Back to Journals » Journal of Asthma and Allergy » Volume 16
How Does Mild Asthma Differ Phenotypically from Difficult-to-Treat Asthma?
Authors Naftel J , Mistry H, Mitchell FA, Belson J, Kyyaly MA , Barber C, Haitchi HM , Dennison P, Djukanovic R, Seumois G, Vijayanand P, Arshad SH, Kurukulaaratchy RJ
Received 8 August 2023
Accepted for publication 8 December 2023
Published 19 December 2023 Volume 2023:16 Pages 1333—1345
DOI https://doi.org/10.2147/JAA.S430183
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Jennifer Naftel,1,* Heena Mistry,1– 5,* Frances Ann Mitchell,4 Jane Belson,4 Mohammed Aref Kyyaly,2,4 Clair Barber,1,2 Hans Michael Haitchi,1– 3,6 Paddy Dennison,1– 3 Ratko Djukanovic,1– 3,6 Gregory Seumois,5 Pandurangan Vijayanand,2,5 Syed Hasan Arshad,1– 4,6 Ramesh J Kurukulaaratchy1– 4
1National Institute for Health Research (NIHR) Southampton Biomedical Research Centre at University Hospital Southampton NHS Foundation Trust, Southampton, UK; 2Clinical and Experimental Sciences Department, Faculty of Medicine, University of Southampton, Southampton, UK; 3Asthma, Allergy and Clinical Immunology Department, University Hospital Southampton NHS Foundation Trust, Southampton, UK; 4The David Hide Asthma & Allergy Research Centre, St Mary’s Hospital, Newport, Isle of Wight, UK; 5Vijayanand Laboratory, La Jolla Institute of Immunology, San Diego, CA, 92037, USA; 6Institute for Life Sciences, University of Southampton, Southampton, UK
*These authors contributed equally to this work
Correspondence: Ramesh J Kurukulaaratchy, Respiratory Medicine & Allergy, Clinical Experimental Sciences, Mailpoint 810, F-Level, South Academic Block, Southampton General Hospital, Tremona Road, Southampton, Hampshire, SO16 6YD, United Kingdom, Tel +442381 208790, Email [email protected]
Background: Despite most of the asthma population having mild disease, the mild asthma phenotype is poorly understood. Here, we aim to address this gap in knowledge by extensively characterising the mild asthma phenotype and comparing this with difficult-to-treat asthma.
Methods: We assessed two real-world adult cohorts from the South of England using an identical methodology: the Wessex AsThma CoHort of difficult asthma (WATCH) (n=498) and a mild asthma cohort from the comparator arm of the Epigenetics Of Severe Asthma (EOSA) study (n=67). Data acquisition included detailed clinical, health and disease-related questionnaires, anthropometry, allergy and lung function testing, plus biological samples (blood and sputum) in a subset.
Results: Mild asthma is predominantly early-onset and is associated with type-2 (T2) inflammation (atopy, raised fractional exhaled nitric oxide (FeNO), blood/sputum eosinophilia) plus preserved lung function. A high prevalence of comorbidities and multimorbidity was observed in mild asthma, particularly depression (58.2%) and anxiety (56.7%). In comparison to difficult asthma, mild disease showed similar female predominance (> 60%), T2-high inflammation and atopy prevalence, but lower peripheral blood/airway neutrophil counts and preserved lung function. Mild asthma was also associated with a greater prevalence of current smokers (20.9%). A multi-component T2-high inflammatory measure was comparable between the cohorts; T2-high status 88.1% in mild asthma and 93.5% in difficult asthma.
Conclusion: Phenotypic characterisation of mild asthma identified early-onset disease with high prevalence of current smokers, T2-high inflammation and significant multimorbidity burden. Early comprehensive assessment of mild asthma patients could help prevent potential later progression to more complex severe disease.
Keywords: mild asthma, difficult asthma, multimorbidity, type 2 inflammation
Introduction
Asthma is a common heterogeneous inflammatory airway condition that places significant burden at individual, societal and health economic levels.1
Difficult asthma is defined as asthma that remains poorly controlled despite maximal inhaled therapies.2 This is often due to the complex interplay of comorbidities, poor inhaler technique and medication adherence.3,4 Approximately 17% of individuals with asthma have difficult asthma with only 3.7% having severe asthma, where disease control remains poor despite optimising other contributory factors.2 Though affecting a minority, difficult/severe asthma has attracted significantly more research focus than milder disease.
Much effort has been placed on characterising difficult asthma and identifying distinct clinical, physiological and pathological traits.5–9 This improved understanding of difficult/severe asthma has been pivotal in driving more personalised medicine approaches.10 Recent work has indicated that most difficult asthma is associated with type 2 (T2)-predominant inflammation that may be amenable to higher level T2-targeting biologics.11,12 Such treatments are becoming commonplace for difficult/severe asthma with good efficacy in most patients.12 In parallel, there is growing recognition that difficult asthma is associated with numerous comorbidities within a complex multimorbidity model of ill-health.13
Conversely, mild asthma remains poorly defined but is typically described as asthma that is well controlled after several months of treatment with low to medium dose inhaled corticosteroid (ICS)/long-acting beta-agonist (LABA) or ICS alone.2 Serra-Batlles et al found that the mean annual drugs cost per patient with mild asthma in Northern Spain was US$253, amounting to a significant economic burden given that 50–75% of the asthma population have mild disease.2,14 Similarly, the United Kingdom (UK) National Review of Asthma Deaths (NRAD) found 58% of asthma deaths were in those with mild or moderate disease.15 Such findings emphasise the importance of better understanding mild asthma.
Key questions that merit further attention are as follows: when compared to difficult/severe asthma, does mild asthma a) show phenotypic differences, b) have similar degrees of T2-high inflammation, and c) exhibit different prevalence of comorbidities and multimorbidity? We sought to answer these questions by assessing two real-world adult cohorts from the same geographical population in the South of England, UK; the Wessex AsThma CoHort of difficult asthma (WATCH) and a mild asthma cohort from the comparator arm of the Epigenetics of Severe Asthma (EOSA) study.16
Methods
Data collection methodology is identical for the difficult and mild asthma cohorts as briefly outlined below and published elsewhere.16 Data acquisition included detailed clinical, health and disease-related questionnaires, anthropometry, allergy and lung function testing, plus a collection of biological samples (blood and sputum in a subset). A modified version of the 2021 Global Initiative for Asthma (GINA) T2-high classification algorithm was used to define T2-high status as follows: 1) fractional exhaled nitric oxide (FeNO)≥20 parts per billion (ppb) and/or 2) blood eosinophil count (BEC)≥0.15×109/L (to 1 decimal place as reported by University Hospital Southampton NHS Foundation Trust (UHSFT) pathology system), and/or 3) maintenance oral corticosteroid (mOCS) use, and/or 4) clinically allergen-driven disease (ie ≥1 positive aeroallergen on skin prick testing (SPT) and reported allergen trigger).2 Of note, the sputum parameter (sputum eosinophilia ≥2%) from the GINA guideline was excluded from the T2-high definition due to limited sputum sample size for each cohort. Comorbidities are defined by conventional clinical diagnostic criteria (Table S1). Multimorbidity counts were calculated for participants with complete comorbidity data (difficult asthma, n=378; mild asthma, n=65). Participants were awarded 1-point for each comorbidity out of a total of 14 with equal weighting.
Sputum induction was performed in a subset of difficult asthma participants (n=139) at the UHSFT NIHR Clinical Research Facility, and mild asthma participants (n=7) at David Hide Asthma & Allergy Centre (DHAAC). Detailed sputum induction methodology is published elsewhere.16 Differential cell counts were obtained from sputum samples.
Difficult Asthma Cohort
The WATCH study is an ongoing “real-world” prospective observational study of patients under the UHSFT Difficult Asthma clinical service.16 Patients attending the Adult or Transitional Regional Asthma Clinic at UHSFT or satellite outreach clinics on the Isle of Wight and were managed with ‘high-dose therapies’ and/or ‘continuous or frequent use of oral steroids’, according to the British Thoracic Society (BTS) Adult Asthma Management guidelines 2016 (consistent with GINA asthma management steps 4 or 5), were eligible for inclusion in the WATCH study.2,16,17 Patients were only excluded if they were unable to provide informed consent or were not receiving treatment in keeping with these pre-determined thresholds.16 Data is recorded at enrolment as previously described with prospective longitudinal data captured in parallel to clinical follow-up appointments. Details of the study protocol are published elsewhere.16 Given their asthma severity, post-bronchodilator lung function tests were performed to prevent participants withholding regular inhaled therapy. Ethical approval was obtained from the West Midlands – Solihull Research Ethics Committee (REC reference: 14/WM/1226). Here, we present data from the first 498 WATCH participants.
Mild Asthma Cohort
The EOSA study used identical data collection and assessments to the WATCH study. It is a cross-sectional observational study comparing clinical and endotypic features of participants with mild asthma to a subset with difficult/severe asthma from WATCH. The comparator mild asthma cohort from EOSA was established from the Isle of Wight Whole Population Birth Cohort (IOWBC, n=22),18 and from DHAAC clinics or the Isle of Wight (IOW) community (n=45), ensuring participants were broadly from the same geographical area as WATCH.18 All children born on IOW between January 1989 and February 1990 were eligible for inclusion in the IOWBC.18 Participants were eligible for inclusion in the EOSA study if on GINA asthma management step 1 or 2 and were excluded if unable to provide informed consent.2 Sixty-seven participants with mild asthma were enrolled. Pre-bronchodilator lung function tests were performed in this cohort. The EOSA study received ethical approval from the South-Central Hampshire B – Southampton Research Ethics Committee (REC reference: 18/SC/0105).
Statistical Analysis
Statistical analysis was performed using SPSS 26 (NY, USA) and figures were generated in GraphPad Prism 9.1.4 (La Jolla, California, USA). For categorical variables, statistical significance was assessed using Chi-square test and Fisher’s exact test for categorical variables where low cell counts were expected. Categorical variables are presented as frequencies and percentages. For continuous variables, the Shapiro–Wilk test of normality was used to determine if data sets are normally distributed. Mann Whitney U-tests were applied for non-parametric (skewed) continuous data with results displayed as median and interquartile ranges and unpaired t-test for parametric (normally distributed) continuous data with results displayed as mean and standard deviations. P-value of <0.05 was regarded as statistically significant.
Results
Demographic Characteristics
There was no significant difference in ethnicity between the mild and difficult asthma cohorts, with the majority of participants being Caucasian (98.6% vs 92.6%, P = 0.0711, Table 1). Both showed female predominance of >60% (62.7% vs 65.3%, P>0.9999). Mean age at study enrolment was significantly lower (by 12.5-years) in the mild asthma cohort compared to the difficult asthma cohort (38.3-years vs 50.8-years, P<0.0001). One-third of the mild asthma cohort were recruited from the IOWBC and were of similar age (median 28.8-years). Therefore, the mild asthma cohort was stratified by recruitment pool (ie IOWBC vs IOW clinic/community; Table S2) to determine if this affected the nature of mild asthma. Compared to mild asthma participants from the IOW clinic/community, the IOWBC participants were significantly younger (28.8-years vs 43.0-years, P<0.001) and had earlier asthma-onset (6.8-years vs 23.5-years, P<0.001), but did not differ in asthma duration. Additionally, IOWBC participants had significantly lower blood neutrophil counts (3.4 × 109/L vs 4.1 × 109/L, P = 0.047) and pre-bronchodilator (BD) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio (98.2% vs 100.7%, P = 0.021) and lower prevalence of gastro-oesophageal reflux disease (GORD) (36.4% vs 64.4%, P = 0.038) than IOW clinic/community participants (Table S2).
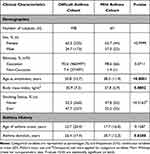 |
Table 1 Comparison of Clinical Characteristics Between Difficult and Mild Asthma Cohorts |
Body mass index (BMI) was significantly lower in mild asthma participants, within the overweight category, whilst difficult asthma participants were obese (27.8 kg/m2 vs 30.9 kg/m2, P = 0.0002, Table 1).
Mild asthma participants had significantly lower proportions of never smokers (47.8% vs 52.3%) and ex-smokers (31.3% vs 42.1%) but notably higher current smokers compared to the difficult asthma cohort (20.9% vs 5.6%, P = 0.0001; Table 1). There was no significant difference in the number of ever (ex-combined with current smokers) and never smokers.
Asthma Morbidity, Medication and Physiological Characteristics
Compared to difficult asthma, mild asthma participants had significantly lower asthma morbidity at study enrolment including health-care utilisation, acute oral corticosteroid (OCS) courses (0.1 vs 3.5, P<0.0001) and lost work-days (0.56 days vs 23.4 days, P<0.0001) in the past 12-months (Table 2). Consistent with the treatment-based definition for their mild status, mean ICS dose was significantly lower (447.4μg BDP equiv. vs 2588.6μg BDP equiv., P<0.0001) and they had no need for mOCS or T2-targeted biologics compared to the difficult asthma cohort where 30.0% were on mOCS and 17.1% were on an asthma biologic at enrolment (Table S3). Furthermore, lung function was preserved in the mild asthma group (Figure 1 and Table S4). Conversely, difficult asthma participants had significantly lower post-BD FEV1%predicted (%pred., 91.6% vs 91.5%, P = 0.974), FVC %pred. (98.2% vs 100.7%, P = 0.457), FEV1/FVC ratio % (78.4% vs 73.5%, P = 0.021) and forced expiratory flow between 25% and 75% exhalation (FEF25-75%) %pred. (79.8% vs 77.3%, P = 0.436), consistent with moderate airflow limitation (Figure 1 and Table S4).
 |
Table 2 Comparison of Asthma Morbidity Between Difficult and Mild Asthma Cohorts |
 |
Figure 1 Lung function characteristics between the difficult and mild asthma cohorts. Post-BD spirometry values shown for difficult asthma and pre-BD spirometry values shown for mild asthma. (A) Clinic FEV1% predicted., (B) Clinic FVC % predicted, (C) Clinic FEV1/FVC ratio %, (D) Clinic FEF25-75% % predicted. Data presented as median ± interquartile range. ****P <0.0001. A full breakdown of the results and statistics is available in Table S4. Abbreviations: FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEF 25–75%, forced expiratory flow between 25% and 75% exhalation. |
A subgroup analysis of the difficult asthma cohort stratified by lung function was performed (Table S5) since, as indicated in Figure 1, a proportion of difficult asthma patients had relatively preserved lung function. Of 338 difficult asthma participants with available spirometry, 56.8% had a post-BD FEV1 <80%pred. and showed higher male frequency (45.3% vs 22.6% P<0.0001), older age at enrolment (54.8 years vs 47.8 years, P<0.0001), longer asthma duration (30.4 years vs 22.3 years, P = 0.0003), fewer days lost from work (20.5 days vs 30.1 days, P = 0.0061), greater mOCS use (36.4% vs 21.8%, P = 0.0065), higher blood and sputum neutrophils (6.4 × 109/L vs 6.0 × 109/L, P = 0.0149 and 44.0% vs 34.1%, P = 0.0427, respectively) and A. fumigatus prevalence of structural disease (non-CF bronchiectasis 19.4% vs 6.3%, P = 0.0006 and COPD 17.8% vs 4.2%, P = 0.0001).
T2 Biomarkers and Inflammometry Characteristics
Blood neutrophil counts (3.85x109/L vs 6.27x109/L, P<0.0001) and % sputum neutrophils (24.1% vs 43.1%, P = 0.0450) were significantly lower in mild asthma participants (Table 3). Aspergillus fumigatus (A. fumigatus) -specific immunoglobulin E (IgE) levels were also significantly lower in the mild asthma cohort (0.18 IU/L vs 1.46 IU/L, P = 0.0041) consistent with low A. fumigatus sensitisation. Furthermore, mild asthma participants had significantly higher FeNO levels (35.0 ppb vs 30.6 ppb, P = 0.0053). Other T2 biomarkers did not differ including BECs (0.22 x109/L vs 0.26 x109/L, P = 0.5185), total IgE levels (267.1 IU/L vs 298.2 IU/L, P = 0.5475) and % sputum eosinophils (6.0% vs 8.5%, P = 0.7636). GINA T2-high status did not significantly differ between cohorts (88.1% vs 93.5%, P = 0.1238); both showed overwhelming T2-high status. Lastly, no significant differences were seen in atopic status (ie number of positive SPTs and subjects with ≥1 positive SPT, 73.8% vs 68.3%, P = 0.4684). When comparing atopy based on allergy skin prick tests, the mild asthma cohort showed greater Cladosporium herbarum (70.8% vs 12.3%, P<0.0001) and Dermatophagoides farinae (37.0% vs 56.9%, P = 0.0036) sensitisation (Table S6). As expected, the difficult asthma cohort showed greater A. fumigatus sensitisation (4.6% vs 15.8%, P = 0.0183)
 |
Table 3 Comparison of T2 Biomarkers and Inflammometry Between Difficult and Mild Asthma Cohorts |
Comorbidity and Health and Disease-Related Questionnaire Characteristics
Several comorbidities were common to both cohorts (mild vs difficult asthma) including GORD (55.2 vs 65.1, P = 0.0068), dysfunctional breathing (31.3 vs 48.6, P = 0.6024), inducible laryngeal obstruction (6.0 vs 14.6, P = 0.0554), and sulphite sensitivity (7.5 vs 7.8, P>0.9999, Figure 2 and Table S7). Difficult asthma participants showed significantly greater prevalence of obesity (31.3 vs 48.6, P = 0.0089), nasal polyposis (9.1 vs 23.4, P = 0.0064) and salicylate sensitivity (7.5 vs 25.0, P = 0.0009). Structural lung diseases including allergic bronchopulmonary aspergillosis/severe asthma with fungal sensitisation (ABPA/SAFS), non-cystic fibrosis bronchiectasis and chronic obstructive pulmonary disease (COPD) were present in the difficult asthma cohort but absent in the mild asthma cohort (Table S7). In contrast, mild asthma participants had a significantly higher prevalence of rhinitis (83.6% vs 67.6%, P = 0.0068) and eczema (49.3% vs 25.8%, P = 0.0001). Over 50% of the mild asthma cohort had depression and anxiety, which was significantly greater than that observed in difficult asthma participants (36.5% and 32.7% respectively) (Figure 2 and Table S7). Both cohorts had a median multimorbidity count of 4.0 (Table S7).
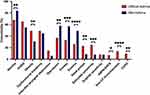 |
Figure 2 Comparison of physician and/or clinically diagnosed comorbidity characteristics between the difficult and mild asthma cohorts. Comorbidities are represented as percentages (%). Fisher’s exact test was applied for categorical variables. *P <0.05, **P <0.01, ***P <0.001, ****P <0.0001. A full breakdown of the results and statistics is available in Table S7. Abbreviations: GORD, gastro-oesophageal reflux disease; ABPA/SAFS, allergic bronchopulmonary aspergillosis/severe asthma with fungal sensitisation; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease. |
Several differences were observed in health and disease-related questionnaire scores (Table 4). Mean Asthma Control Questionnaire-6 (ACQ-6) score was significantly lower in mild asthma (0.72 vs 2.50, P<0.0001), whereas >76% of difficult asthma participants exhibited poor asthma control (defined by an ACQ-6 score ≥1.5, 16.7% vs 76.9%, P<0.0001). Health-related quality of life (HRQoL) outcome measures (ie EuroQoL5D-5L visual analogue scale (EQ5D-5L VAS) and St George’s Respiratory Questionnaire (SGRQ) total score) showed significantly better HRQoL (ie higher EQ5D-5L VAS, 84.2% vs 63.1%, P<0.0001 and lower SGRQ scores, 17.7 vs 50.7, P<0.0001) in mild asthma participants compared to the difficult asthma cohort. In addition, significantly lower scores for the Sino-Nasal Outcome Test-22 (SNOT22, >7 suggests prevalence of rhinosinusitis, 21.3 vs 35.1 P<0.0001), Hull Cough Hypersensitivity (>13 suggests GORD-related cough, 11.6 vs 26.3, P<0.0001) and Nijmegen questionnaires (>23 suggests dysfunctional breathing, 15.1 vs 22.1, P<0.0001), and Hospital Anxiety and Depression Scale Depression component (HADS-D, ≥11 suggests depression, 2.7 vs 5.4, P<0.0001) were observed in mild asthma participants compared to the difficult asthma cohort (Table 4). Of note, difficult asthma participants had clinically significant SNOT22, Hull Cough Hypersensitivity and Nijmegen questionnaires scores that were all above the pre-defined cut-off. No significant difference was observed in the HADS-Anxiety component (HADS-A) score (≥11 suggest anxiety), contrary to physician diagnosed anxiety, which was more prevalent in mild asthma (Figure 2 and Table S7).
 |
Table 4 Comparison of Asthma and Comorbidity-Related Questionnaire Characteristics Between Difficult and Mild Asthma Cohorts |
Discussion
We undertook one of the most comprehensive assessments of the phenotypic nature of mild asthma and compared this with more difficult disease. The mild asthma phenotype was predominantly female, atopic, and associated with early-onset disease, T2-high inflammation, raised FeNO and preserved lung function. Most strikingly, some comorbidities, but not multimorbidity, were significantly higher in mild asthma, particularly depression and anxiety. When compared to difficult asthma, mild disease showed similar female predominance, atopy and T2-high status, but better lung function, higher FeNO level, lower peripheral blood/airway neutrophil counts and higher prevalence of current smoking (Figure 3).
NRAD found that 58% of those who died from asthma were being treated for either mild or moderate disease.15 In a large observational study, 8% of mild asthma participants developed severe asthma over a 10-year period.19 Thus, improving our understanding of the mild asthma phenotype is crucial to risk stratify the asthma population to prevent future disease progression.
Baseline demographics in our mild asthma cohort were consistent with existing knowledge showing female predominance, overweight BMI and an early age of disease-onset.5–9 Asthma has consistently been shown to be a female-predominant disease in adulthood but the literature varies for early-onset asthma. The Severe Asthma Research Program (SARP) III cohort found male predominance in childhood-onset asthma regardless of severity.6 Female asthma predominance with increasing age is thought to be down to differential effects of male and female sex hormones during reproductive years.20 Additionally, the prevalence of obesity in asthma populations is known to increase with age and is associated with more severe disease.21
Our study found a significantly higher proportion of current smokers in the mild asthma cohort (20.9%) compared to the difficult asthma cohort (5.6%). Of note, percentages of ever-smokers were not significantly different between asthma cohorts. While we have no data on smoking duration, we might assume that both cohorts started smoking at a similar age, usually adolescence. Eisner et al found that in ever-smokers with asthma, the median time to smoking cessation was 17-years perhaps explaining the lower number of current smokers and higher number of former smokers in our difficult asthma cohort who had an older age at study enrolment.22 Alternatively, this may reflect increased motivation to stop smoking in the difficult asthma group given their greater asthma morbidity. Smoking in asthma has been associated with accelerated lung function decline and greater fixed airflow obstruction.23–25 One study reported a 10.1% decline in FEV1 over 10-years in non-smokers with asthma versus a 17.8% decline in smokers with asthma, suggesting a synergistic effect.24 Smoking in people with asthma has also been shown to increase neutrophil counts and lower sputum eosinophils, suggesting a shift in the inflammatory phenotype, which may have implications on clinical disease manifestations.25 Smoking can also reduce efficacy of ICS in mild asthma, meaning higher doses are needed to achieve asthma control.26 Chaudhuri et al showed significant improvements in FEV1 and asthma control as early as 1-week post-smoking cessation.27 This suggests a reversible component to the harmful effects of cigarette smoking and a potentially modifiable risk factor for disease progression. Focusing efforts on smoking cessation within primary care may therefore prevent future morbidity in mild asthma.
Our study demonstrated significant comorbidity in mild asthma. Comparable multimorbidity burden was observed in the two cohorts. Some comorbidities were similarly prevalent in mild and difficult asthma; GORD, dysfunctional breathing and inducible laryngeal obstruction. However, rhinitis, eczema, depression and anxiety were more prevalent in mild asthma. Difficult asthma was associated with greater prevalence of obesity,5,6,21 COPD,7,21 nasal polyps (7.23), salicylate sensitivity (23.30) and ABPA/SAFS.28 We previously demonstrated that A. fumigatus sensitisation is associated with more severe airways disease, greater morbidity, impaired lung function and structural airway damage.28 Early screening for A. fumigatus sensitisation should also be incorporated into the assessment of mild asthma.
One striking feature was that >50% of our mild asthma cohort suffered from psychological comorbidities, namely depression and anxiety, which has not been widely reported before with most prior studies showing significant psychological burden in patients with difficult/severe asthma.29–31 Rimington et al looked at the relationship between anxiety and depression and asthma morbidity in patients in a primary care setting.32 Just over 70% of their cohort were on BTS step 1 or 2 for their asthma management suggestive of milder disease.32 They demonstrated a weak correlation between lung function decline and asthma symptom burden but much stronger correlations with HADS-A and HADS-D scores suggesting the assessment of a patient’s asthma control in primary care is complex and under-acknowledged psychological morbidity may lead to increased asthma symptom reporting.32
Whilst the prevalence of depression was higher in the mild asthma cohort, depression severity (as measured by HADS-D scores) was significantly higher in the difficult asthma cohort. Fong et al suggested a severity-graded relationship between HADS-A and HADS-D and difficult asthma outcomes.31 They reported association of anxiety and/or depression in difficult asthma with poorer asthma control, more lost work-days, higher Nijmegen scores, and greater FeNO.31 Therefore, a worsening psychological state may either drive asthma severity or be a consequence of high disease burden feeding into the complexities of asthma multimorbidity. Earlier psychological interventions in primary care may minimise the multimorbidity burden in mild asthma and potentially interrupt pathways to more complex disease.
Comorbidity in difficult asthma is strongly associated with poorer asthma control, greater symptom burden, frequent exacerbations, mOCS dependency and non-adherence to asthma treatment.29–31,33,34 We found that severity of comorbidities based on questionnaire scores was lower and HRQoL was better in mild asthma compared to difficult disease. To et al found 6% of hospitalisations were related to asthma and asthma comorbidity in their population-based cohort study of 12 million residents in Ontario, Canada.33 Optimising comorbidity in asthma management is well acknowledged in severe asthma services. It has recently been proposed that we shift our focus to the concept of complex asthma as a multimorbidity “Difficult Breathing Syndrome” rather than a severe asthma-centric state, recognising the constellation of conditions with multiple “treatable traits” contributing to difficult asthma.34 Whether such notions are relevant to mild asthma too is worth consideration.
The importance of distinguishing T2-high from T2-low asthma is well established with a growing focus on use of T2-targeting biologics in difficult/severe asthma. Definitions of T2-high status vary. For example, in the ALLIANCE cohort, T2-high inflammation was defined by BEC ≥360 cells/µL and serum IgE ≥0.7 kU/L derived from blood counts above the 90th percentile in healthy participants.35 Alternatively, in the UK severe asthma registry, biomarker cut-offs of FeNO >25 ppb BEC >150 cells/µL were used, based on Phase 3 trials showing little efficacy of biologics below these thresholds.36 They reported greater T2-high predominance in their severe asthma registry (mean BECs 0.3 × 109/L, FeNO 39ppb, and IgE 181 IU/mL).36 Previous work from our group highlighted the importance of longitudinal BEC monitoring and unmasked a high proportion of T2-high severe asthma patients.37
Atopy prevalence and coexisting allergic rhinitis and eczema observed in our mild asthma cohort has been described elsewhere.5–9 However, the T2-high inflammatory phenotype is less well described in mild asthma. Previous studies in mild asthma suggested a T2-high phenotype prevalence of 50%.38 We found comparable T2-high biomarker signals between mild and difficult asthma cohorts in relation to BECs and total IgE levels, however FeNO levels were significantly higher in mild asthma participants. These results may be confounded by higher use of ICS and mOCS in the difficult asthma cohort (2588.6μg BDP equiv. vs 447.4μg BDP equiv. in mild asthma). Similarly, blood and sputum neutrophil counts were significantly higher in the difficult asthma cohort, likely reflecting the pathophysiological consequences of higher steroid use as previously described.7 In turn, associations of blood and sputum neutrophilia with difficult asthma were found to be relevant only for those with more severe disease as defined by impaired lung function. Aligned to an overwhelmingly T2-high status, this indicates that a proportion of such patients show a mixed inflammatory response. Nonetheless, 88.1% of the mild asthma cohort also had a GINA T2-high status, suggesting a greater degree of T2-inflammation in mild asthma than previously described.2,38 This emphasises the importance of early ICS prescribing in all asthma patients regardless of disease severity, as reflected in recent international guidelines.2
Our study had limitations including small sample size of the mild asthma cohort. The IOWBC recruitment pool meant a proportion of mild asthma participants were all of similar age, but this was the minority, and sub-analysis suggested no significant differences in phenotypic traits. Data collection was also limited with respect to smoking pack years, medication adherence and small sputum sample size. Similarly, lung function characteristics compared pre-bronchodilator values in the mild asthma cohort with post-bronchodilator values in the difficult asthma cohort, reflecting the real-world limitation of withholding regular inhaler therapy in a high-risk population. A key strength of our study is the identical methodology used in both cohorts allowing detailed comparisons. Moreover, this study provides one of the most comprehensive assessments of mild asthma and its phenotype. Replication in other diverse ethnic populations is warranted given both cohorts were mainly Caucasian. Similarly, validation of our findings in other mild asthma cohorts is essential but currently there are no populations with similar data variables readily available for use as comparators.
In conclusion, this study extensively characterised the phenotypic nature of the EOSA mild asthma cohort. Mild asthma is characterised as a female-predominant, early-onset, significantly multimorbid disease with a high prevalence of current smokers, atopy and T2-high inflammation. The nature of mild asthma has not previously been well described. Identifying a mild asthma phenotype with the characterisation presented warrants further attention in clinical practice as it affords a framework with which to better understand mild asthma and offers steps to address factors that might limit potential progression to more complex severe diseases including early smoking cessation intervention and addressing multimorbidity.
Abbreviations
Data Sharing Statement
We confirm that data accessed complied with relevant data protection and privacy regulations with reference to the General Data Protection Regulations 2018.
Acknowledgments
The authors wish to thank the patients who are participating in this study. They also wish to acknowledge the contributions of the wider WATCH study team including Matthew Harvey, Mae Felongco, Yvette Thirlwall, Kim Bentley, Laura Presland, Yueqing Cheng, Josune Olza Meneses, Adnan Azim, Anna Freeman, Kerry Day, David Hill and Peter Howarth. The authors also wish to acknowledge the support of Southampton NIHR BRC and Clinical Research Facility. Southampton Clinical Research Facility and BRC are funded by NIHR and are a partnership between the University of Southampton and University Hospital Southampton NHS Foundation Trust. The authors also acknowledge funding support from Novartis, NIH and the AAIR Charity.
Funding
The Wessex AsThma CoHort of difficult asthma (WATCH) study has been supported by the NIHR Southampton Biomedical Research Centre (BRC) and Clinical Research Facility at University Hospital Southampton NHS Foundation Trust (UHSFT), UK. The WATCH study itself is not externally funded. Funding assistance for database support for the WATCH study was initially obtained from a non-promotional grant from Novartis (£35,000). Funding assistance for patient costs (eg parking) was initially provided by a charitable grant (£3500) from the Asthma, Allergy & Inflammation Research (AAIR) Charity. Lastly, funding assistance for the Epigenetics of Severe Asthma (EOSA) study was obtained from a National Institutes of Health (NIH) grant (£400,000) in collaboration with La Jolla Institute of Immunology, La Jolla, California, USA.
Disclosure
The authors, JN, HM, FM, JB, MAK, CB, HMH, PD, RD, GS, PV, SHA, RJK, declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Since completion of this work, CB now works for Aztra Zeneca. Professor Ratko Djukanovic reports personal fees, has shares in the company and is a consultant to Synairgen, and personal fees from GlaxoSmithKline and Kymab, outside the submitted work.
References
1. Asthma + Lung UK What is asthma?; 2021. Available from: https://www.asthma.org.uk/advice/understanding-asthma/what-is-asthma/.
2. GINA Difficult to Treat & Severe Asthma in adolescent and adult patients. Diagnosis and Management. A GINA pocket guide for health professionals. Version 3 0.April 2021; 2023. Available from: https://ginasthma.org/wp-content/uploads/2021/08/SA-Pocket-guide-v3.0-SCREEN-WMS.pdf.
3. Barnes P, Woolcock A. Difficult asthma. Eur Respir J. 1998;12(5):1209–1218. doi:10.1183/09031936.98.12051209
4. Patel GB, Peters AT. Comorbidities associated with severe asthma. J Precis Respir Med. 2019;2(1):5–9. doi:10.2500/jprm.2019.190006
5. Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–224. doi:10.1164/rccm.200711-1754OC
6. Teague WG, Phillips BR, Fahy JV, et al. Baseline features of the severe asthma research program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract. 2018;6(2):545–554.e4. doi:10.1016/j.jaip.2017.05.032
7. Shaw DE, Sousa AR, Fowler SJ, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46(5):1308–1321. doi:10.1183/13993003.00779-2015
8. Heaney LG, Brightling CE, Menzies-Gow A, Stevenson M, Niven RM. on behalf of the British thoracic society difficult asthma network. Refractory asthma in the UK: cross-sectional findings from a UK multicentre registry. Thorax. 2010;65(9):787–794. doi:10.1136/thx.2010.137414
9. FitzGerald JM, Tran TN, Alacqua M, et al. International severe asthma registry (ISAR): protocol for a global registry. BMC Med Res Methodol. 2020;20(1):212. doi:10.1186/s12874-020-01065-0
10. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377(10):965–976.
11. Azim A, Newell C, Barber C, et al. Clinical evaluation of type 2 disease status in a real‐world population of difficult to manage asthma using historic electronic healthcare records of blood eosinophil counts. Clin Exp Allergy. 2021;51(6):811–820. doi:10.1111/cea.13841
12. Brusselle GG, Koppelman GH. Biologic therapies for severe asthma. N Engl J Med. 2022;386(2):157–171. doi:10.1056/NEJMra2032506
13. Chen W, Safari A, FitzGerald JM, Sin DD, Tavakoli H, Sadatsafavi M. Economic burden of multimorbidity in patients with severe asthma: a 20-year population-based study. Thorax. 2019;74(12):1113–1119. doi:10.1136/thoraxjnl-2019-213223
14. Serra-Batlles J, Plaza V, Morejon E, Comella A, Brugues J. Costs of asthma according to the degree of severity. Eur Respir J. 1998;12(6):1322–1326. doi:10.1183/09031936.98.12061322
15. National Review of Asthma Deaths. Confidential Enquiry Report; 2014. Available from: https://www.rcplondon.ac.uk/projects/national-review-asthma-deaths.
16. Azim A, Mistry H, Freeman A, et al. Protocol for the Wessex AsThma CoHort of difficult asthma (WATCH): a pragmatic real-life longitudinal study of difficult asthma in the clinic. BMC Pulm Med. 2019;19(1):99. doi:10.1186/s12890-019-0862-2
17. BTS/SIGN. SIGN 158: British guideline on the management of asthma; 2022. Available from: https://www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/.
18. Arshad SH, Holloway JW, Karmaus W, et al. Cohort profile: the isle of wight whole population birth cohort (IOWBC). Int J Epidemiol. 2018;47(4):1043–1044i. doi:10.1093/ije/dyy023
19. Chen W, FitzGerald JM, Lynd LD, Sin DD, Sadatsafavi M. Long-term trajectories of mild asthma in adulthood and risk factors of progression. J Allergy Clin Immunol Pract. 2018;6(6):2024–2032.e5. doi:10.1016/j.jaip.2018.04.027
20. Jenkins CR, Boulet LP, Lavoie KL, Raherison-Semjen C, Singh D. Personalized treatment of asthma: the importance of sex and gender differences. J Allergy Clin Immunol Pract. 2022;10(4):963–971.e3. doi:10.1016/j.jaip.2022.02.002
21. Azim A, Freeman A, Lavenu A, et al. New perspectives on difficult asthma; sex and age of asthma-onset based phenotypes. J Allergy Clin Immunol Pract. 2020;8(10):3396–3406.e4. doi:10.1016/j.jaip.2020.05.053
22. Eisner MD, Yelin EH, Katz PP, et al. Predictors of cigarette smoking and smoking cessation among adults with asthma. Am J Public Health. 2000;90(8):1307–1311. doi:10.2105/AJPH.90.8.1307
23. Arshad SH, Hodgekiss C, Holloway JW, et al. Association of asthma and smoking with lung function impairment in adolescence and early adulthood: the isle of wight birth cohort study. Eur Respir J. 2020;55(3):1900477. doi:10.1183/13993003.00477-2019
24. Apostol GG, Jacobs DR, Tsai AW, et al. Early life factors contribute to the decrease in lung function between ages 18 and 40: the coronary artery risk development in young adults study. Am J Respir Crit Care Med. 2002;166(2):166–172. doi:10.1164/rccm.2007035
25. Hylkema MN, Sterk PJ, de Boer WI, Postma DS. Tobacco use in relation to COPD and asthma. Eur Respir J. 2007;29(3):438–445. doi:10.1183/09031936.00124506
26. Lazarus SC, Chinchilli VM, Rollings NJ, et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med. 2007;175(8):783–790. doi:10.1164/rccm.200511-1746OC
27. Chaudhuri R, Livingston E, McMahon AD, et al. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am J Respir Crit Care Med. 2006;174(2):127–133. doi:10.1164/rccm.200510-1589OC
28. Mistry H, Ajsivinac Soberanis HM, Kyyaly MA, et al. The clinical implications of aspergillus fumigatus sensitization in difficult-to-treat asthma patients. J Allergy Clin Immunol Pract. 2021;9(12):4254–4267.e10. doi:10.1016/j.jaip.2021.08.038
29. Bender BG. Risk taking, depression, adherence, and symptom control in adolescents and young adults with asthma. Am J Respir Crit Care Med. 2006;173(9):953–957. doi:10.1164/rccm.200511-1706PP
30. Sastre J, Crespo A, Fernandez-Sanchez A, et al. Anxiety, depression, and asthma control: changes after standardized treatment. J Allergy Clin Immunol Pract. 2018;6(6):1953–1959. doi:10.1016/j.jaip.2018.02.002
31. Fong WCG, Rafiq I, Harvey M, et al. The detrimental clinical associations of anxiety and depression with difficult asthma outcomes. J Pers Med. 2022;12(5):686. doi:10.3390/jpm12050686
32. Rimington LD. Relationship between anxiety, depression, and morbidity in adult asthma patients. Thorax. 2001;56(4):266–271. doi:10.1136/thorax.56.4.266
33. To T, Simatovic J, Zhu J, et al. Asthma deaths in a large provincial health system. A 10-year population-based study. Ann Am Thorac Soc. 2014;11(8):1210–1217. doi:10.1513/AnnalsATS.201404-138OC
34. Varkonyi-Sepp J, Freeman A, Ainsworth B, Kadalayil LP, Haitchi HM, Kurukulaaratchy RJ. Multimorbidity in difficult asthma: the need for personalised and non-pharmacological approaches to address a difficult breathing syndrome. J Pers Med. 2022;12(9):1435. doi:10.3390/jpm12091435
35. Maison N, Omony J, Illi S, et al. T2-high asthma phenotypes across lifespan. Eur Respir J. 2022;60(3):2102288. doi:10.1183/13993003.02288-2021
36. Jackson DJ, Busby J, Pfeffer PE, et al. Characterisation of patients with severe asthma in the UK severe asthma registry in the biologic era. Thorax. 2021;76(3):220–227. doi:10.1136/thoraxjnl-2020-215168
37. Barber C, Azim A, Newell C, et al. Validation and further insight into the International Severe Asthma Registry (ISAR) eosinophil gradient algorithm in the Wessex AsThma CoHort of difficult asthma (WATCH) using historical blood eosinophil counts and induced sputum. Clin Exp Allergy. 2022;52(6):792–796. doi:10.1111/cea.14109
38. Woodruff PG, Modrek B, Choy DF, et al. T-helper Type 2–driven Inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–395. doi:10.1164/rccm.200903-0392OC
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

