Back to Journals » International Journal of Nanomedicine » Volume 18
How Key Alterations of Mesoporous Silica Nanoparticles Affect Anti-Lung Cancer Therapy? A Comprehensive Review of the Literature
Authors Budiman A, Rusdin A, Subra L, Aulifa DL
Received 6 July 2023
Accepted for publication 14 September 2023
Published 25 September 2023 Volume 2023:18 Pages 5473—5493
DOI https://doi.org/10.2147/IJN.S426120
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Arif Budiman,1,* Agus Rusdin,2,* Laila Subra,3 Diah Lia Aulifa2,*
1Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, West Java, Indonesia; 2Department of Pharmaceutical Analysis and Medicinal Chemistry, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, West Java, Indonesia; 3Department of Pharmacy, faculty of Bioeconomic, Food and Health Sciences, Universiti Geomatika Malaysia, Kuala Lumpur, Malaysia
*These authors contributed equally to this work
Correspondence: Diah Lia Aulifa, Department of Pharmaceutical Analysis and Medicinal Chemistry, Faculty of Pharmacy, Universitas Padjadjaran, Jl. Raya Bandung Sumedang Km. 21, Sumedang, West Java, 45363, Indonesia, Email [email protected]
Abstract: In 2020, there were 2.21 million new instances of lung cancer, making it the top cause of mortality globally, responsible for close to 10 million deaths. The physicochemical problems of chemotherapy drugs are the primary challenge that now causes a drug’s low effectiveness. Solubility is a physicochemical factor that has a significant impact on a drug’s biopharmaceutical properties, starting with the rate at which it dissolves and extending through how well it is absorbed and bioavailable. One of the most well-known methods for addressing a drug’s solubility is mesoporous silica, which has undergone excellent development due to the conjugation of polymers and ligands that increase its effectiveness. However, there are still very few papers addressing the success of this discovery, particularly those addressing its molecular pharmaceutics and mechanism. Our study’s objectives were to explore and summarize the effects of targeting mediator on drug development using mesoporous silica with and without functionalized polymer. We specifically focused on highlighting the molecular pharmaceutics and mechanism in this study’s innovative findings. Journals from the Scopus, PubMed, and Google Scholar databases that were released during the last ten years were used to compile this review. According to inclusion and exclusion standards adjusted. This improved approach produced very impressive results, a very significant change in the characteristics of mesoporous silica that can affect effectiveness. Mesoporous silica approaches have the capacity to greatly enhance a drug’s physicochemical issues, boost therapeutic efficacy, and acquire superb features.
Keywords: mesoporous silica, polymer, ligand, lung cancer
Graphical Abstract:
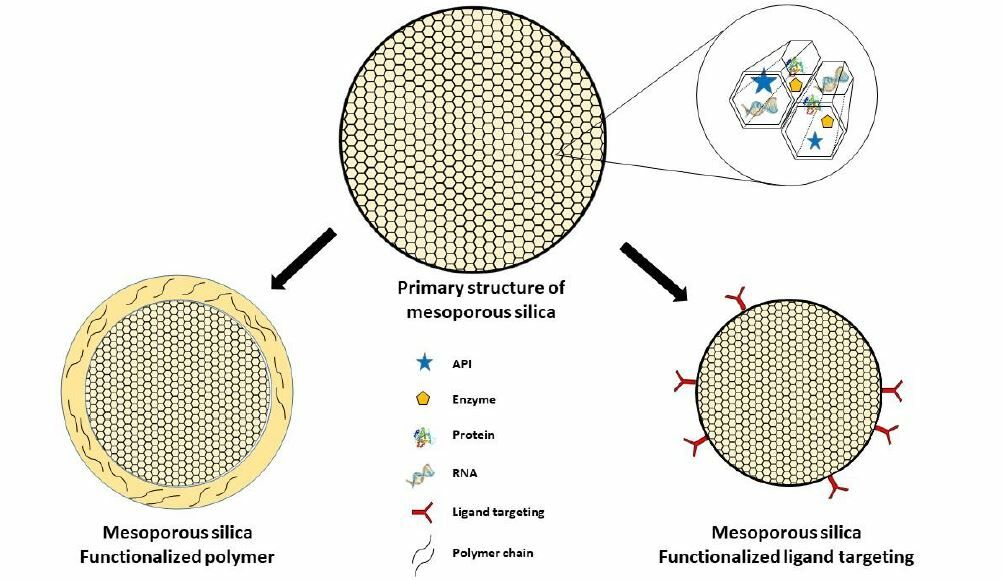
Introduction
Lung cancer, also known as bronchogenic carcinoma, refers to malignancies that begin in the lung parenchyma or within the bronchi. The most prevalent and lethal form of cancer in the world, lung cancer is expected to become more prevalent globally as tobacco use rises. Small-cell lung cancer (SCLC) and non-small-cell lung tumors (NSCLC), the latter of which is further split, are the two categories of lung cancer based on the cell of origin.1–5
A variety of modern therapies are used to treat lung cancer, including surgery, radiofrequency ablation, radiation therapy, chemotherapy, targeted medication therapy, and immunotherapy. Chemotherapy, which is used to inhibit the growth of cancer cells, frequently consists of several different drugs. It may be used prior to, following, or in conjunction with the use of other medications, such as immunotherapy. Usually, lung cancer patients receive their chemotherapy through an IV. However, there are other chemotherapeutic medication restrictions that affect their efficacy, including as their physicochemical properties.6–12
Physicochemical properties are an essential component of a drug’s pharmacokinetic and pharmacodynamic profile, and they have a significant impact on the success rate of drug candidates during the preclinical development process. Poor solubility, chemical stability, or permeability are some examples of stated poor physicochemical qualities of drugs that can affect DMPK and/or medication safety. These properties result in decreased GI tract absorption, which decreases oral bioavailability. This may influence productivity or the ability to obtain enough high exposures to support preclinical safety assessment investigations. High plasma protein binding, promiscuous binding, and off-target toxicity have all been associated with high lipophilicity.13–17
Mesoporous silica nanoparticles (MSNs) have been shown to be a superb platform for the delivery of therapeutic molecules, improve bioavailability of poorly soluble drugs, improve stability, provide modified release like sustained or extended, and even provide targeted drug delivery system. A type of silica known as mesoporous silica is distinguished by its mesoporous structure, which consists of pores with a diameter ranging from 2 nm to 50 nm. The word “mesoporosity” is used by the IUPAC to describe the region between microporous (2 nm) and macroporous (>50 nm). A relatively recent advancement in nanotechnology is mesoporous silica.18–24
Mesoporous silica has been developed in the most recent year to overcome the physical and chemical limitations of a few chemotherapeutic drugs as well as to increase their efficacy. Many research papers indicate the effectiveness of the mesoporous silica system in improving anti-lung cancer medications. There is not a study or article review that discusses and summarizes this subject, though.25–29
In order to provide the primary source, which specifically discusses this subject, authors are encouraged to conduct study review articles. We also try to draw attention to the novelty of this development of functionalized polymer and targeting ligand in mesoporous silica, as well as how the system improves efficacy and its mechanism from a molecular pharmaceutic point of view.
Methodology
This review is based on articles retrieved from Scopus, PubMed, and Google Scholar using the precise keywords “mesoporous silica”, “lung cancer”, “functionalized polymer”, and “functionalized ligand”. We then omitted reviews, opinions, and unrelated subjects. The databases are only able to acquire information on a certain topic, such as “mesoporous silica in pharmaceutical formulations with and without functionalized polymer or ligand”. Figure 1 displays the methodology’s flowchart.
 |
Figure 1 Flowchart of the methodology. |
Mesoporous Silica
How Mesoporous Silica Nanoparticles are Synthesized?
Mesoporous silicas were discovered in the early 1990s by scientists at Mobil Oil Company and Kuroda et al, partly in response to the need to broaden the applications of zeolites.30–32 The new zeolites were created to handle molecules that were too large to fit in the micropores of the existing zeolites. These new zeolites have well-ordered pores that range in width from 2 to 10 nm. The range of molecules that might fit within the pores was further broadened by Stucky et al creation of materials with pores that were even wider (up to 30 nm).33 Mesoporous materials have the ability to carry out the same functions as zeolites, which was immediately realized. They quickly found use in the areas of adsorption, ion exchange, molecular sieving, and catalysis.34–39 It did not take long to recognize that the well-ordered pores’ new dimension could result in whole new kinds of applications. Mesoporous silicas, for instance, were utilized as templates for some of the first synthetic processes of conductive carbon nanowires or to accelerate the production of conductive polymers within their pores.40–44 The use of synthetic mesoporous silicas for environmental cleanup was also suggested; after being functionalized with mercaptopropyl groups, the materials demonstrated their ability to remove heavy metals from water.45,46 Daz and Balkus showed that even molecules as large as proteins might be immobilized in mesoporous materials.47 Vallet-Regi et al demonstrated the loading and gradual release of ibuprofen from the materials in solution before recommending the usage of mesoporous materials for drug release.48,49 Other significant applications included the creation of sensors and energy transfer devices.50–55 All of these early applications, albeit intriguing, were still constrained by an inability to control particle morphology and size. The range of uses for mesoporous silicas would expand further if it was possible to create particles with well-defined forms and uniform sizes. Figure 2 illustrates the fundamental structure of mesoporous silica nanoparticles.56
 |
Figure 2 The introduction of functional groupings in various MSN regions: (a) at the external surface, (b) at the pore entrances, or (c) within the walls. Data from Slowing II et al.56 |
Fabrication of Silica Mesoporous
Sol Gel Method
The wet chemical method known as the sol-gel process is popular in the fields of ceramic engineering and materials science. This procedure is also known as the chemical solution deposition method. The sol-gel method begins with the preparation of a colloidal suspension (also known as sol) for the formation of an inorganic network and continues with the gelation of the sol to create the network in a continuous liquid phase (also known as gel). The precursors that are utilized to create these colloids typically include a metal or metalloid element that is encircled by a variety of reactive ligands. When the beginning material comes into contact with water or diluted acid, the processed starting material transforms into a sol. The gel is created by removing the liquid from the sol, and the sol/gel transition determines the particle size and form. Oxide is created during the calcination of the gel. The majority of the sol-gel chemistry processes are based on the hydrolysis of metal alkoxides, which is followed by the condensation of matching oxides and mixed oxides with various stoichiometry.57,58 These days, many diverse morphologies of mesoporous materials are synthesized using this approach. Different templates, including cat-ion surfactants, triblock copolymers, and organic small molecules, can be utilized as the structure-directing agents in the sol-gel method for the creation of mesoporous materials.59
Template Assisted Technique
The well-known and less expensive template assisted approach can be used to create ordered mesoporous materials. This method creates mesoporous materials using a template. It can be divided into two groups: endotemplate (soft matter templating) and exotemplate technique (hard matter templating). A surfactant (a structure-directing agent) is employed as a template in endotemplate to create ordered mesoporous materials60 Endotemplate, which is depicted in Figure 3,56 is a soft matter templating approach because no hard template solids are utilized. A porous solid is utilized as the template in the exotemplate method (also known as “nanocasting”) in place of the surfactant. Thus, “hard-matter templating” is another name for this technique.An inorganic precursor is used to fill the hollow areas that serve as the framework for the exotemplate, and it is then converted (cured) under the right circumstances. After removing the filled exotemplate framework,60 the pore system of the template is therefore replicated as a “negative image”, and integrated material is created with a significant specific surface area.
 |
Figure 3 The endotemplate approach for porous materials (soft matter templating). Data from Niesz et al.59 |
Microwave Assisted Technique
Since its discovery in 1992, the microwave hydrothermal process has been used to quickly synthesize a variety of ceramic oxides, hydroxylated phases, porous materials, and metal powders. In terms of research technology, the process of creating molecular sieves with microwave assistance is relatively new. Several different forms of zeolite, including zeolite A, Y, Mobil Composition of Matter No. 41 (MCM-41), are produced using this method. This method has many advantages over conventional processes, including quick heating to crystallization temperature, quick super saturation through the quick dissolution of precipitated gels, and a shorter crystallization time than traditional autoclave heating, which has been acknowledged.61
Chemical Etching Technique
This method uses structural variations between a silica core/mesoporous silica structure’s core and shell to create hollow type mesopores, which are then used to construct hollow interiors. This method allows for the synthesis of highly dispersed hollow mesoporous silica with adjustable pore sizes, which can be employed as a transporter for anticancer medication (doxorubicin) with a high loading capacity (1222 mg/g). With mesoporous silica serving as the shell and inorganic nanocrystals like Au, Fe2O3, and Fe3O4 as the core, this method can be utilized to create many types of heterogeneous hollow type nanostructures. To manage particle/pore size and shape, classic approaches such as soft/hard templating techniques and materials self-templating to generate mesoporous materials have had mixed results. The fabrication of uniform soft/hard templates is necessary for the traditional soft/hard templating methods for hollow materials, and their surface functionalization is accomplished through the deposition of heterogeneous shells. The traditional soft/hard templating methods’ calcination process for removing the cores also heavily depends on compositional variations.62
Functionalized of Silica Mesoporous
One of the effective methods for surface functionalizing MSNs is the grafting of polymers from their surfaces. In the field of materials science, the development of polymeric chains from MSNs is crucial since it gives MSNs the appropriate characteristics for a variety of applications.63 The most popular grafting methods are based on a variety of surface-initiated polymerization processes, including nitroxide-mediated radical polymerization (NMP), atom transfer radical polymerization (ATRP), and reversible addition-fragmentation chain transfer polymerization (RAFT). The fields of medication distribution, catalysis, and sensing are just a few where the polymer functionalized MSNs can be applied with ease. Both physical and covalent attachments of polymer chains to MSNs are depicted in Figure 3 (physisorption and chemisorption, respectively). Since it is noncovalent in nature and reversible, the physio sorption method is typically not a good methodology. The irreversible compatibility of covalent bonding between organic and inorganic groups makes it a preferred approach for chemical grafting. There are two ways to perform chemical grafting, including “grafting-to” and “grafting-from”. While grafting-from entails altering the surface of MSNs with an initiator, followed by surface-initiated polymerization, grafting-to uses covalent bonding by reacting pre-synthesized macromolecules containing specified groups with specific groups affixed on the inorganic surface. Controlled radical polymerization techniques, such as RAFT, ATRP, or NMP (Figure 4),64 can be used to build polymer chains.
 |
Figure 4 Schematic illustration for the production of MSNs grafted with a copolymer of positively charged quaternary amines and PEG using RAF and addition-fragmentation equilibria during RAFT polymerization. (I) Immobilization of the RAFT agent using the equal molar quantity of diisopropylcarbodiimide(DIC) and diisopropylethylamine(DIPEA) as catalysts, (II) The RAFT polymerization of PEGMA and PDMAEA at 70 °C under an argon atmosphere in the presence of 2.2′-azobis(isobutyronitrile) (AIBN), and (III) The final product, namely as MSN-PEG+. Data from Sahoo et al.65 |
Mesoporous silica nanoparticles have a wide range of applications, including drug delivery, coating, sensing, and the growing subject of catalysis. MSNs are able to withstand a variety of alterations that are easily anchored on their surface because of their large surface area, biocompatibility, chemical stability, and customizable porosity design.65–67 As a result, methods for modification and functionalization, such as the co-condensation method, the encapsulation process, and post-synthesis procedures like grafting to and grafting-from, are currently attracting a lot of attention. MSNs are being thoroughly researched as smart systems that can be used for controlled and targeted medication delivery systems because of their low toxicity and high drug loading capacity. Finding effective ways to direct ligands toward the specific sick location has proven to be challenging. The idea of a stimuli-responsive drug release system considers the controlled, targeted release of the necessary dosage of medication.68 In many situations, it is possible to use both internal and exterior stimuli, including pH, temperature, light, magnetic fields, electric fields, ultrasounds, and redox potential.
By utilizing hybrid MSNs, Paris et al demonstrated a novel method for stimuli-responsive systems. These hybrid-MSNs have a lower critical solution temperature (LCST) below 37°C, operate as nano-gates sensitive to ultrasound stimulations, and are covered with dual temperature and ultrasound-responsive copolymer. When ultrasound is applied, copolymer alters its physical state at the appropriate temperature, allowing loaded molecules to escape from carriers. In order to demonstrate their ability to exclusively cause cell death when this hybrid system was exposed to ultrasound, doxorubicin-loaded hybrid MSNs were incubated with LNCaP (androgen-sensitive human prostate cancer) cells, as shown in Figure 5.69
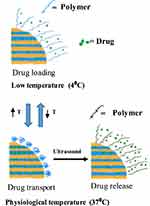 |
Figure 5 An example of how a dual-responsive system behaves in watery medium. Data from Paris et al.69 |
According to Zheng et al, block copolymer serves as the shell and thermo- and pH-responsive nanocarrier silica acts as the core. Methacrylic acid (MAA) and N-isopropyl acrylamide (NIPAM) were polymerized on the silica surface using surface-initiated RAFT (SI-RAFT). Doxorubicin (DOX) was more effectively loaded onto the polymer-grafted MSNs, and these dual-responsive nanoparticles were employed as a drug carrier. The surrounding media’s pH and temperature also affect how quickly drugs are released. While particles were distributed in an aqueous medium at high pH and low temperatures, agglomeration was only seen under acidic and raised temperature conditions (Figure 6). 70
 |
Figure 6 Synthesis of a dual-responsive drug delivery system illustrated. Data from Zheng et al.70 |
The Use of Mesoporous Silica in Cancer Treatment
Although chemotherapy is a significant anti-tumor treatment in clinical practice today, its efficacy is constrained by its low selectivity, substantial systemic toxicity, and multidrug resistance. Due to its distinct features, such as simple large-scale manufacture, changeable uniform pore size, huge surface area, and pore volumes, mesoporous silica nanoparticles (MSNs) have recently become intriguing drug delivery systems (DDSs). While mesoporous silica-based DDS can enhance chemotherapy to some amount, MSN-based chemotherapy has a synergistic impact when combined with other cancer medicines, significantly enhancing therapeutic results.71
MSNs with wide pore widths, a variety of functionalities, simplicity of modification, and strong biocompatibility are the ideal materials to create such synergistic nanoplatforms because they can act as therapeutic agents in treatments that are added on top of chemotherapy as well as drug carriers. MSN-based nano-systems highlighted the benefits of dual- or multi-modal therapy by integrating chemotherapy with other therapeutic modalities such as immunotherapy, gene therapy, phototherapy, magnetic hyperthermia therapy, and sonodynamic therapy. According to expectations, the majority of the reported cases show that MSN-mediated combination therapy produced at least a 1 + 1 > 1 effect in cells or animals, offering experimental support for additional interesting MSN-based delivery system uses. There is not a combination that is objectively better because each combination includes pros and cons. However, given that immunotherapy using PD-1/PD-L1 antibodies, CTAL-4 antibodies, and CAR-T treatment has recently been disclosed as a potent clinical method for treating cancer, the chemo-immuno combination therapy may have the most promising future for further clinical translation.71
Though preliminary results from in vitro and in vivo testing of MSN-based combination chemotherapies have been promising, a number of obstacles must yet be overcome before these nanoparticles may be used in clinical settings. The multimodal therapeutic platforms used in this review, nanocomposites, call for extensive biosafety testing as well as more thorough pharmacokinetic/pharmacodynamic assessments of each contributing component in the complexes. Further research must be done on the ideal dosage ratio between chemotherapeutics and other therapeutic agents. In order to maximize the synergistic benefits, combination medicines should also be strategically integrated. To do this, they should achieve interlocking effects and intelligent drug delivery and release mechanisms. Last but not least, increasing the ability of MSN-based nanocomplexes to target cancer and creating simpler ways to make them are both top priorities. The study on MSN-based chemotherapeutic combination medicines is still in its infancy, but as our knowledge of materials and illnesses grows, so are the potential MSN-based DDS applications. Chemotherapy-based nano-systems that make use of biocompatible MSNs will undoubtedly have a bright future and considerable promise for clinical translation.71
Result and Discussion
The Use of Silica Mesoporous in Lung Cancer Treatment
The non-functionalized mesoporous silica Co-delivery has been suggested to maximize the effectiveness of cancer treatments while using the fewest possible doses of each medicine (Table 1). For a comparative therapeutic efficacy in lung cancer, this study have investigated the effectiveness of co-delivery of anticancer medicines and survivin siRNA in the MSNs. Findings showed that such a combinational strategy led to increased therapeutic efficacy via synergistic medication activity while inhibiting the activity of survivin protein. In light of this, lung cancer therapy in the future may benefit greatly from evolving strategy.72
 |
Table 1 Poorly Water-Soluble Anticancer Drugs Encapsulated into Mesoporous Silica |
Another study of non-functionalized mesoporous silica had been conducted for the cancer treatment with ruthenium based. The drugs show promise, but they lack precise targeting and controlled release capabilities. The purpose of this study was to design and manufacture a new class of bioreducible mer-Ru(III)Cl3(dmso)(L) prodrugs with high DNA binding affinity for nucleus-targeted treatment and selective activation in response to the tumor microenvironment. In this context, increased intracellular glutathione levels caused ruthenium(III) prodrugs to be converted into highly active ruthenium(II) drugs, which led to tumor apoptosis via synergistic intercalation that prevented DNA replication and coordination that cleaved nuclear DNA. To enable multiple targeting and eGFP-based fluorescence imaging of nonsmall cell lung cancer, a multifunctional nanodrug (Ru-MSN-PLip) was created by combining RuDPNI prodrug-loaded mesoporous silica nanoparticles with a fusion protein-incorporated liposome. Ru-MSN-PLip accomplished sequential delivery and increased anticancer effectiveness thanks to nuclear DNA targeting by Ru(III) prodrugs and fusion protein-based cancer cell recognition. Additionally, Ru-MSN-PLip demonstrated sustained blood flow and selective accumulation in tumors, which greatly reduced tumor progression in vivo while exhibiting no discernible damage. Our research demonstrated the potential uses of these bio reducible and traceable nano prodrugs for effective and secure non-small cell lung cancer treatment.73
A proteasome inhibitor known as bortezomib (BTZ) encapsulated hollow mesoporous silicon nanospheres (HMSNs) has been developed as a biocompatible and efficient drug-delivery system for the treatment of non-small cell lung cancer (NSCLC). Then, researchers discovered that in p53 null/mutant NSCLC, the tumor-suppressing efficacy of BTZ or HMSNs-BTZ was diminished. Clinical trials have demonstrated a positive impact of p53 gene therapy and chemotherapeutic medicines in treating NSCLC patients, particularly those with the p53 gene null/mutant NSCLC. But using viral vectors for gene delivery raises certain safety and toxicity issues. For the treatment of p53 signal deficient NSCLC, HMSN-based co-delivery of BTZ and the tumor suppressor gene p53 was created. The half-maximum inhibitory concentration (IC50) of HMSN-PEI-BTZ-p53 was 51% or 25% of that for HMSN-BTZ in 72 or 96 h treatment, respectively, according to the results of the cell viability assay on p53-mutant H1299 cells. The HMSNs-PEI-BTZ-p53 group showed increased dead staining when compared to the HMSNs-BTZ group with a comparable BTZ concentration, which was consistent with accumulating p53 expression. The live/dead staining assay for treated H1299 cells showed wider distribution. Additionally, the Annexin V-FITC/PI assay revealed that HMSNs-PEI-BTZ-p53-induced restored p53 expression caused early apoptosis to be activated, which accelerated later cell death. Results from Western blotting and real-time PCR demonstrated that multiple p53 downstream genes (accumulation of p21 and bax, activation of caspase 3, downregulation of Bcl-2, etc.) responded substantially and synergistically to BTZ action and restored p53 expression. The primary molecular mechanism for the increased effectiveness of HMSNs-PEI-BTZ-p53 on p53-mutant NSCLC cells was the restoration and reactivation of the p53 signal pathway.29
Doxorubicin hydrochloride (DOX) loaded MSNs (MSNs@DOX) were employed in a study to carry and administer the drug in vivo. The findings demonstrated that DOX was successfully loaded into MSNs with an average diameter of 88–11 nm, which considerably improved DOX’s anticancer efficacy against lung cancer both in vitro and in vivo when compared to a conventional DOX treatment. Through the caspase family and the release of cytochrome C, MSNs@DOX significantly increased apoptosis. Furthermore, both in vitro and in vivo cell invasion and migration were severely suppressed. The higher permeability and retention period of the nanoparticles, according to the study, may have contributed to the improved effect of MSNs@DOX on tumor cells. The molecular mechanism behind the anti-metastasis action may be related to the reduction of VEGF-mediated angiogenesis, according to Matrigel plug assays and Western blotting experiments. Results present a fresh viewpoint on the use of nanoparticles in metastasis prevention.74
To enhance the solubility of paclitaxel (PAC) and enhance its therapy of lung cancer, paclitaxel-mesoporous silica nanoparticles with a core-shell structure (PAC-csMSN) were employed as a nanodrug delivery system. By using the adsorption equilibrium approach, PAC was loaded into core-shell mesoporous silica nanoparticles (csMSN), which had an amorphous mesoporous structure. Studies conducted in the lab and on animals revealed that csMSN boosted PAC’s lung absorption and accelerated its breakdown. The PAC-csMSN used for pulmonary administration in rabbits had an area under concentration-time curve (AUC) value that was 2.678 times greater than that obtained with the PAC. A lung biopsy performed 3 days after continuous treatment revealed no evidence of irritation. According to results of cell apoptosis obtained by flow cytometry, PAC-csMSN was more effective than pure PAC at causing cell death. Transmission electron microscopy (TEM) and laser scanning confocal microscopy (LSCM) were used to study PAC-csMSN’s absorption in A549 cells. The acquired results showed that the cellular absorption of csMSN into the cytoplasm was time dependent. All of these findings show that csMSN can be used to provide medications that are poorly water-soluble via pulmonary inhalation for the treatment of lung cancer.75
Mesoporous silica particles that have been functionalized with recently extracted fish oil (Omeg@Silica) had greater anti-proliferative and pro-apoptotic effects on non-small cell lung cancer (NSCLC) cell lines than fish oil alone. Unknown mechanisms may be at work to explain this effectiveness. To understand the anti-cancer effects of a formulation of Omeg@Silica in aqueous ethanol (FOS) in adenocarcinoma (A549) and mucoepidermoid (NCI-H292) lung cancer cells, as well as to evaluate cell migration, as well as to assess the expression of IL-8, NF-B, and miRNA-21, is the purpose of the current study. The findings demonstrate that FOS was more effective than oil alone in reducing cell migration and IL-8 gene production in both cell lines. FOS decreased IL-8 protein release in both cell lines, but only in A549 did it have a greater impact than the oil alone. FOS was able to lower the nuclear expression of miRNA-21 and the transcription factor NF-B in A549. Together, these findings suggest the Omeg@Silica’s prospective usage as an adjunctive therapy for NSCLC. There is a need for focused investigations that demonstrate clinical efficacy.76
The use of hollow mesoporous silicon nanospheres (HMSNs) to encapsulate BTZ for drug delivery is described in this work. The half-maximum inhibitory concentration (IC50) of HMSNseBTZ was 42% of that for free BTZ in 48 h treatments in an in vitro cell viability experiment on human NSCLC H1299 cells. Additionally, an in vivo tumor-suppression experiment revealed that HMSNseBTZ (0.3 mg/kg) exhibited almost 1.5 times the anti-tumor effectiveness of free BTZ. Additionally, it has been suggested that the enhanced anticancer efficacy of HMSNseBTZ may be caused by more robust induction of cell cycle arrest and apoptotic cell death, as well as accelerated activation of Caspase 3 and autophagy. At the very least, the presence of wild-type p53 signaling increased the tumor-suppressing effects of HMSNseBTZ, indicating a possible improvement in therapeutic efficacy with p53 gene therapy and BTZ-based chemotherapy. The HMSNs-based nanoparticles are thus emerging as a promising platform to deliver therapeutic agents for favorable clinical outcomes by lowering doses and frequency of drug administration as well as reducing potential side effects.77
Here, curcumin is added to the mesoporous substance SBA-15 to create porous composite particles. The therapeutic impact of curcumin on lung cancer has also been studied. The composite material’s decreased surface area and pore diameter provided evidence that curcumin had been added to the host material SBA-15, and FT-4 and NGI examined the composite material’s aerodynamic performance. The mesoporous materials displayed good biocompatibility at 10–400 g/mL, as demonstrated by phagocytosis assays on RAW264.7, the toxicity of material extracts on BEAS-2B cells, and the hemolysis experiments. The therapeutic impact of lung cancer following inhalable administration was studied using the B16F10 melanoma metastatic lung mice model. It was discovered that the group receiving curcumin composite particles lost body weight more gradually and acquired lung disease more gradually than the group receiving curcumin crude, suggesting that the composite particles may have a tumor-inhibiting function.28
Mesoporous silica was employed in a distinct study to show, for the first time, that differential interference contrast (DIC) microscopy may be used to see the whole endocytosis process of MSN into living human lung cancer cells (A549) without the need of fluorescence staining. Motion, shape, and vertical position are three physical observables that describe the locations of MSN and the stages of the endocytosis procedure. The MSN experienced considerable Brownian motion in the cell growth media when it was outside the cell. The MSN’s range of motion was severely constrained when it became caught on the cell membrane. The cytoplasm is more viscous than the cell growth medium, and the cellular cytoskeleton networks function as impediments, thus after the MSN entered the cell, it resumed mobility at a much slower rate. The creation of a vesicle after the MSN was caught on the cell membrane and before it entered the cell also caused shape changes around the MSN. Finally, we captured the MSN’s location in three dimensions by connecting a motorized vertical stage to the DIC microscope. Studies of selectively targeted drug administration based on endocytosis require such precise 3D particle tracking capability in living cells.78
The Impact of Polymer Addition on Mesoporous Silica for Lung Cancer Treatment
A new siRNA delivery vector based on magnetic mesoporous silica nanoparticles (MMSNs) has been developed using functionalized mesoporous silica. The M-MSNs’ mesopores were used to hold the siRNAs, which were then modified using fusogenic peptide KALA, polyethylenimine (PEI) capping, and PEGylation to create the nanocarrier. The resulting delivery system demonstrated improved tissue biocompatibility and biosafety, increased cell membrane translocation and endosomal escapability, and prolonged half-life in bloodstream. In subdermal and orthotopic lung cancer models, systemic delivery of vascular endothelial growth factor (VEGF) siRNA via this nanocarrier led to exceptional tumor suppression, tumor metastasis was also greatly decreased, and overall survival was enhanced. Additionally, the functionalized fluorescent markers and the magnetic core of the particles made it simple to image the target tissues in vivo. All things considered, this M-MSNs-based siRNA delivery method has demonstrated excellent potential for use in the treatment of cancer.25
To increase doxorubicin efficacy, a second research of functionalized mesoporous silica polymers was conducted. In this study, doxorubicin (DOX)-loaded mesoporous silica nanocontainer Si-SS-CD-PEG was subjected to hyperthermia to increase its anti-cancer effectiveness. The cyclodextrin gatekeeper’s glutathione-mediated breakdown was thought to be stimulated by heat, leading to the release of DOX from the carrier and cell death brought on by DOX. Only when GSH was present in the suspension did DOX-loaded Si-SS-CD-PEG in PBS begin to release from the carriers, and heating at 42C marginally accelerated this process. Heating considerably boosted both the release of DOX from the internalized carriers and the GSH content in A549 cells. Clonogenic death and apoptosis were significantly elevated in the cancer cells treated with carriers when heated at 42C. A549 cells had a higher GSH content than L-132 cells, and they were much more susceptible to Si-SS-CD-PEG loaded with DOX at both 37C and 42C. The GSH-mediated release of DOX from DOX-loaded Si-SS-CD-PEG was accelerated by hyperthermia. Additionally, heat significantly increased the amount of GSH in cancer cells, which increased the release of DOX from internalized carriers and facilitated DOX-induced clonogenic and apoptotic cell death.26
The development of a functionalized polymer research on mesoporous silica. Doxorubicin (DOX), a model drug for the treatment of drug-resistant non-small cell lung cancer, is to be delivered by a system of mesoporous silicon nanoparticles (NPs) coated with polydopamine and functionalized with d-a-tocopheryl polyethylene glycol 1000 succinate (TPGS). MSNs-DOX@PDA-TPGS has superior in vivo therapeutic efficacy when compared to free DOX and DOX-loaded NPs without TPGS ligand alteration. It also has an exceptional capacity to overcome multidrug resistance. Other hydrophilic and hydrophobic medications can also be delivered using this wonderful drug delivery system.79
The subsequent work involved the creation of functionalized polymer of Mesoporous Silica MCM-41 nanoparticles carrying the stable isotope holmium-165 (165Ho). The 400 nm particles were exposed to an aqueous solution of 165Ho acetylacetonate for 24 hours at room temperature. Holmium-166-containing mesoporous silica (166Ho-MCM-41) nanoparticles (20.8 ± 1.9% w/w 166Ho) were created by thermal neutron irradiation of the obtained solid in a PULSTAR nuclear reactor (reactor power = 1 MW; thermal neutron flux of approximately 5.5 or 7.7 1012 n/cm2 s). Orthotopic non-small cell lung cancer A549-luciferase tumor-bearing mice were given the 166Ho-MCM-41 nanoparticles intravenously (i.v.). Tumors were shown to have an initial dosage per gram (ID/g) of tissue of 4.5 ± 3.9% after 24 hours, and this value grew to 58.8 ± 34.7% ID/g after one week.80
The Impact of Targeting Mediator Addition on Mesoporous Silica for Lung Cancer Treatment
A study of functionalized ligand targeting on mesoporous nanoparticle silica was created for cetuximabcapped (MP-SiO2 NP) as the drug carrier to effectively target EGFR mutant lung cancer cells and release loaded medications like doxorubicin and gefitinib. Instead of lung cancer cells with low EGFR levels, this novel nanomedicine can precisely target those with high EGFR expression. A considerable suppression of cell growth was seen when the gefitinib-loaded cetuximab-capped MP-SiO2 NP was used to treat a gefitinib-resistant cell line derived from PC9 cells (PC9-DR). Additionally, the PC9-DR xenograft tumors were successfully stopped in their tracks by this nanomedicine. This tumor suppression resulted from the efficient release of gefitinib caused by elevated glutathione (GSH) levels in PC9-DR cells and the endocytosis of significant amounts of nanomedicine. Overall, our study offers a unique method for treating EGFR-TKI resistance utilizing cetuximab modified MP-SiO2 NP, which has a high potential for successfully treating lung cancer with an EGFR mutation.81
For the myricetin (Myr) molecule, further research of functionalized ligand targeting on mesoporous silica was proposed. Multidrug resistance protein (MRP-1) siRNA was coupled with drug-loaded mesoporous silica nanoparticles (MSN). To increase the therapeutic effectiveness of Myr for the treatment of NSCLC, folic acid (FA) was added to the surface of the produced nanoparticles. The gathered nanoparticles demonstrated a continuous release of Myr under physiological circumstances. Compared to non-targeted nanoparticles, FA-conjugated nanoformulations showed a considerable uptake in lung cancer cells. The findings of the in vitro drug release tests indicated that FA-conjugated MSN with Myr and MRP-1 nanoparticles released the drug more slowly than free Myr and MSN mixed with Myr. Treatments with FA-conjugated MSN in combination with Myr and MRP-1 significantly decreased the cell viability of lung cancer cell lines, including A549 and NCI-H1299, and were also associated with a reduction in colony formation. Additionally, FA-conjugated MSN loaded with Myr and MRP-1 dramatically increased the expression levels of cleaved Caspase-3 and PARP as well as triggered apoptosis in lung cancer cells. Results from in vivo fluorescence experiments showed that FA-conjugated MSN with Myr and MRP-1 nanoparticles may aggregate preferentially at tumor locations. FA-conjugated MSN loaded with Myr and MRP-1 nanoparticles could more successfully and with fewer negative effects inhibit tumor growth as compared to free Myr and MSN mixed with MRP-1/Myr nanoparticles. Overall, FA-conjugated nanoparticulate system might offer a cutting-edge and efficient platform for treating NSCLC.27
Another outstanding work for functionalized ligand targeting on mesoporous silica was designed to produce the peptides in binding particularly to MT2-MMP, a phage-displayed 12 peptide library was used, and the affinity of peptides toward MT2-MMP was found by multitest methods. The findings demonstrated that a particular peptide with the sequence HHRLHSAPPPQA (MT2-AF5p), which targets the MT2-MMP, has a high selectivity and great affinity for lung cancers. To create a chemotherapeutic drug-targeted delivery system (DOX-laden FMSN@MT2-AF5p), MT2-AF5p was coupled onto fluorescent mesoporous silica nanoparticles (FMSN-NH2) and loaded with doxorubicin (DOX) to further accomplish specific targeting and precise therapeutic effects. The DOX-loaded FMSN@MT2-AF5p increased DOX release in an acidic environment. Most importantly, as demonstrated by the fluorescence imaging ex vivo, FMSN@MT2-AF5p effectively targeted the tumor location. The innovative peptide-functionalized nanoparticles are potential for clinical usage as a precise targeting nanodrug for lung cancer diagnosis and therapy because they have good biocompatibility.82
The subsequent study used metformin as a drug payload to functionalize ligands targeting on mesoporous silica for the treatment of lung cancer. In this study, hyaluronic acid (HA) coated mesoporous silica nanoparticles (MSNs) were used for the active targeting and efficient delivery of metformin because HA has the ability to specifically target CD44 receptors that are overexpressed on the surface of non-small lung cancer cells. Due to Metf@MSNs-HA’s effective CD44-targeting ability and distribution, MTT and qPCR results also showed that Metf-loaded MSNs-HA (Metf@MSNs-HA) had superior cytotoxicity and pro-apoptotic effects against the A549 lung cancer cells compared to free Metf and MSNs@Metf. In addition, it was shown that Metf@MSNs-HA could effectively activate the AMP-activated protein kinase (AMPK) pathway, inhibit the mammalian target rapamycin (mTOR), and increase growth inhibition. This exploratory research demonstrated the enormous potential of Metf@MSNs-HA in lung cancer cell targeting treatment.83
Targeted medication delivery techniques for cancer therapy were developed using manufactured epidermal growth factor receptor antibody (EGFRAb) conjugated silica nanorattles (SNs). According to the results of the current study, lung cancer cells internalize silica nanoparticles at a higher rate than normal lung cells. Additionally, in vitro research amply demonstrated that pyrrolidine-2 loaded EGFRAb-SN significantly decreased cPLA2 activity, arachidonic acid production, and cell proliferation in H460 cells. When compared to free pyrrolidine-2 and pyrrolidine-2 loaded SNs, pyrrolidine-2 loaded EGFRAb-SN dramatically increased cytotoxicity, cell cycle arrest, and death in human non-small cell lung cancer cells. Silica nanorattles and EGFRAb-SN-pyrrolidine-2 displayed negligible systemic toxicity in healthy Balb/c mice, according to an in vivo toxicity study. On the non-small cell lung cancer subcutaneous model, the EGFRAb-SN-pyrrolidine-2 demonstrated significantly greater antitumor efficacy (38%) and increased tumor inhibition rate than the pyrrolidine-2. The low toxicity and strong therapeutic potentials of EGFRAb-SN-pyrrolidine-2 were thus confirmed by the current research, which may offer compelling evidence for silica nanorattles as novel potential carriers for targeted drug delivery systems.84
The final one in this method used folate as a ligand target for mesoporous silica. The monitoring of folate receptor levels in active cancer cells has gained growing attention, although the currently employed fluorescent probes frequently encounter interference from non-target cell surface adsorption. In this study, folic acid is used as a template molecule, a mesoporous silica nanoparticle serves as an imprinting medium, and a CdTe quantum dot serves as a signal transducer for the detection of the folate receptor. Based on the high affinity between folic acid and the folate receptor, this method is intended to provide selective recognition of the folate receptor over other compounds. The probe can be used for targeted A549 cell imaging since it is stable, monodispersed in aqueous suspension, and has low toxicity to living cells. Because of its improved biocompatibility, lower cytotoxicity, and higher membrane permeability, the living cell imaging investigations further highlight its utility in bioimaging cell-surface folate receptor.86
Comparison
Mesoporous silica is used throughout the system, and its primary objective is to improve the physicochemical properties of active pharmaceutical ingredients, such as solubility and stability. These ingredients can take the form of isolate compounds, proteins, enzymes, RNA, and other active ingredients; however, in this case, they can also be in the form of these or other forms. Additionally, some of the methods included in this analysis were designed primarily to target the delivery system and boost effectiveness.
According to the active ingredient content, non-functionalized mesoporous silica can be made with a variety of active ingredients, including genes like the Si-RNA gene and the P53 gene, conventional drugs like paclitaxel, bortezomib, doxorubicin, and ruthenium, and even fish oil that has been extracted. The functionalized polymer or ligand targeting mesoporous silica is very similar to the initial system and contains an active component that can be either the Si-RNA gene or more conventional anticancer medications like doxorubicin, gefitinib, myricetin, and metformin. Judging from other parameters, such as methods, there are also no specific pattern differences related to the synthesis strategy applied for each system. However, throughout, the solvent evaporation method is probably the most widely used synthesis technique, followed by sol gel, templates, and precipitation.
The distinction is clear in the objective of the modification, non-functionalized mesoporous silica is typically to improve the physicochemical properties of the active components and for drug combination delivery systems. The design of functionalized polymers, in general, differs in that the polymer can regulate the release of the active therapeutic ingredient through a variety of release mechanisms, including swelling and erosion. Polymer addition is sometimes used in systems as a bridge for other conjugates. The functionalized ligand system on mesoporous silica then explicitly seeks to acquire a targeted delivery system, which in this case is mediated by each targeting mediator that conjugates directly on mesoporous silica and has an affinity for receptors or enzymes expressed by lung cancer.
Limitation and Challenge
Controlled Synthesis
Precisely creating silica mesoporous nanoparticles involves using templates like surfactant micelles or organic templates to shape the silica structure. Changes in factors such as temperature, pH, and surfactant concentration can influence the formation kinetics of these templates, leading to variations in pore structures, sizes, and shapes. The mechanisms driving these changes are intricate, involving interactions among the template, precursors, and reaction conditions.87–90
Stability
Silica nanoparticles typically remain stable under normal circumstances, but exposure to extreme pH, temperature, or specific chemicals can cause their structure to dissolve and alter. This alteration arises from the disruption of chemical bonds within the silica lattice. Acidic or basic environments and high temperatures can expedite this bond-breaking process.87–90
Biocompatibility and Toxicity
The compatibility of silica mesoporous nanoparticles with biological systems depends on aspects like nanoparticle size, surface charge, and surface chemistry. Nanoparticles of particular sizes can enter cells via endocytosis, potentially leading to negative cellular responses. The mechanisms involved encompass interactions between nanoparticles and cells, including uptake, trafficking within cells, and the potential release of harmful substances from the nanoparticle surface.87–90
Cargo Loading and Release
Incorporating therapeutic agents into mesoporous silica nanoparticles involves capillary condensation, where the nanopores are filled with liquid. The release rate hinges on variables like pore size, interactions between the nanoparticle surface and the cargo, and the nature of the cargo (hydrophobic or hydrophilic). The mechanisms include cargo diffusion through nanopores and detachment from the surface.87–90
Surface Functionalization
Modifying the surface of silica mesoporous nanoparticles entails attaching molecules to the nanoparticle exterior. Achieving consistent and enduring functionalization can be complex due to steric hindrance, where functional groups compete for available surface spots. Weak bonds between functional groups and the nanoparticle surface can also result in detachment or instability over time.87–90
Scale-Up
Expanding the synthesis of nanoparticles can introduce fluctuations in reaction kinetics and mass transfer. These fluctuations may lead to disparities in nanoparticle attributes such as size, shape, and properties. The mechanisms include alterations in mixing dynamics, heat and mass movement, and interactions among reagents and templates.87–90
Characterization Techniques
Analyzing mesoporous nanoparticles necessitates specialized techniques that provide insight into their attributes. These techniques rely on interactions between particles and various forms of energy (eg, electrons in TEM, X-rays in XRD) to generate data or images. However, accessibility to these techniques and the precision of measurements can vary, leading to limitations in characterization.87–90
Aggregation and Dispersion
Nanoparticle aggregation results from attractive forces between particles. Counteracting this aggregation involves introducing repulsive forces through modifications to the surface or using dispersants. Mechanisms encompass van der Waals forces, electrostatic interactions, and steric hindrance.87–90
Challenges Specific to Applications
Different applications possess distinct requirements. In catalysis, mechanisms encompass reactant diffusion into nanopores and interaction with active sites. In sensing, mechanisms involve interactions between target analytes and functional groups on the nanoparticle surface, inducing signal changes.87–90
Regulatory Approval
Obtaining regulatory approval involves comprehensive testing to ascertain nanoparticle safety. Nanoparticles can interact with biomolecules within cells, potentially inducing immune responses or cellular stress. Mechanisms encompass cellular uptake, pathways of internalization, and interactions between nanoparticles and cells.87–90
Adverse Effect and Biologic Response
Inflammatory Reaction
Silica nanoparticles hold the capacity to incite an inflammatory reaction within the body, particularly upon introduction into the bloodstream or various tissues. This response involves activating immune cells, such as macrophages, leading to the emission of inflammatory cytokines. The underlying mechanism of this reaction revolves around immune cells recognizing the nanoparticles as foreign agents, prompting efforts to eliminate them from the body.85,91–93
Activation of Immune Cells
Upon encountering silica nanoparticles, immune cells like macrophages can internalize them through a process called phagocytosis. This internalization initiates immune responses as the cells strive to break down and clear the nanoparticles. During this process, the release of reactive oxygen species (ROS) contributes to oxidative stress and inflammation.85,91–93
Cellular Toxicity
Silica nanoparticles possess the potential to exert direct toxicity on cells, particularly under conditions of elevated concentration or prolonged exposure. These effects stem from interactions between nanoparticles and cellular components, which can disrupt normal cellular processes and ultimately lead to cell death.85,91–93
Immune Recognition
Silica nanoparticles have the potential to be identified by the immune system as foreign entities, prompting the activation of mechanisms for immune surveillance. This recognition triggers immune responses with the goal of neutralizing and eliminating these nanoparticles from the body.85,91–93
Fibrosis Reaction
Extended exposure to elevated concentrations of specific types of silica nanoparticles can trigger a fibrotic response, characterized by excessive connective tissue formation. This reaction has the potential to impede tissue function and impact organ health.85,91–93
Impact of Size and Surface Area
Nanoparticle size and surface area wield significant influence over their interactions with the immune system. Smaller nanoparticles are more prone to entering cells and tissues, potentially intensifying immune responses.85,91–93
Surface Chemistry Effects
Surface chemistry, encompassing factors like surface charge and functional groups, can shape how silica nanoparticles interact with immune cells and biomolecules. Alterations to the nanoparticle surface can modify these interactions and subsequently impact the resulting immune responses.85,91–93
Distribution and Elimination in the Body
The body’s distribution, processing, and elimination of nanoparticles significantly contribute to their potential negative effects. Silica nanoparticles that remain within the body for extended durations might elevate the likelihood of triggering immune responses or other undesirable outcomes.85,91–93
Author Perspective
The high concentration or supersaturation level of drug within mesoporous silica was formed because the monomolecularly dispersed drug on the surface of the mesopores can rapidly dissolve into the dissolution medium through the interaction of water molecules with the hydrophilic group of drugs. Thus, the dissolved drug can be rapidly released from the pore of mesoporous into the bulk dissolution medium. Moreover, the inhibition of drug crystallization within mesoporous silica can contributed to the improvement of solubility and stability from poorly water-soluble drugs (Figure 7). 94–96 Two mechanisms can be used to directly explain how non-functionalized mesoporous silica inhibit drug crystallization leading to improvement of solubility and stability. The first is through molecular interactions that are formed between the active ingredients in drugs and the hydroxyl groups on the surface porous of mesoporous silica. This enables changes in the active ingredients’ properties, which have low solubility in water become more soluble due to the intervention of the properties of amorphous silica. The second is to keep the drug in an amorphous state; the active ingredient is located in silica pores, preventing drug molecules from interacting with one another and preventing the process of recrystallization, in addition, the small particle size on this system also can influence the solubility and dissolution rate. Because of this action, it can also be argued that mesoporous silica can maintain the active ingredient’s stability; this is one of the purposes of the nanoconfinement system.
 |
Figure 7 The mechanism of drug release improvement from the drug within non-functionalized mesoporous silica. Note: Data from these studies.94–96 |
This design is slightly different from the functionalized polymer mesoporous silica design in that it typically uses additional polymer on the surface of mesoporous silica, where the polymer is the outermost (Figure 8). 97–99 Due to this polymer’s sensitivity to solvent presence, pH, and other medium conditions, this polymer can act as an agent that can control drug release. This modified delivery system is able to increase drug effectiveness by extending the duration of action. Normally, hydrophilic or a combination of hydrophilic and lipophilic polymers are used; however, using a pH sensitive polymer, such as eudragit, allows this system to have delayed release properties. The use of polymers as co-carriers in modification of mesoporous silica, also often functions as a connecting agent for targeting mediators in targeted delivery systems.
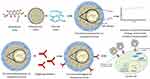 |
Figure 8 Functionalized polymer/ligand on mesoporous silica. Note: Data from these studies.97–99 |
Functionalized mesoporous silica ligand is the third design, and it is primarily suited for targeted delivery systems. Targeting mediators that are especially able to bind with transmembrane receptors that are overexpressed by cancer cells results in the targeted delivery system in this system. The efficiency of therapy is increased because to this process, which enables the medications created to have selective cell activity.
The Future Prospective
Three system designs have been developed for the modification of mesoporous silica for the treatment of lung cancer. The first is a simple design that only uses mesoporous silica as the main carrier and aims to improve the physicochemical issues of the active ingredients and their stability. The second is functionalized mesoporous silica, which has the primary goal of providing a changed delivery system to a specific delivery system. This review discovered that polymers and ligands are additional agents in the mesoporous silica formula. Based on this analysis, we draw the conclusion that mesoporous silica may one day prove to be an effective alteration for treating lung cancer.
One intriguing aspect to contemplate for future advancement is the presence of limitations and challenges. Challenges arise at various stages of the lifecycle of silica mesoporous nanoparticles. Achieving precise synthesis involves the manipulation of parameters such as temperature and pH, which in turn impact the formation of pore structures. Despite their inherent stability, exposure to extreme conditions can disturb their structural integrity. The compatibility with biological systems is contingent on factors like dimensions, charge, and composition, influencing their interactions with cells. Incorporation of therapeutic substances entails capillary condensation, and the subsequent release process is dictated by factors including pore size and the properties of the loaded cargo. The modification of their surface encounters obstacles due to factors like steric hindrance and the fragility of bonds. Scaling up the synthesis introduces variability, and the accurate characterization necessitates the use of specialized techniques with varying levels of precision. The prevention of particle aggregation involves the introduction of repulsive forces. Different applications present distinct challenges. The acquisition of regulatory approval demands comprehensive testing due to the potential for unfavorable interactions.
Another topic under consideration pertains to a significant obstacle in the treatment of lung cancer, which is drug resistance. Addressing this concern, the utilization of a targeted drug delivery system involving silica mesoporous materials emerges as a potential strategy for tackling the issue of treatment inefficacy linked to multiple drug resistance in clinical treatment.
Acknowledgments
We would like to thank the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia (KEMENDIKBUDRISTEK, Penelitian Fundamental) for supporting this work and Universitas Padjadjaran for APC.
Funding
This research was funded by the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia (KEMENDIKBUDRISTEK, Penelitian Fundamental) to Diah Lia Aulifa (no: 3018/UN6.3.1/PT.00/2023, Tanggal 20 Juni 2023) (CC-BY-NC).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tao MH. Epidemiology of lung cancer. Lung Cancer Imaging. 2019;2019:4.
2. Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Prim. 2021;7(1):3. doi:10.1038/s41572-020-00235-0
3. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Heal. 2019;85:1.
4. de Groot PM, Wu CC, Carter BW, Munden RF. The epidemiology of lung cancer. Transl Lung Cancer Res. 2018;7(3):220. doi:10.21037/tlcr.2018.05.06
5. Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J. 2016;48(3):889–902. doi:10.1183/13993003.00359-2016
6. Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–649. doi:10.1056/NEJMoa1916623
7. Lemjabbar-Alaoui H, Hassan OUI, Yang YW, Buchanan P. Lung cancer: biology and treatment options. Biochim Biophys Acta Rev Cancer. 2015;1856(2):189–210.
8. Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5(3):288. doi:10.21037/tlcr.2016.06.07
9. Rosell R, Bivona TG, Karachaliou N. Genetics and biomarkers in personalisation of lung cancer treatment. Lancet. 2013;382(9893):720–731. doi:10.1016/S0140-6736(13)61715-8
10. Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27(8):1345–1356. doi:10.1038/s41591-021-01450-2
11. Cryer AM, Thorley AJ. Nanotechnology in the diagnosis and treatment of lung cancer. Pharmacol Ther. 2019;198:189–205. doi:10.1016/j.pharmthera.2019.02.010
12. Hoy H, Lynch T, Beck M. Surgical treatment of lung cancer. Crit Care Nurs Clin. 2019;31(3):303–313. doi:10.1016/j.cnc.2019.05.002
13. Gigliobianco MR, Casadidio C, Censi R, Di Martino P. Nanocrystals of poorly soluble drugs: drug bioavailability and physicochemical stability. Pharmaceutics. 2018;10(3):134. doi:10.3390/pharmaceutics10030134
14. Censi R, Di Martino P. Polymorph impact on the bioavailability and stability of poorly soluble drugs. Molecules. 2015;20(10):18759–18776. doi:10.3390/molecules201018759
15. Vo CL, Park C, Lee BJ. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm. 2013;85(3):799–813. doi:10.1016/j.ejpb.2013.09.007
16. Ashford M. Bioavailability–physicochemical and dosage form factors. Aulton’s Pharm Des Manuf Med. 2013;314:1.
17. Dai XL, Chen JM, Lu TB. Pharmaceutical cocrystallization: an effective approach to modulate the physicochemical properties of solid-state drugs. CrystEngComm. 2018;20(36):5292–5316. doi:10.1039/C8CE00707A
18. Manzano M, Vallet‐Regí M. Mesoporous silica nanoparticles for drug delivery. Adv Funct Mater. 2020;30(2):1902634. doi:10.1002/adfm.201902634
19. Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system: a review. Int J Pharm Investig. 2015;5(3):124. doi:10.4103/2230-973X.160844
20. Wang Y, Zhao Q, Han N, et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanotechnol Biol Med. 2015;11(2):313–327. doi:10.1016/j.nano.2014.09.014
21. Vallet-Regí M, Colilla M, Izquierdo-Barba I, Manzano M. Mesoporous silica nanoparticles for drug delivery: current insights. Molecules. 2017;23(1):47. doi:10.3390/molecules23010047
22. Argyo C, Weiss V, Bräuchle C, Bein T. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem Mater. 2014;26(1):435–451. doi:10.1021/cm402592t
23. Maggini L, Cabrera I, Ruiz-Carretero A, Prasetyanto EA, Robinet E, De Cola L. Breakable mesoporous silica nanoparticles for targeted drug delivery. Nanoscale. 2016;8(13):7240–7247. doi:10.1039/C5NR09112H
24. Wen J, Yang K, Liu F, Li H, Xu Y, Sun S. Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems. Chem Soc Rev. 2017;46(19):6024–6045. doi:10.1039/C7CS00219J
25. Chen Y, Gu H, Zhang DSZ, Li F, Liu T, Xia W. Highly effective inhibition of lung cancer growth and metastasis by systemic delivery of siRNA via multimodal mesoporous silica-based nanocarrier. Biomaterials. 2014;35(38):10058–10069. doi:10.1016/j.biomaterials.2014.09.003
26. Cheng W, Liang C, Xu L, et al. TPGS‐functionalized polydopamine‐modified mesoporous silica as drug nanocarriers for enhanced lung cancer chemotherapy against multidrug resistance. Small. 2017;13(29):1700623. doi:10.1002/smll.201700623
27. Song Y, Zhou B, Du X, et al. Folic acid (FA)-conjugated mesoporous silica nanoparticles combined with MRP-1 siRNA improves the suppressive effects of myricetin on non-small cell lung cancer (NSCLC). Biomed Pharmacother. 2020;125:109561. doi:10.1016/j.biopha.2019.109561
28. Su W, Wei T, Lu M, et al. Treatment of metastatic lung cancer via inhalation administration of curcumin composite particles based on mesoporous silica. Eur J Pharm Sci. 2019;134:246–255. doi:10.1016/j.ejps.2019.04.025
29. Li C, Hu J, Li W, Song G, Shen J. Combined bortezomib-based chemotherapy and p53 gene therapy using hollow mesoporous silica nanospheres for p53 mutant non-small cell lung cancer treatment. Biomater Sci. 2017;5(1):77–88. doi:10.1039/C6BM00449K
30. Kresgea CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359(6397):710–712. doi:10.1038/359710a0
31. Beck JS, Vartuli JC, Roth WJ, et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc. 1992;114(27):10834–10843. doi:10.1021/ja00053a020
32. Inagaki S, Fukushima Y, Kuroda K. Synthesis of highly ordered mesoporous materials from a layered polysilicate. J Chem Soc Chem Commun. 1993;680–682. doi:10.1039/c39930000680
33. Zhao D, Feng J, Huo Q, et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science. 1998;279(5350):548–552. doi:10.1126/science.279.5350.548
34. Corma A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem Rev. 1997;97(6):2373–2420. doi:10.1021/cr960406n
35. Sayari A, Hamoudi S. Periodic mesoporous silica-based organic− inorganic nanocomposite materials. Chem Mater. 2001;13(10):3151–3168. doi:10.1021/cm011039l
36. Ying JY, Mehnert CP, Wong MS. Synthesis and applications of supramolecular‐templated mesoporous materials. Angew Chemie Int Ed. 1999;38(1‐2):56–77. doi:10.1002/(SICI)1521-3773(19990115)38:1/2<56::AID-ANIE56>3.0.CO;2-E
37. Liu XW, Li JW, Zhou L, Huang DS, Zhou YP. Mesoporous silica adsorbents synthesis, characterization, and their adsorption equilibrium properties for CO2, N2 and CH4. Chem Phys Lett. 2005;415:198–201. doi:10.1016/j.cplett.2005.09.009
38. Grün M, Kurganov AA, Schacht S, Schüth F, Unger KK. Comparison of an ordered mesoporous aluminosilicate, silica, alumina, titania and zirconia in normal-phase high-performance liquid chromatography. J Chromatogr A. 1996;740(1):1–9. doi:10.1016/0021-9673(96)00205-1
39. Raja R, Thomas JM. Catalyst design strategies for controlling reactions in microporous and mesoporous molecular-sieves. J Mol Catal a Chem. 2002;181(1–2):3–14. doi:10.1016/S1381-1169(01)00345-4
40. Polarz S, Kuschel A. Chemistry in confining reaction fields with special emphasis on nanoporous materials. Chem Eur J. 2008;14(32):9816–9829. doi:10.1002/chem.200800674
41. Wu CG, Bein T. Conducting polyaniline filaments in a mesoporous channel host. Science. 1994;264(5166):1757–1759. doi:10.1126/science.264.5166.1757
42. Llewellyn PL, Ciesla U, Decher H, Stadler R, Schüth F, Unger KK. MCM-41 and related materials as media for controlled polymerization processes. In: Studies in Surface Science and Catalysis. Vol. 84. Elsevier; 1994:2013–2020.
43. Cardin DJ, Constantine SP, Gilbert A, et al. Polymerization of alkynes in the channels of mesoporous materials containing Ni and Zn cations: almost complete filling of the voids. J Am Chem Soc. 2001;123(13):3141–3142. doi:10.1021/ja002921f
44. Lin VSY, Radu DR, Han MK, et al. Oxidative polymerization of 1, 4-diethynylbenzene into highly conjugated poly (phenylene butadiynylene) within the channels of surface-functionalized mesoporous silica and alumina materials. J Am Chem Soc. 2002;124(31):9040–9041. doi:10.1021/ja025925o
45. Feng X, Fryxell GE, Wang LQ, Kim AY, Liu J, Kemner KM. Functionalized monolayers on ordered mesoporous supports. Science. 1997;276(5314):923–926. doi:10.1126/science.276.5314.923
46. Mercier L, Pinnavaia TJ. Access in mesoporous materials: advantages of a uniform pore structure in the design of a heavy metal ion adsorbent for environmental remediation. Adv Mater. 1997;9(6):500–503. doi:10.1002/adma.19970090611
47. Diaz JF, Balkus KJ. Enzyme immobilization in MCM-41 molecular sieve. J Mol Catal B Enzym. 1996;2(2–3):115–126. doi:10.1016/S1381-1177(96)00017-3
48. Vallet-Regi M, Rámila A, Del Real RP, Pérez-Pariente JJC. A new property of MCM-41: drug delivery system. Chem Mater. 2001;13(2):308–311. doi:10.1021/cm0011559
49. Vallet-Regí M, Ruiz-González L, Izquierdo-Barba I, González-Calbet JM. Revisiting silica based ordered mesoporous materials: medical applications. J Mater Chem. 2006;16(1):26–31. doi:10.1039/B509744D
50. Bearzotti A, Bertolo JM, Innocenzi P, Falcaro P, Traversa E. Humidity sensors based on mesoporous silica thin films synthesised by block copolymers. J Eur Ceram Soc. 2004;24(6):1969–1972. doi:10.1016/S0955-2219(03)00521-1
51. Casasús R, Marcos MD, Martínez-Máñez R, et al. Toward the development of ionically controlled nanoscopic molecular gates. J Am Chem Soc. 2004;126(28):8612–8613. doi:10.1021/ja048095i
52. Yantasee W, Lin Y, Li X, Fryxell GE, Zemanian TS, Viswanathan VV. Nanoengineered electrochemical sensor based on mesoporous silica thin-film functionalized with thiol-terminated monolayer. Analyst. 2003;128(7):899–904. doi:10.1039/b303973k
53. Walcarius A, Bessière J. Electrochemistry with mesoporous silica: selective mercury (II) binding. Chem Mater. 1999;11(11):3009–3011. doi:10.1021/cm990410q
54. Hirano T, Yui T, Okazaki K, et al. Photo-induced electron migrations in the nano-cavities of mesoporous silica sensitized by a cationic porphyrin dye. J Nanosci Nanotechnol. 2009;9(1):495–500. doi:10.1166/jnn.2009.J007
55. Nguyen TQ, Wu J, Doan V, Schwartz BJ, Tolbert SH. Control of energy transfer in oriented conjugated polymer-mesoporous silica composites. Science. 2000;288(5466):652–656. doi:10.1126/science.288.5466.652
56. Slowing II, Slowing II, Vivero-escoto JL, Trewyn BG, Lin VS. Mesoporous silica nanoparticles: structural design and applications. J Mater Chem. 2010;20(37):7924. doi:10.1039/c0jm00554a
57. Qi K, Chen X, Liu Y, Xin JH, Mak CL, Daoud WA. Facile preparation of anatase/SiO 2 spherical nanocomposites and their application in self-cleaning textiles. J Mater Chem. 2007;17(33):3504–3508. doi:10.1039/b702887c
58. Schmidt HK, Geiter E, Mennig M, Krug H, Becker C, Winkler RP. The sol-gel process for nano-technologies: new nanocomposites with interesting optical and mechanical properties. J Sol-Gel Sci Technol. 1998;13:397–404. doi:10.1023/A:1008660909108
59. Niesz K, Yang P, Somorjai GA. Sol-gel synthesis of ordered mesoporous alumina. Chem Commun. 2005;1986–1987. doi:10.1039/b419249d
60. Hoffmann F, Cornelius M, Morell J, Fröba M. Silica‐based mesoporous organic–inorganic hybrid materials. Angew Chemie Int Ed. 2006;45(20):3216–3251.
61. Newalkar BL, Komarneni S, Katsuki H. Rapid synthesis of mesoporous SBA-15 molecular sieve by a microwave–hydrothermal process. Chem Commun. 2000;2000:2389–2390.
62. Chen Y, Chen H, Guo L, et al. Hollow/rattle-type mesoporous nanostructures by a structural difference-based selective etching strategy. ACS Nano. 2010;4(1):529–539. doi:10.1021/nn901398j
63. Barbey R, Lavanant L, Paripovic D, et al. Polymer brushes via surface-initiated controlled radical polymerization: synthesis, characterization, properties, and applications. Chem Rev. 2009;109(11):5437–5527. doi:10.1021/cr900045a
64. Nebhani L, Mishra S, Joshi T. Polymer functionalization of mesoporous silica nanoparticles using controlled radical polymerization techniques. Adv Microporous Mesoporous Mater. 2020. doi:10.5772/intechopen.92323
65. Sahoo S, Bordoloi A, Halligudi SB. Ordered mesoporous silica as supports in the heterogeneous asymmetric catalysis. Catal Surv from Asia. 2011;15:200–214. doi:10.1007/s10563-011-9122-z
66. Möller K, Bein T. Degradable drug carriers: vanishing mesoporous silica nanoparticles. Chem Mater. 2019;31(12):4364–4378. doi:10.1021/acs.chemmater.9b00221
67. Guillet-Nicolas R, Marcoux L, Kleitz F. Insights into pore surface modification of mesoporous polymer–silica composites: introduction of reactive amines. New J Chem. 2010;34(2):355–366. doi:10.1039/b9nj00478e
68. Tarn D, Ashley CE, Xue MIN, Carnes EC, Zink JI, Brinker CJ. Mesoporous silica nanoparticle nanocarriers: biofunctionality and biocompatibility. Acc Chem Res. 2013;46(3):792–801. doi:10.1021/ar3000986
69. Paris JL, Cabañas MV, Manzano M, Vallet-Regí M. Polymer-grafted mesoporous silica nanoparticles as ultrasound-responsive drug carriers. ACS Nano. 2015;9(11):11023–11033. doi:10.1021/acsnano.5b04378
70. Zheng Y, Wang L, Lu L, Wang Q, Benicewicz BC. pH and thermal dual-responsive nanoparticles for controlled drug delivery with high loading content. Acs Omega. 2017;2(7):3399–3405. doi:10.1021/acsomega.7b00367
71. Gao Y, Gao D, Shen J, Wang Q. A review of mesoporous silica nanoparticle delivery systems in chemo-based combination cancer therapies. Front Chem. 2020;8:598722. doi:10.3389/fchem.2020.598722
72. Dilnawaz F, Sahoo SK. Augmented anticancer efficacy by si-RNA complexed drug-loaded mesoporous silica nanoparticles in lung cancer therapy. ACS Appl Nano Mater. 2018;1(2):730–740. doi:10.1021/acsanm.7b00196
73. Chen F, Zhang F, Shao D, et al. Bioreducible and traceable Ru(III) prodrug-loaded mesoporous silica nanoparticles for sequentially targeted nonsmall cell lung cancer chemotherapy. Appl Mater Today. 2020;19:100558. doi:10.1016/j.apmt.2020.100558
74. Zhang M, Jiang L. Doxorubicin hydrochloride-loaded mesoporous silica nanoparticles inhibit non-small cell lung cancer metastasis by suppressing VEGF-mediated angiogenesis. J Biomed Nanotechnol. 2016;12(11):1975–1986. doi:10.1166/jbn.2016.2290
75. Wang T, Liu Y, Wu C, Yu L, Yu Z, Ye H. Effect of paclitaxel-mesoporous silica nanoparticles with a core-shell structure on the human lung cancer cell line A549. Nanoscale Res Lett. 2017;12(1):1–8. doi:10.1186/s11671-017-1826-1
76. D’Anna C, Di Sano C, Di Vincenzo S, et al. Mesoporous silica particles functionalized with newly extracted fish Oil (Omeg@Silica) Reducing IL-8 counteract cell migration in NSCLC cell lines. Pharmaceutics. 2022;14(10):2079. doi:10.3390/pharmaceutics14102079
77. Shen J, Song G, An M, et al. The use of hollow mesoporous silica nanospheres to encapsulate bortezomib and improve efficacy for non-small cell lung cancer therapy. Biomaterials. 2014;35(1):316–326. doi:10.1016/j.biomaterials.2013.09.098
78. Sun W, Fang N, Trewyn BG, et al. Endocytosis of a single mesoporous silica nanoparticle into a human lung cancer cell observed by differential interference contrast microscopy. Anal Bioanal Chem. 2008;391(6):2119–2125. doi:10.1007/s00216-008-2162-1
79. Lee H, Kim S, Choi BH, et al. Hyperthermia improves therapeutic efficacy of doxorubicin carried by mesoporous silica nanocontainers in human lung cancer cells. Int J Hyperth. 2011;27(7):698–707. doi:10.3109/02656736.2011.608217
80. Di Pasqua AJ, Miller ML, Lu X, Peng L, Jay M. Tumor accumulation of neutron-activatable holmium-containing mesoporous silica nanoparticles in an orthotopic non-small cell lung cancer mouse model. Inorganica Chim Acta. 2012;393:334–336. doi:10.1016/j.ica.2012.06.016
81. Wang Y, Huang HY, Yang L, Zhang Z, Ji H. Cetuximab-modified mesoporous silica nano-medicine specifically targets EGFR-mutant lung cancer and overcomes drug resistance. Sci Rep. 2016;6(May):1–10. doi:10.1038/srep25468
82. Ren L, Ma Z, Li Q, et al. Identifying a membrane-type 2 matrix metalloproteinase-targeting peptide for human lung cancer detection and targeting chemotherapy with functionalized mesoporous silica. ACS Appl Bio Mater. 2019;2(1):397–405. doi:10.1021/acsabm.8b00633
83. Zhang F, Liu W, Long Y, Peng H. Targeted delivery of metformin against lung cancer cells via hyaluronan-modified mesoporous silica nanoparticles. Appl Biochem Biotechnol. 2023;195:4067–4083. doi:10.1007/s12010-022-04289-6
84. Sundarraj S, Thangam R, Sujitha MV, Vimala K, Kannan S. Ligand-conjugated mesoporous silica nanorattles based on enzyme targeted prodrug delivery system for effective lung cancer therapy. Toxicol Appl Pharmacol. 2014;275(3):232–243. doi:10.1016/j.taap.2014.01.012
85. AbouAitah K, Lojkowski W. Delivery of natural agents by means of mesoporous silica nanospheres as a promising anticancer strategy. Pharmaceutics. 2021;13(2):143. doi:10.3390/pharmaceutics13020143
86. Zhou S, Huo D, Hou C, et al. Mesoporous silica-coated quantum dots functionalized with folic acid for lung cancer cell imaging. Anal Methods. 2015;7(22):9649–9654. doi:10.1039/c5ay01760b
87. Perego C, Millini R. Porous materials in catalysis: challenges for mesoporous materials. Chem Soc Rev. 2013;42(9):3956–3976. doi:10.1039/C2CS35244C
88. Alvarez-Berríos MP, Sosa-Cintron N, Rodriguez-Lugo M, Juneja R, Vivero-Escoto JL. Hybrid nanomaterials based on iron oxide nanoparticles and mesoporous silica nanoparticles: overcoming challenges in current cancer treatments. J Chem. 2016;2016:1–15. doi:10.1155/2016/2672740
89. Rosenholm JM, Sahlgren C, Lindén M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles–opportunities & challenges. Nanoscale. 2010;2(10):1870–1883. doi:10.1039/c0nr00156b
90. Nigro A, Pellegrino M, Greco M, et al. Dealing with skin and blood-brain barriers: the unconventional challenges of mesoporous silica nanoparticles. Pharmaceutics. 2018;10(4):250. doi:10.3390/pharmaceutics10040250
91. Ravinayagam V, Jermy BR. Nanomaterials and their negative effects on human health. Appl Nanomater Hum Heal. 2020;2020:249–273.
92. Hosseinpour S, Walsh LJ, Xu C. Modulating osteoimmune responses by mesoporous silica nanoparticles. ACS Biomater Sci Eng. 2021;8(10):4110–4122. doi:10.1021/acsbiomaterials.1c00899
93. Heidegger S, Gößl D, Schmidt A, et al. Immune response to functionalized mesoporous silica nanoparticles for targeted drug delivery. Nanoscale. 2016;8(2):938–948. doi:10.1039/C5NR06122A
94. Karagianni A, Kachrimanis K, Nikolakakis I. Co-amorphous solid dispersions for solubility and absorption improvement of drugs: composition, preparation, characterization and formulations for oral delivery. Pharmaceutics. 2018;10(3):98. doi:10.3390/pharmaceutics10030098
95. Al-Obaidi H, Granger A, Hibbard T, Opesanwo S. Pulmonary drug delivery of antimicrobials and anticancer drugs using solid dispersions. Pharmaceutics. 2021;13(7):1056. doi:10.3390/pharmaceutics13071056
96. Okawa S, Sumimoto Y, Masuda K, Ogawara K, Maruyama M, Higaki K. Improvement of lipid solubility and oral bioavailability of a poorly water-and poorly lipid-soluble drug, rebamipide, by utilizing its counter ion and SNEDDS preparation. Eur J Pharm Sci. 2021;159:105721. doi:10.1016/j.ejps.2021.105721
97. Chen C, Yao W, Sun W, et al. A self-targeting and controllable drug delivery system constituting mesoporous silica nanoparticles fabricated with a multi-stimuli responsive chitosan-based thin film layer. Int J Biol Macromol. 2019;122:1090–1099. doi:10.1016/j.ijbiomac.2018.09.058
98. Nik AB, Zare H, Razavi S, et al. Smart drug delivery: capping strategies for mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2020;299:110115. doi:10.1016/j.micromeso.2020.110115
99. Yang Y, Zhao W, Tan W, et al. An efficient cell-targeting drug delivery system based on aptamer-modified mesoporous silica nanoparticles. Nanoscale Res Lett. 2019;14:1–10. doi:10.1186/s11671-019-3208-3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
