized in the sera of patients with H. pylori infection with high sensitivity and specificity.91
FliD
At the distal end of flagellar filaments, there is a flagellar cap protein (FliD) of 56 kDa encoded by the fliD gene, which participates in the growth of flagellar filaments and protects the structure of flagellar tip from destruction.92–94 The fliD gene encodes the structural component hook-associated protein 2 (HAP2) of the flagellum cap at the end of the filament, which is involved in the assembly of flagella.92 FliD has been proved to be an antigen that can induce immunoresponse to H. pylori.95 Recently, Zhao et al studied the crystal structure of HpFliD at the molecular level. Serological tests showed that the D4 and D5 domains of HpFliD had stronger antigenicity than the D2 and D3 domains and could stimulate stronger immunoresponse, so the D4 and D5 domains were expected to be targets for an H. pylori vaccine or diagnosis of related diseases.94
Lipopolysaccharide
H. pylori lipopolysaccharide is a glycolipid component on the cell surface of H. pylori, and it is also an endotoxin.96 It consists of the three different regions: O-chain polysaccharide (PS) with variable structure, conservative core oligosaccharide, and lipid A.96 H. pylori lipopolysaccharide has been proved that it performs an important role in the pathogenic mechanism of H. pylori and it has the ability to proliferate gastric epithelial cells.97 It can also bind to the protein receptor on the mucous membrane and enhance the binding ability of bacteria to the gastric mucosa.98 O-chain polysaccharides have an aspecific immunostimulatory effect, and play a part in inducing immunoresponse and gastric injury in the host.99 Tian et al synthesized a unique α-(1→3)-linked tri-D-glycero-D-manno-heptose antigen from lipopolysaccharide of H. pylori serogroups O3 and O6 and strains MO19, D2, D4, and D5. This antigen can induce very strong T cell–dependent antigen-specific immunoresponse and induce the body to produce high titers of the antigen-specific antibodies IgG1 and IgG2b.100
Lpp20
Lipoprotein 20 (Lpp20) is a conserved lipoprotein with apparent molecular weight of 18 kDa, which is unique to H. pylori and related to membrane.101 Lpp20 is expressed in almost all tested H. pylori strains.101 By virtue of its strong immunogenicity, many studies have identified it as a dominant candidate for H. pylori vaccine.101–104 Ning et al identified two dominant epitopes of Lpp20, and proved that these two epitopes can stimulate the proliferation of CD4+ T cells.105 Sun et al successfully expressed Lpp20 in recombinant L. lactis strains. Oral administration of the engineered bacteria increased serum IgG and decreased gastric urease activity in mice.106
Heat-Shock Protein
Heat-shock protein A (HspA) is a homologue of GroES chaperone protein family with relative molecular weight of 13 kDa and is found in H. pylori.107,108 Its C-terminal domain is rich in histidine and cysteine, which is different from the GroES protein family.108 This unique domain is conducive to the binding of proteins to nickel ions.109 Some researchers have studied it as a vaccine component of H. pylori and proved its potential as a candidate antigen.110,111 Zhang et al successfully identified two highly conserved B-cell epitopes of HspA with good immunogenicity.112 The recombinant measles virus (MV) vaccine expressing H. pylori HspA antigen has strong antitumor activity and strong immunogenicity.113
GroEL
GroEL is a chaperone protein secreted by H. pylori, which belongs to the molecular chaperone family, which is also described as a homologue of Hsp60.114 It is highly conserved and has been identified as a virulence factor of H. pylori and a risk predictor of gastric cancer.115,116 It has been reported that GroEL antigen was found in all tested strains of H. pylori.117 Khan et al designed a new type of H. pylori multiepitope subunit vaccine containing helper T-lymphocyte epitopes (HTL) of CagA, OipA, GroEL, and VacA antigens using a variety of immunoinformatic methods and other computational methods. Although the stability and immunity of the vaccine have been verified, its effectiveness vaccine still needs to be tested in further clinical trials.118
52 kDa H. pylori Membrane Peptide
The isolation and purification of Hcp is facing great challenges, so the synthetic peptide designed by immunogenic proteins has become an alternative for diagnosis and prevention.119 Espinosa-Ramos et al designed and synthesized an H. pylori 50–52 kDa immunogen-derived peptide antigen with the sequence Met-Val-Thr-Leu-Ile-Asn-Asn-Glu (MVTLINNE). Compared with the untreated group, the MVTLINNE polypeptide vaccine mediated the body to produce specific antiserum IgG and IgA, reduced H. pylori colonization significantly in mice, and combated the occurrence of gastric ulcer effectively.119
Adjuvants
LTB
Heat-labile enterotoxin (LT) is a diarrhea-causing toxin produced by E. coli (ETEC) and a member of the AB5 bacterial toxin family.120,121 LT is related to the cholera toxin in chemical characterization and immunology.122 It consists of one enzyme active subunit (LTA) and five subunits (LTB).120 LTB has been widely used in immunity experiments owing to the fact that it is no catalytic activity or toxicity. There is considerable evidence that vaccines with LTB as an immunoadjuvant can stimulate the body to produce immunoresponse.123–125 Recently, Peng et al expressed the napA gene and LTB through Lactobacillus lactis, and proposed that LTB can enhance the protective effect induced by oral vaccine by aggravating mucosal inflammatory injury and leukocyte leakage.126 LTB has been selected as an immunoadjuvant in most clinical studies of H. pylori vaccine and has good immunoeffect, but it has some side effects on the human body.15,18,20,21,25 A clinical study has shown that low-dose LTB can reduce toxicity and maintain immunogenicity.21
CpG ODNs
The interaction between CpG oligodeoxynucleotides (CpG ODNs) and the expression of Toll-like receptor 9 triggers a cascade of signals to activate B and T lymphocytes, monocytes, natural killer cells, macrophages, and dendritic cells.127 It can also improve the host’s ability to resist pathogen attacks by initiating immunomodulatory cascades.128 CpG ODNs have been widely used as a vaccine adjuvant and have been proved to be safe for humans.129,130 Paydarnia et al demonstrated that the rCagA protein carried by CpG adjuvant not only maintains the antigenicity of the recombinant protein throughout an experiment but also induces a strong Th1-biased immunoresponse.48
cGAMP
cGAMP is the first endogenous cyclic dinucleotides discovered. It is synthesized by cyclase in response to the stimulation of DNA ligands, which can directly bind to STING receptor protein and activate STING to trigger downstream signal cascade, including TBK1, IRF3 activation, IFNβ, and other cytokines.131,132 STING agonists have proved to be a promising immunoadjuvant, including cGAMP.133,134 In a recent study, H. pylori UreA, UreB, and NAP adjuvanted with cGAMP induced immunoresponse in mice. The reaction of antigen-specific immunoglobulin and mucosal IgA in serum of mice immunized after nasal cavity and subcutaneous immunization increased significantly, while gastric mucosal colonization decreased significantly.135
Plant Polysaccharides
Plant polysaccharides (PPSs) are active components extracted from natural plants, and have unique characteristics and low toxicity.136 Many studies have shown that polysaccharide adjuvants are effective vaccine adjuvants that can improve humoral or cellular immunity, such as in Astragalus polysaccharides, Epimedium polysaccharides, LBPs, chitosan, Taiwan Ganoderma formosanum polysaccharides PS-F2, Chinese yam polysaccharides, isatis root polysaccharides, and Achyranthes bidentata polysaccharides.137–140 Liu et al have proved that Astragaluspolysaccharides combined with rUreB can induce mixed Th1 and Th17 immunoresponses, which may help mice to resist H. pylori infection.141 Similarly, Guo et al showed that the combination of polysaccharide mucosal adjuvant-containing LBPs and chitosan with a multivalent epitope vaccine enhanced the protective effect of this vaccine.40
α-GalCer
α-Galactosylceramide (α-GalCer) is a kind of glycolipid originally extracted from marine sponge.142 α-GalCer is considered an effective multifunctional vaccine adjuvant, and can induce humoral and cellular immunoresponses.143,144 The results of prophylactic intragastric immunization with whole-cell inactivated antigen of H. pylori combined with nontoxic oral adjuvant α-GalCer showed strong intestinal and systemic Th1 cellular immunoresponse, as well as significant antigen-specific mucosal and systemic antibody response. The effect of α-GalCer adjuvant is similar to that of the standard adjuvant cholera toxin.145
Cytokines
Many studies have shown that the combination of cytokine protein or cytokine gene-encoding plasmid with DNA vaccine can make DNA vaccine prefer to trigger Th1 immunoresponse, such as IL2, IL1, IL6, IL15, and IL12.146 Nemattalab et al proposed for the first time that the use of IL18, IL17A, and IL22 as molecular adjuvants in a DNA vaccine regimen can change immunoresponse and improve the efficacy of DNA vaccine. It was proved that the coexpression of oipA gene and IL17A molecular adjuvant can effectively combat H. pylori infection.147
Propolis
Propolis, a kind of resin compound collected by bees from developing flowers, has complex chemical composition and different biological and pharmacological properties.148 Propolis also shows the characteristics of immunostimulation and immunomodulation.148 It has been proved to be an adjuvant in mouse-model vaccination.149,150 Soudi et al found that the recombinant OipA vaccine with propolis as adjuvant can induce more IFNγ and induce stronger cellular immunoresponse than the recombinant OipA vaccine without adjuvant.82
Chemically Synthesized Adjuvants
An important intracellular signal molecule of bacteria, 3’,5’-cyclic diguanylic acid (c-di-GMP) plays an important role in bacterial movement, adhesion, and virulence, so it has been considered an effective immunostimulator and a useful mucosal adjuvant.151,152 Introduction of fluorine into therapeutic agents has been well recognized as a useful modification to modulate pharmacological properties.153 The chemically synthesized bis-(3’-5’)-cyclic dimeric 2’-deoxy-2’-fluoroguanosine monophosphate (20-F-c-di-GMP) as an adjuvant in combination with the ultrasonic extract of H. pylori can induce the production of antigen-specific antibodies in mice and reduce the colonization of H. pylori in the stomach of immunized mice.154
Vaccine-Delivery Vectors
Bacterial Carrier-Delivery System
Live bacteria, including attenuated bacteria and probiotics, can be modified into delivery systems for delivering target antigens. The advantage of these live bacterial vaccine vectors is that they stimulate long-lasting humoral and cellular immunity.155 Pathogens such as bacteria and viruses are usually pathogenic to humans, but their pathogenic genes can be mutated by chemical or molecular biological techniques, which can reduce their toxicity while maintaining their invasiveness to the mucosa. The application of bacterial delivery systems in H. pylori vaccine will be briefly described below.
Lactic Acid Bacteria
Lactic acid bacteria (LAB) are nonpathogenic, non-colonized, and Gram-positive and rated Generally Recognized as Safe (GRAS) by the US Food and Drug Administration.156 Target proteins delivered by LAB via mucosal routes can successfully induce systemic humoral immunoresponses.157 Recently, there have also been some new developments in LAB as vaccine carriers. Recombinant Lb. acidophilus expressing H. pylori adhesin Hp0410 can induce high levels of mucosal SIgA antibody and enhance the effect of mice against H. pylori infection.158 Gou et al designed an M cell–targeting L. lactis surface display system — plSAM. The system can successfully deliver recombinant antigen, promote the phagocytosis and transport of antigens by M cells in the gastrointestinal tract, and induce protective immunoresponse.159 Likewise, recombinant L. lactis MG1363 carrying antigen CagL can induce antigen-specific antibodies in mice.54
Saccharomyces cerevisiae
Saccharomyces cerevisiae is a single-celled eukaryotic microorganism with a size of about 5–10 μm that was awarded GRAS organism status by the US Food and Drug Administration.160 S. cerevisiae has achieved results in the vaccine research on diverse viruses, bacteria, parasites, cancers.161 In H. pylori vaccine development, researchers applied S. cerevisiae to expressing recombinant UreB and VacA, and gained an oral vaccine against H. pylori, which showed significant humoral and mucosal immunoresponses and significantly reduced the colonization of H. pylori after vaccination in mice.162
Listeria monocytogenes
Listeria monocytogenes is a Gram-positive, facultative anaerobe with a broad host spectrum, and is considered as a potential vaccine vector that can induce a wide range of immunoresponses.163 With L. monocytogenes as the vaccine carrier, the attenuated live vaccine containing multiple epitope chimeric antigens (MECU) of H. pylori has been constructed. The results indicated that the vaccine can stably express and secrete MECU. After intragastric and intravenous immunization, it can significantly reduce the colonization of H. pylori and induce a high level of anti-H. pylori antibody.155
Shigella
Shigella, also known as Shigella dysenteriae, is a non-capsular, non-athletic filamentum that is highly contagious. Noninvasive Shigella strains without plasmid invasion genes may be used as carriers of oral vaccines for short-term immunization.164 Oral attenuated Shigella vector vaccine expressing the H. pylori fusion protein rUreB–HspA and subcutaneous injection of rUreB–HspA protein can induce antigen-specific serum immunoresponse, mucosal SIgA immunoresponse, and T-cell immunoresponse.165
Nanometer Material-Delivery Systems
As the protein degrades after the oral vaccine enters the gastrointestinal tract, the antigen-uptake rate may be low, which leads to the oral vaccine having no immunogenicity or weak immunogenicity.166 NP-delivery systems are increasingly used in vaccine design to overcome the weak immunogenicity of oral vaccines.167 Some NPs themselves have adjuvant properties, which can improve vaccine efficacy.168 D,L-lactide-co-glycolic acid (PLGA) NPs are qualified vaccine-delivery devices and drug controlled-release systems that have been widely used in vaccine delivery.169 HP55 is a special type of enteric coating agent that can prevent drugs from being degraded by gastric acid.166 PLGA-NPs can be modified with HP55 to obtain HP55/PLGA NPs, which are acid-resistant with excellent stability.166 Tan et al encapsulated the H. pylori recombinant antigen in acid-resistant HP55/PLGA NPs, which protected the recombinant H. pylori antigen against the complex gastrointestinal environment and improved the protective effect of the recombinant vaccine.166 As a kind of biocompatible carrier, solid-lipid NPs have been widely used in delivering siRNA and plasmid DNA in vitro.170–172 MPL-A is an effective TLR4 agonist and an attenuated analogue of lipopolysaccharide that has been used as vaccine adjuvant in some studies.173,174 SLN-A nanoliposome is made by mixing the adjuvant MPL-A into SLNs, which can effectively express recombinant H. pylori antigen UreA in mouse immune cells, stimulate human macrophage-like cells to express proinflammatory cytokine TNFα, and make cells absorb particles and localize in the intracellular cavity within 3 hours.167
Measles Virus
MV is a pure human pathogen and an envelope virus belonging to the genus Morbillivirus of the paramyxovirus family.175 MV has two surface glycoproteins — hemagglutinin (H) and fusion protein (F) — which are responsible for virus attachment and entry into host cells.113 MV is considered a promising carrier platform for vaccine development, owing to its stability, low cost, and high security.176 Iankov et al constructed a recombinant MV Edmonston vaccine strain expressing HspA antigen of H. pylori. The results proved that the recombinant MV-HspA strain has strong antitumor activity and strong immunogenicity to H. pylori HspA antigen.113
Plant Expression System
Transgenic plants are used in the production of biological products and vaccines, and have been widely used in the development of edible vaccines.177 Leafy crops, including vegetables, are considered suitable candidates for recombinant vaccines.178
Outer-Membrane Vesicles
Outer-membrane vesicles (OMVs) are a spherical bilayer membrane structure naturally released from the outer membrane of Gram-negative bacteria with the participation of the VacJ/Yrb ATP-binding cassette xtrasport system.179,180 The main components are periplasmic proteins, toxins, outer-membrane proteins, and lipids.179,181 OMVs have been proved to play a significant role in the interaction between host and pathogen.182 Due to their nanometer size, good plasticity, and safety, OMVs have been described as a promising antigen-delivery system.183,184 Chen et al successfully transported heterologous proteins to vesicles by fusing the natural bacterial protein ClyA with heterologous proteins.183 Recently, Liu et al proved that oral administration of H. pylori OMVs can induce strong humoral immunoresponse and significant mucosal immunoresponse in mice.185
Clinical Trials of H. pylori Vaccines
Currently, most candidate vaccines are in the early stage. According to reports, there are more than 10 H. pylori vaccines that have completed clinical trials or are undergoing clinical trials. A research summary of H. pylori vaccines entering the clinical experiment stage is shown in Table 1.
 |
Table 1 List of vaccines and completed clinical trials |
Among these candidate vaccines that have entered the clinical research stage, few have shown satisfactory immunization results. The earliest clinical trial, by Kreiss et al in 1996, showed that all volunteers were still infected with H. pylori after oral administration of recombinant H. pylori urease vaccine.14 Later, researchers speculated that adding an effective adjuvant might significantly enhance the immunoeffect. Michetti et al added LT to urease as an immunoadjuvant, and oral administration of this vaccine significantly reduced the density of H. pylori in the stomach, but could not eradicate H. pylori infection.15 These results mean more powerful or extensive antigens may be needed to participate in immunization. Banerjee et al proved that enteric coated urease is safe and may have more immunogenicity than soluble urease.21 Rectal administration of LT has a reliable safety profile, but immunoresponse to the target antigen urease is very poor and does not improve with increased LT dose. This may be due to urease degradation in the rectum, improper selection of urease dose levels, failure of the basic immunomechanism to coordinate the appropriate response, or inadequate antigen presentation and lack of appropriate T cells to help or induce tolerance.20 A recombinant H. pylori oral vaccine combined with UreB and LTB has completed phase III clinical trials. It can significantly reduce the incidence of H. pylori infection in children and is fairly safe. One year after vaccination, the natural acquisition rate of H. pylori decreased by 71.8% (95% CI 48.2%–85.6%),25 but the protection rate had dropped to 55% 2 years later. Unfortunately, the development of this vaccine has stopped.
A series of clinical trials have also been carried out on the use of S. enterica Typhi as a vaccine-delivery carrier. PhoP/phoQ-deleted S. enterica Typhi Ty800 vaccine strains expressing UreA and UreB of H. pylori could not stimulate humoral or mucosal immunoresponse to urease. The reason may be that the amount of antigen inoculation is small and needs to be taken orally many times. Angelakopoulos et al thought that enhanced plasmid stability can prolong antigen presentation and enhance immunogenicity. A more highly attenuated S. enterica serovar Typhi strain given at larger doses could result in fewer adverse events and more consistent and vigorous immunoresponses to urease. This may be related to the enhancement of plasmid stability and the prolongation of intestinal colonization time.17 Bumamn et al speculated that the reason that S. enterica serovar Typhi Ty21a expressing H. pylori urease could not stimulate humoral immunoresponse to urease may be related to the low expression of heterologous antigen, and also the good immunoresponse of bacterial carrier itself may make the weak humoral or mucosal response of antigen ignored.19 Follow-up clinical trials further proved that attenuated S. enterica Typhi vaccine did not show a satisfactory protective effect,22 so Aebischer et al proposed that prime–boost regimen combined with live vaccine and other vaccine preparations may be more effective.23
Malfertheiner et al designed an H. pylori vaccine containing three antigens (VacA, CagA, and NAP) associated with H. pylori virulence and aluminium hydroxide adjuvant. The vaccine had good tolerance and strong immunogenicity, but did not provide additional protection against H. pylori infection.24 H. pylori colonization usually occurs in early childhood, and the immune system of adults is more likely to eliminate H. pylori spontaneously than children. Therefore, the challenge experiment may be more suitable for children. The research on whole-cell inactivated vaccine of H. pylori has also been going on for some time. Oral formalin inactivated whole-cell vaccine (HWC) of H. pylori can stimulate mucosal and systemic immunoresponse to H. pylori antigen in humans, but there is no evidence that vaccination can eradicate H. pylori in infected volunteers.18
Discussion
Reasons for the Lack of Clinical Trials on H. pylori Vaccine
Although putative H. pylori vaccines have been studied for 30 years, none has been put on the market, and most of the published clinical trials have ended at phase I. There are many reasons for this phenomenon, which are discussed below.
Immunotolerance Mechanism of H. pylori
T-regulatory (Treg) cells can maintain the benign interaction between H. pylori and host to some extent.186 Treg-cell response can be observed after H. pylori infection, and can drive immunotolerance and inhibit the activity of Th1 and Th17 cells.187 Therefore, the immunoregulation and -tolerance mechanism caused indirectly by the activation of Treg cells may ensure the continuous colonization of H. pylori.
Genetic Diversity of H. pylori
The high spontaneous mutation rate and recombination frequency of the genome leads to 20%–30% genomic variation among different H. pylori strains.4 One study showed that not only were H. pylori isolates from different individuals highly polymorphic but that variation in H. pylori strains was observed in the same host.188
Presence of H. pylori in Cells
It has been reported that after extracellular H. pylori is cleared by gentamicin, the bacteria will re-gather in the extracellular environment, indicating that H. pylori may be a facultative intracellular bacteria that can exist and hide in the cells.189 H. pylori can exist in the gastric lamina propria, gastric epithelial cells, and immune cells of patients with gastric diseases.190–192 The number of H. pylori cells is often more than the number of phagocytes, resulting in the persistence of H. pylori in gastric epithelial cells.193 All in all, the presence of H. pylori in cells may be one of the reasons for the persistence of bacteria in the body.
Limitations of Mouse Model
Although it must be admitted that the mouse H. pylori model has some value for the preliminary evaluation of the immunoefficacy of candidate vaccines, it has limitations, because mice are not natural hosts of H. pylori.194 Therefore, the immunoeffect observed in mouse models may not reflect the level of protection of the vaccine in humans.4 Studies have shown that H. pylori carried by captive rhesus monkeys is the same as that found in humans, so it may indicate that large animals such as rhesus monkeys are more suitable for vaccine efficacy testing than mice.195,196
Lack of Attention and Investment
Despite the high coverage of H. pylori and the huge burden of disease, as far as we know, there are no large biological companies that have plans to develop H. pylori vaccines. The urgency of vaccine demand, public attention, and capital investment may also affect the speed of the vaccine reaching the market. From this point of view, an H. pylori vaccine is different from other much-needed infectious disease vaccines (such as COVID-19 vaccines). These reasons can lead to poor immunoeffect of vaccines, low efficiency of vaccine development, and difficulty in marketing a vaccine, so researchers need to overcome these difficulties in the future.
Improvement of Vaccine-Design Strategy
Many studies have shown that immunity against H. pylori induced by a single antigen does not produce effective protection, and effective immunity against H. pylori infection is often achieved by the combination of various antigens.197 Therefore, many research groups are committed to developing H. pylori vaccines based on multiple antigens. Epitope-based vaccines are more cost-effective than mixed proteins and can cover more protein targets, so multiepitope vaccines receive more attention.91
Clinical studies have proved that most of the adverse reactions in tested populations are caused by adjuvants,15,21 so it is necessary to explore an a toxic or low-toxicicty but effective adjuvant. Aluminum hydroxide and LT are widely used adjuvants of H. pylori vaccine in clinical research, but aluminum hydroxide does not enhance cellular immunity. It is well known that LT is toxic, so some studies choose to reduce the dosage of LT to reduce the toxic side effects and maintain the adjuvant activity. Some studies have also proposed using LT mutants, such as double-mutant LT,198 but the production of mutant toxins requires sophisticated gene manipulation. Cytokines such as IL18, IL17A, and IL22 as molecular adjuvants can improve the efficacy of DNA vaccine.146,147 α-GalCer as a mucosal adjuvant has been proved to be as effective as the standard adjuvant cholera toxin.145,199 cGAMP can enhance antigen-specific cellular immunoresponse and is a promising molecular adjuvant.133,200 When CpG is used as an adjuvant of H. pylori vaccine, it can induce biased Th1 immunoresponse.48 Fluorinated c-di-GMP analogues have been shown to be excellent adjuvants both intranasally and orally.154 However, the safety and efficacy of these adjuvants in humans need to be evaluated. In addition, some PPSs and propolis are considered candidates for a low-toxicity H. pylori vaccine adjuvant because of their safety.40,82,136,141 However, the related immunostimulation mechanism of PPSs needs further research. Propolis can indeed improve the effectiveness of the vaccine to a certain extent, but it can be used as adjuvant only after its chemical composition is analyzed and its quality-control process checked. OMV is more effective than cholera toxin adjuvant in eradicating H. pylori infection, and compared with toxic adjuvants such as CpG, OMV has the advantage of natural atoxicity, but the production of OMV requires certain technology, so its application is limited. These findings provide more ideas and innovations for the development of vaccine adjuvants in the future.
Live pathogens cannot only be used as vaccine vectors but also stimulate long-lasting humoral and cellular immunity. Although the use of live vectors for antigen delivery is a promising method, the stability of antigen-delivery vectors is an important variable of human response to carrier antigens. Prolonging the intestinal colonization time of live vaccine allows for longer antigen transmission,17 and studies have shown that some antigens may be more immunogenic when secreted from S. enterica Typhi than expressed in cells, because the transmission time of the surface antigen may be longer than that of the plasma antigen.16 The expression level of heterologous antigen in S. enterica Typhi may play a vital part in the strength of immunoresponse, and less antigen expression may mean more regular vaccination.
Nanometer material-delivery systems have good tolerance and biocompatibility, can prolong antigen contact time, and overcome the shortcomings of weak immunogenicity of oral vaccines. Therefore, the nanopreparation-delivery system has been used by researchers to treat a variety of diseases, including H. pylori-related diseases.166,167 The shape and size of NPs usually change with the loading of antigen, and the stability of NPs is usually independent of temperature, but related to time, so improving the uniformity and stability of NPs is a direction for future study. At the same time, accurate control of antigen release and cell targeting should also be paid attention.
Prospects
An effective H. pylori vaccine for human should not only be effective and safe but also needs good patient compliance and a long period of protection. With regard to candidate antigens, subsequent scientific research can be devoted to the development of multiepitope vaccines with multiple targets, and some advanced modern immunoinformatics methods can also be used in the design of multiepitope vaccines. Due to a variety of side effects of adjuvants, the safety of adjuvants has always been an issue of concern to researchers. Excellent adjuvants should be stable, cheap, easy to prepare, without unacceptable side effects, and compatible with vaccine ingredients. We should continue to develop safe and atoxic adjuvants to improve the immunoeffect of the vaccine.
Many live-vaccine bacterial vectors have the problem of low expression, and secretory antigens may allow longer antigen transmission than cytoplasmic antigens, so maybe we can try to explore a vector system that can efficiently secrete and express foreign antigens. NPs are also a promising antigen protection strategy, but the uniformity and stability of NPs need to be further optimized. MV-attenuated strains are also a good vaccine platform for expressing bacterial protective antigens that can not only induce anti-MV immunoresponse but also target H. pylori antigen, but the safety and efficacy of this vaccine in humans need to be further studied.
Many studies on H. pylori vaccine have also revealed problems in the dosage form of the vaccine. H. pylori infection usually occurs in early childhood, so vaccine development should be targeted more at children. We also need to consider children’s medication, such as the oral dose of the vaccine not being too high. Otherwise, it is difficult for children to take. Therefore, it is necessary for us to study other better formulations or more convenient vaccination methods. We may be able to develop vaccines with higher antigen loads to solve the problem of high oral doses. In addition to oral and intravenous injections, nasal and rectal administration have been shown to be at least as effective as oral administration. Oral enhanced vaccination after parenteral injection may be a more effective scheme.20
In conclusion, through the efforts of researchers in recent years, some discoveries have been made in the screening and discovery of candidate antigens. The exploration of delivery systems and adjuvants provides novel ideas for the development of an H. pylori vaccine. However, the vast majority of H. pylori vaccine research is still in the early stages. The development of an H. pylori vaccine that can be used in humans still needs much exploration and effort. We need to know more about the mechanism of immunosuppression in order to better design vaccine strategies, and exploring the optimal combination of antigens, adjuvants, and delivery carriers may be the key to vaccine design. In addition, we believe there is a need to raise awareness of H. pylori infection in low- and middle-income countries to scale up the introduction of talent and funding so as to better promote the development of an H. pylori vaccine.
Disclosure
Songhui Li and Wenfeng Zhao are-co-first authors for this study. Lingyi Kong and Lei Yang are co-correspondence authors for this study. Lei Xia is affiliated with Bloomage Biotechnology Corporation Limited. The authors report no other conflicts of interest in this work.
References
1. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273–1275.
2. Marshall B, Royce H, Annear D, et al. Original isolation of Campylobacter pyloridis from human gastric mucosa. Microbios Lett. 1984;25(98):83–88.
3. Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22(2):283–297. doi:10.1093/oxfordjournals.epirev.a018040
4. Maleki Kakelar H, Barzegari A, Dehghani J, et al. Pathogenicity of Helicobacter pylori in cancer development and impacts of vaccination. Gastric Cancer. 2019;22(1):23–36. doi:10.1007/s10120-018-0867-1
5. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429. doi:10.1053/j.gastro.2017.04.022
6. Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47(7):868–876. doi:10.1111/apt.14561
7. Zagari RM, Frazzoni L, Marasco G, Fuccio L, Bazzoli F. Treatment of Helicobacter pylori infection: a clinical practice update. Minerva Med. 2021;112(2):281–287. doi:10.23736/S0026-4806.20.06810-X
8. Lu C, Sang J, He H, et al. Probiotic supplementation does not improve eradication rate of Helicobacter pylori infection compared to placebo based on standard therapy: a meta-analysis. Sci Rep. 2016;6:23522. doi:10.1038/srep23522
9. Goderska K, Agudo Pena S, Alarcon T. Helicobacter pylori treatment: antibiotics or probiotics. Appl Microbiol Biotechnol. 2018;102(1):1–7. doi:10.1007/s00253-017-8535-7
10. Lee YC, Dore MP, Graham DY. Diagnosis and treatment of Helicobacter pylori infection. Annu Rev Med. 2022;73:183–195. doi:10.1146/annurev-med-042220-020814
11. Pallen MJ, Clayton CL. Vaccination against Helicobacter pylori urease. Lancet. 1990;336(8708):186–187. doi:10.1016/0140-6736(90)91716-N
12. Czinn SJ, Nedrud JG. Oral immunization against Helicobacter pylori. Infect Immun. 1991;59(7):2359–2363. doi:10.1128/iai.59.7.2359-2363.1991
13. Czinn SJ, Cai A, Nedrud JG. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine. 1993;11(6):637–642. doi:10.1016/0264-410X(93)90309-L
14. Kreiss C, Buclin T, Cosma M, Corthésy-Theulaz I, Michetti P. Safety of oral immunisation with recombinant urease in patients with Helicobacter pylori infection. Lancet. 1996;347(9015):1630–1631. doi:10.1016/S0140-6736(96)91119-8
15. Michetti P, Kreiss C, Kotloff KL, et al. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology. 1999;116(4):804–812. doi:10.1016/S0016-5085(99)70063-6
16. DiPetrillo MD, Tibbetts T, Kleanthous H, Killeen KP, Hohmann EL. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine. 1999;18(5–6):449–459. doi:10.1016/S0264-410X(99)00246-7
17. Angelakopoulos H, Hohmann EL. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect Immun. 2000;68(4):2135–2141. doi:10.1128/IAI.68.4.2135-2141.2000
18. Kotloff KL, Sztein MB, Wasserman SS, Losonsky GA, DiLorenzo SC, Walker RI. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect Immun. 2001;69(6):3581–3590. doi:10.1128/IAI.69.6.3581-3590.2001
19. Bumann D, Metzger WG, Mansouri E, et al. Safety and immunogenicity of live recombinant Salmonella enterica serovar Typhi Ty21a expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine. 2001;20(5–6):845–852. doi:10.1016/S0264-410X(01)00391-7
20. Sougioultzis S, Lee CK, Alsahli M, et al. Safety and efficacy of E coli enterotoxin adjuvant for urease-based rectal immunization against Helicobacter pylori. Vaccine. 2002;21(3–4):194–201. doi:10.1016/S0264-410X(02)00467-X
21. Banerjee S, Medina-Fatimi A, Nichols R, et al. Safety and efficacy of low dose Escherichia coli enterotoxin adjuvant for urease based oral immunisation against Helicobacter pylori in healthy volunteers. Gut. 2002;51(5):634–640. doi:10.1136/gut.51.5.634
22. Metzger WG, Mansouri E, Kronawitter M, et al. Impact of vector-priming on the immunogenicity of a live recombinant Salmonella enterica serovar typhi Ty21a vaccine expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine. 2004;22(17–18):2273–2277. doi:10.1016/j.vaccine.2003.11.020
23. Aebischer T, Bumann D, Epple HJ, et al. Correlation of T cell response and bacterial clearance in human volunteers challenged with Helicobacter pylori revealed by randomised controlled vaccination with Ty21a-based Salmonella vaccines. Gut. 2008;57(8):1065–1072. doi:10.1136/gut.2007.145839
24. Malfertheiner P, Schultze V, Rosenkranz B, et al. Safety and immunogenicity of an intramuscular Helicobacter pylori vaccine in noninfected volunteers: a Phase I study. Gastroenterology. 2008;135(3):787–795. doi:10.1053/j.gastro.2008.05.054
25. Zeng M, Mao XH, Li JX, et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2015;386(10002):1457–1464. doi:10.1016/S0140-6736(15)60310-5
26. Malfertheiner P, Selgrad M, Wex T, et al. Efficacy, immunogenicity, and safety of a parenteral vaccine against Helicobacter pylori in healthy volunteers challenged with a Cag-positive strain: a randomised, placebo-controlled Phase 1/2 study. Lancet Gastroenterol Hepatol. 2018;3(10):698–707. doi:10.1016/S2468-1253(18)30125-0
27. Ha NC, Oh ST, Sung JY, Cha KA, Lee MH, Oh BH. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nat Struct Biol. 2001;8(6):505–509. doi:10.1038/88563
28. Mayo K, Held M, Wadström T, Mégraud F. Helicobacter pylori-human polymorphonuclear leucocyte interaction in the presence of ammonia. Eur J Gastroenterol Hepatol. 1997;9(5):457–461. doi:10.1097/00042737-199705000-00009
29. Suzuki M, Miura S, Suematsu M, et al. Helicobacter pylori-associated ammonia production enhances neutrophil-dependent gastric mucosal cell injury. Am J Physiol. 1992;263(5 Pt 1):G719–725. doi:10.1152/ajpgi.1992.263.5.G719
30. Lytton SD, Fischer W, Nagel W, Haas R, Beck FX. Production of ammonium by Helicobacter pylori mediates occludin processing and disruption of tight junctions in Caco-2 cells. Microbiology. 2005;151(Pt 10):3267–3276. doi:10.1099/mic.0.28049-0
31. Wroblewski LE, Shen L, Ogden S, et al. Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology. 2009;136(1):236–246. doi:10.1053/j.gastro.2008.10.011
32. Kuwahara H, Miyamoto Y, Akaike T, et al. Helicobacter pylori urease suppresses bactericidal activity of peroxynitrite via carbon dioxide production. Infect Immun. 2000;68(8):4378–4383. doi:10.1128/IAI.68.8.4378-4383.2000
33. Schoep TD, Fulurija A, Good F, et al. Surface properties of Helicobacter pylori urease complex are essential for persistence. PLoS One. 2010;5(11):e15042. doi:10.1371/journal.pone.0015042
34. Scott DR, Marcus EA, Wen Y, Singh S, Feng J, Sachs G. Cytoplasmic histidine kinase (HP0244)-regulated assembly of urease with UreI, a channel for urea and its metabolites, CO2, NH3, and NH4(+), is necessary for acid survival of Helicobacter pylori. J Bacteriol. 2010;192(1):94–103. doi:10.1128/JB.00848-09
35. Nasr-Esfahani M, Doosti A, Sazegar H. Evaluation of the immune response against Helicobacter pylori; in infused BALB/c mice by pcDNA3.1(+)-ureA. Folia Med (Plovdiv). 2020;62(1):37–45. doi:10.3897/folmed.62.e47932
36. Liu PF, Wang Y, Ulrich RG, et al. Leaf-encapsulated vaccines: agroinfiltration and transient expression of the antigen staphylococcal endotoxin B in radish leaves. J Immunol Res. 2018;2018:3710961. doi:10.1155/2018/3710961
37. Leunk RD, Johnson PT, David BC, Kraft WG, Morgan DR. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26(2):93–99. doi:10.1099/00222615-26-2-93
38. Foegeding NJ, Caston RR, McClain MS, Ohi MD, Cover TL. An overview of Helicobacter pylori VacA toxin biology. Toxins. 2016;8(6). doi:10.3390/toxins8060173
39. Chauhan N, Tay ACY, Marshall BJ, Jain U. Helicobacter pylori VacA, a distinct toxin exerts diverse functionalities in numerous cells: an overview. Helicobacter. 2019;24(1):e12544. doi:10.1111/hel.12544
40. Guo L, Hong D, Wang S, et al. Therapeutic protection against H. pylori infection in Mongolian gerbils by oral immunization with a tetravalent epitope-based vaccine with polysaccharide adjuvant. Front Immunol. 2019;10:1185. doi:10.3389/fimmu.2019.01185
41. Censini S, Lange C, Xiang Z, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996;93(25):14648–14653. doi:10.1073/pnas.93.25.14648
42. Tummuru MK, Cover TL, Blaser MJ. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61(5):1799–1809. doi:10.1128/iai.61.5.1799-1809.1993
43. Covacci A, Censini S, Bugnoli M, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993;90(12):5791–5795. doi:10.1073/pnas.90.12.5791
44. Hatakeyama M, Higashi H. Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis. Cancer Sci. 2005;96(12):835–843. doi:10.1111/j.1349-7006.2005.00130.x
45. Esmaeili D, Mobarez A, Salmanian A, Zavaran Hosseini A, Doust H. Immune responses of conserved 32 KD fragment from N-terminal of H. pylori cagA gene. J Pure Appl Microbio. 2010;4:117–123.
46. Shapouri Moghaddam A, Mansouri S, Neshani A, et al. Construction, cloning, and expression of CagA recombinant protein of Helicobacter pylori. Avicenna J Med Biotechnol. 2020;12(2):135–138.
47. Mohabatimobarez A, Salmanian AH, Hosseini AZ, Esmaeili D. Clearance of Helicobacter pylori with formulation rCagA and LPS in a mouse model. Gene Reports. 2020;19:100588. doi:10.1016/j.genrep.2020.100588
48. Paydarnia N, Mansoori B, Esmaeili D, et al. Helicobacter pylori recombinant CagA regulates Th1/Th2 balance in a BALB/c murine model. Adv Pharm Bull. 2020;10(2):264–270. doi:10.34172/apb.2020.031
49. Kutter S, Buhrdorf R, Haas J, Schneider-Brachert W, Haas R, Fischer W. Protein subassemblies of the Helicobacter pylori Cag type IV secretion system revealed by localization and interaction studies. J Bacteriol. 2008;190(6):2161–2171. doi:10.1128/JB.01341-07
50. Kumari R, Shariq M, Sharma S, Kumar A, Mukhopadhyay G. CagW, a VirB6 homologue interacts with Cag-type IV secretion system substrate CagA in Helicobacter pylori. Biochem Biophys Res Commun. 2019;515(4):712–718. doi:10.1016/j.bbrc.2019.06.013
51. Chehelgerdi M, Doosti A. Effect of the cagW-based gene vaccine on the immunologic properties of BALB/c mouse: an efficient candidate for Helicobacter pylori DNA vaccine. J Nanobiotechnology. 2020;18(1):63. doi:10.1186/s12951-020-00618-1
52. Kwok T, Zabler D, Urman S, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449(7164):862–866. doi:10.1038/nature06187
53. Tegtmeyer N, Hartig R, Delahay RM, et al. A small fibronectin-mimicking protein from bacteria induces cell spreading and focal adhesion formation. J Biol Chem. 2010;285(30):23515–23526. doi:10.1074/jbc.M109.096214
54. Aliramaei MR, Khorasgani MR, Rahmani MR, Zarkesh Esfahani SH, Emamzadeh R. Expression of Helicobacter pylori CagL gene in Lactococcus lactis MG1363 and evaluation of its immunogenicity as an oral vaccine in mice. Microb Pathog. 2019;142:103926. doi:10.1016/j.micpath.2019.103926
55. Hazell SL, Evans DJ, Graham DY. Helicobacter pylori catalase. J Gen Microbiol. 1991;137(1):57–61. doi:10.1099/00221287-137-1-57
56. Harris AG, Wilson JE, Danon SJ, Dixon MF, Donegan K, Hazell SL. Catalase (KatA) and KatA-associated protein (KapA) are essential to persistent colonization in the Helicobacter pylori SS1 mouse model. Microbiology. 2003;149(Pt 3):665–672. doi:10.1099/mic.0.26012-0
57. Basu M, Czinn SJ, Blanchard TG. Absence of catalase reduces long-term survival of Helicobacter pylori in macrophage phagosomes. Helicobacter. 2004;9(3):211–216. doi:10.1111/j.1083-4389.2004.00226.x
58. Harris AG, Hinds FE, Beckhouse AG, Kolesnikow T, Hazell SL. Resistance to hydrogen peroxide in Helicobacter pylori: role of catalase (KatA) and Fur, and functional analysis of a novel gene product designated ‘KatA-associated protein’, KapA (HP0874). Microbiology. 2002;148(Pt 12):3813–3825. doi:10.1099/00221287-148-12-3813
59. Manoochehr M, Neissi N, Tarighi P, Makvandi K, Rashidi N. Evaluation of the genes expression related to the immune system in response to Helicobacter pylori catalase epitopes. Mol Genet Microbiol Virol. 2020;35(1):47–51. doi:10.3103/S089141682001005X
60. Xie W, Zhao W, Zou Z, Kong L, Yang L. Oral multivalent epitope vaccine, based on UreB, HpaA, CAT, and LTB, for prevention and treatment of Helicobacter pylori infection in C57BL / 6 mice. Helicobacter. 2021;26(3):e12807. doi:10.1111/hel.12807
61. Evans DG, Karjalainen TK, Evans DJ, Graham DY, Lee CH. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J Bacteriol. 1993;175(3):674–683. doi:10.1128/jb.175.3.674-683.1993
62. Sutton P, Doidge C, Pinczower G, et al. Effectiveness of vaccination with recombinant HpaA from Helicobacter pylori is influenced by host genetic background. FEMS Immunol Med Microbiol. 2007;50(2):213–219. doi:10.1111/j.1574-695X.2006.00206.x
63. Lundström AM, Blom K, Sundaeus V, Bölin I. HpaA shows variable surface localization but the gene expression is similar in different Helicobacter pylori strains. Microb Pathog. 2001;31(5):243–253. doi:10.1006/mpat.2001.0466
64. Odenbreit S. Adherence properties of Helicobacter pylori: impact on pathogenesis and adaptation to the host. Int J Med Microbiol. 2005;295(5):317–324. doi:10.1016/j.ijmm.2005.06.003
65. Peek RM, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208(2):233–248. doi:10.1002/path.1868
66. Banga Ndzouboukou JL, Lei Q, Ullah N, Zhang Y, Hao L, Fan X. Helicobacter pylori adhesins: hpaA a potential antigen in experimental vaccines for H. pylori. Helicobacter. 2021;26(1):e12758. doi:10.1111/hel.12758
67. Lindgren A, Pavlovic V, Flach CF, Sjöling A, Lundin S. Interferon-gamma secretion is induced in IL-12 stimulated human NK cells by recognition of Helicobacter pylori or TLR2 ligands. Innate Immun. 2011;17(2):191–203. doi:10.1177/1753425909357970
68. Xue RY, Guo MF, Guo L, et al. Synthetic lipopeptide enhances protective immunity against Helicobacter pylori infection. Front Immunol. 2019;10:1372. doi:10.3389/fimmu.2019.01372
69. Tonello F, Dundon WG, Satin B, et al. The Helicobacter pylori neutrophil-activating protein is an iron-binding protein with dodecameric structure. Mol Microbiol. 1999;34(2):238–246. doi:10.1046/j.1365-2958.1999.01584.x
70. Namavar F, Sparrius M, Veerman EC, Appelmelk BJ, Vandenbroucke-Grauls CM. Neutrophil-activating protein mediates adhesion of Helicobacter pylori to sulfated carbohydrates on high-molecular-weight salivary mucin. Infect Immun. 1998;66(2):444–447. doi:10.1128/IAI.66.2.444-447.1998
71. Brisslert M, Enarsson K, Lundin S, et al. Helicobacter pylori induce neutrophil transendothelial migration: role of the bacterial HP-NAP. FEMS Microbiol Lett. 2005;249(1):95–103. doi:10.1016/j.femsle.2005.06.008
72. Teneberg S, Miller-Podraza H, Lampert HC, et al. Carbohydrate binding specificity of the neutrophil-activating protein of Helicobacter pylori. J Biol Chem. 1997;272(30):19067–19071. doi:10.1074/jbc.272.30.19067
73. Wang G, Hong Y, Olczak A, Maier SE, Maier RJ. Dual Roles of Helicobacter pylori NapA in inducing and combating oxidative stress. Infect Immun. 2006;74(12):6839–6846. doi:10.1128/IAI.00991-06
74. Evans DJ, Evans DG, Takemura T, et al. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63(6):2213–2220. doi:10.1128/iai.63.6.2213-2220.1995
75. Satin B, Del Giudice G, Della Bianca V, et al. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med. 2000;191(9):1467–1476. doi:10.1084/jem.191.9.1467
76. Liu M, Zhong Y, Chen J, et al. Oral immunization of mice with a multivalent therapeutic subunit vaccine protects against Helicobacter pylori infection. Vaccine. 2020;38(14):3031–3041. doi:10.1016/j.vaccine.2020.02.036
77. Zhong Y, Chen J, Liu Y, et al. Oral immunization of BALB/c mice with recombinant Helicobacter pylori antigens and double mutant heat-labile toxin (dmLT) induces prophylactic protective immunity against H. pylori infection. Microb Pathog. 2020;145:104229. doi:10.1016/j.micpath.2020.104229
78. Jafari E, Mahmoodi S. Design, expression, and purification of a multi-epitope vaccine against Helicobacter Pylori based on Melittin as an adjuvant. Microb Pathog. 2021;157:104970. doi:10.1016/j.micpath.2021.104970
79. Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci U S A. 2000;97(13):7533–7538. doi:10.1073/pnas.130079797
80. Tabassam FH, Graham DY, Yamaoka Y. OipA plays a role in Helicobacter pylori-induced focal adhesion kinase activation and cytoskeletal re-organization. Cell Microbiol. 2008;10(4):1008–1020. doi:10.1111/j.1462-5822.2007.01104.x
81. Dossumbekova A, Prinz C, Mages J, et al. Helicobacter pylori HopH (OipA) and bacterial pathogenicity: genetic and functional genomic analysis of hopH gene polymorphisms. J Infect Dis. 2006;194(10):1346–1355. doi:10.1086/508426
82. Soudi H, Falsafi T, Mahboubi M, Gharavi S. Evaluation of Helicobacter pylori OipA protein as a vaccine candidate and propolis as an adjuvant in C57BL/6 mice. Iran J Basic Med Sci. 2021;24(9):1220–1230. doi:10.22038/ijbms.2021.56232.12579
83. Mittl PR, Lüthy L, Reinhardt C, Joller H. Detection of high titers of antibody against Helicobacter cysteine-rich proteins A, B, C, and E in Helicobacter pylori-infected individuals. Clin Diagn Lab Immunol. 2003;10(4):542–545. doi:10.1128/cdli.10.4.542-545.2003
84. Krishnamurthy P, Parlow MH, Schneider J, et al. Identification of a novel penicillin-binding protein from Helicobacter pylori. J Bacteriol. 1999;181(16):5107–5110. doi:10.1128/JB.181.16.5107-5110.1999
85. Nasr-Esfahani M, Doosti A, Jami MS. Chitosan nanoparticles-mediated pCDNA3.1(–)-hcpD DNA vaccine against Helicobacter pylori in BALB/c mice. Mol Genet Microbiol Virol. 2019;34(2):131–139. doi:10.3103/S0891416819020083
86. Eaton KA, Suerbaum S, Josenhans C, Krakowka S. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect Immun. 1996;64(7):2445–2448. doi:10.1128/iai.64.7.2445-2448.1996
87. Kostrzynska M, Betts JD, Austin JW, Trust TJ. Identification, characterization, and spatial localization of two flagellin species in Helicobacter pylori flagella. J Bacteriol. 1991;173(3):937–946. doi:10.1128/jb.173.3.937-946.1991
88. Yan J, Liang SH, Mao YF, Li LW, Li SP. Construction of expression systems for flaA and flaB genes of Helicobacter pylori and determination of immunoreactivity and antigenicity of recombinant proteins. World J Gastroenterol. 2003;9(10):2240–2250. doi:10.3748/wjg.v9.i10.2240
89. Gu H. Role of flagella in the pathogenesis of Helicobacter pylori. Curr Microbiol. 2017;74(7):863–869. doi:10.1007/s00284-017-1256-4
90. Ansari H, Tahmasebi-Birgani M, Bijanzadeh M. DNA vaccine containing Flagellin A gene induces significant immune responses against Helicobacter pylori infection: an in vivo study. Iran J Basic Med Sci. 2021;24(6):796–804. doi:10.22038/ijbms.2021.54415.12227
91. Hamzehloo Z, Mosayebi G, Khansarinejad B, Zolfaghari M, Abtahi H. Antigenicity identification of a novel recombinant multi-epitope antigen based on FlaA and UreB antigens of Helicobacter pylori. Jundishapur J Microbiol. 2019. doi:10.5812/jjm.66502
92. Kim JS, Chang JH, Chung SI, Yum JS. Molecular cloning and characterization of the Helicobacter pylori fliD gene, an essential factor in flagellar structure and motility. J Bacteriol. 1999;181(22):6969–6976. doi:10.1128/JB.181.22.6969-6976.1999
93. Tasteyre A, Barc MC, Collignon A, Boureau H, Karjalainen T. Role of FliC and FliD flagellar proteins of Clostridium difficile in adherence and gut colonization. Infect Immun. 2001;69(12):7937–7940. doi:10.1128/IAI.69.12.7937-7940.2001
94. Cho SY, Song WS, Oh HB, Kim HU, Jung HS, Yoon SI. Structural analysis of the flagellar capping protein FliD from Helicobacter pylori. Biochem Biophys Res Commun. 2019;514(1):98–104. doi:10.1016/j.bbrc.2019.04.065
95. Ghasemi A, Mohammad N, Mautner J, Karsabet MT, Ardjmand A, Moniri R. Immunization with recombinant FliD confers protection against Helicobacter pylori infection in mice. Mol Immunol. 2018;94:176–182. doi:10.1016/j.molimm.2018.01.001
96. Monteiro MA, Britton S, Applebee LA, Baqar S. Synthesis and immunogenicity of a Helicobacter pylori lipopolysaccharide-based conjugate. Vaccine. 2011;29(17):3098–3102. doi:10.1016/j.vaccine.2011.02.063
97. Yokota S, Okabayashi T, Rehli M, Fujii N, Amano K. Helicobacter pylori lipopolysaccharides upregulate toll-like receptor 4 expression and proliferation of gastric epithelial cells via the MEK1/2-ERK1/2 mitogen-activated protein kinase pathway. Infect Immun. 2010;78(1):468–476. doi:10.1128/IAI.00903-09
98. Monteiro MA. Helicobacter pylori: a wolf in sheep’s clothing: the glycotype families of Helicobacter pylori lipopolysaccharides expressing histo-blood groups: structure, biosynthesis, and role in pathogenesis. Adv Carbohydr Chem Biochem. 2001;57:99–158.
99. Eaton KA, Logan SM, Baker PE, Peterson RA, Monteiro MA, Altman E. Helicobacter pylori with a truncated lipopolysaccharide O chain fails to induce gastritis in SCID mice injected with splenocytes from wild-type C57BL/6J mice. Infect Immun. 2004;72(7):3925–3931. doi:10.1128/IAI.72.7.3925-3931.2004
100. Tian G, Qin C, Liu Z, et al. Total synthesis of the Helicobacter pylori serotype O2 O-antigen alpha-(1 --> 2)- and alpha-(1 --> 3)-linked oligoglucosides. Chem Commun (Camb). 2020;56(3):344–347. doi:10.1039/C9CC07915G
101. Kostrzynska M, O’Toole PW, Taylor DE, Trust TJ. Molecular characterization of a conserved 20-kilodalton membrane-associated lipoprotein antigen of Helicobacter pylori. J Bacteriol. 1994;176(19):5938–5948. doi:10.1128/jb.176.19.5938-5948.1994
102. Keenan J, Oliaro J, Domigan N, et al. Immune response to an 18-kilodalton outer membrane antigen identifies lipoprotein 20 as a Helicobacter pylori vaccine candidate. Infect Immun. 2000;68(6):3337–3343. doi:10.1128/IAI.68.6.3337-3343.2000
103. Cao P, McClain MS, Forsyth MH, Cover TL. Extracellular release of antigenic proteins by Helicobacter pylori. Infect Immun. 1998;66(6):2984–2986. doi:10.1128/IAI.66.6.2984-2986.1998
104. Hocking D, Webb E, Radcliff F, et al. Isolation of recombinant protective Helicobacter pylori antigens. Infect Immun. 1999;67(9):4713–4719. doi:10.1128/IAI.67.9.4713-4719.1999
105. Ning Y, Ye J, Wen J, et al. Identification of two Lpp20 CD4+ T cell epitopes in Helicobacter pylori-infected subjects. Front Microbiol. 2018;9:884. doi:10.3389/fmicb.2018.00884
106. Sun N, Zhang R, Duan G, et al. A food-grade engineered Lactococcus lactis strain delivering Helicobacter pylori Lpp20 alleviates bacterial infection in H. pylori-challenged mice. Biotechnol Lett. 2019;41(12):1415–1421. doi:10.1007/s10529-019-02740-z
107. Suerbaum S, Thiberge JM, Kansau I, Ferrero RL, Labigne A. Helicobacter pylori hspA-hspB heat-shock gene cluster: nucleotide sequence, expression, putative function and immunogenicity. Mol Microbiol. 1994;14(5):959–974. doi:10.1111/j.1365-2958.1994.tb01331.x
108. Kansau I, Guillain F, Thiberge JM, Labigne A. Nickel binding and immunological properties of the C-terminal domain of the Helicobacter pylori GroES homologue (HspA). Mol Microbiol. 1996;22(5):1013–1023. doi:10.1046/j.1365-2958.1996.01536.x
109. Cun S, Li H, Ge R, Lin MC, Sun H. A histidine-rich and cysteine-rich metal-binding domain at the C terminus of heat shock protein A from Helicobacter pylori: implication for nickel homeostasis and bismuth susceptibility. J Biol Chem. 2008;283(22):15142–15151. doi:10.1074/jbc.M800591200
110. Ferrero RL, Thiberge JM, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci U S A. 1995;92(14):6499–6503. doi:10.1073/pnas.92.14.6499
111. Wu C, Shi Y, Guo H, et al. Protection against Helicobacter pylori infection in Mongolian gerbil by intragastric or intramuscular administration of H. pylori multicomponent vaccine. Helicobacter. 2008;13(3):191–199. doi:10.1111/j.1523-5378.2008.00609.x
112. Zhang X, Sang S, Guan Q, Tao H, Wang Y, Liu C. Identification of B-cell epitopes of HspA from Helicobacter pylori and detection of epitope antibody profiles in naturally infected persons. Vaccines. 2021;10(1). doi:10.3390/vaccines10010065
113. Iankov ID, Kurokawa C, Viker K, et al. Live attenuated measles virus vaccine expressing Helicobacter pylori heat shock protein A. Mol Ther Oncolytics. 2020;19:136–148. doi:10.1016/j.omto.2020.09.006
114. González-López MA, Velázquez-Guadarrama N, Romero-Espejel ME, Olivares-Trejo Jde J. Helicobacter pylori secretes the chaperonin GroEL (HSP60), which binds iron. FEBS Lett. 2013;587(12):1823–1828. doi:10.1016/j.febslet.2013.04.048
115. Gao L, Michel A, Weck MN, Arndt V, Pawlita M, Brenner H. Helicobacter pylori infection and gastric cancer risk: evaluation of 15 H. pylori proteins determined by novel multiplex serology. Cancer Res. 2009;69(15):6164–6170. doi:10.1158/0008-5472.CAN-09-0596
116. Gao L, Weck MN, Michel A, Pawlita M, Brenner H. Association between chronic atrophic gastritis and serum antibodies to 15 Helicobacter pylori proteins measured by multiplex serology. Cancer Res. 2009;69(7):2973–2980. doi:10.1158/0008-5472.CAN-08-3477
117. Macchia G, Massone A, Burroni D, Covacci A, Censini S, Rappuoli R. The Hsp60 protein of Helicobacter pylori: structure and immune response in patients with gastroduodenal diseases. Mol Microbiol. 1993;9(3):645–652. doi:10.1111/j.1365-2958.1993.tb01724.x
118. Khan M, Khan S, Ali A, et al. Immunoinformatics approaches to explore Helicobacter Pylori proteome (Virulence Factors) to design B and T cell multi-epitope subunit vaccine. Sci Rep. 2019;9(1):13321. doi:10.1038/s41598-019-49354-z
119. Espinosa-Ramos D, Caballero-Hernandez D, Gomez-Flores R, et al. Immunization with a synthetic Helicobacter pylori peptide induces secretory IgA antibodies and protects mice against infection. Can J Infect Dis Med Microbiol. 2019;2019:8595487. doi:10.1155/2019/8595487
120. Merritt EA, Zhang Z, Pickens JC, Ahn M, Hol WG, Fan E. Characterization and crystal structure of a high-affinity pentavalent receptor-binding inhibitor for cholera toxin and E. coli heat-labile enterotoxin. J Am Chem Soc. 2002;124(30):8818–8824. doi:10.1021/ja0202560
121. Clements JD, Hartzog NM, Lyon FL. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6(3):269–277. doi:10.1016/0264-410X(88)90223-X
122. Clements JD, Yancey RJ, Finkelstein RA. Properties of homogeneous heat-labile enterotoxin from Escherichia coli. Infect Immun. 1980;29(1):91–97. doi:10.1128/iai.29.1.91-97.1980
123. Ma Y, Luo Y, Huang X, Song F, Liu G. Construction of Bifidobacterium infantis as a live oral vaccine that expresses antigens of the major fimbrial subunit (CfaB) and the B subunit of heat-labile enterotoxin (LTB) from enterotoxigenic Escherichia coli. Microbiology. 2012;158(Pt 2):498–504. doi:10.1099/mic.0.049932-0
124. Jeon BW, Nandre RM, Lee JH. Oral immunization with an attenuated Salmonella Gallinarum mutant as a fowl typhoid vaccine with a live adjuvant strain secreting the B subunit of Escherichia coli heat-labile enterotoxin. BMC Vet Res. 2013;9:96. doi:10.1186/1746-6148-9-96
125. Chaudhari AA, Lee JH. Evaluation of the adjuvant effect of Salmonella-based Escherichia coli heat-labile toxin B subunits on the efficacy of a live Salmonella-delivered avian pathogenic Escherichia coli vaccine. Avian Pathol. 2013;42(4):365–372. doi:10.1080/03079457.2013.811466
126. Peng X, Zhang R, Wang C, et al. E. coli enterotoxin LtB enhances vaccine-induced anti-H. pylori protection by promoting leukocyte migration into gastric mucus via inflammatory lesions. Cells. 2019;8(9). doi:10.3390/cells8090982
127. Hemmi H, Takeuchi O, Kawai T, et al. A toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. doi:10.1038/35047123
128. Klinman DM, Conover J, Coban C. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect Immun. 1999;67(11):5658–5663. doi:10.1128/IAI.67.11.5658-5663.1999
129. Wang R, Doolan DL, Le TP, et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282(5388):476–480. doi:10.1126/science.282.5388.476
130. Klinman DM. Use of CpG oligodeoxynucleotides as immunoprotective agents. Expert Opin Biol Ther. 2004;4(6):937–946. doi:10.1517/14712598.4.6.937
131. Kato K, Omura H, Ishitani R, Nureki O. Cyclic GMP-AMP as an endogenous second messenger in innate immune signaling by cytosolic DNA. Annu Rev Biochem. 2017;86:541–566. doi:10.1146/annurev-biochem-061516-044813
132. Corrales L, Glickman LH, McWhirter SM, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11(7):1018–1030. doi:10.1016/j.celrep.2015.04.031
133. Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341(6152):1390–1394. doi:10.1126/science.1244040
134. Kranzusch PJ, Wilson SC, Lee AS, Berger JM, Doudna JA, Vance RE. Ancient origin of cGAS-STING reveals mechanism of universal 2’,3’ cGAMP signaling. Mol Cell. 2015;59(6):891–903. doi:10.1016/j.molcel.2015.07.022
135. Chen J, Zhong Y, Liu Y, et al. Parenteral immunization with a cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) adjuvanted Helicobacter pylori vaccine induces protective immunity against H. pylori infection in mice. Hum Vaccin Immunother. 2020;16(11):2849–2854. doi:10.1080/21645515.2020.1744364
136. Wang H, Liu YM, Qi ZM, et al. An overview on natural polysaccharides with antioxidant properties. Curr Med Chem. 2013;20(23):2899–2913. doi:10.2174/0929867311320230006
137. Pi CC, Chu CL, Lu CY, et al. Polysaccharides from Ganoderma formosanum function as a Th1 adjuvant and stimulate cytotoxic T cell response in vivo. Vaccine. 2014;32(3):401–408. doi:10.1016/j.vaccine.2013.11.027
138. Yang L, Hu Y, Xue J, et al. Compound Chinese herbal medicinal ingredients can enhance immune response and efficacy of RHD vaccine in rabbit. Vaccine. 2008;26(35):4451–4455. doi:10.1016/j.vaccine.2008.06.075
139. Qiu Y, Hu YL, Cui BA, et al. Immunopotentiating effects of four Chinese herbal polysaccharides administered at vaccination in chickens. Poult Sci. 2007;86(12):2530–2535. doi:10.3382/ps.2007-00076
140. Chen Z, Lu J, Srinivasan N, Tan BK, Chan SH. Polysaccharide-protein complex from Lycium barbarum L. is a novel stimulus of dendritic cell immunogenicity. J Immunol. 2009;182(6):3503–3509. doi:10.4049/jimmunol.0802567
141. Liu C, Luo J, Xue RY, et al. The mucosal adjuvant effect of plant polysaccharides for induction of protective immunity against Helicobacter pylori infection. Vaccine. 2019;37(8):1053–1061. doi:10.1016/j.vaccine.2018.12.066
142. Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi:10.1126/science.278.5343.1626
143. Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005;175(5):3309–3317. doi:10.4049/jimmunol.175.5.3309
144. Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, et al. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195(5):617–624. doi:10.1084/jem.20011889
145. Longet S, Abautret-Daly A, Davitt CJH, et al. An oral alpha-galactosylceramide adjuvanted Helicobacter pylori vaccine induces protective IL-1R- and IL-17R-dependent Th1 responses. NPJ Vaccines. 2019;4:45. doi:10.1038/s41541-019-0139-z
146. Thompson AL, Staats HF. Cytokines: the future of intranasal vaccine adjuvants. Clin Dev Immunol. 2011;2011:289597. doi:10.1155/2011/289597
147. Nemattalab M, Shenagari M, Taheri M, et al. Co-expression of Interleukin-17A molecular adjuvant and prophylactic Helicobacter pylori genetic vaccine could cause sterile immunity in Treg suppressed mice. Cytokine. 2020;126:154866. doi:10.1016/j.cyto.2019.154866
148. Touzani S, Embaslat W, Imtara H, et al. In vitro evaluation of the potential use of propolis as a multitarget therapeutic product: physicochemical properties, chemical composition, and immunomodulatory, antibacterial, and anticancer properties. Biomed Res Int. 2019;2019:4836378. doi:10.1155/2019/4836378
149. Fischer G, Conceição FR, Leite FP, et al. Immunomodulation produced by a green propolis extract on humoral and cellular responses of mice immunized with SuHV-1. Vaccine. 2007;25(7):1250–1256. doi:10.1016/j.vaccine.2006.10.005
150. Fischer G, Paulino N, Marcucci MC, et al. Green propolis phenolic compounds act as vaccine adjuvants, improving humoral and cellular responses in mice inoculated with inactivated vaccines. Mem Inst Oswaldo Cruz. 2010;105(7):908–913. doi:10.1590/S0074-02762010000700012
151. Chen W, Kuolee R, Yan H. The potential of 3’,5’-cyclic diguanylic acid (c-di-GMP) as an effective vaccine adjuvant. Vaccine. 2010;28(18):3080–3085. doi:10.1016/j.vaccine.2010.02.081
152. Karaolis DK, Means TK, Yang D, et al. Bacterial c-di-GMP is an immunostimulatory molecule. J Immunol. 2007;178(4):2171–2181. doi:10.4049/jimmunol.178.4.2171
153. Quartermain MD, Pasquali SK, Hill KD, et al. Variation in prenatal diagnosis of congenital heart disease in infants. Pediatrics. 2015;136(2):e378–385. doi:10.1542/peds.2014-3783
154. Li J, Lee RK, Chen W, Yan H. 2′-fluoro-c-di-GMP as an oral vaccine adjuvant. RSC Adv. 2019;9(71):41481–41489. doi:10.1039/C9RA08310C
155. Wang S, Ma J, Ji Q, Liu Q. Evaluation of an attenuated Listeria monocytogenes as a vaccine vector to control Helicobacter pylori infection. Immunol Lett. 2021;238:68–74. doi:10.1016/j.imlet.2021.07.010
156. Wells JM, Robinson K, Chamberlain LM, Schofield KM, Le Page RWF. Lactic acid bacteria as vaccine delivery vehicles. Antonie van Leeuwenhoek. 1996;70(2):317–330. doi:10.1007/BF00395939
157. Cano-Garrido O, Seras-Franzoso J, Garcia-Fruitós E. Lactic acid bacteria: reviewing the potential of a promising delivery live vector for biomedical purposes. Microb Cell Fact. 2015;14:137. doi:10.1186/s12934-015-0313-6
158. Hongying F, Xianbo W, Fang Y, Yang B, Beiguo L. Oral immunization with recombinant Lactobacillus acidophilus expressing the adhesin Hp0410 of Helicobacter pylori induces mucosal and systemic immune responses. Clin Vaccine Immunol. 2014;21(2):126–132. doi:10.1128/CVI.00434-13
159. Guo L, Zhang F, Wang S, et al. Oral immunization with a M cell-targeting recombinant L. lactis vaccine LL-plSAM-FVpE stimulate protective immunity against H. pylori in mice. Front Immunol. 2022;13:918160. doi:10.3389/fimmu.2022.918160
160. Darby RA, Cartwright SP, Dilworth MV, Bill RM. Which yeast species shall I choose? Saccharomyces cerevisiae versus Pichia pastoris (review). Methods Mol Biol. 2012;866:11–23.
161. Kumar R, Kumar P. Yeast-based vaccines: new perspective in vaccine development and application. FEMS Yeast Res. 2019;19(2). doi:10.1093/femsyr/foz007
162. Cen Q, Gao T, Ren Y, Lu X, Lei H. Immune evaluation of a Saccharomyces cerevisiae-based oral vaccine against Helicobacter pylori in mice. Helicobacter. 2021;26(1):e12772. doi:10.1111/hel.12772
163. Rothman J, Paterson Y. Live-attenuated Listeria-based immunotherapy. Expert Rev Vaccines. 2013;12(5):493–504. doi:10.1586/erv.13.34
164. Venkatesan M, Fernandez-Prada C, Buysse JM, Formal SB, Hale TL. Virulence phenotype and genetic characteristics of the T32-ISTRATI Shigella flexneri 2a vaccine strain. Vaccine. 1991;9(5):358–363. doi:10.1016/0264-410X(91)90064-D
165. Zhang X, Sang S, Guan Q, Tao H, Wang Y, Liu C. Oral administration of a Shigella 2aT32-based vaccine expressing UreB-HspA fusion antigen with and without parenteral rUreB-HspA boost confers protection against Helicobacter pylori in mice model. Front Immunol. 2022;13:894206. doi:10.3389/fimmu.2022.894206
166. Tan Z, Liu W, Liu H, et al. Oral Helicobacter pylori vaccine-encapsulated acid-resistant HP55/PLGA nanoparticles promote immune protection. Eur J Pharm Biopharm. 2017;111:33–43. doi:10.1016/j.ejpb.2016.11.007
167. Francis JE, Skakic I, Dekiwadia C, et al. Solid lipid nanoparticle carrier platform containing synthetic TLR4 agonist mediates non-viral DNA vaccine delivery. Vaccines. 2020;8(3). doi:10.3390/vaccines8030551
168. Lim M, Badruddoza AZM, Firdous J, et al. Engineered nanodelivery systems to improve DNA vaccine technologies. Pharmaceutics. 2020;12(1):30. doi:10.3390/pharmaceutics12010030
169. Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly(D,L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004;22(19):2406–2412. doi:10.1016/j.vaccine.2003.12.032
170. Bondi ML, Azzolina A, Craparo EF, et al. Novel cationic solid-lipid nanoparticles as non-viral vectors for gene delivery. J Drug Target. 2007;15(4):295–301. doi:10.1080/10611860701324698
171. Penumarthi A, Parashar D, Abraham AN, et al. Solid lipid nanoparticles mediate non-viral delivery of plasmid DNA to dendritic cells. J Nanopart Res. 2017;19(6). doi:10.1007/s11051-017-3902-y
172. Kim HR, Kim IK, Bae KH, Lee SH, Lee Y, Park TG. Cationic solid lipid nanoparticles reconstituted from low density lipoprotein components for delivery of siRNA. Mol Pharm. 2008;5(4):622–631. doi:10.1021/mp8000233
173. Del Giudice G, Rappuoli R, Didierlaurent AM. Correlates of adjuvanticity: a review on adjuvants in licensed vaccines. Semin Immunol. 2018;39:14–21. doi:10.1016/j.smim.2018.05.001
174. Wagner R, Hildt E. Composition and mode of action of adjuvants in licensed viral vaccines. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2019;62(4):462–471. doi:10.1007/s00103-019-02921-1
175. Zhdanov VM. The measles virus. Mol Cell Biochem. 1980;29(1):59–66. doi:10.1007/BF00230955
176. Zuniga A, Wang Z, Liniger M, et al. Attenuated measles virus as a vaccine vector. Vaccine. 2007;25(16):2974–2983. doi:10.1016/j.vaccine.2007.01.064
177. Rybicki EP. Plant-made vaccines for humans and animals. Plant Biotechnol J. 2010;8(5):620–637. doi:10.1111/j.1467-7652.2010.00507.x
178. Abdoli-nasab M, Torabi-nia N. Transient expression of UreB of Helicobacter pylori in spinach (Spinacia oleracea). Sci Hortic (Amsterdam). 2019;247:320–326. doi:10.1016/j.scienta.2018.12.018
179. Roier S, Zingl FG, Cakar F, et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat Commun. 2016;7:10515. doi:10.1038/ncomms10515
180. Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181(16):4725–4733. doi:10.1128/JB.181.16.4725-4733.1999
181. Sharndama HC, Mba IE. Helicobacter pylori: an up-to-date overview on the virulence and pathogenesis mechanisms. Braz J Microbiol. 2022;53(1):33–50. doi:10.1007/s42770-021-00675-0
182. Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13(10):605–619. doi:10.1038/nrmicro3525
183. Chen DJ, Osterrieder N, Metzger SM, et al. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc Natl Acad Sci U S A. 2010;107(7):3099–3104. doi:10.1073/pnas.0805532107
184. Chmiela M, Walczak N, Rudnicka K. Helicobacter pylori outer membrane vesicles involvement in the infection development and Helicobacter pylori-related diseases. J Biomed Sci. 2018;25(1):78. doi:10.1186/s12929-018-0480-y
185. Liu Q, Li X, Zhang Y, et al. Orally-administered outer-membrane vesicles from Helicobacter pylori reduce H. pylori infection via Th2-biased immune responses in mice. Pathog Dis. 2019;77(5). doi:10.1093/femspd/ftz050
186. Robinson K, Kenefeck R, Pidgeon EL, et al. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008;57(10):1375–1385. doi:10.1136/gut.2007.137539
187. Oertli M, Noben M, Engler DB, et al. Helicobacter pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci U S A. 2013;110(8):3047–3052. doi:10.1073/pnas.1211248110
188. Israel DA, Salama N, Krishna U, et al. Helicobacter pylori genetic diversity within the gastric niche of a single human host. Proc Natl Acad Sci U S A. 2001;98(25):14625–14630. doi:10.1073/pnas.251551698
189. Amieva MR, Salama NR, Tompkins LS, Falkow S. Helicobacter pylori enter and survive within multivesicular vacuoles of epithelial cells. Cell Microbiol. 2002;4(10):677–690. doi:10.1046/j.1462-5822.2002.00222.x
190. Necchi V, Candusso ME, Tava F, et al. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology. 2007;132(3):1009–1023. doi:10.1053/j.gastro.2007.01.049
191. Semino-Mora C, Doi SQ, Marty A, Simko V, Carlstedt I, Dubois A. Intracellular and interstitial expression of Helicobacter pylori virulence genes in gastric precancerous intestinal metaplasia and adenocarcinoma. J Infect Dis. 2003;187(8):1165–1177. doi:10.1086/368133
192. Engstrand L, Graham D, Scheynius A, Genta RM, El-Zaatari F. Is the sanctuary where Helicobacter pylori avoids antibacterial treatment intracellular? Am J Clin Pathol. 1997;108(5):504–509. doi:10.1093/ajcp/108.5.504
193. Dubois A, Borén T. Helicobacter pylori is invasive and it may be a facultative intracellular organism. Cell Microbiol. 2007;9(5):1108–1116. doi:10.1111/j.1462-5822.2007.00921.x
194. Solnick JV, Canfield DR, Hansen LM, Torabian SZ. Immunization with recombinant Helicobacter pylori urease in specific-pathogen-free rhesus monkeys (Macaca mulatta). Infect Immun. 2000;68(5):2560–2565. doi:10.1128/IAI.68.5.2560-2565.2000
195. Dubois A, Fiala N, Heman-Ackah LM, et al. Natural gastric infection with Helicobacter pylori in monkeys: a model for spiral bacteria infection in humans. Gastroenterology. 1994;106(6):1405–1417. doi:10.1016/0016-5085(94)90392-1
196. Solnick JV, Canfield DR, Yang S, Parsonnet J. Rhesus monkey (Macaca mulatta) model of Helicobacter pylori: noninvasive detection and derivation of specific-pathogen-free monkeys. Lab Anim Sci. 1999;49(2):197–201.
197. Calado CRC. Antigenic and conserved peptides from diverse Helicobacter pylori antigens. Biotechnol Lett. 2022;44(3):535–545. doi:10.1007/s10529-022-03238-x
198. Sjökvist Ottsjö L, Flach CF, Clements J, Holmgren J, Raghavan S. A double mutant heat-labile toxin from Escherichia coli, LT(R192G/L211A), is an effective mucosal adjuvant for vaccination against Helicobacter pylori infection. Infect Immun. 2013;81(5):1532–1540. doi:10.1128/IAI.01407-12
199. Lindqvist M, Persson J, Thörn K, Harandi AM. The mucosal adjuvant effect of alpha-galactosylceramide for induction of protective immunity to sexually transmitted viral infection. J Immunol. 2009;182(10):6435–6443. doi:10.4049/jimmunol.0900136
200. Wang J, Li P, Wu MX. Natural STING agonist as an “ideal” adjuvant for cutaneous vaccination. J Invest Dermatol. 2016;136(11):2183–2191. doi:10.1016/j.jid.2016.05.105
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
