Back to Journals » International Journal of Nanomedicine » Volume 18
How Combined Macrolide Nanomaterials are Effective Against Resistant Pathogens? A Comprehensive Review of the Literature
Authors Siraj EA , Yayehrad AT , Belete A
Received 27 May 2023
Accepted for publication 23 August 2023
Published 15 September 2023 Volume 2023:18 Pages 5289—5307
DOI https://doi.org/10.2147/IJN.S418588
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Ebrahim Abdela Siraj,1,2 Ashagrachew Tewabe Yayehrad,1 Anteneh Belete2
1Department of Pharmacy, School of Health Sciences, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia; 2Department of Pharmaceutics and Social Pharmacy, School of Pharmacy, College of Medicine and Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Ebrahim Abdela Siraj, Department of Pharmacy, Bahir Dar University, PO Box: 79, Bahir Dar, Ethiopia, Tel +251 918020093, Email [email protected]
Abstract: Macrolide drugs are among the broad-spectrum antibiotics that are considered as “miracle drugs” against infectious diseases that lead to higher morbidity and mortality rates. Nevertheless, their effectiveness is currently at risk owing to the presence of devastating, antimicrobial-resistant microbes. In view of this challenge, nanotechnology-driven innovations are currently being anticipated for promising approaches to overcome antimicrobial resistance. Nowadays, various nanostructures are being developed for the delivery of antimicrobials to counter drug-resistant microbial strains through different mechanisms. Metallic nanoparticle-based delivery of macrolides, particularly using silver and gold nanoparticles (AgNPs & AuNPs), demonstrated a promising outcome with worthy stability, oxidation resistance, and biocompatibility. Similarly, macrolide-conjugated magnetic NPs resulted in an augmented antimicrobial activity and reduced bacterial cell viability against resistant microbes. Liposomal delivery of macrolides also showed favorable synergistic antimicrobial activities in vitro against resistant strains. Loading macrolide drugs into various polymeric nanomaterials resulted in an enhanced zone of inhibition. Intercalated nanomaterials also conveyed an outstanding macrolide delivery characteristic with efficient targeting and controlled drug release against infectious microbes. This review abridges several nano-based delivery approaches for macrolide drugs along with their recent achievements, challenges, and future perspectives.
Keywords: macrolides, nanomedicine, drug resistance, macrolide nano-delivery
Introduction
The discovery of antibiotics in the last century can be considered as an ultimate success in pharmaceutical industry as these medications enhanced the clinical outcomes of several infections that led to significant morbidity and mortality rates.1,2 However, misuse of the potent and highly effective antimicrobial agents results in the emergence of multidrug-resistance which consequently restricted the choice of antibiotics for treating mild and severe infections.3,4 The rise of antimicrobial-resistant pathogens in turn causes a significant global health and socio-economic crisis. According to the report from European Union (EU), nearly ten thousand tons of antibiotics are consumed annually, but more than twenty five thousand peoples died because of the ailments related to resistant pathogens.5 The increase in allergic responses linked to antibiotic use is another issue caused by antibiotic overuse, which limits the usage of some antimicrobials in patients and endangers their wellbeing. If this trend continues, 300 million people worldwide are expected to die prematurely due to antibiotic resistance over the next 35 years.6 Antibiotic resistance has been deemed a “major threat to public health” by the World Health Organization (WHO) as it directly and indirectly affects both human and animal health. It has been estimated that antimicrobial resistance is associated with extra health-care costs and productivity losses in the EU of at least €1.5 billion each year.7
Bacteria have evolved an array of mechanisms that enable them to resist the inhibitory action of the antibiotics. Understanding the molecular patterns of these resistance mechanisms is not only an important issue but can also provide strategic intelligence to guide the thoughtful creation of novel delivery methods to counter or prevent the resistance.8,9 Since bacterial infections are major causes of morbidity and mortality worldwide, attention has been focused on emerging novel therapeutic approaches including the application of NP-based materials for antimicrobial delivery.10,11 Some examples of multi-drug resistant microbes and their mechanisms of resistance are shown in Table 1.12–18
 |
Table 1 Some Examples of Multi-Drug Resistant Microbes with Their Mechanism of Resistance |
Macrolide drugs are broad-spectrum antibiotics that inhibit protein synthesis by targeting the bacterial ribosome, specifically inhibiting the conversion of a subset of cellular proteins. Their action crucially depends on the nascent protein sequence and antibiotic structure.19–21 Initially, macrolides were primarily used for the treatment of upper respiratory tract, skin, and soft tissue infections. Further investigations were then stretched for their improved pharmacologic effectiveness and broader microbial coverage.22 Macrolides are now effective in the treatment of infections caused by gram-positive bacteria (Streptococcus pneumoniae, Streptococcus pyogenes, Staphylococcus aureus), some gram-negative bacteria (Haemophilus influenzae), and atypical pathogens (Chlamydia trachomatis, chlamydia, Treponema pallidum, Mycoplasma pneumoniae). It has also been noted that many of the infections that can be treated with next-generation cephalosporin are treatable using macrolides as well.23 Microbial resistance towards various antibiotics can be minimized through firm adherence to the guidelines of various antimicrobial stewardship programs (ASPs) and implementing the recommendations from pharmacologic and clinical study reports.24,25 Collaborative efforts of all stakeholders are needed in the provision of rational use of drugs in a way that can prevent or at least minimize the risk of drug resistance.26
Nanotechnology has recently been given significant attention for its application in pharmaceutical science, particularly in the development and optimization of novel drug delivery systems. A NP is a microscopic particle with at least one dimension less than 100 nm.27,28 On this scale, materials have unique physicochemical properties, including ultrasmall size, large surface-to-mass ratio, high reactivity, and unique interactions with biological systems.29 Drugs in nano formulations exhibit improved pharmacokinetics, therapeutic indices, and serum solubilities than their conventional delivery forms. Additionally, NP-based drug delivery is characterized with additional therapeutic benefits such as prolonged systemic circulation, sustained and controlled drug release, and targeted delivery of drugs to their site of action.3 Recent study reports demonstrated that metallic NPs, liposomes, polymeric NPs, and intercalated structures can be potential alternatives for effective and efficient antimicrobial delivery means against resistant strains. Metallic NPs, particularly silver and gold NPs (AgNPs & AuNPs), revealed a promising outcome owing to their stability, oxidation resistance, and biocompatibility.30 Liposomal delivery of macrolides showed a promising in vitro antimicrobial activity against resistant strains with synergistic activities, enhanced biofilm synthesis inhibition, and better stability.31 Macrolide-loaded polymeric nanomaterials (NMs) resulted in an enhanced antimicrobial activity (zone of inhibition) and anti-inflammatory response.32 Macrolide-conjugated magnetic NPs resulted in an augmented antimicrobial activity and reduced bacterial cell viability.33 Intercalated nanomaterials also conveyed an outstanding macrolide delivery characteristics with efficient targeting and controlled drug release.34,35
The present review mainly focuses on the potential of NMs and related nanotechnology-based drug delivery approaches against drug-resistant microbes with the ultimate goal of conveying the reader up-to-date and suggestive information about countering macrolide resistance using the technology the time permits. The contemporary knowledge and recent study reports on nano-based approaches to overcome the increasing macrolide resistance are summarized and discussed with a comprehensive review of published information. To accomplish this task, literatures for review were collected from hugely reputable electronic databases such as PubMed, Medline, Google Scholar, and Science Direct, which provide access to scientific and medical research in different valuable journals. Books and related documents were also used when necessary.
The Pattern of Macrolide Drug Resistance
Macrolides are synthesized from Saccharopolyspora erythraea, a bacterium found in soil. They prevent bacteria from producing proteins by reversibly interacting with the P-site of the 50S ribosomal unit. Gram-positive cocci and intracellular pathogens such as Mycoplasma, Chlamydia, and Legionella species are the principal targets of macrolides. While the primarily discovered macrolide was erythromycin, some others, like azithromycin, clarithromycin, and roxithromycin were discovered gradually.36 Macrolide antibiotics are of great significance in both medical and clinical practice. Macrolides may exert bacteriostatic or bactericidal action depending on the bacterial type and the strength of the drug concentration. They play imperative roles in the pharmacological management of respiratory infections.37 They are also recommended as alternatives for penicillins and β-lactams in case of allergic history in patients. Awkwardly, their therapeutic outcome is being in question nowadays owing to the emergence of resistant microbes infecting the respiratory system, mainly due to the mis-use and improperly regulated outpatient consumption of these drugs.20,22,24 Currently, there are evolving investigations on macrolide resistance, types of resistant isolates, variations in location, levels of resistance, and the mechanisms of resistance.38
The Prevalence and Factors Associated with Macrolide Resistance
Some study reports indicated that macrolides are being out of use for some particular respiratory infections like pneumonia due to the occurrence of resistance.29 In Europe, a superior level (about 27.5%) of erythromycin resistance has been reported between 1991 and 1992 where an increasing rate of 3.7% and 2.2% was seen among children aged 1–2 and 3–4 years, respectively. Notably, the resistant groups were also reported to be cross-resistant with penicillins.12 A similar event was reported from Finland that the macrolide resistance of Group A streptococci is increasing in an alarming rate with time.39 Reports on macrolide resistance in S. pneumoniae also clearly indicated that varying degrees of resistances (3–74%) occurred with locational varieties among different nations. Additionally, significant differences were observed within a nation depending on the cause of the strains (community or hospital level), patient age, sample source, seasonal conditions, and pneumococcal serotype.27,28 The incidence was typically higher in youngsters, in pneumococci strains, and in middle ear fluid samples. These variations pose a significant impact in the effectiveness of treating patients with MLS B class (macrolide, lincosamide, and streptogramin B) agents.26 Spiramycin is a medium-spectrum 16-membered macrolide drug utilized for respiratory infection management but in vitro assessments indicated that pathogens like F. nucleatum became resistant for this drug in nearly half of the study groups.40 Japan reported macrolide-resistant M. pneumoniae strains earlier in 2000s from the pandemic which occurred between 2010 and 2012 among children in Japan. In 2012, 81.6% of M. pneumoniae cases were macrolide-resistant, followed by 59.3% in 2014, and 43.6% in 2015. Other nations have also experienced similar pandemics with drug resistance.29,41,42
Although numerous factors can be mentioned for the development of macrolide resistance, the extensive and inappropriate utilization had inexorably led to the blowout of resistant microbial isolates. The irrational antibiotic use leads not only to the occurrence of unpredictable adverse effects but also to the rise of health costs as older resistant antibiotic drugs are being replaced by new expensive antibiotics.43 Such complications from microbial resistance are seen in the plasmid-borne New Delhi metallo-b-lactamase-1 (NDM-1) gene that is believed to be transmitted between the bacterial species of Klebsiella pneumoniae and Escherichia coli and a nosocomial infection causing strain called methicillin-resistant staphylococcus aureus (MRSA).13,32,44 Even though the exact mechanism still not well understood, such macrolide-resistant microbial strains are expected to interfere the pharmacologic activity of the drugs which is related to the Erm methyltransferase functions.45 In order to investigate the association of antibiotic use pattern and the rate of drug resistance, the determinant factors such as the specimen type, the study area, the presence of nosocomial resistant strains, the study period, and the sample representation should be well considered.46 Moreover, other factors including the overall antibiotic use pattern, the macrolide type, presence of setback resistance, the regimen, the drug pharmacokinetics and pharmacodynamics, patient adherence, the presence of cross infection, and community deeds can contribute to the emergence of resistance.47 Consumption of antimicrobial agents and the prevalence of antibiotic resistance are intricately related. The two main factors influencing resistance are selective antibiotic pressure and the spread of resistant bacteria with potential of transmission of resistance genes across bacteria. But, only certain bacterial species and antibiotic drug classes are impacted by selective pressure.48,49
Mechanisms of Macrolide Resistance
Various mechanisms are anticipated for resistance of microbes towards macrolides. These include: (i) target site modification by methylases encoded by erm genes; (ii) modifying enzymes such as esterases encoded by ere A and B genes or phosphotransferases encoded by mphA, B, and D genes; (iii) efflux pumps; and (iv) mutations in the rrl and rpl genes encoding ribosomal proteins L22, L4, and 23S rRNA, which confer resistance in gram-positive bacteria. The existence of more than one of the aforementioned genes will convene complete cross-resistance among two different macrolides.50,51 The central modes of acquired resistance to erythromycin encompass either a methylase mediated target site modification at the 23, S rRNA ribosomal subunit, leading to the MLS B resistance phenotype encoded by erm genes, or an efflux mechanism (M phenotype) encoded by mef genes. However, certain isolates may harbor both of these resistance mechanisms. The mef gene is reported to account the highest proportion of the resistance mechanisms like the case of erythromycin-resistant isolates.52 On the other hand, macrolide resistance-associated 23S rRNA gene mutations were discovered in 4.6% and 10% of study groups in Russia and Estonia, respectively.46 These problematic groups hinder the effective use of macrolides for the treatment of disease caused by detrimental pathogens. Moreover, microbes perpetually attained resistance to the majority of antimicrobials shortly after their introduction. Hence, it became mandatory to either escalate doses or search for alternative antibiotic class. Unfortunately, there has been inadequacy of new antibiotic classes in the preceding 20 years.53
Though various resistance mechanisms are enumerated for macrolide drugs, the commonly encountered mechanisms are diversity in MLSB, ribosomal methylation, resistance expression drug modification, target mutation, and antibiotic efflux. According to biochemical investigations, the methylation of the antibiotics’ ribosomal target, or the so-called MLSB phenotype, results in cross-resistance to macrolides, lincosamides, and streptogramins B. Later, a vast number of bacteria were found to have the MLSB phenotype, which is expressed by a variety of erm (erythromycin ribosome methylase) genes.53 Ribosomal methylation is among the most common methods for macrolide and lincosamide resistance by impairing the ability of these drugs to attach to their targets. It is also the responsible mechanism for cross-resistance to the three medication classes macrolide, lincosamide, and streptogramin B due to overlapping at the binding sites in the 23S rRNA.39,54 On the other way, MLSB resistance can be either constitutively expressed or induced. When a bacterial strain develops inducible resistance, its mRNA becomes dormant and unable to encode methylase. Hence, the mRNA can become active only in the presence of a macrolide inducer.55 In contrast, active methylase mRNA is generated during constitutive expression even in the absence of an inducer. The presence of an attenuator upstream of the structural erm gene for methylase is associated with induction. The staphylococcal determinant erm(C), as well as the erm(A) and erm(B) determinants, exhibited post-transcriptional induction, in accordance with the paradigm of translation attenuation.51
Genetic modification is another mechanism of macrolide resistance which is caused by bacterial enzymes and resulting in altered antimicrobial target site. For instance, mutations in erm group C and G streptococci inhibit the affinity of MLS B against the target site.29,56 Similar occasions are predicted for E. coli mutants for their extreme resistance against erythromycin in vitro. Most isolates of M. avium and H. pylori, if not all, exhibit clarithromycin resistance owing to this mechanism. S. pneumoniae, Treponema pallidum and Propioni species have also been linked to similar mutations.53,57 Furthermore, recent studies have shown that efflux mechanism is found to be another most common mechanism for macrolide resistance. The efflux pumps which are encoded by mefE in S. pneumoniae and by mefA in S. pyogenes have been revealed to be determinant for streptococci’s resistance to macrolides. Mef, which is thought to be a membrane protein, is believed to be necessary for the energy-dependent efflux of 14- and 15-membered macrolides out of the cell.39,58 Limited rate of influx and extreme drug efflux properties impede the required drug concentrations at the target. Consequently, there will be a sublethal drug level at the desired location that results in an enhanced target-based resistance.12,41 There are also chromosomally encoded pumps in gram-negative bacteria, frequently originated from a family of proteins with 12 membrane-spanning regions, which facilitate an innate resistance towards hydrophobic drug substances such as macrolides.59 The destruction of antimicrobials by target-related factors such as covalent binding leads to an inherent resistance mechanism to other antimicrobials with analogous structures.41 Enterobacteria have been found to possess esterases and phosphotransferases that confer resistance to lincosamides, 14- and 15-membered macrolides, and erythromycin. Although only a few strains have been documented thus far, the discovery that clinical S. aureus strains produce phosphotransferases encoded by mph(C) genes is concerning.39 Generally, methylases and efflux genes are the furthermost significant and pervasive features in gram-positive microbes.43 The commonly predicted mechanisms of macrolide resistance are shown in Figure 1.1
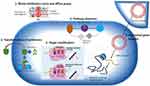 |
Figure 1 Multiple resistance mechanism of bacteria to MDR pathogen. Notes: Reproduced from Imran M, Jha SK, Hasan et al. Overcoming Multidrug Resistance of Antibiotics via Nanodelivery Systems. Pharmaceutics. 2022;14(3):1–25 (https://creativecommons.org/licenses/by/4.0/).1 |
Combating Macrolide Drug Resistance
Despite the efforts exerted incessantly in the discovery of new antibiotics, the treatment of intracellular infections often fails to completely eradicate the causative pathogens. It is indisputable that antibiotic resistance is life-threatening in the same sense as cancer, both in the number of cases and in the likely outcomes; thus, the actions have to be taken as a matter of extreme urgency.60 Indeed, there are many debates and arguments between the development of novel antibacterial agents and the discovery of novel delivery approaches for the available antimicrobials to overcome this situation via collaborative approaches.41 Reports are clearly dictating that the emergence of multi-drug-resistant (MDR) pathogens raises the specter of a future in which we will be out-of a pharmaceutical defense mechanism against infections caused by those pathogens. To date, microbes have been able to stay up with or even surpass human innovations in the antibiotic development.35 In light of this pressing issue, there has been much interest in alternative antimicrobial therapies, including the use of nanotechnology, which has become the most important and impactful innovation to tackle this troublesome situation.45 Beyond the discovery of novel antimicrobials and delivery systems, all-embracing effort should be exerted towards the development of effective strategies to combat multi drug resistance by improving patient compliance and awareness for infection prevention.43,47 Adjuvant antimicrobial therapeutic approaches such as antibiotic–antibiotic combination strategy and antibiotic–adjunct combination strategy can be considered as possible alternative strategies to circumvent antibiotic resistance. However, nanotechnology-based antibacterial formulation and delivery approaches are currently being a marvelously promising strategy, even for the treatment of infections with MDR microbes.7 NPs are used as “magic bullets” to deliver drugs at the desired concentration, location, and timing. Nanotechnology offers a vast opportunity to command and modify molecular structures on nano scale to attain specific target actions.61 Nano-bullet targeting is advantageous over conventional systems because it enhances the therapeutic capacity by preventing microbial resistance, frequent drug intakes, and associated side effects. Therefore, nanoscience enhances patient compliance by protecting their natural microbiome. NPs can also be applied to overcome drug resistance owing to their multifunctionality, as they can tackle simultaneous, multiple gene mutations by the bacteria.53
Nano-Based Macrolide Delivery Innovations Against Resistant Microbes
Application and Mechanisms of Nanomaterials in Countering AMR
Nanotechnology is nowadays being recognized significantly in medicine for its indispensable roles in combating multidrug resistance. The unique characteristics of nano-sized materials such as controllable size, shape, and physicochemistry make them promising for efficient and effective drug development and delivery application.46,48 Nanotechnology provides special drug delivery systems that can address the difficulties related to targeting, resistance, and stability. These nano-sized delivery systems have transformed the pharmaceutical research and development activities towards a more advanced innovation with better treatment outcomes. The superior surface area from the reduced sizes of NPs and nanocarriers enabled targeted drug delivery to the desired disease areas, minimizing harmful effects on healthy tissues.62,63 NMs also resulted in better aqueous solubility, enhanced enzymatic stability, and sustained or controlled release of the drug that collectively bring improved therapeutic outcomes.20 Antimicrobial agents integrated with nanotechnology come-up with promising broad spectrum, synergistic, and flexible antimicrobial activities.5 NPs represent a promising alternative as they can fight against bacteria intrinsically, conjugate with current antimicrobials, or/and serve as transporters or carriers for naturally occurring antimicrobials.29 Common platforms for antibacterial drug delivery include metallic NPs, vesicular delivery systems, solid-lipid NMs, and polymeric NPs.64 Nanomedicine was regarded as one of the front coming agendas in the 2006 report of Food and Drug Administration (FDA) under “The Critical Path Opportunities List and Report” recognizing the significance of encouraging research in the field of nanotechnology. The FDA outlined the need for promotion of science and investment towards advancing nano-regulation, applying nanomedicine for health promotion and protection, innovating qualitative and quantitative analytical techniques for nanomaterial safety, and developing systems for risk prediction and assessment.45,51
The numerous characteristics of NPs make them advantageous carriers of medications for combating disease-causing microorganisms. These include improved drug solubility, enhanced stability, simplicity of synthesis, biocompatibility with the agents used as targets, and regulation of release under stimuli-responsive conditions. Their acquired characteristic of greater surface-to-volume ratio enables their specific functionality in drug delivery.1 The parameters that determine the pharmaceutical application of NPs are shown in Table 2.29,51,65–71 The application of specially designed NMs brings a reasonable benefit compared to the conventional modalities against infections disease instigated from intracellular and MDR microbes.24 Antimicrobials can be functionalized with metallic, organic, polymeric, and magnetic NPs. These functionalizing NPs are reported to have intrinsic antibiotic activities and will elicit synergistic effects when combined with antimicrobial drugs. Although they face challenges with respect to passing through biologic membranes, other types of transport such as vesicular or endocytosis can facilitate their intracellular entry.29 Their special interaction with surface lipids is also predicted as one mechanism of their enhanced intracellular delivery.51,72 Apart from their intracellular delivery mechanisms, various mechanisms are hypothesized for the antimicrobial activities of NPs. They can directly interact and disrupt bacterial cell wall; inhibit biofilm formation; activate and stimulate host immune responses; produce reactive oxygen species (ROS), and interact with the intracellular components. These diverse ways of antimicrobial activities, most of them being different to the drug actions, make them exciting for application in fighting against drug resistance.59 In addition, NPs can possess physical barrier against the drug resistance mechanisms.73 NP-based antibiotic drug delivery is believed to overcome the commonly reported drawbacks of the conventional administrations which are associated with resistance, lower margin of safety, adverse events, poor specificity, and inconvenience.74 They are also promising delivery systems of chemical compounds for nanodiagnostics, nanotherapeutics, and nanotheranostics.62,75
 |
Table 2 Parameters That Determine the Pharmaceutical Application of Nanoparticles |
In a comprehensive view, NMs generally have two main proposed activities in antibacterial drug delivery. Firstly, they can be functionalized with available antibiotics to enable powerful penetration of the drugs through bacterial membranes. Vesicular NMs (nanosomes, dendrimers, and micelles), nano-polymers, and inorganic NPs are applied to functionalize antimicrobial agents by incorporation or surface modification approaches.76 These ways of antibiotic delivery results in boosted drug response through effective microbicidal activities which might be from two directions: from upgraded delivery and drug transportation features as contrasting to the antibiotic drug alone, and/or the synergistic effects of the functionalized drug-nanomaterial combinations.45,77,78 Secondly, NMs can elicit various intrinsic antimicrobial properties. Various inorganic NMs demonstrated well-established and investigable activities against a wide range of microbes. Even though vast similarities with the conventional antimicrobials are reported in their mechanisms, the way antibiotic resistance is occurring is far from those NMs.19,45 Their distinctive physicochemistry is utilized in formulating novel antimicrobials devoid of the short-comings from the conventional drugs. Their resemblance to the bacterial intracellular components with respect to size and surface area provides a multidirectional interaction and loading to the microbe intracellularly.79 Furthermore, suitable physicochemical modifications on the NPs can prevent predetermined mechanisms of drug resistance.80 The different mechanisms underlying the intrinsic antibacterial effects of NPs are summarized in Figure 2.29
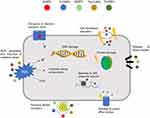 |
Figure 2 Different mechanisms of action of NPs in bacterial. Notes: Reproduced from Baptista PV, McCusker MP, Carvalho A, Ferreira DA. Nano-Strategies to Fight Multidrug Resistant Bacteria – “A Battle of the Titans”. Front Microbiol. 2018;9:1–26 (https://creativecommons.org/licenses/by/4.0/).29 |
Nanomaterials for Macrolide Delivery
Nanotechnology is advancing therapeutic systems against complicated acute and chronic disorders by improving the drug delivery, synergizing pharmacologic responses, and preventing resistance mechanisms. Nanofabrication of therapeutic agents by applying proper organic, inorganic, or polymeric NMs has shown a promising antimicrobial therapy.81 Their vital role in interrupting major microbial cellular functions such as cell wall permeability, efflux activity, reactive species formation, and metabolism and reproduction is of utmost importance in countering AMR. Hence, nano-based approaches should be in place as a potential alternative for particular circumstances where they can bring a thorough impression on quality of life though the expensiveness of nanomedicine makes the conventional therapy more preferable.29 In addition to the cost-effectiveness considerations in applying nanomedicine, integrating the health and safety with the technology led engineering should be done with generous scientific considerations.82 At the moment, about 75% of the market available nanomedicine products reside on drug delivery systems.83 Drug manufacturers are nowadays shifting towards nanomaterial-based drug delivery systems as these systems recognizably advanced the health-care system with numerous merits that were impractical and difficult to achieve previously with the conventional approaches. Common NMs with promising antimicrobial drug delivery outcomes are shown in Figure 3.82 Table 3. 3,27,84–95 also presents some examples of NMs used for the delivery of various macrolide drugs.
 |
Figure 3 Diagrammatic explanation of different Nano carriers. Notes: Reproduced from Chowdhury A, Kunjiappan S, Panneerselvam T. Nanotechnology and nanocarrier-based approaches on treatment of degenerative diseases. Int Nano Lett. 2017;7(2):91–122 (https://creativecommons.org/licenses/by/4.0/).82 |
 |
Table 3 Example of Nanomaterials Based Drug Formulation of Various Macrolide Drugs |
Inorganic NPs
Inorganic NMs were being applied for centuries and are currently under development and investigation as antimicrobials. Noble metals (eg, Ag, Au, Pt, and Pd), carbon-based materials (carbon nano-fibers and different types of carbon nanotubes), semiconducting materials (CdSe, CdS, ZnS, TiO2, PbS, InP, Si/SiO2), magnetic materials (Fe3O4, Co, CoFe2O4, FePt, CoPt, and their composites), and lanthanides (Gd2O3 and Eu2O3) are among the most important inorganic NMs35,96 which are used as antimicrobial agents and delivery systems.97 Inorganic NPs exhibit a variety of activities such as catalysis and sensing to optics, microbicidal effects, cytotoxic properties, and as data storages based on their particle properties.82,86 Presently, metallic NPs, particularly silver and gold NPs (AgNPs & AuNPs), have been investigated for vast medical applications and demonstrated a promising outcome owing to their stability, oxidation resistance, and biocompatibility.30
AuNPs offer countless rewards in drug development studies as their core is chemically inert and non-cytotoxic. Moreover, nano-sized engineering of gold particles is accompanied by efficient drug loading for targeted antimicrobial delivery.82,87 In one study, the macrolide drug clarithromycin demonstrated a better activity against S. aureus and S. pyogen when loaded with CdS & NiO NPs than the free drug and the unloaded NPs.9 Another study on AuNP-based functionalization of azithromycin and clarithromycin reported an improved minimum inhibitory concentrations (MIC) and cytotoxicity profiles against oral pathogenic bacteria and fungi both in vitro and in vivo.61 Another study on azithromycin loaded AuNP and clarithromycin loaded AuNP also exhibited enhanced antibacterial effect with higher mean zone of inhibition than the respective drugs alone against known resistant pathogens.98 AgNPs have size and ionization dependent bacterial cell penetration ability through interactions with the thiol groups of the membrane, which then can disrupt the bacterial DNA and its physiologically important enzymes. These, then, results in inability to reproduction and cell death.64,73 More importantly, AgNPs have multi-target activities and interactions inside the bacterial cell which helps them escape resistance that can enhance antibacterial activities and reduce the microbial resistance when they are functionalized with macrolides.97,99 The diverse mechanisms of antimicrobial action of AgNPs are summarized in Figure 4.3,81 In one study, azithromycin was functionalized with silver NPs and nanoprisms (AgNPs & AgNPrs). Nanoprism conjugates of the drug resulted in a better and efficient antimicrobial activities against E. coli and S. aureus than the non-conjugate forms and AgNP conjugates.100
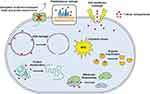 |
Figure 4 General mechanism of action for silver nanoparticles. Notes: Reproduced from Roy A, Bulut O, Some S, Mandal AK, Yilmaz MD. Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Advances. 2019;5:2673–702 (https://creativecommons.org/licenses/by-nc/3.0/).81 |
Some metallic oxide and magnetic NPs provide a discriminatory poisonousness to the microbial cell without significant alterations to the physiologic human cells. ZnO NPs demonstrated an appreciable level of growth inhibition against MRSA, S. epidermidis, K. pneumoniae, L. monocytogenes, S. enteritidis, S. mutans, Lactobacillus, and E. coli.10 Their main antimicrobial activity is described to be disruption of the bacterial membrane due to their interaction with bio-macromolecules inside the membrane and by generating ROS.13 A study on erythromycin-loaded ZnO NPs against macrolide-resistant GABHS isolates confirmed that the functionalization resulted with an enhanced antimicrobial activity and a higher anti-GABHS response with an increase in ZnO NP compositions.79 Magnetic NPs (MNPs) are usually built from a magnetic substance and an element that functionalizes its surface. Hence, they are able to be guided by a magnetic field towards their target sites.101 Commonly utilized magnetic elements include Fe, Co, and Ni. The functionalizing material is selected based on the planned application and outcome to be achieved. Inorganic MNPs such as Fe2O3 and FeO NPs can be coated with a functionalizing layer synthesized from other inorganic materials like silica and gold or using organic macromolecular and bimolecular polymers. The preparation can finally be formulated as NPs (5–500 nm) or microbead forms (500–500 µm).7 The flexible nature of MNPs enables them to be coated in various ways in order to achieve synergistic effects under stimuli responsive conditions with different sensors. Targeted drug delivery, which is a limitation of the conventional, and still for some of the advanced delivery technologies, can also be attained effectively with a well-controlled magnetic filled system.29 For accomplishing this objective, a drug can be conjugated with biocompatible MNPs for delivery, with an external magnetic field applied or focused towards the target sites of therapeutic response in vivo. The magnetic fields will then guide the drug-MNP conjugate towards the target tissue to enhance targeted delivery and physiologic interaction of the drug.83 With this principle, erythromycin-conjugated FeNPs resulted in an augmented antimicrobial activity and reduced bacterial cell viability against S. pneumoniae.33
Solid Lipid Nanocarriers
Solid lipid NPs (SLNPs) have been potential candidates for advanced antimicrobial drug delivery as they are biocompatible, stable, and tolerable. Their formulation process is relatively easier for large-scale production and needs a less strict regulation as they can be formulated from physiologically acceptable lipids while avoiding the use of organic solvents. They are characterized with efficient incorporation of lipophilic drugs within their core and providing an effective shield for products susceptible to degradation. Their nanometric size makes them easier for administration through almost all routes of drug administration and for numerous types of disorders.80,102 They share most of the merits from the orthodox NPs while concurrently excluding several NP-related downsides associated with cost, stability, maintaining drug bioactivity, and leakage of hydrophilic drugs.65 However, they are not completely devoid of limitations as they still need further effort to enhance their drug-loading capacities, to optimize their complex nature of lipid physicochemistry, and for the availability of more advanced vesicular delivery systems.103
Stearic acid SLNPs, loaded with azithromycin, resulted in higher entrapment efficiency and acceptable particle property required for lymphatic absorption. Evaluation of the loaded formulation showed an improved solubility, better oral bioavailability, and higher percentage of in vitro release of that poorly soluble drug.87 In vitro antibacterial study of clarithromycin-loaded SLNPs against E. coli, H. influenza, S. typhi, S. aureus, and S. pneumoniae demonstrated significantly higher zone of inhibition. The cumulative effect of NP characteristics resulted in an improved potency of clarithromycin.3 Another study on azithromycin-loaded SLNPs to determine the associated role of the lipid concentration indicated that the medication-to-polymer ratio had a significant impact on the physicochemical parameters with a drug-to-polymer ratio of 1:3 demonstrating maximum entrapment efficiency. The azithromycin-loaded NPs displayed valuable physicochemical properties for oral administration and enhanced antibacterial capabilities against S. typhi.104 Another group of lipid-based NMs, liposomes, also demonstrated an interesting macrolide delivery characteristics. Liposomal delivery of azithromycin showed a promising in vitro antimicrobial activity against E. coli strains characterized with synergistic activities, efficient inhibition of biofilm synthesis, and enhanced formulation stability.31 Liposomal delivery of macrolides is also reported as an effective means of topical application for skin infections. A study was done on liposomal delivery of azithromycin against infectious microbes on the skin with four different liposomal formulation types: conventional liposomes, deformable liposomes, propylene glycol-containing liposomes, and cationic liposomes. All these formulations offered good entrapment and drug retaining efficiency. The cationic, deformable, and glycol-based liposomes successfully subdued the bacterial strain growth and prevented the biofilm formation which enables them for being a potential approach for improved topical treatment of skin infections from multidrug resistant microbes.105
Polymeric Nanoparticles
Biodegradable NPs synthesized from polymers such as chitosan, gelatin, sodium alginate, polycyanoacrylate or poly (D, L-lactide) and poly (lactide-co-glycolide) (PLGA) are more efficient and effective for drug delivery than the traditional polymeric matrix systems.86 Several foregoing studies invigorated the potential application of this polymeric NMs for effective antimicrobial drug delivery.97,106 A study on an in vitro anti-inflammatory and antimicrobial activity of azithromycin-loaded chitosan reported enhanced antimicrobial activity (zone of inhibition) and anti-inflammatory responses from the study formulations.32,107 Similarly, PLGA nanocapsules loaded with clarithromycin provided enhanced bacterial cell permeation and an apposite aerosol delivery with 1000 times higher reduction in the number of intracellular S. aureus cells in vitro. These formulations also demonstrated 70–80% bactericidal effect against M. abscessus.76 Clarithromycin-loaded chitosan NPs against S. pneumonia also showed more antibacterial property than the intact drug.108 Self-assembling supramolecular amphiphilic polymers have appeared as promising application tools in drug delivery and development due to their superior performance compared to their monomeric forms. These formulations possess integral unoccupied lipophilic hollow spaces for additional guest–host interactions.79,109 Various groups of synthetic amphiphilic macrocycles, such as calixarenes and pillarenes, are nowadays being preferred for the generation of nanocargos for drug delivery because of their stability, potentials for amendment, and inherent extra guest–host interactions. Loading of clarithromycin in calixarene amphiphiles showed an interesting anti-biofilm and bactericidal effects improving the antibacterial activity of clarithromycin.20 Another similar investigation on loading the hydrophobic drug clarithromycin into self-assembling amphiphilic nanostructures demonstrated a well augmented antibacterial activity of clarithromycin against the drug-resistant strains of S. pneumonia with additional immune-regulatory effects and satisfactory safety. The proposed mechanism for this augmented antibacterial activity is illustrated in Figure 5.73
 |
Figure 5 Proposed mechanism of action of AZT-D-LPs. Notes: Reproduced from Liu X, Li Z, Wang et al. Novel antimicrobial peptide—modified azithromycin-loaded liposomes against methicillin-resistantStaphylococcus aureus. Int J Nanomedicine. 2016;11:6781–94 (https://creativecommons.org/licenses/by-nc/3.0/).73 |
Intercalated Nanostructures
Intercalated NMs conveyed an outstanding delivery characteristics for targeted and controlled drug delivery against infectious microbes.34,35 They are currently under substantial consideration in novel drug development due to their promising properties with respect to cost, biocompatibility, safety, and drug loading and release efficiency.98 Several formulations of medicinal products intercalated with constructive organic anions, such as DNA, amino acids, and polymers have been set effectively, thus intensifying their variety of applications.13,110 Intercalated structures incorporated by antibiotic drugs also showed improved antimicrobial effects.77 A preliminary study on docking macrolides to be intercalated in natural biomaterials like actin demonstrated that residues on the actin binding site formed important hydrophobic and hydrogen-bonding interactions with the ligands for the macrolide drug. The binding site possessed three key segments: a lipophilic pocket that can intercalate the huge lipophilic “anchor” of the macrolide; a lipophilic “cleft” to interpolate the lipophilic tail of the drug and depolarize the molecules, and an opposite end of a lipophobic area for integration of hydrophilic components or drugs.111 A biosynthetic and phylogenetic intercalation of a newly discovered macrolide anthracimycin, involving an intramolecular cycloaddition in a natural microorganism-based biosynthetic gene cluster, provided an enhanced antimicrobial activity and development of novel analogues with new structural architectures and novel modes of action against Gram-positive pathogens including B. anthracis and methicillin-resistant and vancomycin-resistant S. aureus (MRSA and VRSA).112 This promising intercalation investigation result might further be boosted by the application of advanced nanostructured delivery systems.
Conclusion
Microbial resistance has become the world’s most jeopardizing public health concern after the nuclear war, as it significantly imparts overall global health and economic challenges. Like that of other antimicrobial drugs, macrolide resistance has been increasing alarmingly, rendering it difficult to treat infections using conventionally available drugs. Hence, it is now the right time to address those challenges by innovating unconventional approaches. In light of these events, integration of biological and nanomaterial sciences is proving itself as an emerging solution to be utilized in medicine for the delivery of antimicrobials and other therapeutic agents in a way that prevents resistance, promotes targeting, reduces non-specific cytotoxicity, and enhances overall treatment outcome. Metallic NPs, polymeric NMs, vesicular delivery systems, and some other nanostructures enhanced the antibacterial activities of macrolide drugs by improving their pharmacokinetics, carrying and delivering more efficiently at target sites, minimizing toxicities, and most importantly, exerting novel mechanisms for combating drug resistance. Among these delivery systems, polymer-based NPs have gained considerable attention due to their possession of enhanced targeting towards the infectious regions and their higher stability in biological environments.
The Future Perspective
Though nanotechnology is spectacularly innovating and advancing the health care delivery systems, there are still challenges that require further efforts for its clinical translation at extraordinary levels. The main areas to be focused include NM safety, biocompatibility, intellectual property, regulations, production time, and cost.51,72,113 Majority of the challenges are associated with the complexity of adjusting size and shape of the NPs with an acceptable level of stability through the production process. Most NPs have an intrinsic ability to infuse through membranes and blowout to diverse body compartments.47,114 Though this property is regarded as beneficial for drug transport, it may induce a risk for non-target accumulation posing possible health intimidations. Enhanced enter-/intracellular membrane transportation, on the other hand, may lead the NPs escape the body’s defense mechanisms and elicit inflammatory and/or toxic effects.115 NPs can produce ROS that cause inflammation and toxicity with other associated acute and chronic major organ complications. Additionally, the biological-NP interactions are not still entirely investigated.24,116 Therefore, it is prudent to conduct a thorough exploration to further characterize the likely mechanisms of the interaction between the NPs and the biological systems. The inconsistent in vitro evaluations on NP activities should be amplified and complemented by thorough in vivo investigations.50 The toxicity of NPs on human cells, particularly neurotoxicity, should be given more emphasis to plainly understand the mechanisms that NPs cross the blood-brain barrier. Although only a few studies have reported that NPs enter bacterial cells through porins, it is crucial to further understand the precise mechanisms for bacterial cell penetration of NPs.117 Researchers should emphasize on using safe and environmentally acceptable natural reducing agents for NP synthesis and they should master translating the nanoformulations into clinical applications.51,115 In sum, dedicated multidisciplinary efforts are still in-need for advanced quality research, assured NM safety, successful therapeutic outcome, cost effective production, well recognized regulatory framework, clinical extrapolation, and commercialization of antimicrobial nanodelivery.
Abbreviations
MLS, Macrolides; FTIR, Fourier Transform Infrared; MIC, Minimum Inhibitory Concentration; NMs, Nanomaterials; FDA, Food and Drug Administration; EU, European Union; ROS, Reticulo-endothelial system; rRNA, Ribosomal Ribose Nucleic Acid; ASPs, Antimicrobial stewardship programs; PK/PD, Pharmacodynamics/Pharmacokinetics; MLS, Macrolides; mRNA, Messenger Ribonucleic Acid; MAPs, Macromolecular antimicrobial polymers; DLs, Deformable liposomes; GABHS, Group A beta hemolyticus; PNP, Polymeric Nanoparticles; MLSB, Macrolide– lincosamide–streptogramin B; MQESD, Modified quasi-emulsion solvent diffusion; MRSA, Methicillin resistant staphylococcus aureus; PGLs, Propylene glycol-containing liposomes; AMR, Antimicrobial Resistance; MDR, Multi Drug Resistant; MNPs, Magnetic Nanoparticles; NPs, Nanoparticles; WHO, World Health Organization; AZM, Azithromycin; GAS, Group A streptococci; CLA, Clarithromycin; ERY, Erythromycin; BCS, Biopharmaceutical classification; CL, Conventional liposomes; DMSO, Dimethyl sulfoxide; CATLs, Cationic liposomes; MNPs, Magnetic nanoparticles; LAN, Acetylcysteine liposomal nanoparticles; LA, Liposomal; Azithromycin; LDH, Layered Hydroxide; NAC, N-acetylcysteine.
Acknowledgments
Addis Ababa University is thankfully acknowledged for providing internet access.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas, took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Imran M, Jha SK, Hasan N, et al. Overcoming multidrug resistance of antibiotics via nanodelivery systems. Pharmaceutics. 2022;14(3):1–25. doi:10.3390/pharmaceutics14030586
2. Fareed N, Nisa S, Bibi Y, et al. Green synthesized silver nanoparticles using carrot extract exhibited strong antibacterial activity against multidrug resistant bacteria. J King Saud Univ. 2023;35(2):102477. doi:10.1016/j.jksus.2022.102477
3. Valizadeh H, Mohammadi G, Ehyaei R, Milani M, Azhdarzadeh M, Lotfipour F. Antibacterial activity of clarithromycin loaded PLGA nanoparticles. Int J Pharm Sci. 2012;2012:63–68.
4. Abou DH, Abbas HS. Antimicrobial activity of biosynthesized Cuo / Se nanocomposite against Helicobacter pylori. Arab J Chem. 2023;16(9):105095. doi:10.1016/j.arabjc.2023.105095
5. Alavi M, Rai M. Expert review of anti-infective therapy recent advances in antibacterial applications of metal nanoparticles (MNPs) and metal nanocomposites (MNCs) against multidrug- resistant (MDR) bacteria. Expert Rev Anti Infect Ther. 2019;17(6):419–428. doi:10.1080/14787210.2019.1614914
6. Thu Dao TA, Webb HK, Malherbe F. Optimization of pectin extraction from fruit peels by response surface method: conventional versus microwave-assisted heating. Food Hydrocoll. 2021;113:106475. doi:10.1016/j.foodhyd.2020.106475
7. Cristea C, Tertis M, Galatus R. Magnetic nanoparticles for antibiotics detection. Nanomaterials. 2017;7(6):119. doi:10.3390/nano7060119
8. Wilson DN, Hauryliuk V, Atkinson GC, O’Neill AJ. Target protection as a key antibiotic resistance mechanism. Nat Rev Microbiol. 2020;18:637–648. doi:10.1038/s41579-020-0386-z
9. Abdulbaqi MR, Maraie NK, Dawood A Loading of clarithromycin and paclitaxel on synthesized CDS/NIO nanoparticles as promising nanocarriers nanoparticles as promising nanocarriers; 2016.
10. Beyth N, Houri-Haddad Y, Domb A, Khan W, Hazan R. Alternative antimicrobial approach: nano-antimicrobial materials. Evid Based Complement Altern Med. 2015;2015:1–16. doi:10.1155/2015/246012
11. Wan F, Jumaa H, Sternberg C, Rades T, Nielsen HM, Kłodzin SN. Utilizing nanoparticles for improving anti-biofilm effects of azithromycin: a head-to-head comparison of modified hyaluronic acid nanogels and coated poly (lactic- co -glycolic acid) nanoparticles. J Colloid Interface Sci. 2019;555:595–606. doi:10.1016/j.jcis.2019.08.006
12. Baquero F. Gram-positive resistance: challenge for the development of new antibiotics. J Antimicrob Chemother. 1997;39(SUPPL. A):1–6. doi:10.1093/jac/39.suppl_1.1
13. Das B, Patra S. Antimicrobials: meeting the challenges of antibiotic resistance through nanotechnology. In: Nanostructures for Antimicrobial Therapy: Nanostructures in Therapeutic Medicine Series. Elsevier Inc.; 2017:1–22.
14. Ergin A, Ercis S, Hasçelik G. Macrolide resistance mechanisms and in vitro susceptibility patterns of viridans group streptococci isolated from blood cultures. J Antimicrob Chemother. 2006;2005:139–141. doi:10.1093/jac/dki404
15. Peche J. Macrolide resistance mechanisms in Gram-positive cocci. Int J Antimicrob Agents. 2001;18:1–4. doi:10.1016/S0924-8579(01)00396-X
16. Miklasi M. Mechanisms of resistance to macrolide antibiotics among Staphylococcus aureus. Antibiotics. 2021;2021:1.
17. Jubeh B, Breijyeh Z, Ismail A. Resistance of gram-positive bacteria to current antibacterial agents and overcoming approaches. Molecules. 2020;26:1–22. doi:10.3390/molecules26010001
18. John JF, Harvin AM. History and evolution of antibiotic resistance in coagulase-negative staphylococci: susceptibility pro fi les of new anti-staphylococcal agents. Ther Clin Risk Manag. 2007;3(14):1143–1152.
19. Vázquez-Laslop N, Mankin AS. How macrolide antibiotics work. Trends Biochem Sci. 2018;43(9):668–684. doi:10.1016/j.tibs.2018.06.011
20. Ali I, Imran M, Saifullah S, Tian H, Guo D, Shah MR. Amphiphilic p-Sulfonatocalix[6]arene based self-assembled nanostructures for enhanced clarithromycin activity against resistant streptococcus pneumoniae. Colloids Surf B. 2019;186:110676. doi:10.1016/j.colsurfb.2019.110676
21. Gómez MA, Bonilla JM, Coronel MA, et al. Antibacterial activity against Staphylococcus aureus of chitosan/chondroitin sulfate nanocomplex aerogels alone and enriched with erythromycin and elephant garlic (Allium ampeloprasum L. var. ampeloprasum) extract. Pure Appl Chem. 2018;90(5):885–900. doi:10.1515/pac-2016-1112
22. Shekunov BY, Chattopadhyay P, Tong HHY, Chow AHL. Particle size analysis in pharmaceutics: principles, methods and applications. Pharm Res. 2007;24(2):203–227. doi:10.1007/s11095-006-9146-7
23. Golkar T, Zielinski M, Berghuis AM. Look and outlook on enzyme-mediated macrolide resistance. Front Microbiol. 2018;9:1–15. doi:10.3389/fmicb.2018.01942
24. Rudramurthy GR, Swamy MK, Sinniah UR, Ghasemzadeh A. Nanoparticles: alternatives against drug-resistant pathogenic microbes. Molecules. 2016;21(7):1–30. doi:10.3390/molecules21070836
25. Zharkova MS, Orlov DS, Golubeva OY, et al. Application of antimicrobial peptides of the innate immune system in combination with conventional antibiotics-a novel way to combat antibiotic resistance? Front Cell Infect Microbiol. 2019;9(APR). doi:10.3389/fcimb.2019.00128
26. Lee H, Jung D, Yeom JS, et al. Evaluation of ceftriaxone utilization at multicenter study. Korean J Intern Med. 2009;24:374–380. doi:10.3904/kjim.2009.24.4.374
27. Koopaei MN, Maghazei MS, Mostafavi SH, et al. Enhanced antibacterial activity of roxithromycin loaded pegylated poly lactide-co-glycolide nanoparticles. J Pharm Sci. 2012;20(1):1–8.
28. Mba IE, Nweze EI. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: research progress, challenges, and prospects. World J Microbiol Biotechnol. 2021;37(6):1–30. doi:10.1007/s11274-021-03070-x
29. Baptista PV, McCusker MP, Carvalho A, Ferreira DA. Nano-strategies to fight multidrug resistant bacteria —“a battle of the titans”. Front Microbiol. 2018;9(July):1–26. doi:10.3389/fmicb.2018.01441
30. Jabri AT, Imran M, Rao K. Fabrication of lecithin-gum tragacanth muco-adhesive hybrid nano-carrier system for in-vivo performance of Amphotericin B. Carbohydr Polym. 2018;194. doi:10.1016/j.carbpol.2018.04.013
31. Aljihani SA, Alehaideb Z, Alarfaj RE, et al. Enhancing azithromycin antibacterial activity by encapsulation in liposomes/liposomal-N-acetylcysteine formulations against resistant clinical strains of Escherichia coli. Saudi J Biol Sci. 2020;27(11):3065–3071. doi:10.1016/j.sjbs.2020.09.012
32. Shunmugaperumal T, Kaur V. In vitro anti-inflammatory and antimicrobial activities of azithromycin after loaded in chitosan- and tween 20-based oil-in-water macroemulsion for acne management. AAPS Pharm Sci Tech. 2016;17(3):700–709. doi:10.1208/s12249-015-0401-2
33. Vignesh K, Rajarajan M, Suganthi A. Photocatalytic degradation of erythromycin under visible light by zinc phthalocyanine-modified titania nanoparticles. Mater Sci Semicond Process. 2014;23(1):98–103. doi:10.1016/j.mssp.2014.02.050
34. Gao P, Nie X, Zou M, Shi Y, Cheng G. Recent advances in materials for extended-release antibiotic delivery system. J Antibiot. 2011;64(9):625–634.
35. Forna N, Damir D, Duceac LD, et al. Nano‐architectonics of antibiotic‐loaded polymer particles as vehicles for active molecules. Appl Sci. 2022;12(4):1998. doi:10.3390/app12041998
36. Periti P, Mazzei T, Mini E, Novelli A. Pharmacokinetic Drug Interactions of Macrolides. Clin Pharmacokinet. 1992;23(2):106–131. doi:10.2165/00003088-199223020-00004
37. More PR, Pandit S, Filippis A, Franci G, Mijakovic I, Galdiero M. Silver Nanoparticles: bactericidal and mechanistic approach against drug resistant pathogens. Microorganisms. 2023;11(2):1.
38. Walsh FM, Amyes SGB. Microbiology and drug resistance mechanisms of fully resistant pathogens. Curr Opin Microbiol. 2004;7(5):439–444. doi:10.1016/j.mib.2004.08.007
39. Leclercq R. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clin Infect Dis. 2002;34:482–492. doi:10.1086/324626
40. Rams TE, Dujardin S, Sautter JD, Degener JE, van Winkelhoff AJ. Spiramycin resistance in human periodontitis microbiota. Anaerobe. 2011;17(4):201–205. doi:10.1016/j.anaerobe.2011.03.017
41. Bush K, Courvalin P, Dantas G, et al. Tackling antibiotic resistance. Nat Rev Microbiol. 2011;9(12):894–896. doi:10.1038/nrmicro2693
42. Miao MX, San QZ, Xin YW, Kun QF, Gu Y. Study on the characteristics of pectin-ketoprofen for colon targeting in rats. Int J Pharm. 2005;298(1):91–97. doi:10.1016/j.ijpharm.2005.04.012
43. Tanwar J, Das S, Fatima Z, Hameed S. Multidrug resistance: an emerging crisis. Interdiscip Perspect Infect Dis. 2014;2014:1–7. doi:10.1155/2014/541340
44. Gaynor M, Mankin AS. Macrolide antibiotics: binding site, mechanism of action. Resistance. 2005;2005:21–35.
45. Aruguete DM, Kim B, Hochella MF, et al. Antimicrobial nanotechnology: its potential for the effective management of microbial drug resistance and implications for research needs in microbial nanotoxicology. Environ Sci Process Impacts. 2013;15(1):93–102. doi:10.1039/C2EM30692A
46. Shipitsyna E, Rumyantseva T, Golparian D, et al. Prevalence of macrolide and fluoroquinolone resistance-mediating mutations in Mycoplasma genitalium in five cities in Russia and Estonia. PLoS One. 2017;12(4):1–11. doi:10.1371/journal.pone.0175763
47. Hetta HF, Ramadan YN, Al-Harbi AI. Nanotechnology as a promising approach to combat multidrug resistant bacteria: a comprehensive review and future perspectives. Biomedicines. 2023;11(2):1.
48. Čižman M. The use and resistance to antibiotics in the community. Int J Antimicrob Agents. 2003;21(4):297–307. doi:10.1016/S0924-8579(02)00394-1
49. Öztürk AA, Aygül A, Şenel B. Influence of glyceryl behenate, tripalmitin and stearic acid on the properties of clarithromycin incorporated solid lipid nanoparticles (SLNs): formulation, characterization, antibacterial activity and cytotoxicity. J Drug Deliv Sci Technol. 2019;54:101240. doi:10.1016/j.jddst.2019.101240
50. Brar B, Marwaha S, Poonia AK, Koul B, Kajla S, Rajput VD. Nanotechnology: a contemporary therapeutic approach in combating infections from multidrug-resistant bacteria. Arch Microbiol. 2023;205(2):1–19. doi:10.1007/s00203-023-03404-3
51. Chakraborty N, Jha D, Roy I, et al. Nanobiotics against antimicrobial resistance: harnessing the power of nanoscale materials and technologies. J Nanobiotechnol. 2022;20(1):1–25. doi:10.1186/s12951-022-01573-9
52. Shibl AM. Patterns of macrolide resistance determinants among S. pyogenes and S. pneumoniae isolates in Saudi Arabia. J Int Med Res. 2005;33(3):349–355. doi:10.1177/147323000503300310
53. Ruddaraju LK, Pammi SVN, Guntuku G, Padavala VS, Kolapalli VRM. A review on anti-bacterials to combat resistance: from ancient era of plants and metals to present and future perspectives of green nano technological combinations. Asian J Pharm Sci. 2020;15(1):42–59. doi:10.1016/j.ajps.2019.03.002
54. Engberg J, Aarestrup FM, Taylor DE, Gerner-smidt P, Nachamkin I. Quinolone and macrolide resistance in campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg Infect Dis. 2001;7(1):24–34. doi:10.3201/eid0701.010104
55. Wang W, Ma X, Jiang P, et al. AC SC. Food Hydrocoll. 2016. doi:10.1016/j.foodhyd.2016.06.019
56. Jain S, Arora P, Popli H. A comprehensive review on Citrus aurantifolia essential oil: its phytochemistry and pharmacological aspects. Brazilian J Nat Sci. 2020;3(2):354. doi:10.31415/bjns.v3i2.101
57. Klugman KP, Lonks JR. Hidden Epidemic of Macrolide- resistant Pneumococci. Emerg Infect Dis. 2005;11(6):802–807. doi:10.3201/eid1106.050147
58. Zavadska D, Berziņa D, Drukaļska L, Pugačova Ņ, Miklaševičs E, Gardovska D. Macrolide resistance in group A beta haemolytic Streptococcus isolated from outpatient children in Latvia. Apmis. 2010;118(5):366–370. doi:10.1111/j.1600-0463.2010.02607.x
59. Singh R, Smitha MS, Singh SP. The role of nanotechnology in combating multi-drug resistant bacteria. J Nanosci Nanotechnol. 2014;14(7):4745–4756. doi:10.1166/jnn.2014.9527
60. Ramteke S, Jain NK. Clarithromycin- and omeprazole-containing gliadin nanoparticles for the treatment of Helicobacter pylori. J Drug Target. 2008;16(January):65–72. doi:10.1080/10611860701733278
61. Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven challenges for nanomedicine. Nat Nanotechnol. 2008;3(May):2–4. doi:10.1038/nnano.2008.114
62. Imran M, Raza M, Ullah F, et al. Glycoside-based niosomal nanocarrier for enhanced in-vivo performance of Ce fi xime. Int J Pharm. 2016;505:122–132. doi:10.1016/j.ijpharm.2016.03.042
63. Mubeen B, Ansar AN, Rasool R, et al. Nanotechnology as a novel approach in combating microbes providing an alternative to antibiotics. Antibiotics. 2021;10(12). doi:10.3390/antibiotics10121473
64. Mi G, Shi D, Wang M, Webster TJ. Reducing bacterial infections and biofilm formation using nanoparticles and nanostructured antibacterial Surfaces. Adv Healthc Mater. 2018;7(13):1–23. doi:10.1002/adhm.201800103
65. Kalhapure RS, Suleman N, Mocktar C, Seedat N, Govender T. Nanoengineered drug delivery systems for enhancing antibiotic therapy. J Pharm Sci. 2014;2014:1–34.
66. Zaidur M, Sabuj R. Nanoscale Advances Inhaled antibiotic-loaded polymeric nanoparticles for the management of lower respiratory tract. Nanoscale Adv. 2021;3:4005–4018. doi:10.1039/D1NA00205H
67. Wu S, Altenried S, Zogg A, Zuber F, Maniura-weber K, Ren Q. Role of the surface nanoscale roughness of stainless steel on bacterial adhesion and microcolony formation. ACS omega. 2018;3:6456–6464. doi:10.1021/acsomega.8b00769
68. Saddik MS, Elsayed MMA, El-Mokhtar MA, et al. Tailoring of novel azithromycin-loaded zinc oxide nanoparticles for wound healing. Pharmaceutics. 2022;14(1):111. doi:10.3390/pharmaceutics14010111
69. Schleh C, Semmler-behnke M, Lipka J, et al. Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in secondary target organs after oral administration. Nanotoxicology. 2012;2012:5390.
70. Mitchell MJ, Billingsley MM, Haley RM, Langer R, Wechsler ME, Peppas NA. Engineering precision nanoparticles. Nat Rev Drug Discov. 2021;20:101–124. doi:10.1038/s41573-020-0090-8
71. Joseph TM, Mahapatra DK, Esmaeili A, et al. Nanoparticles: taking a unique position in medicine. Nanomaterials. 2023;13:574. doi:10.3390/nano13030574
72. Vassallo A, Silletti MF, Faraone I, Milella L. Nanoparticulate antibiotic systems as antibacterial agents and antibiotic delivery platforms to fight infections. J Nanomater. 2020;2020:1–31. doi:10.1155/2020/6905631
73. Liu X, Li Z, Wang X, et al. Novel antimicrobial peptide—modified azithromycin-loaded liposomes against methicillin-resistant Staphylococcus aureus. Int J Nanomedicine. 2016;11:6781–6794. doi:10.2147/IJN.S107107
74. Moritz M, Geszke-moritz M. The newest achievements in synthesis, immobilization and practical applications of antibacterial nanoparticles. J Chem Eng. 2013;228:596–613. doi:10.1016/j.cej.2013.05.046
75. Chatterjee M, Hens A, Mahato K, Jaiswal N. A novel approach to fabricate dye-encapsulated polymeric micro- and nanoparticles by thin film dewetting technique. J Colloid Interface Sci. 2017;506:126–134. doi:10.1016/j.jcis.2017.07.023
76. Schmitt V, Pätzold L, Abed N, et al. PLGA nanocapsules improve the delivery of clarithromycin to kill intracellular Staphylococcus aureus and Mycobacterium abscessus. Nanomedicine. 2019;24:102125. doi:10.1016/j.nano.2019.102125
77. Tewabe A, Marew T, Birhanu G. The contribution of nano-based strategies in overcoming ceftriaxone resistance: a literature review. Pharmacol Res Perspect. 2021;9(4):1–12. doi:10.1002/prp2.849
78. Yayehrad AT, Wondie GB, Marew T. Different nanotechnology approaches for ciprofloxacin delivery against multidrug-resistant microbes. Infect Drug Resist. 2022;15(January):413–426. doi:10.2147/IDR.S348643
79. Fozouni L, Javidmehr S, Barghamadi H, Mazandarani A, Rouhafza S. Antibacterial effect of zinc oxide nanoparticles on group A β-hemolytic Streptococci with macrolide resistance isolated from university student carriers in north of Iran. Int J Infect. 2019; 2019:1–7.
80. Gupta A, Mumtaz S, Li C, Hussain I, Rotello VM. Combatting antibiotic-resistant bacteria using nanomaterials. Chem Soc Rev. 2019;48:415–427. doi:10.1039/C7CS00748E
81. Roy A, Bulut O, Some S, Mandal AK, Yilmaz MD. Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019;9:2673–2702. doi:10.1039/C8RA08982E
82. Chowdhury A, Kunjiappan S, Panneerselvam T. Nanotechnology and nanocarrier-based approaches on treatment of degenerative diseases. Int Nano Lett. 2017;7(2):91–122.
83. Dobson J. Magnetic nanoparticles for drug delivery. Drug Dev Res. 2006;67(1):55–60. doi:10.1002/ddr.20067
84. Li P, Chen X, Shen Y, et al. Mucus penetration enhanced lipid polymer nanoparticles improve the eradication rate of Helicobacter pylori bio fi lm. J Control Release. 2019;300(132):52–63. doi:10.1016/j.jconrel.2019.02.039
85. Masood F, Yasin T, Bukhari H, Mujahid M. Characterization and application of roxithromycin loaded cyclodextrin based nanoparticles for treatment of multidrug resistant bacteria. Mater Sci Eng C. 2016;61:1–7. doi:10.1016/j.msec.2015.11.076
86. Manimekalai P, Manavalan R. Selection of excipients for the formulation of Ceftriaxone sodium loaded chitosan Nanoparticle through drug - Excipient compatibility testing. Int J Res Pharm Sci. 2015;6(2):199–203.
87. Bhattacharyya S, Reddy P. Effect of surfactant on azithromycin dihydrate loaded stearic acid solid lipid nanoparticles. Turkish J Pharm Sci. 2019;16(4):425–431. doi:10.4274/tjps.galenos.2018.82160
88. Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems - a review (part 1). Trop J Pharm Res. 2013;12(April):255–264.
89. Angsantikul P, Thamphiwatana S, Zhang Q, et al. Coating nanoparticles with gastric epithelial cell membrane for targeted antibiotic delivery against helicobacter pylori infection. Adv Ther. 2018;2018:1–9.
90. Alhajlan M, Alhariri M, Omri A. Efficacy and safety of liposomal clarithromycin and its effect on pseudomonas aeruginosa virulence factors. Antimicrob Agents Chemother. 2013;57(6):2694–2704. doi:10.1128/AAC.00235-13
91. Mohd S, Rizvi D, Selim A, et al. Antibiotic-loaded gold nanoparticles: a nano-arsenal against ESBL producer-resistant pathogens. Pharmaceutics. 2023;2023:1–20.
92. Abruzzo A, Parolin C, Rossi M, Vitali B, Cappadone C, Bigucci F. Development and characterization of azithromycin-loaded microemulsions: a promising tool for the treatment of bacterial skin infections. Antibiotics. 2022;11(8):1040. doi:10.3390/antibiotics11081040
93. Owais HM, Baddour MM, El-metwally HAE, Barakat HS, Ammar NS, Meheissen MA. Assessment of the in vitro activity of azithromycin niosomes alone and in combination with levofloxacin on extensively drug-resistant Klebsiella pneumoniae clinical isolates. Braz J Microbiol. 2021;52:597–606. doi:10.1007/s42770-021-00433-2
94. Gao Y, Wang J, Chai M, et al. Size and charge adaptive clustered nanoparticles targeting the bio fi lm microenvironment for chronic lung infection management. ACS nano. 2020;14:5686–5699. doi:10.1021/acsnano.0c00269
95. Bosnjakovic A, Mishra MK, Ren W, et al. Poly (amidoamine) dendrimer-erythromycin conjugates for drug delivery to macrophages involved in periprosthetic inflammation. Biol Med. 2011;7(3):284–294. doi:10.1016/j.nano.2010.10.008
96. Altun E, Aydogdu MO, Chung E, Ren G, Homer-Vanniasinkam S, Edirisinghe M. Metal-based nanoparticles for combating antibiotic resistance. Appl Phys Rev. 2021;8(4). doi:10.1063/5.0060299
97. Sharma A, Kumar Arya D, Dua M, Chhatwal GS, Johri AK. Nano-technology for targeted drug delivery to combat antibiotic resistance. Expert Opin Drug Deliv. 2012;9(11):1325–1332. doi:10.1517/17425247.2012.717927
98. Emmanuel R, Saravanan M, Ovais M, Padmavathy S, Shinwari ZK, Prakash P. Antimicrobial efficacy of drug blended biosynthesized colloidal gold nanoparticles from Justicia glauca against oral pathogens: a nanoantibiotic approach. Microb Pathog. 2017;113:295–302. doi:10.1016/j.micpath.2017.10.055
99. Mansouri F, Saffari M, Moniri R, Abbas Moosavi G, Molaghanbari M, Razavizade M Investigation of the effect of silver nanoparticles alone and their combination with clarithromycin on H. pylori isolates; 2022. Available from: https://www.researchsquare.com/article/rs-1631922/latest.pdf.
100. Yaqub A, Ali S, Allah Ditta S, Tanvir F, Ali S, Naz M. Enhanced bactericidal activity of Azithromycin-coated silver nanoprisms in comparison to their spherical-shaped counterparts. Micro Nano Lett. 2020;15:834–839. doi:10.1049/mnl.2019.0704
101. Liu JF, Issadore D HHS Public Access; 2020.
102. Darbasizadeh B, Fatahi Y, Feyzi-barnaji B, et al. Crosslinked-polyvinyl alcohol-carboxymethyl cellulose/ZnO nanocomposite fibrous mats containing erythromycin (PVA-CMC/ZnO-EM): fabrication, characterization and in-vitro release and anti-bacterial properties. Int J Biol Macromol. 2019;141:1137–1146. doi:10.1016/j.ijbiomac.2019.09.060
103. Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2012;64:83–101. doi:10.1016/j.addr.2012.09.021
104. Vanić Ž, Rukavina Z, Manner S, et al. Azithromycin-liposomes as a novel approach for localized therapy of cervicovaginal bacterial infections Azithromycin-liposomes as a novel approach for localized therapy of cervicovaginal bacterial infections. Int J Nanomedicine. 2023;1:1.
105. Rukavina Z, Šegvić KM, Filipović-Grčić J, Lovrić J, Vanić Ž. Azithromycin-loaded liposomes for enhanced topical treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections. Int J Pharm. 2018;553(1–2):109–119. doi:10.1016/j.ijpharm.2018.10.024
106. Bin-Jumah M, Gilani SJ, Jahangir MA, et al. Clarithromycin-loaded ocular chitosan nanoparticle: formulation, optimization, characterization, ocular irritation, and antimicrobial activity. Int J Nanomedicine. 2020;15:7861–7875. doi:10.2147/IJN.S269004
107. Ouyang J, Huang H, Chen X, Chen J. Biodegradable polymer/TiO2Nanotubes loaded roxithromycin as nanoarray capsules for long-lasting antibacterial properties of titanium implant. J Nanomater. 2020;2020:1–11. doi:10.1155/2020/5432926
108. Ashvini HM, Balla A, Mutta SK. Clarithromycin-loaded chitosan nanoparticles: preparation, characterisation and antibacterial activity on Streptococcus pneumonia. Indian J Pharm Sci. 2019;81(2):302–308. doi:10.36468/pharmaceutical-sciences.511
109. Le H, Karakasyan C, Jouenne T, Cerf D, Dé E. Application of polymeric nanocarriers for enhancing the bioavailability of antibiotics at the target site and overcoming antimicrobial resistance. Appl Sci. 2021;11(22):10695. doi:10.3390/app112210695
110. Al-ahmer SD, Shami AM, Al-saadi BQH. Using of hybrid nanoantibiotics antimicrobial agent as promising antimicrobial agent. Iraqi J Biotechnol. 2018;17(3):1–16.
111. Melville JL, Moal IH, Baker-Glenn C, Shaw PE, Pattenden G, Hirst JD. The structural determinants of macrolide-actin binding: in silico insights. Biophys J. 2007;92(11):3862–3867. doi:10.1529/biophysj.106.103580
112. Alt S, Wilkinson B. Biosynthesis of the novel macrolide antibiotic anthracimycin. ACS Chem Biol. 2015;10(11):2668–2679. doi:10.1021/acschembio.5b00525
113. Hadiya S, Reham A, Rehab M. Nanoparticles based combined antimicrobial drug. Bull Pharm Sci. 2022;45(2):1121–1141.
114. Abo-zeid Y, Amer A, Bakkar MR, El-Houssieny B, Sakran W. Antimicrobial activity of azithromycin encapsulated into PLGA NPs: a potential strategy to overcome efflux resistance. Antibiotics. 2022;11(11):1–20. doi:10.3390/antibiotics11111623
115. Tigabu B, Getachew A. Treatment of antibiotic-resistant bacteria by nanoparticles: current approaches and prospects. Ann Adv Chem. 2022;6(1):001–9. doi:10.29328/journal.aac.1001025
116. Azhdarzadeh M, Lotfipour F, Zakeri-milani P, Mohammadi G, Valizadeh H. Anti-bacterial performance of azithromycin nanoparticles as colloidal drug delivery system against different gram-negative and gram- positive bacteria. Adv Pharm Bull. 2012;2(1):17–24. doi:10.5681/apb.2012.003
117. Khalid UA, Afolabi BL. Nano-carriers based combating approach against antibiotic resistance: an insight into nanoparticles based peptide delivery. Int J Med Microbiol. 2023;1:1–23.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
