Back to Journals » International Journal of Nanomedicine » Volume 19
How Biodegradable Polymers Can be Effective Drug Delivery Systems for Cannabinoids? Prospectives and Challenges
Authors Sobieraj J , Strzelecka K , Sobczak M, Oledzka E
Received 18 January 2024
Accepted for publication 15 April 2024
Published 22 May 2024 Volume 2024:19 Pages 4607—4649
DOI https://doi.org/10.2147/IJN.S458907
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Jan Sobieraj,* Katarzyna Strzelecka,* Marcin Sobczak, Ewa Oledzka
Department of Pharmaceutical Chemistry and Biomaterials, Faculty of Pharmacy, Medical University of Warsaw, Warsaw, 02-097, Poland
*These authors contributed equally to this work
Correspondence: Ewa Oledzka, Department of Pharmaceutical Chemistry and Biomaterials, Faculty of Pharmacy, Medical University of Warsaw, 1 Banacha Street, Warsaw, 02-097, Tel +48-22-572-07-55, Email [email protected]
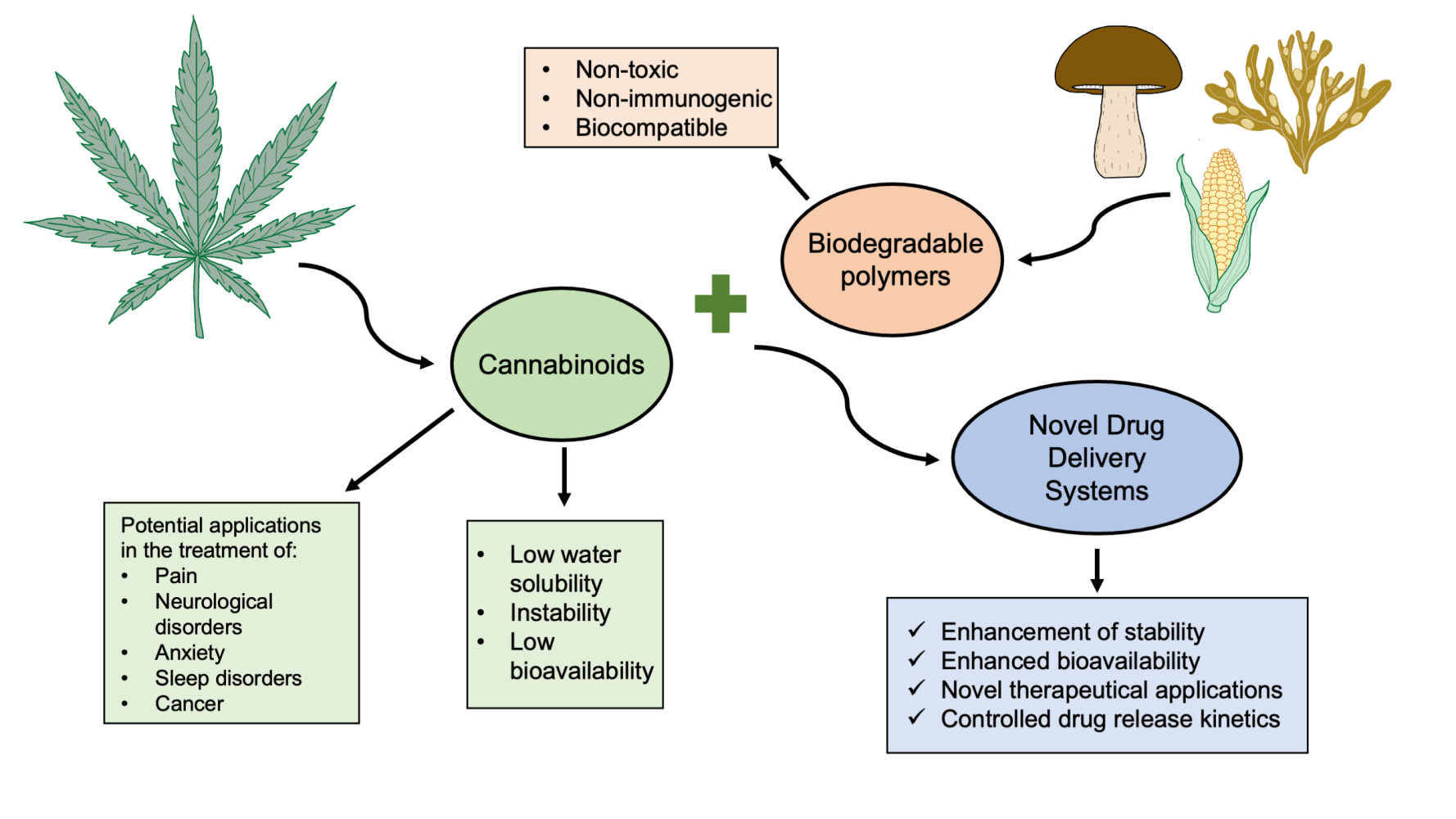
Abstract: Cannabinoids are compounds found in and derived from the Cannabis plants that have become increasingly recognised as significant modulating factors of physiological mechanisms and inflammatory reactions of the organism, thus inevitably affecting maintenance of homeostasis. Medical Cannabis popularity has surged since its legal regulation growing around the world. Numerous promising discoveries bring more data on cannabinoids’ pharmacological characteristics and therapeutic applications. Given the current surge in interest in the medical use of cannabinoids, there is an urgent need for an effective method of their administration. Surpassing low bioavailability, low water solubility, and instability became an important milestone in the advancement of cannabinoids in pharmaceutical applications. The numerous uses of cannabinoids in clinical practice remain restricted by limited administration alternatives, but there is hope when biodegradable polymers are taken into account. The primary objective of this review is to highlight the wide range of indications for which cannabinoids may be used, as well as the polymeric carriers that enhance their effectiveness. The current review described a wide range of therapeutic applications of cannabinoids, including pain management, neurological and sleep disorders, anxiety, and cancer treatment. The use of these compounds was further examined in the area of dermatology and cosmetology. Finally, with the use of biodegradable polymer-based drug delivery systems (DDSs), it was demonstrated that cannabinoids can be delivered specifically to the intended site while also improving the drug’s physicochemical properties, emphasizing their utility. Nevertheless, additional clinical trials on novel cannabinoids’ formulations are required, as their full spectrum therapeutical potential is yet to be unravelled.
Keywords: cannabinoids, drug delivery systems, cannabidiol, tetrahydrocannabinol, biodegradable polymers, nanomedicine
Graphical Abstract:
Introduction
Cannabinoids are obtained from plants belonging to the genus Cannabis, Cannabaceae family. Cannabis, particularly Cannabis sativa, has been known for its therapeutic and psychotomimetic applications for over 6000 years. Every Cannabis sativa plant produces active compounds, but the levels and proportions of these compounds vary among different varieties. These variations cannot be attributed solely to genetic makeup, as environmental factors such as climate and growing conditions may also influence them. Therefore, it is more accurate to refer to these variables as chemical varieties or chemovars, instead of strains.1 The main active components present in Cannabis sativa comprise ∆9-tetrahydrocannabinol ((6aR,10aR)-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydrobenzo[c]chromen-1-ol) (THC) and cannabidiol (2-[(1R,6R)-3-methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol) (CBD).2
The mechanism of action of cannabinoids is dependent on their interaction with two cannabinoid receptors known as CB1 and CB2, which are found throughout the central and peripheral nervous systems. They are present in axons, dendrites, and cell bodies, and they influence neuronal, glial, and microglial activity.3 When these receptors are activated, they inhibit adenylyl cyclase production causing hyperpolarization of neurons, which stops the transmission of electric impulses. CB1 and CB2 receptors are highly concentrated in the hippocampal formation and olfactory bulb, where they impact memory, cognition, smell, and pain perception.4 Additionally, CB1 receptors can be found in the dorsal horn of the spinal cord and on afferent A-β and A-ẟ fibers, suggesting their potential as analgesics for nerve damage.5 CB2 receptors, on the other hand, are unique as their presence is not solely limited to the nervous system and can be found on immune cells, macrophages, and solid tumor tissues, indicating their potential anti-cancer activity.6–8 Furthermore, the activation of CB1 receptors in the brain via THC is responsible for the mind-altering effects of Cannabis sativa, whereas CBD has no psychoactive effects.9
Medicinal Cannabis refers to the use of Cannabis or cannabinoids with an intention to treat a disease or alleviate some of its symptoms. There are several ways, in which cannabinoids can be administered to a patient.10 Smoking is the most widespread form of Cannabis consumption, yet it is not accepted for therapeutic purposes, due to health hazards evoked by pyrolytic by-products.2 Cannabinoids can be administered orally, sublingually, and topically, they can be vaporized and inhaled or even mixed into food.10–12 For instance, CBD inhalation and intranasal administration provides a rapid rise of the drug’s concentration in the plasma, thus reaching the brain faster. On the other hand, transdermal route of CBD administration delivers the drug systemically in a slower manner, gradually over time.13 Prescribed cannabinoids include the oromucosal spray nabiximols (Cannabis-based extract with CBD:THC in a 1:1 ratio) and capsules with the synthetic analogue of THC - dronabinol or nabilone.10–12
Cannabinoids’ beneficial features have given them a new therapeutic meaning in the treatment of a wide range of conditions. These include multiple sclerosis (MS), epilepsy, post-traumatic stress disorder (PTSD), anxiety, cancer, and, most critically, chronic pain.14 Furthermore, Cannabis sativa-based products have gained significant popularity and attention in the areas of cosmetology and dermatology. CBD, in particular, has caught the attention of scientists due to its multiple therapeutic characteristics, including anti-inflammatory and antioxidant properties. Furthermore, CBD has a noteworthy therapeutic potential in the treatment of skin disorders such as atopic dermatitis, pruritus, psoriasis, and acne.15
Despite the various healing benefits that cannabinoids offer, their effectiveness in therapy is significantly limited due to their physical and chemical properties, such as their low solubility in water and instability. Studies performed by Fairbairn et al in 1976 and Pacifici et al in 2018 demonstrated that cannabinoids can undergo degradation when exposed to heat, light, or long-term storage, which can affect their potency.16,17 According to the study conducted by Drooge et al, THC undergoes rapid degradation within a few hours of exposure to air, and this process is accelerated at higher temperatures.18 The Biopharmaceutics Classification System (BCS) classifies cannabinoids such as THC and CBD as drugs with high lipophilicity (logP of around 6.3 and 6.97 respectively) and low water solubility (12.6 and 28.0 mg/L respectively). Additionally, the pKa values of THC and CBD are 9.29 and 10.6, respectively.19–21
The inconsistent and unreliable absorption patterns observed with cannabinoids can be attributed to their physicochemical properties. When taken orally, THC-based medications have a lower bioavailability rate (between 6% and 10%) due to their instability in the acidic environment of the stomach. Additionally, they are heavily metabolized by the CYP450 enzymes in the liver (specifically, CYP3A4 and CYP2C9) into an equally potent metabolite called 11-OH THC, which is then further metabolized into the inactive THC-COOH form.22 According to the reports, the absorption of THC is limited by the excretion of P-glycoprotein (P-gp) from the enterocytes.23 Like THC, CBD also exhibits low oral bioavailability in humans, typically falling in the range of 9% to 13%. This is owing to its low solubility in water and extensive initial metabolism by CYP enzymes (specifically, CYP3A4 and CYP2C19) into a 7-OH metabolite, which results in the loss of roughly 75% of the drug that enters systemic circulation.24,25 The inhalation of cannabinoids through smoking has been found to have the highest bioavailability, ranging from 2% to 56%. The reason for this is that smoking enables swift and effective delivery of drugs to the brain via the lungs and bloodstream. Likewise, the use of smoking for medicinal purposes cannot be endorsed because it poses health risks from the combustion by-products produced.2 Given the limitations of cannabinoids, innovative drug delivery strategies that protect these compounds from oxidation, while increasing their potency and bioavailability, are required.26
What Do We Know About Clinically Important Cannabinoids?
In recent years, the scientific community has exhibited a growing interest in investigating Cannabis’ possible therapeutic applications. Many researchers have presented encouraging results from preclinical and clinical studies, indicating that cannabinoids have the potential to treat a wide range of conditions, including pain, cancer, neurological, and psychiatric illnesses. Considering cannabinoids’ pharmacological targets are not only limited to CB1 and CB2 receptors, their physiological activities and potential therapeutic applications are still being investigated and expanded.27
In the subsequent sections of this review, the therapeutic potential of cannabinoids in the treatment of various disorders will be addressed.
Cannabinoids in Pain Management
Pain can be defined as an unpleasant sensory and emotional experience, which is concurrent with actual or potential tissue damage. Such discomfort can also occur without any physical derangement, as stated in the definition proposed by the International Association for the Study of Pain (IASP).28 Despite significant advances in pain management, it remains a challenging burden both for the patients and their doctors. The most pain-affected group of patients comprises cancer patients, who suffer from pain chronically.29 Every patient who suffers from pain is more susceptible to immune and metabolic upset, thus slowing down the regeneration process. Inadequate pain relief caused by insufficient analgesic dosage or worse, complete lack of pain management therapy leads to augmented morbidity and mortality.30
Pain-managing strategies are most commonly based on the three-step World Health Organization (WHO) analgesic ladder scheme. As the first-line treatment of mild pain, physicians use non-opioid analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen with or without adjuvants. If the analgesia is insufficient or the pain aggravates to moderate intensity, the doctors continue the treatment with the drugs from the first step with the addition of weak opioids such as hydrocodone, codeine or tramadol.31 The management of severe and persistent pain is the most troublesome due to the high-risk profiles of potent opioid drugs administered as the third step of the pain ladder. These drugs include eg morphine, fentanyl, oxycodone, hydromorphone or buprenorphine.32
NSAIDs are the first-line treatment of pain in the world due to their wide availability. Often, patients self-administer these drugs without consulting their doctors, which can lead to improper or excessive use. The overuse of NSAIDs can lead to minor gastrointestinal (GI) side effects such as dyspepsia, but utter abuse can induce serious complications like bleeding from ulcers or perforation of the GI tract. Furthermore, NSAIDs can cause cardiovascular or nephrological adverse effects.33
Adjuvants can be used additionally to primary analgesic medications on every step of the WHO pain ladder. The term “adjuvant analgesics” refers to any drug with a primary indication other than pain, that can have an analgesic effect in certain painful conditions.34 Some adjuvants are known for their multipurpose analgesic properties in diverse pain syndromes, eg tricyclic antidepressants, corticosteroids, α2-adrenergic agonists or neuroleptics. On the other hand, adjuvants may have specific conditions, in which they can be successfully used. Anticonvulsants and local anaesthetics aid in neuropathic pain; bisphosphonates, calcitonin and radiopharmaceuticals are widely administered in conditions associated with bone pain; muscle relaxants are useful during musculoskeletal pain and anticholinergics relieve patients with symptoms of bowel obstructions. Furthermore, ketamine, capsaicin and cannabinoids are also known for their adjuvant properties.35
As the global market and interest in herbal medicine surges, more attention is being directed towards the use of plant-derived drugs.36 In the United States, it is estimated that almost twenty million Americans use herbal medicine.37 An annual turnover of more than 1.5 billion dollars and yearly growth of almost 25% is noted.38
Herbal products are very important in Chinese traditional medicine. A wide variety of medicinal plants have been associated with anticancer effects. It is speculated that these plants could become a rich source of natural antioxidants and helpful chemopreventive substances.39 Furthermore, many plants comprise natural analgesics that target various pathological mechanisms involved in the perception of pain, contrary to traditional analgesics, which usually act on a single pathway.40
However, the most common reason why American adults seek herbal medicine is pain management. In some cases of chronic pain, such as musculoskeletal pain, arthritis or migraine, biological changes to the central nervous system (CNS) or peripheral tissues are permanently established. Patients are condemned to chronic use of NSAIDs or opioids, which can lead to serious side effects, especially considering the elderly. To limit the dosage of administered traditional drugs and improve the analgesic effect, patients often turn to herbal medicines.36
The pain-relieving effects of THC are due to a variety of mechanisms of action that involve cannabinoid receptors CB1 and CB2. Additionally, THC interacts with other pathways, such as delta and kappa opioid receptors, the GABA-ergic/glutamatergic system, and the noradrenergic system.40 These interactions influence the way the pain is perceived.3,41,42 Furthermore, THC acts as an agonist on the transient-receptor potential vanilloid 1 receptor, which helps to block hyperalgesia and allodynia and regulates stimuli that cause thermal and mechanical pain. Other receptors sensitive to cannabinoids include calcitonin gene-related peptide, nuclear factor kappa-light-chain-enhancer of activated B-cells, G protein-coupled receptor 18, and peroxisome proliferator-activated receptors. These interactions contribute to vasodilatation, regulation of the host’s immune response during infection as well as the induction of apoptosis of proinflammatory macrophages.43 Cannabinoids also affect adenosine, serotonin, and dopamine receptors, which is followed by pain alleviation and sensation of relief.44 Moreover, cannabinoids can inhibit cyclooxygenase (preferably COX-2 than COX-1) and block the production of pain and inflammatory mediators at the location of the tumor, explaining its anti-inflammatory action. Activation of CB1 receptors in the central nervous system produces an analgesic effect, while activation of peripheral CB2 receptors amplifies this effect.45
The analgesic role of cannabinoids is studied in various pain conditions.46 A double-blind randomised clinical trial was conducted to assess the impact of nabiximols on pain in advanced cancer patients, who suffer from opioid-resistant pain. Patients, who were administered with nabiximols presented a statistically significant improvement in baseline scores on the numerical pain rating scale, compared with placebo.45 There is low-quality data regarding the influence of cannabinoids on neuropathic pain. In a Cochrane systematic review, 16 clinical trials have been evaluated. A moderate improvement of neuropathic pain sensation among studied participants was observed when treated with cannabinoids, compared with placebo (21% vs 17%).47 There are no high-quality clinical trials focused on the application of cannabinoids in patients suffering from musculoskeletal pain. The use of cannabinoids is recommended as an adjunctive after first and second-line recommended treatments.48 Cannabis has been found to aid in chronic pain management in patients with fibromyalgia. Although clinical data is limited, there are placebo-controlled clinical trials suggesting a potential positive effect of THC-rich Cannabis oil in patients presenting with severe symptoms.49,50 In a systematic review, Stockings et al concluded that cannabinoids significantly reduce MS related neuropathic pain when compared to placebo, yet they do not treat MS-related musculoskeletal pain.51
Regardless of positive aspects of analgesic treatment of chronic pain disorders, the physicians must not neglect the side effects when prescribing medical Cannabis for such application. After reviewing the Danish nationwide registers, Holt et al conducted an analysis to determine the relationship between Cannabis use and the incidence of specific cardiovascular side effects. First-time arrhythmia and acute coronary syndrome incidence was compared between medical Cannabis users and non-users. The study results revealed that patients suffering from chronic pain, who had used prescribed medical Cannabis, are at greater risk of developing a new-onset arrhythmia in the 180 days following the beginning of the treatment. No such association was found regarding the acute coronary syndrome.52
Cannabinoids in the Treatment of Neurological Disorders
Alzheimer’s disease (AD) is the most prevalent neurodegenerative condition worldwide and accounts for around 60–80% of all instances of dementia.53 The main features of AD include the presence of β-amyloid plaques, phosphorylated tau proteins, neurofibrillary tangles, glial activation, and the loss of neurons.54 Preclinical research has shown that CBD can be effective in animal models of AD due to its serotonergic activity. A study conducted on mice demonstrated that administering CBD intraperitoneally could regulate the activation of microglia by beta-amyloid and improve cognitive function. To mimic cognitive decline related to AD, β-amyloid was injected intracerebroventricularly in mice. The study showed that mice administered with CBD had reduced latencies in the Morris water maze compared to those given a control substance.55 Moreover, when given to a transgenic mouse model of AD that expresses β-amyloid, THC demonstrates neuroprotective properties. It leads to a decrease in neuronal loss and a reduction in the buildup of β-amyloid compared to the control group receiving vehicle controls.56 Recent clinical research has demonstrated that nabilone shows potential as a therapy for neurodegenerative and neuroinflammatory diseases. In a double-blind, randomised crossover study involving AD patients, the administration of nabilone at a daily dose of 0.5–2 mg resulted in a decrease in markers associated with oxidative stress and neuroinflammation, such as tumor necrosis factor-alpha (TNF-α). Additionally, this reduction suggested a positive correlation between the use of nabilone and a decrease in agitation.57,58
Parkinson’s disease (PD) ranks as the second most prevalent neurodegenerative disorder, following AD. PD is distinguished by the degeneration of dopaminergic neurons in the substantia nigra pars compacta. This neural loss disrupts the flow of dopamine in the striatum, resulting in the characteristic symptoms of PD. These symptoms encompass diminished motor function, including resting tremor, bradykinesia, postural instability, and rigidity. In addition to motor symptoms, PD can also lead to cognitive impairment, mood disorders, and sensory disturbances related to pain perception.53,59 Proinflammatory signalling is believed to contribute to disease progression. As a result, cannabinoids are considered to have therapeutic potential due to their anti-inflammatory properties.60 Clinical studies have shown that when CBD is given to patients with PD, there is a decrease in the frequency of psychotic symptoms such as sleep problems, hallucinations, and delusions. Additionally, patients experience a reduction in the intensity of tremors and an overall improvement in their well-being and motor function.61–63 In a recent clinical trial using CBD (Epidiolex®), researchers observed similar positive outcomes in symptoms related to PD. Patients in the trial reported good tolerability with no significant side effects when following a daily dosage of 5–25 mg/kg.64 In a recent randomised, double-blind, placebo-controlled Phase II study, researchers investigated the potential of nabilone in alleviating non-motor adverse effects associated with PD. The study has found that PD patients who were administered doses of nabilone up to 1 mg reported positive responses. Clinical assessments and self-scoring methods used in the study indicated that patients receiving nabilone experienced improvements in non-motor adverse effects compared to the placebo group, which reported increased disturbances caused by non-motor adverse effects.65
Further, seizures occur when there is an abnormal synchronized activity among neurons in the brain. The causes of seizures can vary and may include factors such as genetic susceptibility, brain injuries, the presence of brain tumors, and neurodegenerative disorders.66 Cannabinoid compounds have shown the ability to reduce spasms in various neurodegenerative diseases. This provides additional evidence for the use of cannabinoids in treating epileptic seizures. The anticonvulsant effects of THC are believed to be due to the stimulation of CB1 receptors. The conducted studies proved that mice lacking functional CB1 receptors or having genetic changes affecting the activity of the endogenous cannabinoid system are more susceptible to seizures.67,68 Furthermore, clinical studies have shown positive results when using cannabinoids to manage epilepsy. However, much of the research has focused on CBD due to its well-tolerated nature and lack of psychoactive effects.69 The utilization of CBD in clinical trials for treatment-resistant epilepsy and Dravet syndrome, specifically with the use of the clinically approved Epidiolex®, resulted in a notable decrease in the frequency and duration of epileptic seizures. Additionally, long-term safety and quality-of-life studies with this drug demonstrated that CBD offers effective and well-tolerated long-term treatment while enhancing the overall quality of life for patients.70–73 The neuroprotective potential of CBD consequently suggests its applicability in becoming an alternative remedy for patients suffering from epilepsy.74
MS is a chronic and disabling neurological disease that primarily impacts individuals in their early adulthood. Pathologically, it exhibits distinct features such as inflammation, loss of neuronal and axonal cells, demyelination, and the presence of astrocytic gliosis in the brain stem and spinal cord. Physiologically, MS is characterized by intermittent episodes of sensory and motor dysfunction, primarily caused by neurodegeneration.75,76 In the initial clinical trials, nabiximols demonstrated potential in effectively reducing spasticity associated with MS when compared to placebo controls, by decreasing the frequency and intensity of muscle spasms experienced by individuals undergoing treatment.77,78 In a more recent clinical research, nabiximols demonstrated greater effectiveness in improving spasticity caused by MS compared to solely adjusting the dosage of standard anti-spasticity medication.79 Dronabinol, however, despite being well tolerated by the patients, was found to have no positive impact on cognitive function. In fact, there were indications that cognitive function may even decline over time with the use of this drug.80 Additionally, it was discovered that a whole Cannabis extract provided relief from muscle stiffness in individuals with MS.81 Lastly, it was determined that medicinal Cannabis contributed to a slight decrease in fatigue among individuals with MS, in comparison to age and sex-matched controls who had no history of Cannabis usage.82
A recently published three-arm, randomized, double-blind clinical trial by Walczynska-Dragon et al provides new data on the efficacy of CBD formulations in temporomandibular disorders. The emphasis was put on the myorelaxing, pain-relieving and bruxism-reducing properties of CBD in patients suffering from muscle-related temporomandibular disorders. The study discovered that oral administration of CBD formulations successfully reduced the pain reported by patients while also reducing the muscle spasticity and thereby minimising the bruxing activity. A concentration of 10% CBD was found to induce superior effects, when compared to the formulation containing 5% CBD.83
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative condition where muscle control progressively diminishes due to muscle weakness and deterioration. This ultimately causes a decline in the ability to perform tasks such as chewing, swallowing, talking, and breathing, eventually resulting in death.84,85 Although the exact cause and mechanisms that lead to the onset of ALS are yet to be fully understood, it is believed that factors such as excitotoxicity, oxidative stress, and neuroinflammation contribute to the development of the condition. In particular, sporadic cases which make up 90–95% of all ALS cases, have no known specific cause.86,87 A multicenter, randomised placebo-controlled clinical trial on the ALS patients indicated that nabiximols reduced spasticity symptoms. Furthermore, no notable adverse effects were reported during the study.88 In addition, a clinical trial was conducted to assess the effectiveness of THC in relieving cramps in ALS patients. However, the trial did not find any significant reduction in cramp intensity based on patient feedback. Despite this, the use of THC at a daily oral dose of 10 mg was found to be well tolerated, and no significant adverse effects were reported.89
Cannabinoids in the Treatment of Anxiety
Fear and anxiety are natural biological processes that help individuals prepare for potential harm. They trigger various responses in behaviour, physiology, the autonomic nervous system, and hormones. In the short term, this is advantageous since it promotes quick detection of potential threats through heightened vigilance and enables a swift response. However, prolonged, unnecessary, or exaggerated fear and anxiety can have significant adverse effects on health. Anxiety tends to persist even when the threat has diminished.90 Clinical experiments with rimonabant, a CB1 receptor antagonist initially studied as an obesity treatment, revealed the importance of endocannabinoid system (ECS) signalling in controlling human anxiety. During a meta-analysis of four randomised, double-blind, placebo-controlled studies, it was found that the continuous administration of rimonabant, at a dosage of 20 mg per day, resulted in a significant elevation of anxiety levels measured by the Hospital Anxiety and Depression Scale. As a result, many individuals in the rimonabant group decided to discontinue their participation in the studies.91
In general, small amounts of exogenous cannabinoids typically produce calming effects similar to anti-anxiety medications, while larger doses often trigger the opposite effect. However, inhibiting the breakdown of endocannabinoids appears to overcome these dual effects by boosting CB1 receptor activity in a limited and specific way, resulting in reduced anxiety-related behaviours.92 THC exhibits a two-phase response regarding anxiety in animals, which is dependent on the dosage and the form of administration. Specifically, when given in high doses, THC has an anxiety-inducing effect during acute administration,93,94 whereas low doses are anxiolytic.95,96
Habitual Cannabis use has been thought to be related to a higher possibility of developing anxiety disorders. Beletsky et al conducted a critical systematic review of literature on the relation between Cannabis use and anxiety disorders. The researchers concluded that the hypothesized correlation may be explained by the anxiety predisposing individuals who seek for Cannabis as a remedy, rather than the Cannabis use itself, that leads to anxiety development. Despite numerous studies suggesting the causal relation between Cannabis use and progression of anxiety, such association appears to be less likely.97
More recently, scientists have been utilizing more targeted pharmacological treatments to address anxiety disorders. In a double-blind placebo-controlled study, the daily use of the fatty acid amide hydrolase (FAAH) inhibitor JNJ-42165279 for 12 weeks showed improvement in the severity of symptoms related to social anxiety disorder (SAD). The effects of the treatment were even more pronounced in individuals with higher levels of FAAH inhibition.98,99 This observation in especially intriguing given preliminary data indicating that persons with SAD may have increased levels of central FAAH expression.100 Importantly, this data suggests that using pharmacological approaches to target the ECS could be a realistic and successful therapy option for anxiety disorders.90
Cannabinoids in the Treatment of Sleep Disorders
Sleep is a crucial biological process that serves a critical function in replenishing and restoring necessary bodily functions for optimal performance during wakeful hours.101 Achieving optimal sleep health involves ensuring sufficient duration, appropriate timing, efficient sleep patterns, and restful sleep that leaves individuals feeling alert and capable during the day.102 About 30 to 35% of the population experiences insufficient sleep.103
The activation of CB1 receptors located in the pons and basal forebrain is thought to contribute to the initiation of sleep. This is believed to happen by stimulating cholinergic neurons in the basal forebrain and pons through CB1 receptors, which helps facilitate the process of sleep induction.104 The sleep-wake cycle is also influenced by the serotonergic transmitter system in the brainstem’s dorsal raphe nucleus.105 Some studies suggest that the ECS may regulate the serotonin system through the activation of CB1 receptors, potentially affecting sleep-wake cycles.106
Anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are the endogenous ligands for both CB1 and CB2 receptors.107 Both clinical and preclinical studies have reported the presence of a daily rhythm in the levels of endocannabinoids circulating in the body.108 The level of 2-AG in the human blood plasma gradually rises from the middle of sleep to the early afternoon. This increase is more significant when sleep is restricted. Medications that inhibit the activity of monoacylglycerol lipase (MAGL), the enzyme responsible for breaking down 2-AG, have been found to increase the levels of 2-AG in the brain. This leads to wakefulness in rats, resulting in a decrease in both non-rapid eye movement (NREM) and rapid eye movement (REM) sleep.109 As compared to 2-AG, AEA has been found to have a positive impact on sleep. Researchers have discovered that by administering drugs that inhibit the FAAH enzyme and increase the levels of natural AEA, they could improve the sleep quality of males who were experiencing withdrawal symptoms due to Cannabis dependency. This treatment specifically helped to normalize the “slow wave” sleep patterns.110
There is limited data available on the impact of CBD on sleep. However, studies conducted on rats showed some interesting findings. When given lower doses of CBD via injection, the rats had an increase in the overall percentage of sleep and a decrease in the time taken to enter the REM phase. On the other hand, higher doses of CBD resulted in an increase in the amount of time it took the rats to enter REM sleep.111,112 In a recent double-blinded, randomised controlled trial, 1793 participants took part in a 5 week observation period, in which their sleep disturbances were assessed. Each participant received a 4-week supply of randomly assigned capsules containing either 15 mg of CBD or 5 mg of melatonin, alone or in combination with other cannabinoids. The sleep disturbance data was collected via weekly online surveys. The scientists discovered that continuous usage of low-dose CBD is safe and may improve sleep quality. The improvement, however, did not exceed the effects of administration of 5 mg of melatonin.113 Some individuals who report the use of Cannabis products for mild-to-moderate anxiety claim to experience a better quality of sleep on the following night. Interestingly, the highest perceived quality of sleep was noted by the respondents who ingested Cannabis products containing high CBD concentration.114
When THC is used for a short period, it shows certain effects on sleep. Studies conducted on rabbits and cats suggest that these effects include improved sleep continuity, reduced sleep onset latency (SOL), increased overall sleep duration (OSD), and decreased wake after sleep onset (WASO). Moreover, taking THC for a short duration has been associated with a decrease in REM sleep and an increase in slow-wave sleep (SWS).115,116 Unlike previously mentioned, continuous use of THC has demonstrated a decrease in SWS, indicating the potential development of tolerance over time.117 Additionally, there is an indication of heightened sleep disturbances characterized by an increase in SOL, an increase in WASO, and a decrease in OSD.118 During a four-armed crossover study, which was conducted in a double-blinded and placebo-controlled environment, the researchers used electroencephalography monitoring to observe the effects of combining CBD and THC. The study concluded that the combination of CBD and THC resulted in more stimulating properties. Furthermore, CBD had a tendency to reduce the sedative effects of THC, especially when larger doses were administered.119
Many people in the community use medicinal cannabinoid products to aid sleep without seeking guidance from healthcare professionals. However, the evidence supporting the use of cannabinoids for insomnia and other sleep disorders is not comprehensive enough to definitively support their clinical use. Nonetheless, the increasing knowledge of how the ECS regulates sleep-wake cycles gives a strong reason to explore and refine Cannabis products for potential therapeutic benefits.106,109,120
Cannabinoids in the Treatment of Cancer
Cancer is a condition that impacts around 40% of individuals during their lifetime.121 According to the American Cancer Society, it is predicted that in 2024 there will be over 2 million new cancer cases and the death toll will exceed 610,000 among cancer patients in the United States. Almost half (48%) of newly diagnosed cancer cases in men are attributed to prostate, lung and colorectal cancers, with prostate cancer alone accounting for 29% of diagnoses. In women, breast cancer, lung cancer and colorectal cancers constitute 51% of all new diagnoses, and breast cancer alone is responsible for 32% of overall female cancer cases.122,123 For a long time, oncological patients had limited choice when it came to the treatment options. These options primarily involved surgery, radiation therapy and chemotherapy, either used individually or in combination.124 Due to high toxicity of commonly used chemotherapeutic drugs and their low targeting specificity, their activity does not limit to cancer cells, but also causes harm to healthy tissues. These toxic agents destroy not only the abruptly proliferating cancer cells, but also the healthy cells, what leads to serious side effects such as reduced appetite, nausea, difficulty sleeping, and heightened anxiety and may even result in patients’ death.121,125 Untargeted radiotherapy faces the same issue of lacking specificity. This form of treatment induces DNA damage, leading to fragmentation of DNA strands, which ultimately results in the death of cells. Acute radiation damage primarily affects rapidly dividing cells, such as cancer cells, but also the stem cells of the skin and the lining of the digestive tract. This causes a disruption in the protective barrier, commonly observed in the skin, oral mucosa and in the GI tract. These effects are most commonly occurring within 5 years after radiotherapy completion. Eventually, the stem cells undergo compensatory hyperplasia, leading to recovery and resolution of symptoms within a few weeks. However, if the acute damage is not completely healed and persists for an extended period, the resulting lesions are considered late effects with long-term consequences.126 Innovation is sought after for novel forms of drug delivery that can release drugs selectively and efficiently target cells at specific sites. This is crucial for enhancing patients’ well-being and reducing the risk of toxicity.127
Cancer is a condition characterized by abnormal and unregulated cell division and growth. The development of cancer relies on the presence of mutations in multiple genes.128 Numerous studies have been conducted to demonstrate the expression or overexpression of CB1 and/or CB2 receptors in various types of human cancers such as glioma, lymphoma, leukaemia, breast, lung, ovarian, pancreatic, prostate, skin, thyroid cancers, endometrial, esophageal, head and neck, hepatocellular, renal, and mobile tongue carcinomas. These studies have utilized various techniques such as immunohistochemical staining, Western blotting, qRT-PCR, or a combination of methods to determine the expression levels of these receptors.121 Components found in Cannabis extracts, such as cannabinoids and terpenes, could offer an alternative approach to managing side effects. These compounds also have the potential for use alongside synthetic cytostatic drugs to assist in tumor disappearance.121
There have been numerous in vitro and in vivo studies demonstrating that cannabinoids have the ability to impact nearly all critical aspects of cancer. They can hinder cell growth, decrease inflammation, promote programmed cell death, impede the spread and growth of tumors, as well as their angiogenesis and metastasis.129,130 Autophagy and apoptosis play a crucial role in regulating excessive cell growth. It has been observed that cannabinoids have the ability to induce autophagy in various types of cancer.131,132 Inflammation plays a significant role in the development of cancer. The ECS regulates immune system function and controls inflammation. Some cannabinoids effectively reduce inflammation locally or systemically.133,134 There have been several reports indicating that cannabinoids have the ability to inhibit the migration, invasion, and spread of cancer cells.135 A recently published review on glioblastoma treatment with cannabinoids, suggests that glioblastoma cells express CB1 and CB2 receptors, through which cannabinoids may mediate signals leading to inhibition of proliferation and migration of tumor cells. Thus, utilizing cannabinoids might become a beneficial treatment option for patients suffering from glioblastoma.136,137 Lately, it has also been described that in vitro oral cancer cells’ exposure to cannabinoids triggers apoptosis and inhibits cell proliferation. Furthermore, the downregulation of multiple signalling pathways has been associated with anti-cancer features of cannabinoids.138
CBD has displayed promising potential in managing cancer in preclinical studies and some human clinical trials. However, there is still a limited understanding of the mechanisms behind its anticancer effects. To explore CBD’s potential as a cancer treatment and unravel its underlying mechanisms of action, further research is needed, especially in large-scale clinical trials. Additionally, the variations among CBD products available in the market pose a significant challenge, along with the limited knowledge of CBD’s efficacy in treating cancer and its potential side effects.121,139
Although there is not enough data to directly confirm the anti-cancer effect of cannabinoids, their potential in managing cancer symptoms has received significant attention. According to a report published by the National Academies of Sciences, Engineering, and Medicine: The Health Effects of Cannabis and Cannabinoids, one of the most significant clinical findings indicates that oral cannabinoids have helpful antiemetic properties. They can effectively alleviate chemotherapy-induced nausea and vomiting in adults.140,141 A randomised study was conducted involving 469 adults suffering from advanced cancer and weight loss. This study is one of the largest controlled trials of dronabinol. The trial compared the effects of three different treatments namely dronabinol 2.5 mg, megestrol acetate (a progestational agent) 800 mg, or a combination of both. The results showed that 49% of those who received dronabinol experienced an increase in their appetite, while 75% of those who received megestrol and 66% of those who received both also experienced an increase in appetite. Only 3% of the Cannabis recipients and 11% of those on megestrol gained more than 10% of their body weight. Interestingly, although dronabinol was found to be effective in increasing appetite, it was found to be ineffective in promoting weight gain.142 Synthetic cannabinoids have shown potential as a future treatment for cancer-associated cachexia syndrome (CACS). However, limited high-quality trials have been conducted in recent years, hindering the evaluation of their uses. Theoretical evidence suggests that cannabinoids could be an ideal treatment option for CACS patients. However, further high-quality research is necessary to determine the appropriate dosage and the applicability of these treatments.143 Enhanced levels of the CB1 receptor can be found in specific regions of the brain that regulate pain signal processing. Opioid medications are widely used among oncology patients. Initially, it was believed that opioids and cannabinoids affect similar pathways. However, they operate on distinct receptors, and unlike opioids, cannabinoid pain-relieving effects are not inhibited by opioid antagonists. Additionally, both CB1 and CB2 receptor agonists demonstrate analgesic properties both centrally and peripherally. Cannabinoids, along with terpenoids, may also exhibit anti-inflammatory effects, which contribute to their analgesic properties. The report by the US National Academies of Sciences, Engineering, and Medicine concludes that there is compelling evidence for the therapeutic benefits of Cannabis in pain relief.141,144
A recent research study explored the potential use of CBD as a self-assembly inducer for the production of nanoparticle (NP) structures that contain anticancer drugs. These drug-loaded NP complexes were formed by linking the CBD to various anticancer medications (namely N-desacetylthiocolchicine, podophyllotoxin, and paclitaxel) through a linker that enhances drug release. The NPs were created using a technique called solvent displacement, which resulted in consistently sized and stable structures with hydrodynamic diameters ranging from 160 to 400 nm. In the study, the researchers evaluated the potential of NPs complexes to prevent the growth of three different human tumor cell lines - biphasic mesothelioma cell line (MSTO-211H), colorectal adenocarcinoma cell line (HT-29), and hepatocellular carcinoma cell line (HepG2). The results showed that the concentration required to inhibit cell growth, known as GI50 values, was in the low micromolar range. These findings provide additional evidence that NPs can deliver the drug into the cells, enabling their cytotoxic effects. Furthermore, the research suggests that it may be possible to adjust the activity of anticancer drugs by changing the type of linker.145
Cannabinoids Use in Dermatology and Cosmetology
Recent research indicates that CB1 and CB2 receptors have natural ligands present in the skin, suggesting the existence of an ECS specific to the skin (Figure 1).146
 |
Figure 1 Scheme of endocannabinoid system (ESC) specific to the skin. |
Cannabinoids have the ability to either activate or inhibit ECS, which impacts various processes including sebum production, keratinocyte proliferation, inflammation, and hair growth. The activation of CB1 in specific epidermal layers and CB2 in the basal layer may result in increased DNA methylation in keratinocytes, which ultimately inhibits their proliferation. The discovery of a potential ECS in the skin suggests that it is possible to use selective agonists and antagonists for CB1 and CB2 receptors to manipulate the cannabinoid receptors in the treatment of various dermatological conditions.147 The presence of cannabinoid receptors on nerve fibers and mast cells in the skin suggests that cannabinoid receptor agonists may have anti-inflammatory and pain-relieving effects. This implies that these agonists have a wide spectrum of therapeutic potential.148 The transient receptor potential (TRP) channels are another set of skin receptors that can be targeted by cannabinoids. The TRPs are channels that allow cations to pass through. They have six transmembrane segments labelled as S1-S6, and a hydrophilic loop. The pore for ions is located between the S5 and S6 transmembrane segments. These channels can be divided into six subgroups by their amino acid sequences. The subgroups include TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPP (polycystin), and TRPML (mucolipin).149 The topical application of substances that activate TRPA1 and TRPM8 receptors demonstrated positive effects on both epidermal proliferation and regaining epidermal permeability after injuries.150,151 The inhibition of TRPV1 by AEA, has been demonstrated by several research studies to have antipruritic effects. TRPV1 ion channel is mainly expressed in nociceptive neurons in the peripheral nervous system and plays a significant role in causing skin irritation, such as burning pruritus.152,153
In a study conducted by Stander et al, a cream containing palmitoylethanolamide (PEA) has been administered to 22 patients suffering from pruritus, prurigo, and lichen simplex. PEA acts on the cannabinoid receptor by inhibiting the FAAH enzyme, thereby activating AEA. The application of PEA resulted in a reduction of itch by 86.4% in these patients.154 Cannabinoids have the potential to hinder the excessive growth of keratinocytes in psoriasis. According to Wilkinson et al, it is suggested that the main mechanism through which THC inhibits keratinocyte proliferation is by targeting the peroxisome proliferative-activated receptor gamma (PPARγ).155 Another way in which cannabinoids can inhibit keratinocyte proliferation is through the downregulation of keratin K6 and K16 expression, which is achieved through the activation of CB1 receptors.156,157 In March 2016, AXIM Biotechnologies initiated human clinical trials on a topical ointment that includes cannabigerol and other cannabinoids in different concentrations. The ointment, known as RenecannTM, aims to treat psoriasis. If these tests are a success, RenecannTM will be the first cannabinoid treatment for psoriasis to gain approval of the US Food and Drug Administration (FDA).147
Endocannabinoids are crucial in the modulation of pain perception. When the peripheral CB1 receptor is activated, it effectively reduces hyperalgesia caused by mild heat injury in a dose-dependent manner. Moreover, the selective activation of the peripheral CB2 receptor demonstrated antiallodynic effects in a rodent model of post-incisional pain. When both the peripheral CB1 and CB2 receptors are activated simultaneously, they have a synergistic effect on preventing pain transmission in the peripheral nervous system.158
CBD demonstrates antioxidant properties, most likely due to the presence of a phenolic ring within its chemical structure. It interrupts and disrupts the chain reactions that lead to the creation of harmful free radicals. In addition, CBD either captures or transforms these radicals into less reactive forms. It also binds to transition metal ions that play a role in the Fenton reaction, preventing the generation of hydroxyl radicals. Additionally, CBD has the potential to influence the levels and activity of other antioxidants.15 Apart from antioxidant properties of cannabinoids, literature describes their lipostatic, anti-inflammatory and anti-proliferative effects. CBD expresses remarkable anti-acne features, which is why it is pursued as a reassuring treatment of acne vulgaris.159
The possibility of topical administration of CBD and its favourable cutaneous biodistribution throughout epidermis and dermis highlights it as a patient-friendly therapeutic formulation to treat multiple dermatological conditions.160 For instance, a variety of in vitro and in vivo studies exposed that cannabinoids may potentially aid in the healing process of postsurgical and chronic wounds.161 The effectiveness of using oil with a high concentration of CBD topically for hair loss and thinning has been assessed through clinical trials. These issues tend to be connected to androgenetic alopecia. ECS receptors that are located in hair follicles play a role in the growth of cells and control the various phases of the hair growth cycle, which include anagen, catagen, and telogen phases. Through interaction with appropriate receptors, CBD has shown potential to elongate the hair shaft. A study conducted by Szabó et al on cultured hair follicle cells found that lower doses of CBD contributed to hair growth, while higher doses resulted in an earlier entry into the catagen phase, which halts hair growth.162
Studies that focus solely on isolated THC and CBD without considering the complete range of cannabinoids and other important substances found in Cannabis may overlook the potential biological advantages offered by whole Cannabis extracts that contain a diverse range of compounds like terpenes, carotenoids, or flavonoids.163 The high concentration of essential fatty acids (EFA) in hemp seed oil is thought to have beneficial effects on conditions such as atopic dermatitis, psoriasis, and especially acne. However, there have been numerous studies that have yielded contradictory findings, suggesting that the effects of EFA depend on the dose and duration of use.164 Both α-linolenic acid and linoleic acid have been shown to decrease the harmful effects of UV radiation and minimize hyperpigmentation.165 Furthermore, hemp seed oil, which is a non-comedogenic dry oil, does not produce a greasy or sticky residue on the skin. As a result, it has been utilized in manufacturing long-lasting moisturizing patches and stable emulsions in sunscreen cosmetics.166,167 The full-spectrum Cannabis extracts contain carotenoids, particularly β-carotene, lutein, and zeaxanthin. These carotenoids have antioxidant and UV-filtering properties because they are highly solubilised in the lipid bilayer membrane. Carotenoids can enhance skin hydration, support skin rejuvenation, and stimulate fibroblasts to produce collagen and elastin.168 Terpenes are unsaturated hydrocarbons that have a volatile nature and constitute the most significant category of organic compounds found in plants. Although more than 200 terpenes have been discovered in Cannabis, there are three monoterpenes (β-myrcene, D-limonene, and α-pinene) and one sesquiterpenoid (β-caryophyllene) that have been identified to exhibit important biological significance. β-myrcene, α-pinene and β-caryophyllene enhance CBD’s anti-inflammatory effects by inhibiting the production of prostaglandin E2 through the COX-2 pathway.163 Apart from their individual effects, these Cannabis-derived terpenoids are believed to influence the effects of cannabinoids through the entourage effect. The term refers to the ability of two or more cannabinoids or non-cannabinoids to have a more potent synergistic effect when used together compared to when used separately.168,169
What are Drug Delivery Systems?
The term “drug delivery systems” (DDSs) refers to a wide range of carriers designed to improve the selectivity of action and the pharmacodynamic and pharmacokinetic properties of the drug. The vast majority of drugs currently in use are low molecular weight compounds. They are distinguished by rapid metabolism and excretion from the human body, as well as low selectivity of pharmacodynamic properties, necessitating the search for formulations capable of overcoming the aforementioned challenges.170 Furthermore, DDSs allow the modification of drug properties by increasing solubility, preventing the drug from being transformed into an inactive form, resulting in a beneficial modification of pharmacokinetics and biodistribution parameters.171 The matrix material used as a carrier for the active substance must meet a number of criteria, including nontoxicity, biocompatibility, non-immunogenicity, and lack of accumulation in the body.170 Furthermore, the size, surface character, drug bonding type, and presence of functional groups on the surface all play important roles in DDSs action.172
Due to the type of connection between the polymer carrier and the active substance, two types of DDSs may be distinguished; namely those obtained by the physical incorporation/absorption of the drug and the systems obtained by conjugation of the drug to the carrier. The concept of the drug-polymer carrier conjugates was proposed by Helmut Ringsdorf in 1975. The Ringsdorf’s model included a framework made of a biocompatible polymer combined with a solubility-modifying group, a labelling element (antibody or another tropic group) ensuring transport to the target site of action, and a drug (Figure 2).173
 |
Figure 2 Scheme of a polymer-drug conjugate structure (the Ringsdorf’s model).172 |
The type of bonding between the polymer carrier and the drug is a critical parameter for the controlled drug release characteristic, as it determines conjugates’ susceptibility to hydrolytic and enzymatic degradation.174
It is worthwhile to mention the DDSs obtained through physical incorporation/adsorption carriers such as micelles, NPs and liposomes. Polymeric micelles (PMs) (Figure 3) are spherical, colloidal nanosystems that spontaneously form in aqueous solutions when the critical micelle concentration (CMC) of amphiphilic block copolymers is exceeded.175 In PMs, the hydrophilic part of the copolymer faces outwards, while the lipophilic (hydrophobic) part forms the core. Conversely, in reverse micelles, the lipophilic part is directed outwards, while the hydrophilic component faces the core.176
 |
Figure 3 Schematic structure of polymeric micelles (PMs).177 |
PMs are a promising group of carriers, particularly in the field of oncology. In preclinical studies, PMs demonstrated significant potential as a vehicle for lipophilic drugs and small molecules in cancer treatment, as well as in tumor imaging.178 The physicochemical properties of PMs primarily depend on the type of used polymer together with the sizes of their hydrophilic and lipophilic blocks. In PMs, the drug can be bonded to the core via electrostatic interactions with a lipophilic drug molecule or it can be covalently bonded to the polymer chains. The perfect PM should possess an excellent drug loading capacity, precise control over drug release, and exhibit biocompatibility and stability.14
One of the most prominent DDSs are NPs. Similarly to the PMs, these are also spherical, colloidal structures with at least one dimension below 100 nm.179 Due to the mechanism of drug encapsulation, two types of carriers among NPs may be distinguished - nanospheres and nanocapsules. Nanospheres are matrix structures, in which the active substance is evenly dispersed (Figure 4A), while nanocapsules are systems, in which the active substance is enclosed in a polymeric shell (Figure 4B).180
 |
Figure 4 Schematic structure of (A) polymeric nanosphere and (B) polymeric nanocapsule.181 |
Various physical interactions such as absorption, adsorption, as well as chemical bonding including covalent, ionic and van der Waals forces, are employed to associate the active substance with NPs.182
Polymeric NPs have the ability to regulate drug release, protect the drug from adverse conditions, control bioavailability, and improve therapeutic efficacy, making them promising carriers for a variety of drugs.183 Furthermore, surface modification of NPs enables precise drug targeting to specific sites, while avoiding interactions with blood morphotic elements. This modification can be achieved by attaching monoclonal antibodies, antigens or other ligands capable of influencing specific receptors.184
One of the most popular methods of surface functionalization of NPs is PEGylation. PEGylation was first described in 1977 when Abuchowski et al described the covalent association of polyethylene glycol (PEG) with bovine serum albumin. This combination reduced the immunogenicity of the protein and established the basis for enzyme therapy, which is often associated with severe side effects.185 Furthermore, Abuchowski and colleagues found that while using bovine liver catalase covalently bound to PEG, the obtained complexes had a longer residence time in the blood (without a significant reduction in enzyme activity) than self-administered catalase.186
Until now, many active substances and carriers modified with PEG have been described in the literature, including proteins, peptides, enzymes, antibodies, and NPs. PEGylation improves the drug solubility and protects them from enzymatic degradation and antibody recognition. Furthermore, it reduces renal elimination and extends the drugs’ residence time in the body.187
Last but not least, liposomes are considered very promising carriers for pharmaceutical purposes. Due to their exceptional characteristics that encompass prolonging the half-life of the active substance, exerting control over drug release kinetics, safeguarding the encapsulated molecules against physiological degradation as well as demonstrating excellent biocompatibility.188,189 Additionally, liposomes express the capability of selective transport to the target site via active and/or passive targeting strategies, thereby diminishing systemic side effects, enhancing the maximal tolerated dose and in consequence augmenting the therapeutic advantages.190
Liposomes, initially identified by a British researcher Alec D. Bangham in the 1960s at the University of Cambridge, are comprised of one or multiple lipid bilayers that encapsulate the hydrophilic core (Figure 5). Originally constituted solely of natural lipids, liposomes nowadays encompass a combination of natural or/and synthetic lipids and surfactants.191 The lipophilic (hydrophobic) coating may be neutral in nature or contain modifying elements in the structure, ensuring specific targeting.192 The functionalization of liposomes can occur as a result of PEGylation, attachment of antibodies, peptides or aptamers.193
 |
Figure 5 Schematic structure of liposome.193 |
Despite various aforementioned benefits that liposomes offer as DDSs, they also possess three crucial drawbacks - instability, high potential of accumulation in the spleen and liver, and finally slow drug release.194 Numerous techniques can be applied to enhance the stability of liposomes including modification of liposomal membrane, implementation of protective coating and utilization of surfactants. Furthermore, with the use of biodegradable polymers as surface coatings for liposomes it is possible to achieve enhanced delivery and release of the drugs, decreased oxygen exposure, thereby higher stability, and consequently, prolonged circulation time.195
Recently, the primary application of polymers has been directed to the development of highly advanced DDSs, where polymers serve as carriers for various active substances. Biodegradable polymers, in particular, are highly appealing for the DDSs advancement due to their ability to decompose naturally within the human body. This distinguishing feature eliminates the need for their removal or any additional procedures once they have been introduced. Furthermore, biodegradable polymers provide the opportunity to create novel DDSs with highly specialized properties (physical, chemical, and biological) through simple structural or preparation method modifications.196 At this point, it is worthwhile to start addressing the most common biodegradable polymers used in the development of the DDSs.
What is the Role of Biodegradable Polymers in the Development of Drug Delivery Systems?
Polymers are commonly divided into three categories: synthetic, semi-synthetic and natural (also known as biopolymers). Biopolymers can be further classified into those of plant and animal origin. Notable examples of biopolymers derived from plants include cellulose (which serves as the fundamental building block of plant cell walls) and alginic acid (a gelling agent found in species of red algae such as Gelidium amansii). Biopolymers originating from animals include inter alia chitosan, which acts as the primary component of Invertebrate bone tissue.197 All quoted biopolymers belong to the group of polysaccharides, which is one of the most widespread groups of natural polymers. Due to the fact that they expose noteworthy physicochemical and physiological properties, such as biodegradability and biocompatibility and, thus can be utilized in the development of novel DDSs, they are worthwhile meticulous characterization.198
Cellulose is a natural polymer found abundantly in nature and is composed of repeating glucose units. It is the most prevalent organic material and polysaccharide on Earth. Cellulose is commonly found in the form of microfibrils in wood and plant cell walls, algae tissues, and the epidermal cell membranes of tunicates.199 Bacteria can also produce cellulose in the form of nanofiber networks. Cellulosic materials exhibit a hierarchical structure that spans from the nanoscale to macroscopic dimensions, including fibril aggregates, fibrils, nanocrystallites, and nanoscale-disordered domains.200 Cellulose has a complex multi-level structure consisting of bundles or aggregates of superfine fibrils. Each superfine fibril contains multiple cellulose chains. The fibril itself is comprised of both large crystalline domains and small disordered, amorphous domains. The cross-sectional dimension of the fibril ranges from 2 to 20 nm, depending on the synthesis source. Within a cellulose fibril, a single cellulose chain passes through numerous crystalline and disordered domains, connected by strong β(1-4)-glycosidic bonds. The crystalline domain of a cellulose fibril exhibits an impressive alignment of cellulose chains.201
Due to its physical and mechanical properties, cellulose and its derivatives have garnered significant attention for biomedical applications as biocompatible polymers. Cellulose naturally demonstrates functionality, flexibility, and high specific strength due to its hierarchical structure. Additionally, it offers advantages such as low density, affordability, and biodegradability. These cellulose-based materials allow for the manipulation of porosity and interconnectivity, which are desirable in various biomedical applications. However, cellulose has some disadvantages for biomedical applications, such as moisture sensitivity, insolubility in water and common solvents, and low resistance to microbial attack. Nonetheless, this compound can be chemically modified by substituting its native hydroxyl groups with other functional groups like acids, chlorides, and oxides. This modification addresses the less desirable properties of cellulose or creates new desired characteristics.202
Another natural polymer widely used in biomedical applications is an alginate. This compound belongs to the heteropolysaccharides and is composed of 1,4 linked β-D-mannuronic acid (M residue) and 1.4 linked α-L-guluronic acid (G residue). It naturally occurs in the cell walls of Phaeophyceae sp., providing resilience and elasticity.203 Alginate possesses the unique property of forming hydrogel, which makes it an excellent thickener, stabilizer, emulsifier and gelling agent.204 Depending on the content of G and M residues in the alginate molecule, obtained hydrogel may be respectively stiffer (at higher content of G residues) or softer.205 Generally, alginate is considered to be non-toxic, non-immunogenic and biocompatible, however, molecules with a higher content of M residues may cause an immune response.206
One of the first biomedical applications of alginate involved dressing materials, which prevent wound drying by providing a moist environment, resulting in faster healing. At the same time, alginate dressings improve patient comfort by reducing pain during dressing changes.207 In recent years alginate has gained importance in the scientific community due to its capability to appear in different forms. Through easy modifications, it can form microspheres, microcapsules, hydrogels, fibers and foams. This versatile characteristic expands the potential biomedical applications of alginate in fields like drug delivery and tissue engineering.208 To achieve specific desired properties and functions such as cell compatibility, gelation capability and appropriate mechanical strength, various chemical and physical modifications can be applied.209
The final natural polymer worth mentioning is chitosan (CS). CS is a plentiful biopolymer sourced from natural chitin, which is widely found in the exoskeletons of arthropods, insects, crustacean shells and fungal cell walls.198 In terms of its structure, CS is a polysaccharide that contains native amine groups, which possess a positive charge. It is composed of D-glucosamine and N-acetyl-D-glucosamine, that are randomly distributed in the chain via β-(1→4) linkages. The presence of D-glucosamine is responsible for conferring its cationic nature at physiological pH.210
CS belongs to the group of biodegradable and biocompatible polymers. In the human body, it can be degraded by endogenous enzymes, such as chitosanases and lysozymes, into smaller molecules such as oligosaccharides and monosaccharides, which can be further absorbed by the organism. Moreover, CS demonstrates antibacterial properties, as well as mucoadhesion, film formation capacity and lack of toxicity.211,212 Regardless of its exceptional biological and physicochemical features, its use in biomedical applications is highly limited due to poor solubility and weak mechanical properties.210 Nevertheless, several strategies have been devised in order to overcome these limitations by modifying the CS structure. The presence of free amino and hydroxyl groups has been exploited to create diverse CS derivatives, which exhibited enhanced water solubility.213,214 Consequently, CS has found widespread application in numerous pharmaceutical purposes, including controlled drug delivery, tissue engineering and creating novel cosmetic commodities.210
Furthermore, CS has gained significant attention as a coating material for NPs, liposomes and other DDSs. The main advantages of CS-coating include improvement of physicochemical stability, controlled drug release, increased mucoadhesion, increased cellular uptake and improvement of antimicrobial features.212
To summarize, the main advantages of natural polymers are their biocompatibility, lack of toxic effects, safety, low cost, and widespread availability. Unfortunately, natural polymers are also characterized by extremely high volatility, the possibility of heavy metal contamination, and microbiological contamination.215
As a result, synthetic biodegradable polyesters are a unique class of polymers that exhibit no cytotoxic, immunologic, systemic, cardiogenic, or teratogenic effects when used in vivo. Their characteristics are intricately linked to their chemical composition, morphology, and an average molecular weight (Mn). By judiciously choosing these parameters, it is possible to create a drug carrier based on biodegradable polyester with distinct structural, microstructural and physicochemical properties. This group comprises polymers such as polylactide (PLA), poly(ε-caprolactone) (PCL), polyglycolide (PGA) or lactide (LA), ε-caprolactone (ε-CL) and glycolide copolymers.216,217
The most widely used biodegradable polyesters are poly(lactic acid) and PLA. These polymers are composed of the same repeating units, however, they are obtained through different chemical reactions. Poly(lactic acid) is synthesized via direct polycondensation of lactic acid, while PLA is obtained through the Ring-Opening Polymerization (ROP) of a cyclic dimer of lactic acid (so-called lactide).218
Currently, PLA has gained popularity in the production of biomedical materials and disposable goods such as food packaging. PLA consists of two enantiomeric polymers, poly(D-lactide) (PDLA) and poly(L-lactide) (PLLA), which can form stereocomplex crystals.219
PLLA belongs to the group of compounds that have been approved by the FDA.220 Its use in biomedical applications has risen due to its biocompatibility and biodegradability. Both in vitro and in vivo, PLLA undergoes hydrolytic degradation, which results in the formation of lactic acid and its short oligomers as by-products. The degradation products of PLLA can later be integrated into Krebs’s cycle and be eliminated as carbon dioxide and water.221
Another biodegradable polymer worth mentioning is PCL, which is a semi-crystalline, linear aliphatic polyester obtained by ROP of ε-CL. PCL possesses a low melting point (Tm = 59–64°C) and glass transition temperature (Tg around −60°C), which both contribute to its favorable plastic properties.222
Compared to other aliphatic polyesters, PCL exhibits relatively higher elasticity, with Young’s modulus of 0.4–0.6 GPa (more than two times lower than that of PLA).222,223 As a result, PCL has inferior mechanical properties, which is why it is very often modified by copolymerization with other monomers. PCL is a stable polyester that takes approximately 2 to 4 years to degrade, making it one of the slowest degrading biodegradable polyesters. Its resistance to hydrolytic degradation can be attributed to the presence of repetitive CH2 groups within structural units, which delays the degradation process unless exposed to long-term conditions favoring hydrolysis.224 In the human body, PCL is hydrolyzed to 6-hydroxycaproic acid, which can be further metabolized through the citric acid cycle and eliminated. Due to its properties, PCL has been used in the design of various DDSs and medical products such as sutures, subcutaneous contraceptive implants, dressings and materials for filling root canal cavities in dentistry.225
Last but not least, PGA is another important biodegradable polymer obtained also by ROP. It is a thermoplastic, semi-crystalline polyester characterized by a low glass transition temperature (Tg ~ 40°C) and a relatively high melting point (Tm ~ 230°C).222 PGA exhibits good mechanical properties, also it is both biodegradable and biocompatible. Its hydrolytic degradation occurs within 6 weeks. Unfortunately, due to its high price, PGA has been a material less frequently used than PLA and PCL. Furthermore, its highly crystalline structure makes it very poorly soluble in popular organic solvents.226 Nevertheless, PGA still remains one of the most promising biodegradable polyesters, therefore being a point of interest for many researchers involved in the design of innovative DDSs.196
In order to obtain biodegradable materials with specific structural, amphiphilic, mechanical or physicochemical properties, copolymers prove to be useful. By varying the molar ratios of the monomers used, it is possible to obtain a copolymer carrier with the desired specification utile for biomedical applications.227
One of the most important biodegradable copolymers, which has been approved both by the FDA and the European Medicines Agency (EMA), is poly(lactide-co-glycolide) (PLGA).228 In an aqueous environment, PLGA is degraded by the hydrolysis of ester bonds, resulting in the formation of lactic and glycolic acids, which can later be incorporated into Krebs’s cycle. This fact makes PLGA both a biocompatible and non-toxic copolymer.127 Furthermore, the properties of PLGA are closely related to the ratio of lactide to glycolide units in the copolymer chain, its Mn and chain microstructure. All these factors can affect the degradation profile of PLGA, thus its potential for use in the development of DDSs.229
Another biodegradable copolymer worth mentioning is poly(lactide-co-ε-caprolactone) (PLACL). PLACL combines the mechanical properties of PCL with the rapid degradation of PLA.127 These factors make PLACL widely used in the design of membranes and scaffolds, which have found application in regenerative medicine.230 Furthermore, the unique features of PLACL make it an excellent material in the development of DDSs, such as sirolimus loaded polymer films,231 doxorubicin and ciprofloxacin pH-responsive fiber scaffolds232 and protein loaded nanocapsules for oral delivery.233
Last but not least, poly(glycolide-co-ε-caprolactone) (PGACL) is a material that is stable in mechanically dynamic environments and induces proper intercellular activities. Furthermore, PGACL is a significantly more flexible polymer than PLGA, making it a suitable material for the development of scaffolds for blood vessels and other smooth muscle tissues.234
To sum up, biodegradable polymers are an emerging group of materials used in the design of novel DDSs. By providing biodegradability, biocompatibility and lack of toxic side effects combined with the possibility of achieving controlled release of the drugs, they offer an interesting opportunity for the versatile administration routes of cannabinoids.
How to Unravel Cannabinoids’ Therapeutical Potential with the Use of Biodegradable Polymeric Carriers?
Since cannabinoids exhibit high therapeutic potential in the treatment of various disorders, they have become a point of interest for many researchers. Unfortunately, their use in classical pharmaceutical forms is limited by their physicochemical properties, rapid degradation, and reduced bioavailability. For that reason, many scientists have attempted to create DDSs that would overcome the aforementioned difficulties.
The current review presents latest information gathered from an extensive literature investigation on biodegradable polymers as carriers for cannabinoids delivery. The data was compiled using keywords and advanced search techniques, as well as databases such as PubMed, Web of Science, Scopus, and Google Scholar.
In the table below (Table 1), the details of innovative biodegradable polymer-based DDSs for cannabinoid delivery have been collected. Importantly, both in vitro and in vivo data, as well as additional studies performed by the authors, were summarized. Furthermore, the most interesting examples were acknowledged and investigated, with a particular emphasis on the results obtained from in vitro and in vivo studies.
 |
Table 1 The Innovative Biodegradable Polymers-Based DDSs for Cannabinoids Delivery |
Poor oral bioavailability is a result of the low aqueous solubility of CBD when administered orally. Recently, Shreiber‐Livne et al conducted a study, in which the researchers managed to encapsulate CBD within NPs of a highly hydrophobic PEG-b-PCL block copolymer. Furthermore, the pharmacokinetic properties of CBD were investigated in male Sprague Dawley rats administered with the formulation orally. When compared to the free form of CBD, the use of CBD-loaded NPs resulted in a ~20-fold increase of Cmax. Furthermore, the use of loaded NPs reduced the time to achieve Cmax (Tmax) from 4 to 0.3 hours and increased the AUC of oral bioavailability by 14 times. This data emphasizes the nanotechnology strategy’s ability to enhance CBD’s oral performance with minimal systemic side effects.239
Muresan et al examined the anti-nociceptive effects of two distinct CBD formulations with an emphasis on intrathecal delivery of the CBD. Two formulations were used: triblock star co-polymer 3-arm PEG1014-(LA)100 NPs and an oil-in-water nanoemulsion (NE). The researchers observed that both of the CBD formulations maintained in the spinal cord and reached high concentrations in the brain within 10 minutes of intrathecal treatment during pharmacokinetics investigations on adult male Sprague Dawley rats. While the polymeric NPs established their Tmax at 30 minutes, the CBD NE reached its Cmax in the brain in 120 minutes. In comparison to blank formulations, the two CBD formulations discussed above demonstrated immediate anti-nociceptive effects. This study reveals the potential advantages of CBD encapsulation in a variety of cannabinoid applications once again.238
Researchers have focused on the anti-tumorigenic properties of CBD in bladder cancer in a different study carried out by Chen et al. Using an emulsion solvent evaporation approach, researchers have developed two nano-sized CBD carriers: PLGA NPs and PLGA CS-coated NPs. The obtained PLGA NPs and PLGA-CS coated NPs were spherical in shape, had an average size of 192.90 ± 2.41 nm and 287.20 ± 0.90 nm, zeta potential (ζ) of −6.27 ± 0.93 mV and 3.37 ± 0.16 mV, and entrapment efficiency (EE) of 70.3 ± 0.7% and 78.5 ± 0.8% respectively. Furthermore, researchers have performed in vitro drug release studies in two different pH media - pH 5.0, which represented liposome and pH 6.5, which mimicked urine and tumor microenvironment. In both media, PLGA NPs showed an initial burst release within 15 h, followed by a sustained release lasting up to 110 h. The drug release kinetics in the case of PLGA-CS coated NPs showed a sustained pattern, with each release profile pointing to a slow release of the drug. During cytotoxicity studies of CBD PLGA NPs, evaluated in the human urothelial bladder cancer cell line (T24) and human uroepithelial cell line (SV-HUC-1), researchers established that both of the formulations can successfully inhibit the proliferation of T24 cells, at the same time causing no adverse impact on SV-HUC-1 cells. Moreover, the CS coating allowed the PLGA NPs to adhere to the bladder wall, creating a new and effective long-term treatment for uro-oncology.235
The research conducted by Monou et al exemplified the potential of cannabinoids in antimicrobial and wound-healing formulations. Scientists have developed NPs encapsulated CBD and cannabigerol (CBG) using Pluronic-F127 (PF127). NPs showed a high value of EE and were uniformly sized, with a diameter of less than 200 nm. The obtained NPs were additionally added to 3D printed films containing sodium alginate. In vitro release studies have shown a prolonged release of both, CBD and CBG, whereas CBG NPs loaded films exhibited zero-order kinetics. Further, aneuploid immortal keratocyte cell lines (HaCaT) were subjected to an in vitro cell scratch assay using various concentrations of cannabinoid-loaded NPs. In the first 6 hours, the percentage of wound area decreased for both cannabinoids and all concentrations applied. Nonetheless, following that, the wound areas for all concentrations were larger than the initial measurements. The results show that CBD and CBG NPs do not have significant wound healing capabilities in vitro, but they do have a brief impact on the healing process. To verify the potential wound-healing properties of CBD and CBG, more investigations are necessary.240 In addition, the antibacterial efficacy against three common pathogens (E. coli, S. aureus, and a Bacillus spp.) of the CBG and CBD formulations (5 mg/mL) was assessed using the agar diffusion method with filter paper discs. It is worth noting that both formulations exhibited inhibition of S. aureus and Bacillus spp. strains, suggesting that the two cannabinoid formulations were released into the medium. However, there was no inhibition observed on E. coli. Moreover, it was observed that CBG exhibited a slightly stronger antibacterial effect against S. aureus, while CBD demonstrated a better impact on the tested Bacillus spp. strains. The authors concluded that additional specialized research is necessary to elucidate the differences in antibacterial activity between these two cannabinoids.240
In continuity, Fraguas-Sánchez and coauthors established CBD-loaded PLGA NPs using the emulsion solvent evaporation technique for intraperitoneal application in the treatment of ovarian cancer.222 The products obtained had an average particle size of 236 ± 12 nm and ζ value of −16.6 ± 1.2 mV. The biodegradable NPs delivered high CBD concentrations in a controlled manner for more than 96h during the in vitro release study and exhibited stability for at least three months of storage. Furthermore, obtained NPs were assessed in the in vitro cell culture experiments with the use of an epithelial ovarian cancer cell line (SKOV-3). The encapsulated form of CBD maintained its ability to prevent the growth of ovarian cancer cells and had a lower IC50 compared to the solution form. During Western Blot analysis both CBD in its solution form as well as CBD NPs triggered the activation of poly(ADP-ribose) polymerase (PARP), indicating the initiation of cell apoptosis. In an experimental model using SKOV-3-derived tumors in the chorioallantoic membrane (CAM) of fertilized chicken eggs, there was an insignificantly higher level of inhibition of tumor growth observed with CBD NPs compared to CBD solution.237
An interesting study was described by Kamali et al, which investigated the potential of CBD-loaded PLGA microspheres incorporated into gelatin/nano-hydroxyapatite scaffolds in the treatment of critical-sized bone defects. The obtained microspheres were spherical, with an average size of 11.6 ± 1.3 μm and EE of 70.1 ± 4.5%. The in vitro release characteristics showed that both PLGA microspheres and scaffold incorporated with PLGA microspheres could continuously release the drug for 25 days. In in vivo studies, gross views of the harvested radial bones from adult male Wistar rats were evaluated after 4- and 12-weeks post-surgery and the macroscopical score was assessed based on newly formed tissues at the 12th-week post-surgery. The researchers discovered that bone defects treated with a gelatin/nano-hydroxyapatite scaffold but not with CBD were replaced by fibrous and cartilaginous-like tissue. The defects treated with CBD-PLGA-gelatin/nano-hydroxyapatite scaffolds were completely filled with bone-like tissue, just like the autograph. The migration of bone marrow mesenchymal stem cells towards the injury site, as well as their differentiation into osteoblasts, was observed. This suggests that the created scaffold is biocompatible and capable of promoting the formation of new bone tissue.242,243
Furthermore, Demisli et al focused their research on the transdermal CBD delivery in the form of NE-filled CS-hydrogel.248 The goal of that study was to develop a new method of delivering lipophilic bioactive substances through the skin that would minimise the irritation caused by the burst release on the surface. A lab-controlled permeation test was performed to determine how effectively CBD penetrates the skin from hydrogels containing NEs. As a substitute for human skin, the test used the intact porcine ear skin. While compared to the NEs alone, the hydrogels loaded with NEs showed a higher rate of CBD penetration and permeation coefficient. This suggests that the NE-filled hydrogels improve transdermal absorption. These findings were expected because CS is widely recognised as a penetration enhancer. It interacts with the stratum corneum in a variety of ways, including modifying the protein structure of the stratum corneum and acting as a moisturising agent to increase the water content of the stratum corneum. These systems have the potential to be excellent options for transdermal delivery of highly lipophilic bioactive compounds. More research is needed in the future to investigate their ability to encapsulate both lipophilic and hydrophilic bioactive substances at the same time, with the goal of achieving synergistic effects and improving biological outcomes.248
Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone (CB13) shows significant therapeutic promise as an analgesic for chronic pain conditions that have limited response to traditional medications. Nonetheless, its use in humans is hampered by the occurrence of mild-to-moderate dose-dependent adverse effects, as well as its pharmacokinetic properties. As a result, using an appropriate carrier system for CB13 oral administration appears to be an appealing approach to fostering the development of a valuable treatment option.250
Durán-Lobato and colleagues produced CS- and PEG-modified polymeric PLGA NPs and lipid NPs (LNPs). They subsequently carried out a comparative evaluation of these carriers under the same experimental conditions to determine their suitability for oral administration of CB13. When compared to LNPs, polymeric PLGA NPs had significantly longer release profiles. PEG-coated formulations had limited uptake in GI model cells and strongly inhibited uptake by monocytes. CS-coated NPs, on the other hand, showed the highest uptake in GI model cells and minimal uptake in THP1 cells. The coated PLGA NPs had slightly reduced GI tract uptake and more significant phagocytic uptake than the LNPs, which was likely due to the larger particle size of the formulations investigated in this study. Although more research is needed to understand how these carriers interact with biological entities, this study provides a comparative understanding of how PLGA NPs and LNPs perform under identical experimental conditions. Finally, this knowledge will help in determining which formulation is best suited for oral delivery of cannabinoids.250
In recent years, Δ9-THC has gained recognition for its therapeutic potential in treating different medical conditions.258,259 However, the main barrier of using cannabinoids, such as THC, for medicinal purposes is determining a safe and efficient method of administration. The currently used soft gelatin capsules administered orally have limitations due to the significant first-pass metabolism of THC and the difficulty for patients experiencing severe nausea to retain the capsules in the stomach for an adequate amount of time for absorption and effectiveness. Using the nasal mucosa to deliver drugs into the body systemically is an efficient way to avoid the initial breakdown of drugs in the liver. Furthermore, the nasal mucosa is advantageous for drug absorption due to its large epithelial surface area created by numerous microvilli. Al-Ghananeem et al investigated the feasibility of administering dissolved Δ9-THC via the intranasal route. They were additionally interested to find out how the use of a CS-based nasal bioadhesive gel affected THC bioavailability. This formulation strategy was designed to reduce drug clearance by the mucociliary system. The researchers used conscious rabbits to administer the THC formulations via the nasal route and compare them to intravenous administration. After being administered nasally, the THC nasal solution showed a Cmax of 20 ± 3 ng/mL at 20 minutes. Interestingly, the THC loaded in the CS gel formulation exhibited a similar profile initially and later reached a higher Cmax of 31 ± 4 ng/mL (reached at 45 minutes). The researchers additionally examined into the absolute bioavailability of THC after nasal administration, comparing the concentration of THC in blood plasma after nasal administration to that after intravenous injection. THC nasal solution and gel formulations showed absolute bioavailability values of 13.3 ± 7.8% and 15.4 ± 6.5%, respectively. Based on the findings of this study, it appears that both solubilized THC administered intranasally and THC in a mucoadhesive CS gel formulation could be promising methods for delivering THC throughout the body. However, more research is needed to fully assess the clinical effectiveness and safety of this intranasal delivery system.253
However, Villate and colleagues conducted a study, in which they obtained full-spectrum Cannabis extract CS-coated alginate microcapsules using the vibration microencapsulation nozzle technique. This was carried out in order to produce an edible pharmaceutical-grade product. The synthesized microcapsules predominantly contained cannabinoids of the ∆9-THC-type and CBD-type. These microcapsules had an average size of 460 ± 260 μm and an average sphericity of 0.5 ± 0.3. According to the in vitro GI release experiment, the rapid release of cannabinoids in the intestinal phase results in a moderate to high bioaccessibility (57–77%) of medically significant compounds. When compared to alternative methods and materials for encapsulation, the vibration microencapsulation nozzle technique stands out for its use of natural materials that are readily available in abundance, resulting in a low cost of the process. Furthermore, this technique does not rely on expensive technology, involves any potentially hazardous steps, or produces any harmful waste that could have an impact on health or the environment. Furthermore, it does not necessitate the use or manufacture of any organic solvents. The performed study opens the door to the development of numerous new formulations based on full-spectrum Cannabis extracts. Allowing the creation of formulations with specific desired properties, any Cannabis extract can be encapsulated using a customizable synthesis pathway.256
Lastly, Uziel et al pursued the idea of full spectrum Cannabis extract administration. An extended-release formulation, in the form of polymeric microdepots, that had been created through melt printing, was developed for a full-spectrum extract rich in CBD. Once microdepots were injected subcutaneously into mice, they allowed for the continuous release of the enclosed extract for two weeks. Mice were given a single injection of microdepots containing Cannabis extract or a Cannabis extract solution with the same dosage to determine how effectively the formulation functioned within a living organism. Microdepots were extracted from the tissue beneath the skin at various time intervals throughout the experiment to determine the amount of cannabinoids released. Blood samples were also analysed to obtain pharmacokinetics profiles of cannabinoid levels in the serum. This prolonged administration results in a higher concentration of various significant and minor cannabinoids in the bloodstream, surpassing the effects of injecting regular Cannabis extract. In terms of seizure prevention, the microdepots reduced the occurrence of tonic-clonic seizures by 40%, increased the survival rate by 50%, and delayed the onset of the first tonic-clonic seizure by 170% one week after administration. These findings suggest that a long-term delivery system that includes the entire Cannabis spectrum has the potential to offer a novel method of Cannabis administration and treatment.257
Future Prospectives
As the interest in biodegradable polymers is thriving, they become utilized towards increasingly sophisticated issues. The discovery of biodegradable polymer-based DDSs opens new opportunities for clinical application of drugs, whose poor physicochemical, pharmacodynamic, or pharmacokinetic characteristics limit their administration routes and deployment in applied medicine.
One of the promising substances, which have caught the attention of researchers around the world are cannabinoids. With ample amount of new research, that indicate effectiveness of cannabinoids in various conditions, there emerges the need for a proper way of their administration. Cannabinoids exhibit poor bioavailability, low water solubility and significant instability, which is why it is troublesome to properly study their effectiveness in treatment of different conditions. With the use of polymeric drug vehicles, not only are the cannabinoids’ pharmacokinetic properties improved, but also the controlled release of the drug may be achieved.
The current review gathered and described both the cannabinoids individually and the DDSs that encapsulate them. The main objective was to underline how the biodegradable DDSs promote the controlled drug release and augment the drug’s bioavailability. With a big heterogeneity of the polymers that constitute the matrix of the carriers, the administration perspective broadens.
Even though there has been extensive research in that area, the use of biodegradable carriers with cannabinoids still encounters several uncertainties and concerns. More clinical trials need to be performed to properly study every aspect of medical cannabinoid therapies in vivo, although thus far observed results emphasize the future optimism in the development of that field.
As a last note, it should be acknowledged that scientists could explore the use of biodegradable polymeric DDSs containing cannabinoids for combined therapy (delivering cannabinoids at the same time or combining with other treatments). Additionally, these systems could also deliver cannabinoids or their analogues alongside other compounds. This approach would make better use of the flexibility of the suggested systems and their ability to overcome limitations in drug availability, ultimately improving the overall effectiveness of the treatment. Furthermore, with the use of biodegradable polymers a targeted therapy towards different specific disorders may be achieved.
The analysis of the literature allows to believe that in the coming years there will be a further increase in interest in new cannabinoid delivery systems. Intensification of work in this area will probably lead to the commercialization of the new type DDSs.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lewis M, Russo E, Smith K. Pharmacological Foundations of Cannabis Chemovars. Planta Med. 2018;84(04):225–233. doi:10.1055/s-0043-122240
2. Huestis MA. Human Cannabinoid Pharmacokinetics. C&b. 2007;4(8):1770–1804. doi:10.1002/cbdv.200790152
3. Ward SJ, Lichtman AH, Piomelli D, Parker LA. Cannabinoids and Cancer Chemotherapy-Associated Adverse Effects. JNCI Monogr. 2021;2021(58):78–85. doi:10.1093/jncimonographs/lgab007
4. Mackie K. Distribution of Cannabinoid Receptors in the Central and Peripheral Nervous System. In: Pertwee RG editor. Cannabinoids. Vol 168. Handbook of Experimental Pharmacology. Springer-Verlag; 2005:299–325. doi:10.1007/3-540-26573-2_10
5. Pertwee RG. Cannabinoid receptors and pain. Progress Neurobiol. 2001;63(5):569–611. doi:10.1016/S0301-0082(00)00031-9
6. Galiegue S, Mary S, Marchand J, et al. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur J Biochem. 1995;232(1):54–61. doi:10.1111/j.1432-1033.1995.tb20780.x
7. Ofek O, Karsak M, Leclerc N, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA. 2006;103(3):696–701. doi:10.1073/pnas.0504187103
8. Sarfaraz S, Adhami VM, Syed DN, Afaq F, Mukhtar H. Cannabinoids for Cancer Treatment: progress and Promise. Cancer Res. 2008;68(2):339–342. doi:10.1158/0008-5472.CAN-07-2785
9. Fernández Ó. THC:CBD in Daily Practice: available Data from UK, Germany and Spain. Eur Neurol. 2016;75(Suppl. 1):1–3. doi:10.1159/000444234
10. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for Medical Use: a Systematic Review and Meta-analysis. JAMA. 2015;313(24):2456. doi:10.1001/jama.2015.6358
11. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Internal Med. 2018;49:12–19. doi:10.1016/j.ejim.2018.01.004
12. Pagano C, Navarra G, Coppola L, Avilia G, Bifulco M, Laezza C. Cannabinoids: therapeutic Use in Clinical Practice. IJMS. 2022;23(6):3344. doi:10.3390/ijms23063344
13. O’Sullivan SE, Jensen SS, Kolli AR, Nikolajsen GN, Bruun HZ, Hoeng J. Strategies to Improve Cannabidiol Bioavailability and Drug Delivery. Pharmaceuticals. 2024;17(2):244. doi:10.3390/ph17020244
14. Legare CA, Raup-Konsavage WM, Vrana KE. Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals. Pharmacology. 2022;107(3–4):131–149. doi:10.1159/000521683
15. Grymel M, Grabiec P, Nurkowska K. Cannabidiol - characteristic and application in cosmetology and dermatology. Aesth Cosmetol Med. 2021;10(6):299–303. doi:10.52336/acm.2021.10.6.06
16. Fairbairn JW, Liebmann JA, Rowan MG. The stability of cannabis and its preparations on storage. J Pharm Pharmacol. 2011;28(1):1–7. doi:10.1111/j.2042-7158.1976.tb04014.x
17. Pacifici R, Marchei E, Salvatore F, Guandalini L, Busardò FP, Pichini S. Evaluation of long-term stability of cannabinoids in standardized preparations of cannabis flowering tops and cannabis oil by ultra-high-performance liquid chromatography tandem mass spectrometry. Clin Chem Lab Med. 2018;56(4):94–96. doi:10.1515/cclm-2017-0758
18. van Drooge DJ, Hinrichs WLJ, Wegman KAM, Visser MR, Eissens AC, Frijlink HW. Solid dispersions based on inulin for the stabilisation and formulation of Δ9-tetrahydrocannabinol. Eur. J. Pharm. Sci. 2004;21(4):511–518. doi:10.1016/j.ejps.2003.11.014
19. Cherniakov I, Izgelov D, Domb AJ, Hoffman A. The effect of Pro NanoLipospheres (PNL) formulation containing natural absorption enhancers on the oral bioavailability of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in a rat model. Eur. J. Pharm. Sci. 2017;109:21–30. doi:10.1016/j.ejps.2017.07.003
20. Cherniakov I, Izgelov D, Barasch D, Davidson E, Domb AJ, Hoffman A. Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. J Control Release. 2017;266:1–7. doi:10.1016/j.jconrel.2017.09.011
21. Stella B, Baratta F, Della Pepa C, Arpicco S, Gastaldi D, Dosio F. Cannabinoid Formulations and Delivery Systems: current and Future Options to Treat Pain. Drugs. 2021;81(13):1513–1557. doi:10.1007/s40265-021-01579-x
22. Grotenhermen F. Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 2003;42(4):327–360. doi:10.2165/00003088-200342040-00003
23. Bonhomme-Faivre L, Benyamina A, Reynaud M, Farinotti R, Abbara C. Disposition of Δ 9 tetrahydrocannabinol in CF1 mice deficient in mdr1a P-glycoprotein. Addict. Biol. 2008;13(3–4):295–300. doi:10.1111/j.1369-1600.2008.00096.x
24. Millar SA, Stone NL, Yates AS, O’Sullivan SE. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front Pharmacol. 2018;9:1365. doi:10.3389/fphar.2018.01365
25. Nelson KM, Bisson J, Singh G, et al. The Essential Medicinal Chemistry of Cannabidiol (CBD). J Med Chem. 2020;63(21):12137–12155. doi:10.1021/acs.jmedchem.0c00724
26. Reddy TS, Zomer R, Mantri N. Nanoformulations as a strategy to overcome the delivery limitations of cannabinoids. Phytother Res. 2023;37(4):1526–1538. doi:10.1002/ptr.7742
27. Maurya N, Velmurugan BK. Therapeutic applications of cannabinoids. Chem. Biol. Interact. 2018;293:77–88. doi:10.1016/j.cbi.2018.07.018
28. Phadke A, Amin P. A Recent Update on Drug Delivery Systems for Pain Management. J Pain Palliative Care Pharmacother. 2021;35(3):175–214. doi:10.1080/15360288.2021.1925386
29. Mercadante S. Cancer Pain Treatment Strategies in Patients with Cancer. Drugs. 2022;82(13):1357–1366. doi:10.1007/s40265-022-01780-6
30. Al Malyan M, Becchi C, Nikkola L, et al. Polymer-Based Biodegradable Drug Delivery Systems in Pain Management. J Craniofacial Surgery. 2006;17(2):302–313. doi:10.1097/00001665-200603000-00018
31. Anekar AA, Hendrix JM, Cascella M. WHO Analgesic Ladder. StatPearls Publishing; 2023.
32. Yang J, Bauer BA, Wahner-Roedler DL, Chon TY, Xiao L. The Modified WHO Analgesic Ladder: is It Appropriate for Chronic Non-Cancer Pain? JPR. 2020;13:411–417. doi:10.2147/JPR.S244173
33. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging and Disease. 2018;9(1):143. doi:10.14336/AD.2017.0306
34. Mitra R, Jones S. Adjuvant Analgesics in Cancer Pain: a Review. Am J Hosp Palliat Care. 2012;29(1):70–79. doi:10.1177/1049909111413256
35. Lussier D, Huskey AG, Portenoy RK. Adjuvant Analgesics in Cancer Pain Management. oncologist. 2004;9(5):571–591. doi:10.1634/theoncologist.9-5-571
36. Jahromi B, Pirvulescu I, Candido KD, Knezevic NN. Herbal Medicine for Pain Management: efficacy and Drug Interactions. Pharmaceutics. 2021;13(2):251. doi:10.3390/pharmaceutics13020251
37. Nahin R, Barnes P, Stussman B, Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;30(18):1–14.
38. Weiner DK, Ernst E. Complementary and Alternative Approaches to the Treatment of Persistent Musculoskeletal Pain. Clin J Pain. 2004;20(4):244–255. doi:10.1097/00002508-200407000-00006
39. Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. doi:10.1016/j.lfs.2003.09.047
40. Li SH, Li L, Yang RN, Liang SD. Compounds of traditional Chinese medicine and neuropathic pain. Chinese J Nat Med. 2020;18(1):28–35. doi:10.1016/S1875-5364(20)30002-9
41. Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2004;74(11):1317–1324. doi:10.1016/j.lfs.2003.09.038
42. Finn DP, Haroutounian S, Hohmann AG, Krane E, Soliman N, Rice AS. Cannabinoids, the endocannabinoid system, and pain: a review of preclinical studies. Pain. 2021;162(1):5–25. doi:10.1097/j.pain.0000000000002268
43. Console-Bram L, Marcu J, Abood ME. Cannabinoid receptors: nomenclature and pharmacological principles. Prog Neuro Psychopharmacol Biol Psychiatry. 2012;38(1):4–15. doi:10.1016/j.pnpbp.2012.02.009
44. Meng H, Deshpande A. Cannabinoids in chronic non-cancer pain medicine: moving from the bench to the bedside. BJA Educ. 2020;20(9):305–311. doi:10.1016/j.bjae.2020.05.002
45. Jose A, Thomas L, Baburaj G, Munisamy M, Rao M. Cannabinoids as an alternative option for conventional analgesics in cancer pain management: a pharmacogenomics perspective. Indian J Palliat Care. 2020;26(1):129. doi:10.4103/IJPC.IJPC_155_19
46. Safi K, Sobieraj J, Błaszkiewicz M, Żyła J, Salata B, Dzierżanowski T. Tetrahydrocannabinol and Cannabidiol for Pain Treatment—An Update on the Evidence. Biomedicines. 2024;12(2):307. doi:10.3390/biomedicines12020307
47. Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;2020(7):182. doi:10.1002/14651858.CD012182.pub2
48. Johal H, Vannabouathong C, Chang Y, Zhu M, Bhandari M. Medical cannabis for orthopaedic patients with chronic musculoskeletal pain: does evidence support its use? Therapeutic Adv Musculoskeletal. 2020;12. doi:10.1177/1759720X20937968
49. van de Donk T, Niesters M, Kowal MA, Olofsen E, Dahan A, van Velzen M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain. 2019;160(4):860–869. doi:10.1097/j.pain.0000000000001464
50. Chaves C, Bittencourt PCT, Pelegrini A. Ingestion of a THC-Rich Cannabis Oil in People with Fibromyalgia: a Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pain Med. 2020;21(10):2212–2218. doi:10.1093/pm/pnaa303
51. Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159(10):1932–1954. doi:10.1097/j.pain.0000000000001293
52. Holt A, Nouhravesh N, Strange JE, et al. Cannabis for chronic pain: cardiovascular safety in a nationwide Danish study. Eur Heart J. 2024;45(6):475–484. doi:10.1093/eurheartj/ehad834
53. Erkkinen MG, Kim MO, Geschwind MD. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb Perspect Biol. 2018;10(4):a033118. doi:10.1101/cshperspect.a033118
54. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577–1590. doi:10.1016/S0140-6736(20)32205-4
55. Martín-Moreno AM, Reigada D, Ramírez BG, et al. Cannabidiol and Other Cannabinoids Reduce Microglial Activation In Vitro and In Vivo: relevance to Alzheimer’s Disease. Mol Pharmacol. 2011;79(6):964–973. doi:10.1124/mol.111.071290
56. Franke T, Irwin C, Beindorff N, Bouter Y, Bouter C. Effects of tetrahydrocannabinol treatment on brain metabolism and neuron loss in a mouse model of sporadic Alzheimer’s disease. Nuklearmedizin. 2019;58(2):9. doi:10.1055/s-0039-1683689
57. Ruthirakuhan MT, Herrmann N, Gallagher D, et al. Investigating the safety and efficacy of nabilone for the treatment of agitation in patients with moderate-to-severe Alzheimer’s disease: study protocol for a cross-over randomized controlled trial. Contemporary Clin Trials Commun. 2019;15:100385. doi:10.1016/j.conctc.2019.100385
58. Ruthirakuhan M, Herrmann N, Andreazza AC, et al. Agitation, Oxidative Stress, and Cytokines in Alzheimer Disease: biomarker Analyses From a Clinical Trial With Nabilone for Agitation. J Geriatr Psychiatry Neurol. 2020;33(4):175–184. doi:10.1177/0891988719874118
59. Davie CA. A review of Parkinson’s disease. Br Med Bul. 2008;86(1):109–127. doi:10.1093/bmb/ldn013
60. Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener. 2015;4(1):19. doi:10.1186/s40035-015-0042-0
61. Zuardi A, Crippa J, Hallak J, et al. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J Psychopharmacol. 2009;23(8):979–983. doi:10.1177/0269881108096519
62. De Faria SM, De Morais Fabrício D, Tumas V, et al. Effects of acute cannabidiol administration on anxiety and tremors induced by a Simulated Public Speaking Test in patients with Parkinson’s disease. J Psychopharmacol. 2020;34(2):189–196. doi:10.1177/0269881119895536
63. Chagas MHN, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol. 2014;28(11):1088–1098. doi:10.1177/0269881114550355
64. Leehey MA, Liu Y, Hart F, et al. Safety and Tolerability of Cannabidiol in Parkinson Disease: an Open Label, Dose-Escalation Study. Cannabis Cannabinoid Res. 2020;5(4):326–336. doi:10.1089/can.2019.0068
65. Peball M, Krismer F, Knaus H, et al. Non‐Motor Symptoms in Parkinson’s Disease are Reduced by Nabilone. Ann. Neurol. 2020;88(4):712–722. doi:10.1002/ana.25864
66. Falco-Walter J. Epilepsy-Definition, Classification, Pathophysiology, and Epidemiology. Semin Neurol. 2020;40(6):617–623. doi:10.1055/s-0040-1718719
67. Clement AB, Hawkins EG, Lichtman AH, Cravatt BF. Increased Seizure Susceptibility and Proconvulsant Activity of Anandamide in Mice Lacking Fatty Acid Amide Hydrolase. J Neurosci. 2003;23(9):3916–3923. doi:10.1523/JNEUROSCI.23-09-03916.2003
68. Marsicano G, Goodenough S, Monory K, et al. CB1 Cannabinoid Receptors and On-Demand Defense Against Excitotoxicity. Science. 2003;302(5642):84–88. doi:10.1126/science.1088208
69. Jones NA, Hill AJ, Smith I, et al. Cannabidiol Displays Antiepileptiform and Antiseizure Properties In Vitro and In Vivo. J Pharmacol Exp Ther. 2010;332(2):569–577. doi:10.1124/jpet.109.159145
70. Devinsky O, Nabbout R, Miller I, et al. Long‐term cannabidiol treatment in patients with Dravet syndrome: an open‐label extension trial. Epilepsia. 2019;60(2):294–302. doi:10.1111/epi.14628
71. Devinsky O, Patel AD, Thiele EA, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90(14):e1204–e1211. doi:10.1212/WNL.0000000000005254
72. Gaston TE, Szaflarski M, Hansen B, Bebin EM, Szaflarski JP. Quality of life in adults enrolled in an open-label study of cannabidiol (CBD) for treatment-resistant epilepsy. Epilepsy Behav. 2019;95:10–17. doi:10.1016/j.yebeh.2019.03.035
73. Laux LC, Bebin EM, Checketts D, et al. Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox-Gastaut syndrome or Dravet syndrome: expanded access program results. Epilepsy Res. 2019;154:13–20. doi:10.1016/j.eplepsyres.2019.03.015
74. Shrivastava A, Gupta JK. Enlightening Pharmacological Mechanisms of Cannabidiol in Epilepsy: a Comprehensive Review on their Neuroprotective Potential. Ind J Pharm Edu Res. 2023;58(1):15–20. doi:10.5530/ijper.58.1.2
75. Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816–1821. doi:10.1177/1352458520970841
76. Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391(10130):1622–1636. doi:10.1016/S0140-6736(18)30481-1
77. Novotna A, Mares J, Ratcliffe S, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex ®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis: Sativex for refractory spasticity in MS. Eur J Neurol. 2011;18(9):1122–1131. doi:10.1111/j.1468-1331.2010.03328.x
78. Collin C, Davies P, Mutiboko IK, Ratcliffe S. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis: cannabis-based medicine in spasticity by multiple sclerosis. Eur J Neurol. 2007;14(3):290–296. doi:10.1111/j.1468-1331.2006.01639.x
79. Markovà J, Essner U, Akmaz B, et al. Sativex ® as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: a double-blind, placebo-controlled randomised clinical trial. Int J Neurosci. 2019;129(2):119–128. doi:10.1080/00207454.2018.1481066
80. University of California, Davis. Cannabis for Spasticity in Multiple Sclerosis: a Placebo-Controlled Study (Clinical Trial Registration No. NCT00682929); 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT00682929.
81. Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG, on behalf of the MUSEC Research Group. MUltiple Sclerosis and Extract of Cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012;83(11):1125–1132. doi:10.1136/jnnp-2012-302468
82. Rudroff T Medical Marijuana and its Effects on Motor Function in People with Multiple Sclerosis: an Observational Case-Control Study (Clinical Trial Registration No. NCT02898974); 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT02898974.
83. Walczyńska-Dragon K, Kurek-Górecka A, Niemczyk W, et al. Cannabidiol Intervention for Muscular Tension, Pain, and Sleep Bruxism Intensity—A Randomized, Double-Blind Clinical Trial. JCM. 2024;13(5):1417. doi:10.3390/jcm13051417
84. Longinetti E, Fang F. Epidemiology of amyotrophic lateral sclerosis: an update of recent literature. Curr Opinion Neurol. 2019;32(5):771–776. doi:10.1097/WCO.0000000000000730
85. Zarei S, Carr K, Reiley L, et al. A comprehensive review of amyotrophic lateral sclerosis. Surg Neurol Int. 2015;6(1):171. doi:10.4103/2152-7806.169561
86. Liu J, Wang F. Role of Neuroinflammation in Amyotrophic Lateral Sclerosis: cellular Mechanisms and Therapeutic Implications. Front Immunol. 2017;8:1005. doi:10.3389/fimmu.2017.01005
87. Batra G, Jain M, Singh R, et al. Novel therapeutic targets for amyotrophic lateral sclerosis. Indian J Pharmacol. 2019;51(6):418. doi:10.4103/ijp.IJP_823_19
88. Riva N, Mora G, Sorarù G, et al. Safety and efficacy of nabiximols on spasticity symptoms in patients with motor neuron disease (CANALS): a multicentre, double-blind, randomised, placebo-controlled, Phase 2 trial. Lancet Neurol. 2019;18(2):155–164. doi:10.1016/S1474-4422(18)30406-X
89. Weber M, Goldman B, Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg. 2010;81(10):1135–1140. doi:10.1136/jnnp.2009.200642
90. Petrie GN, Nastase AS, Aukema RJ, Hill MN. Endocannabinoids, cannabinoids and the regulation of anxiety. Neuropharmacology. 2021;195:108626. doi:10.1016/j.neuropharm.2021.108626
91. Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370(9600):1706–1713. doi:10.1016/S0140-6736(07)61721-8
92. Moreira FA, Wotjak CT. Cannabinoids and Anxiety. In: Stein MB, Steckler T, editors. Behavioral Neurobiology of Anxiety and Its Treatment. Vol 2. Current Topics in Behavioral Neurosciences. Berlin Heidelberg: Springer; 2009:429–450. doi:10.1007/7854_2009_16
93. Rock EM, Limebeer CL, Petrie GN, Williams LA, Mechoulam R, Parker LA. Effect of prior foot shock stress and Δ9-tetrahydrocannabinol, cannabidiolic acid, and cannabidiol on anxiety-like responding in the light-dark emergence test in rats. Psychopharmacology. 2017;234(14):2207–2217. doi:10.1007/s00213-017-4626-5
94. Todd SM, Arnold JC. Neural correlates of interactions between cannabidiol and Δ 9 -tetrahydrocannabinol in mice: implications for medical cannabis: THC and CBD interactions. Br. J. Pharmacol. 2016;173(1):53–65. doi:10.1111/bph.13333
95. Rubino T, Sala M, Viganò D, et al. Cellular Mechanisms Underlying the Anxiolytic Effect of Low Doses of Peripheral Δ9-Tetrahydrocannabinol in Rats. Neuropsychopharmacol. 2007;32(9):2036–2045. doi:10.1038/sj.npp.1301330
96. Braida D, Limonta V, Malabarba L, Zani A, Sala M. 5-HT1A receptors are involved in the anxiolytic effect of Δ9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague-Dawley rats. Eur. J. Pharmacol. 2007;555(2–3):156–163. doi:10.1016/j.ejphar.2006.10.038
97. Beletsky A, Liu C, Lochte B, Samuel N, Grant I. Cannabis and Anxiety: a Critical Review. Med Cannabis Cannabinoids. 2024;7(1):19–30. doi:10.1159/000534855
98. Schmidt ME, Liebowitz MR, Stein MB, et al. The effects of inhibition of fatty acid amide hydrolase (FAAH) by JNJ-42165279 in social anxiety disorder: a double-blind, randomized, placebo-controlled proof-of-concept study. Neuropsychopharmacol. 2021;46(5):1004–1010. doi:10.1038/s41386-020-00888-1
99. Keith JM, Jones WM, Tichenor M, et al. Preclinical Characterization of the FAAH Inhibitor JNJ-42165279. ACS Med Chem Lett. 2015;6(12):1204–1208. doi:10.1021/acsmedchemlett.5b00353
100. Ahmed M, Tyndale RF, Rubin-Kahana DS, et al. Investigating Fatty Acid Amide Hydrolase Levels in Social Anxiety Disorder: a Positron Emission Tomography (PET) Study Using [C-11]CURB. Biol. Psychiatry. 2020;87(9):S299. doi:10.1016/j.biopsych.2020.02.770
101. Buxton OM, Broussard JL, Zahl AK, Hall M. Effects of Sleep Deficiency on Hormones, Cytokines, and Metabolism. In: Redline S, Berger NA editors. Impact of Sleep and Sleep Disturbances on Obesity and Cancer. Springer New York; 2014:25–50. doi:10.1007/978-1-4614-9527-7_2
102. Murawski B, Wade L, Plotnikoff RC, Lubans DR, Duncan MJ. A systematic review and meta-analysis of cognitive and behavioral interventions to improve sleep health in adults without sleep disorders. Sleep Med Rev. 2018;40:160–169. doi:10.1016/j.smrv.2017.12.003
103. Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of Healthy Sleep Duration among Adults — United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(6):137–141. doi:10.15585/mmwr.mm6506a1
104. Murillo-Rodríguez E. The role of the CB1 receptor in the regulation of sleep. Prog Neuro Psychopharmacol Biol Psychiatry. 2008;32(6):1420–1427. doi:10.1016/j.pnpbp.2008.04.008
105. Maejima T, Masseck OA, Mark MD, Herlitze S. Modulation of firing and synaptic transmission of serotonergic neurons by intrinsic G protein-coupled receptors and ion channels. Front Integr Neurosci. 2013;7. doi:10.3389/fnint.2013.00040
106. Kaul M, Zee PC, Sahni AS. Effects of Cannabinoids on Sleep and their Therapeutic Potential for Sleep Disorders. Neurotherapeutics. 2021;18(1):217–227. doi:10.1007/s13311-021-01013-w
107. Marzo VD, Bifulco M, Petrocellis LD. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3(9):771–784. doi:10.1038/nrd1495
108. Hanlon EC, Tasali E, Leproult R, et al. Sleep Restriction Enhances the Daily Rhythm of Circulating Levels of Endocannabinoid 2-Arachidonoylglycerol. Sleep. 2016;39(3):653–664. doi:10.5665/sleep.5546
109. Suraev AS, Marshall NS, Vandrey R, et al. Cannabinoid therapies in the management of sleep disorders: a systematic review of preclinical and clinical studies. Sleep Med Rev. 2020;53:101339. doi:10.1016/j.smrv.2020.101339
110. D’Souza DC, Cortes-Briones J, Creatura G, et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry. 2019;6(1):35–45. doi:10.1016/S2215-0366(18)30427-9
111. Chagas MHN, Crippa JAS, Zuardi AW, et al. Effects of acute systemic administration of cannabidiol on sleep-wake cycle in rats. J Psychopharmacol. 2013;27(3):312–316. doi:10.1177/0269881112474524
112. Monti JM. Hypnoticlike effects of cannabidiol in the rat. Psychopharmacology. 1977;55(3):263–265. doi:10.1007/BF00497858
113. Saleska JL, Bryant C, Kolobaric A, et al. The Safety and Comparative Effectiveness of Non-Psychoactive Cannabinoid Formulations for the Improvement of Sleep: a Double-Blinded, Randomized Controlled Trial. J Am Nutrition Assoc. 2024;43(1):1–11. doi:10.1080/27697061.2023.2203221
114. Bidwell L, Sznitman S, Martin-Willett R, Hitchcock L. Daily associations with cannabis use and sleep quality in anxious cannabis users. Behav. Sleep Med. 2024;22(2):150–167. doi:10.1080/15402002.2023.2217969
115. Fujimori M, Himwich HE. Δ9-Tetrahydrocannabinol and the sleep-wakefulness cycle in rabbits. Physiol Behav. 1973;11(3):291–295. doi:10.1016/0031-9384(73)90003-6
116. Wallach MB, Gershon S. The effects of Δ8-THC on the EEG, reticular multiple unit activity and sleep of cats. Eur. J. Pharmacol. 1973;24(2):172–178. doi:10.1016/0014-2999(73)90068-X
117. Barratt ES, Adams PM. Chronic marijuana usage and sleep-wakefulness cycles in cats. Biol Psychiatry. 1973;6(3):207–214.
118. Pacek LR, Herrmann ES, Smith MT, Vandrey R. Sleep continuity, architecture and quality among treatment-seeking cannabis users: an in-home, unattended polysomnographic study. Exp. Clin. Psychopharmacol. 2017;25(4):295–302. doi:10.1037/pha0000126
119. Nicholson AN, Turner C, Stone BM, Robson PJ. Effect of Δ-9-Tetrahydrocannabinol and Cannabidiol on Nocturnal Sleep and Early-Morning Behavior in Young Adults. J Clin Psychopharmacol. 2004;24(3):305–313. doi:10.1097/01.jcp.0000125688.05091.8f
120. Lavender I, McGregor IS, Suraev A, Grunstein RR. Cannabinoids, Insomnia, and Other Sleep Disorders. Chest. 2022;162(2):452–465. doi:10.1016/j.chest.2022.04.151
121. Cherkasova V, Wang B, Gerasymchuk M, Fiselier A, Kovalchuk O, Kovalchuk I. Use of Cannabis and Cannabinoids for Treatment of Cancer. Cancers. 2022;14(20):5142. doi:10.3390/cancers14205142
122. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA a Cancer J Clinicians. 2024;74(1):12–49. doi:10.3322/caac.21820
123. Rahman T. Erratum to Cancer statistics, 2024. CA a Cancer J Clinicians. 2024;74(2):203. doi:10.3322/caac.21830
124. Debela DT, Muzazu SG, Heraro KD, et al. New approaches and procedures for cancer treatment: current perspectives. SAGE Open Medicine. 2021:9. doi:10.1177/20503121211034366
125. Chakraborty S, Rahman T. The difficulties in cancer treatment. Ecancermedicalscience. 2012;6(16). doi:10.3332/ecancer.2012.ed16
126. Majeed H, Gupta V. Adverse Effects of Radiation Therapy StatPearls. StatPearls Publishing LLC.; 2023.
127. Strzelecka K, Piotrowska U, Sobczak M, Oledzka E. The Advancement of Biodegradable Polyesters as Delivery Systems for Camptothecin and Its Analogues—A Status Report. IJMS. 2023;24(2):1053. doi:10.3390/ijms24021053
128. Loeb LA, Loeb KR, Anderson JP. Multiple mutations and cancer. Proc Natl Acad Sci USA. 2003;100(3):776–781. doi:10.1073/pnas.0334858100
129. Pyszniak M, Tabarkiewicz J, Łuszczki J. Endocannabinoid system as a regulator of tumor cell malignancy - biological pathways and clinical significance. OTT. 2016;9:4323–4336. doi:10.2147/OTT.S106944
130. Ramer R, Bublitz K, Freimuth N, et al. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule‐1. FASEB j. 2012;26(4):1535–1548. doi:10.1096/fj.11-198184
131. Salazar M, Carracedo A, Salanueva ÍJ, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest. 2009;119(5):1359–1372. doi:10.1172/JCI37948
132. Donadelli M, Dando I, Zaniboni T, et al. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2011;2(4):e152–e152. doi:10.1038/cddis.2011.36
133. Tubaro A, Giangaspero A, Sosa S, et al. Comparative topical anti-inflammatory activity of cannabinoids and cannabivarins. Fitoterapia. 2010;81(7):816–819. doi:10.1016/j.fitote.2010.04.009
134. Anil SM, Peeri H, Koltai H. Medical Cannabis Activity Against Inflammation: active Compounds and Modes of Action. Front Pharmacol. 2022;13:908198. doi:10.3389/fphar.2022.908198
135. Winkler K, Ramer R, Dithmer S, Ivanov I, Merkord J, Hinz B. Fatty acid amide hydrolase inhibitors confer anti-invasive and antimetastatic effects on lung cancer cells. Oncotarget. 2016;7(12):15047–15064. doi:10.18632/oncotarget.7592
136. Buchalska B, Kamińska K, Owe-Larsson M, Cudnoch-Jędrzejewska A. Cannabinoids in the treatment of glioblastoma. Pharmacol Rep. 2024;8:850. doi:10.1007/s43440-024-00580-x
137. Dasram MH, Naidoo P, Walker RB, Khamanga SM. Targeting the Endocannabinoid System Present in the Glioblastoma Tumour Microenvironment as a Potential Anti-Cancer Strategy. IJMS. 2024;25(3):1371. doi:10.3390/ijms25031371
138. Cretu B, Zamfir A, Bucurica S, et al. Role of Cannabinoids in Oral Cancer. IJMS. 2024;25(2):969. doi:10.3390/ijms25020969
139. Heider CG, Itenberg SA, Rao J, Ma H, Wu X. Mechanisms of Cannabidiol (CBD) in Cancer Treatment: a Review. Biology. 2022;11(6):817. doi:10.3390/biology11060817
140. Abrams DI. The therapeutic effects of Cannabis and cannabinoids: an update from the National Academies of Sciences, Engineering and Medicine report. Eur J Internal Med. 2018;49:7–11. doi:10.1016/j.ejim.2018.01.003
141. Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda, Board on Population Health and Public Health Practice, Health and Medicine Division, National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. National Academies Press; 2017:24625. doi:10.17226/24625
142. Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol Versus Megestrol Acetate Versus Combination Therapy for Cancer-Associated Anorexia: a North Central Cancer Treatment Group Study. JCO. 2002;20(2):567–573. doi:10.1200/JCO.2002.20.2.567
143. Seymour-Jackson E, Laird BJA, Sayers J, Fallon M, Solheim TS, Skipworth R. Cannabinoids in the treatment of cancer anorexia and cachexia: where have we been, where are we going? Asia PacJ Oncol Nurs. 2023;10:100292. doi:10.1016/j.apjon.2023.100292
144. Vučković S, Srebro D, Vujović KS, Vučetić Č, Prostran M. Cannabinoids and Pain: new Insights From Old Molecules. Front Pharmacol. 2018;9:1259. doi:10.3389/fphar.2018.01259
145. Colombo E, Coppini DA, Polito L, et al. Cannabidiol as Self-Assembly Inducer for Anticancer Drug-Based Nanoparticles. Molecules. 2022;28(1):112. doi:10.3390/molecules28010112
146. Baswan SM, Klosner AE, Glynn K, et al. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. CCID. 2020;13:927–942. doi:10.2147/CCID.S286411
147. Sheriff T, Lin MJ, Dubin D, Khorasani H. The potential role of cannabinoids in dermatology. J Dermatological Treat. 2020;31(8):839–845. doi:10.1080/09546634.2019.1675854
148. Ständer S, Schmelz M, Metze D, Luger T, Rukwied R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatological Sci. 2005;38(3):177–188. doi:10.1016/j.jdermsci.2005.01.007
149. Río CD, Millán E, García V, Appendino G, DeMesa J, Muñoz E. The endocannabinoid system of the skin. A potential approach for the treatment of skin disorders. Biochem. Pharmacol. 2018;157:122–133. doi:10.1016/j.bcp.2018.08.022
150. Denda M, Tsutsumi M, Goto M, Ikeyama K, Denda S. Topical Application of TRPA1 Agonists and Brief Cold Exposure Accelerate Skin Permeability Barrier Recovery. J Invest Dermatol. 2010;130(7):1942–1945. doi:10.1038/jid.2010.32
151. Denda M, Tsutsumi M, Denda S. Topical application of TRPM8 agonists accelerates skin permeability barrier recovery and reduces epidermal proliferation induced by barrier insult: role of cold-sensitive TRP receptors in epidermal permeability barrier homoeostasis: TRPM8 agonists accelerate barrier recovery. Experimental Dermatol. 2010;19(9):791–795. doi:10.1111/j.1600-0625.2010.01154.x
152. Soliman E, Henderson KL, Danell AS, Van Dross R. Arachidonoyl‐ethanolamide activates endoplasmic reticulum stress‐apoptosis in tumorigenic keratinocytes: role of cyclooxygenase‐2 and novel J‐series prostamides. Mol, Carcinog. 2016;55(2):117–130. doi:10.1002/mc.22257
153. Soliman E, Van Dross R. Anandamide-induced endoplasmic reticulum stress and apoptosis are mediated by oxidative stress in non-melanoma skin cancer: receptor-independent endocannabinoid signaling. Mol, Carcinog. 2016;55(11):1807–1821. doi:10.1002/mc.22429
154. Ständer S, Reinhardt HW, Luger TA. Topische Cannabinoidagonisten: eine effektive, neue Möglichkeit zur Behandlung von chronischem Pruritus. Hautarzt. 2006;57(9):801–807. doi:10.1007/s00105-006-1180-1
155. Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatological Sci. 2007;45(2):87–92. doi:10.1016/j.jdermsci.2006.10.009
156. Carbone A, Siu A, Patel R. Pediatric Atopic Dermatitis: a Review of the Medical Management. Ann Pharmacother. 2010;44(9):1448–1458. doi:10.1345/aph.1P098
157. Soliman E, Ladin D, Van Dross R. Cannabinoids as Therapeutics for Non-Melanoma and Melanoma Skin Cancer. J Dermatol Clin Res. 2016;4(2):1069.
158. Kupczyk P, Reich A, Szepietowski JC. Cannabinoid system in the skin - a possible target for future therapies in dermatology. Experimental Dermatol. 2009;18(8):669–679. doi:10.1111/j.1600-0625.2009.00923.x
159. Ferreira I, Lopes CM, Amaral MH. Treatment Advances for Acne Vulgaris: the Scientific Role of Cannabinoids. Cosmetics. 2024;11(1):22. doi:10.3390/cosmetics11010022
160. Lapteva M, Faro Barros J, Kalia YN. Cutaneous Delivery and Biodistribution of Cannabidiol in Human Skin after Topical Application of Colloidal Formulations. Pharmaceutics. 2024;16(2):202. doi:10.3390/pharmaceutics16020202
161. Parikh AC, Jeffery CS, Sandhu Z, Brownlee BP, Queimado L, Mims MM. The effect of cannabinoids on wound healing: a review. Health Sci Rep. 2024;7(2):e1908. doi:10.1002/hsr2.1908
162. Smith G, Satino J. Hair Regrowth with Cannabidiol (CBD)-rich Hemp Extract - A Case Series. Cannabis. 2021;4(1):53–59. doi:10.26828/cannabis/2021.01.003
163. Maayah ZH, Takahara S, Ferdaoussi M, Dyck JRB. The molecular mechanisms that underpin the biological benefits of full-spectrum cannabis extract in the treatment of neuropathic pain and inflammation. Mol Basis Dis. 2020;1866(7):165771. doi:10.1016/j.bbadis.2020.165771
164. Balić A, Vlašić D, Žužul K, Marinović B, Bukvić Mokos Z. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. IJMS. 2020;21(3):741. doi:10.3390/ijms21030741
165. Moore EM, Wagner C, Komarnytsky S. The Enigma of Bioactivity and Toxicity of Botanical Oils for Skin Care. Front Pharmacol. 2020;11:785. doi:10.3389/fphar.2020.00785
166. Pei L, Luo Y, Gu X, Wang J. Formation, Stability and Properties of Hemp Seed Oil Emulsions for Application in the Cosmetics Industry. Tenside Surfactants Detergents. 2020;57(6):451–459. doi:10.3139/113.110712
167. Metwally S, Ura DP, Krysiak ZJ, Kaniuk L, Szewczyk PK, Stachewicz U. Electrospun PCL Patches with Controlled Fiber Morphology and Mechanical Performance for Skin Moisturization via Long-Term Release of Hemp Oil for Atopic Dermatitis. Membranes. 2020;11(1):26. doi:10.3390/membranes11010026
168. Mnekin L, Ripoll L. Topical Use of Cannabis sativa L. Biochemicals. Cosmetics. 2021;8(3):85. doi:10.3390/cosmetics8030085
169. Anand U, Pacchetti B, Anand P, Sodergren MH. Cannabis-based medicines and pain: a review of potential synergistic and entourage effects. Pain Management. 2021;11(4):395–403. doi:10.2217/pmt-2020-0110
170. Nevozhay D, Kańska U, Budzyńska R, Boratyński J. Current status of research on conjugates and related drug delivery systems in the treatment of cancer and other diseases. Postepy higieny medycyny doswiadczalnej. 2007;61:350–360.
171. Allen TM, Cullis PR. Drug Delivery Systems: entering the Mainstream. Science. 2004;303(5665):1818–1822. doi:10.1126/science.1095833
172. Zoltowska K, Sobczak M, Oledzka E. Polyurethanes in pharmacy — current state and perspectives of the development. Polimery. 2014;59(10):689–698. doi:10.14314/polimery.2014.689
173. Ringsdorf H. Structure and properties of pharmacologically active polymers. J Polym Sci, C Polym Symp. 1975;51(1):135–153. doi:10.1002/polc.5070510111
174. Elvira C, Gallardo A, Roman J, Cifuentes A. Covalent Polymer-Drug Conjugates. Molecules. 2005;10(1):114–125. doi:10.3390/10010114
175. Larson N, Ghandehari H. Polymeric Conjugates for Drug Delivery. Chem Mater. 2012;24(5):840–853. doi:10.1021/cm2031569
176. Negut I, Bita B. Polymeric Micellar Systems—A Special Emphasis on “Smart” Drug Delivery. Pharmaceutics. 2023;15(3):976. doi:10.3390/pharmaceutics15030976
177. Perumal S, Atchudan R, Lee W. A Review of Polymeric Micelles and Their Applications. Polymers. 2022;14(12):2510. doi:10.3390/polym14122510
178. Kaur J, Gulati M, Jha NK, et al. Recent advances in developing polymeric micelles for treating cancer: breakthroughs and bottlenecks in their clinical translation. Drug Discovery Today. 2022;27(5):1495–1512. doi:10.1016/j.drudis.2022.02.005
179. Jung A. Nanoparticles in medical applications - a direction of the future? Pediatr Med Rodz. 2014;10(2):104–110. doi:10.15557/PiMR.2014.0015
180. Mohanraj VJ, Chen Y. Nanoparticles - A review. Trop J Pharm Res. 2007;5(1):561–573. doi:10.4314/tjpr.v5i1.14634
181. Budnicka M, Gadomska-Gajadhur A, Ruskowski P, Synoradzki L. Biodegradable polymers for the treatment of tuberculosis Part I. Epidemiology, therapy and treatment methods. Polimery. 2017;62(10):711–719. doi:10.14314/polimery.2017.711
182. Niemirowicz K, Car H. Nanonośniki jako nowoczesne transportery w kontrolowanym dostarczaniu leków. CHEMIK. 2012;66(8):868–881.
183. Tiwari G, Tiwari R, Bannerjee S, et al. Drug delivery systems: an updated review. Int J Pharma Investig. 2012;2(1):2. doi:10.4103/2230-973X.96920
184. Bajracharya R, Song JG, Patil BR, et al. Functional ligands for improving anticancer drug therapy: current status and applications to drug delivery systems. Drug Delivery. 2022;29(1):1959–1970. doi:10.1080/10717544.2022.2089296
185. Abuchowski A, van Es T, Palczuk NC, Davis FF. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem. 1977;252(11):3578–3581. doi:10.1016/S0021-9258(17)40291-2
186. Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252(11):3582–3586. doi:10.1016/S0021-9258(17)40292-4
187. Ikeda Y, Nagasaki Y. PEGylation Technology in Nanomedicine. In: Kunugi S, Yamaoka T, editors. Polymers in Nanomedicine. Vol 247. Advances in Polymer Science. Berlin Heidelberg: Springer; 2011:115–140. doi:10.1007/12_2011_154
188. Wang N, Wang T, Li T, Deng Y. Modulation of the physicochemical state of interior agents to prepare controlled release liposomes. Colloids Surf. B. 2009;69(2):232–238. doi:10.1016/j.colsurfb.2008.11.033
189. Niu M, Lu Y, Hovgaard L, et al. Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: the effect of cholate type, particle size and administered dose. Eur. J. Pharm. Biopharm. 2012;81(2):265–272. doi:10.1016/j.ejpb.2012.02.009
190. Liu P, Chen G, Zhang J. A Review of Liposomes as a Drug Delivery System: current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules. 2022;27(4):1372. doi:10.3390/molecules27041372
191. Bozzuto G, Molinari A. Liposomes as nanomedical devices. IJN. 2015;10(1):975–999. doi:10.2147/IJN.S68861
192. Sarecka-Hujar B, Jankowski A, Wysocka J. Liposomy-postać modyfikująca transport substancji aktywnych przez skórę Część 2. Zastosowanie w transporcie leków o działaniu ogólnoustrojowym. Ann Acad Med Silesiensis. 2011;65(4):45–50.
193. Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022;8(5):e09394. doi:10.1016/j.heliyon.2022.e09394
194. Goik U, Załęska-Żyłka I, Pietrzycka A. Liposomes as carriers for the delivery of active substances to the skin. Eng Biomaterials. 2015;18(130):27–39.
195. Pasarin D, Ghizdareanu AI, Enascuta CE, et al. Coating Materials to Increase the Stability of Liposomes. Polymers. 2023;15(3):782. doi:10.3390/polym15030782
196. Jerbic IS. Biodegradable synthetic polymers and their application in advanced drug delivery systems (DDS). J Chem Eng Process Technol. 2018;1(1):1–9. doi:10.4172/2157-7048-C1-011
197. Doppalapudi S, Jain A, Khan W, Domb AJ. Biodegradable polymers-an overview. Polym Adv Technol. 2014;25(5):427–435. doi:10.1002/pat.3305
198. Prasher P, Sharma M, Mehta M, et al. Current-status and applications of polysaccharides in drug delivery systems. Colloid Interface Sci. Commun. 2021;42:100418. doi:10.1016/j.colcom.2021.100418
199. Chanthathamrongsiri N, Petchsomrit A, Leelakanok N, Siranonthana N, Sirirak T. The comparison of the properties of nanocellulose isolated from colonial and solitary marine tunicates. Heliyon. 2021;7(8):e07819. doi:10.1016/j.heliyon.2021.e07819
200. Ioelovich M. Cellulose as a nanostructured polymer: a short review. BioRes. 2008;3(4):1403–1418. doi:10.15376/biores.3.4.Ioelovich
201. Klemm D, Cranston ED, Fischer D, et al. Nanocellulose as a natural source for groundbreaking applications in materials science: today’s state. Mater Today. 2018;21(7):720–748. doi:10.1016/j.mattod.2018.02.001
202. Seddiqi H, Oliaei E, Honarkar H, et al. Cellulose and its derivatives: towards biomedical applications. Cellulose. 2021;28(4):1893–1931. doi:10.1007/s10570-020-03674-w
203. Ahmad Raus R, Wan Nawawi WMF, Nasaruddin RR. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021;16(3):280–306. doi:10.1016/j.ajps.2020.10.001
204. Szekalska M, Puciłowska A, Szymańska E, Ciosek P, Winnicka K. Alginate: current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int J Polym Sci. 2016;2016:1–17. doi:10.1155/2016/7697031
205. Fu S, Thacker A, Sperger DM, et al. Relevance of Rheological Properties of Sodium Alginate in Solution to Calcium Alginate Gel Properties. AAPS Pharm Sci Tech. 2011;12(2):453–460. doi:10.1208/s12249-011-9587-0
206. Otterlei M, Østgaard K, Skjåk-Bræk G, Smidsrød O, Soon-Shiong P, Espevik T. Induction of Cytokine Production from Human Monocytes Stimulated with Alginate. J Immunother. 1991;10(4):286–291. doi:10.1097/00002371-199108000-00007
207. Thomas S. Alginate dressings in surgery and wound management: part 2. J Wound Care. 2000;9(3):115–119. doi:10.12968/jowc.2000.9.3.25959
208. Venkatesan J, Bhatnagar I, Manivasagan P, Kang KH, Kim SK. Alginate composites for bone tissue engineering: a review. Int J Biol Macromol. 2015;72:269–281. doi:10.1016/j.ijbiomac.2014.07.008
209. Sun J, Tan H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials. 2013;6(4):1285–1309. doi:10.3390/ma6041285
210. Li J, Cai C, Li J, et al. Chitosan-Based Nanomaterials for Drug Delivery. Molecules. 2018;23(10):2661. doi:10.3390/molecules23102661
211. Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Delivery Rev. 2010;62(1):3–11. doi:10.1016/j.addr.2009.09.004
212. Frank LA, Onzi GR, Morawski AS, Pohlmann AR, Guterres SS, Contri RV. Chitosan as a coating material for nanoparticles intended for biomedical applications. React Funct Polym. 2020;147:104459. doi:10.1016/j.reactfunctpolym.2019.104459
213. Ali A, Ahmed S. A review on chitosan and its nanocomposites in drug delivery. Int J Biol Macromol. 2018;109:273–286. doi:10.1016/j.ijbiomac.2017.12.078
214. Ways M, Lau T, Khutoryanskiy W. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers. 2018;10(3):267. doi:10.3390/polym10030267
215. Bhatia S. Natural Polymers vs Synthetic Polymer. In: Natural Polymer Drug Delivery Systems. Springer International Publishing; 2016:95–118. doi:10.1007/978-3-319-41129-3_3
216. Piskin E. Biodegradable polymers as biomaterials. J biomater sci Poly ed. 1995;6(9):775–795. doi:10.1163/156856295X00175
217. Chandra R. Biodegradable polymers. Prog Polym Sci. 1998;23(7):1273–1335. doi:10.1016/S0079-6700(97)00039-7
218. Duda A, Penczek S. Polylactide [poly(lactic acid)]: synthesis, properties and applications. p. 2003;48(1):16–27.
219. Luo F, Fortenberry A, Ren J, Qiang Z. Recent Progress in Enhancing Poly(Lactic Acid) Stereocomplex Formation for Material Property Improvement. Front Chem. 2020;8:688. doi:10.3389/fchem.2020.00688
220. Capuana E, Lopresti F, Ceraulo M, La Carrubba V. Poly-l-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: a Review on Processing and Applications. Polymers. 2022;14(6):1153. doi:10.3390/polym14061153
221. Casalini T, Rossi F, Castrovinci A, Perale G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front Bioeng Biotechnol. 2019;7:259. doi:10.3389/fbioe.2019.00259
222. Andrzejewska A, Topoliński T. Biodegradable polymers for biomedical applications. Dev Mech Engi. 2015;6(3):5–12.
223. Ragaert K, De Somer F, Van de Velde S, Degrieck J, Cardon L. Methods for Improved Flexural Mechanical Properties of 3D-Plotted PCL-Based Scaffolds for Heart Valve Tissue Engineering. SV-JME. 2013;59(11):669–676. doi:10.5545/sv-jme.2013.1003
224. Znajewska Z. Biodegradacja polikaprolaktonu przez grzyby Trichoderma viride. Chem Rev. 2018;1(10):78–81. doi:10.15199/62.2018.10.8
225. Woodruff MA, Hutmacher DW. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog Polym Sci. 2010;35(10):1217–1256. doi:10.1016/j.progpolymsci.2010.04.002
226. Budak K, Sogut O, Aydemir Sezer U. A review on synthesis and biomedical applications of polyglycolic acid. J Polym Res. 2020;27(8):208. doi:10.1007/s10965-020-02187-1
227. Kumar N, Ravikumar MNV, Domb AJ. Biodegradable block copolymers. Adv. Drug Delivery Rev. 2001;53(1):23–44. doi:10.1016/S0169-409X(01)00219-8
228. Surya N, Bhattacharyya S. PLGA-THE SMART POLYMER FOR DRUG DELIVERY. Farm Farmakol. 2021;9(5):334–345. doi:10.19163/2307-9266-2021-9-5-334-345
229. Machatschek R, Schulz B, Lendlein A. The influence of pH on the molecular degradation mechanism of PLGA. MRS Adv. 2018;3(63):3883–3889. doi:10.1557/adv.2018.602
230. Chandrasekaran AR, Venugopal J, Sundarrajan S, Ramakrishna S. Fabrication of a nanofibrous scaffold with improved bioactivity for culture of human dermal fibroblasts for skin regeneration. Biomed Mater. 2011;6(1):015001. doi:10.1088/1748-6041/6/1/015001
231. Li F, Li X, He R, Cheng J, Ni Z, Zhao G. Preparation and evaluation of poly(D, L-lactic acid)/poly(L-lactide-co-ε-caprolactone) blends for tunable sirolimus release. Colloids Surf. A. 2020;590:124518. doi:10.1016/j.colsurfa.2020.124518
232. Sang Q, Li H, Williams G, Wu H, Zhu LM. Core-shell poly(lactide-co-ε-caprolactone)-gelatin fiber scaffolds as pH-sensitive drug delivery systems. J Biomater Appl. 2018;32(8):1105–1118. doi:10.1177/0885328217749962
233. Abu Abed OS, Chaw C, Williams L, Elkordy AA. Lysozyme and DNase I loaded poly (D, L lactide-co-caprolactone) nanocapsules as an oral delivery system. Sci Rep. 2018;8(1):13158. doi:10.1038/s41598-018-31303-x
234. Lee SH, Kim BS, Kim SH, et al. Elastic biodegradable poly(glycolide-co-caprolactone) scaffold for tissue engineering. J Biomed Mater Res. 2003;66A(1):29–37. doi:10.1002/jbm.a.10497
235. Chen S, Deng C, Zheng W, et al. Cannabidiol Effectively Promoted Cell Death in Bladder Cancer and the Improved Intravesical Adhesion Drugs Delivery Strategy Could Be Better Used for Treatment. Pharmaceutics. 2021;13(9):1415. doi:10.3390/pharmaceutics13091415
236. Chevalier MT, Al-Waeel M, Alsharabasy AM, Rebelo AL, Martin-Saldaña S, Pandit A. Therapeutic Polymer-Based Cannabidiol Formulation: tackling Neuroinflammation Associated with Ischemic Events in the Brain. Mol Pharm. 2024;
237. Fraguas-Sánchez AI, Torres-Suárez AI, Cohen M, et al. PLGA Nanoparticles for the Intraperitoneal Administration of CBD in the Treatment of Ovarian Cancer: in Vitro and In Ovo Assessment. Pharmaceutics. 2020;12(5):439. doi:10.3390/pharmaceutics12050439
238. Muresan P, Woodhams S, Smith F, et al. Evaluation of cannabidiol nanoparticles and nanoemulsion biodistribution in the central nervous system after intrathecal administration for the treatment of pain. Nanomed Nanotechnol Biol Med. 2023;49:102664. doi:10.1016/j.nano.2023.102664
239. Shreiber-Livne I, Sulimani L, Shapira A, Procaccia S, Meiri D, Sosnik A. Poly(ethylene glycol)-b-poly(epsilon-caprolactone) nanoparticles as a platform for the improved oral delivery of cannabidiol. Drug Deliv Transl Res. 2023;13:3192–3203. doi:10.1007/s13346-023-01380-1
240. Monou PK, Mamaligka AM, Tzimtzimis EK, et al. Fabrication and Preliminary In Vitro Evaluation of 3D-Printed Alginate Films with Cannabidiol (CBD) and Cannabigerol (CBG) Nanoparticles for Potential Wound-Healing Applications. Pharmaceutics. 2022;14(8):1637. doi:10.3390/pharmaceutics14081637
241. Hernán Pérez de la Ossa D, Ligresti A, Gil-Alegre ME, et al. Poly-ε-caprolactone microspheres as a drug delivery system for cannabinoid administration: development, characterization and in vitro evaluation of their antitumoral efficacy. J Control Release. 2012;161(3):927–932. doi:10.1016/j.jconrel.2012.05.003
242. Kamali A, Oryan A, Hosseini S, et al. Cannabidiol-loaded microspheres incorporated into osteoconductive scaffold enhance mesenchymal stem cell recruitment and regeneration of critical-sized bone defects. Mater Sci Eng C. 2019;101:64–75. doi:10.1016/j.msec.2019.03.070
243. Kamali A, Oryan A, Hosseini S, et al. Corrigendum to Cannabidiol-loaded microspheres incorporated into osteoconductive scaffold enhance mesenchymal stem cell recruitment and regeneration of critical-sized bone defects. Mater Sci Eng C. 2021;126:112179. doi:10.1016/j.msec.2021.112179
244. Hernán Pérez De La Ossa D, Lorente M, Gil-Alegre ME, et al. Local Delivery of Cannabinoid-Loaded Microparticles Inhibits Tumor Growth in a Murine Xenograft Model of Glioblastoma Multiforme. PLoS One. 2013;8(1):e54795. doi:10.1371/journal.pone.0054795
245. Fraguas-Sánchez AI, Fernández-Carballido A, Simancas-Herbada R, Martin-Sabroso C, Torres-Suárez AI. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int J Pharm. 2020;574:118916. doi:10.1016/j.ijpharm.2019.118916
246. David C, De Souza JF, Silva AF, et al. Cannabidiol-loaded microparticles embedded in a porous hydrogel matrix for biomedical applications. J Mater Sci Mater Med. 2024;35(1):14. doi:10.1007/s10856-023-06773-9
247. Toncheva-Moncheva N, Dimitrov E, Grancharov G, Momekova D, Petrov P, Rangelov S. Cinnamyl-Modified Polyglycidol/Poly(ε-Caprolactone) Block Copolymer Nanocarriers for Enhanced Encapsulation and Prolonged Release of Cannabidiol. Pharmaceutics. 2023;15(8):2128. doi:10.3390/pharmaceutics15082128
248. Demisli S, Galani E, Goulielmaki M, et al. Encapsulation of cannabidiol in oil-in-water nanoemulsions and nanoemulsion-filled hydrogels: a structure and biological assessment study. J Colloid Interface Sci. 2023;634:300–313. doi:10.1016/j.jcis.2022.12.036
249. Durán-Lobato M, Muñoz-Rubio I, Holgado MÁ, Álvarez-Fuentes J, Fernández-Arévalo M, Martín-Banderas L. Enhanced Cellular Uptake and Biodistribution of a Synthetic Cannabinoid Loaded in Surface-Modified Poly(lactic-co-glycolic acid) Nanoparticles. J Biomed Nanotechnol. 2014;10(6):1068–1079. doi:10.1166/jbn.2014.1806
250. Durán-Lobato M, Martín-Banderas L, Gonçalves LMD, Fernández-Arévalo M, Almeida AJ. Comparative study of chitosan- and PEG-coated lipid and PLGA nanoparticles as oral delivery systems for cannabinoids. J Nanopart Res. 2015;17(2):61. doi:10.1007/s11051-015-2875-y
251. Berrocoso E, Rey-Brea R, Fernández-Arévalo M, Micó JA, Martín-Banderas L. Single oral dose of cannabinoid derivate loaded PLGA nanocarriers relieves neuropathic pain for eleven days. Nanomed Nanotechnol Biol Med. 2017;13(8):2623–2632. doi:10.1016/j.nano.2017.07.010
252. Martín-Banderas L, Muñoz-Rubio I, Prados J, et al. In vitro and in vivo evaluation of Δ9-tetrahidrocannabinol/PLGA nanoparticles for cancer chemotherapy. Int J Pharm. 2015;487(1–2):205–212. doi:10.1016/j.ijpharm.2015.04.054
253. Al-Ghananeem AM, Malkawi AH, Crooks PA. Bioavailability of Δ 9 -tetrahydrocannabinol following intranasal administration of a mucoadhesive gel spray delivery system in conscious rabbits. Drug Dev. Ind. Pharm. 2011;37(3):329–334. doi:10.3109/03639045.2010.513009
254. Román-Vargas Y, Porras-Arguello JD, Blandón-Naranjo L, Pérez-Pérez LD, Benjumea DM. Evaluation of the Analgesic Effect of High-Cannabidiol-Content Cannabis Extracts in Different Pain Models by Using Polymeric Micelles as Vehicles. Molecules. 2023;28(11):4299. doi:10.3390/molecules28114299
255. Porras JD, Román Y, Palacio J, Blandón-Naranjo L, Benjumea D, Pérez LD. Amphiphilic block copolymers bearing fatty acid derivatives as vehicles for THC in the development of analgesic oral formulations. React Funct Polym. 2024;195:105811. doi:10.1016/j.reactfunctpolym.2023.105811
256. Villate A, San Nicolas M, Olivares M, Aizpurua-Olaizola O, Usobiaga A. Chitosan-Coated Alginate Microcapsules of a Full-Spectrum Cannabis Extract: characterization, Long-Term Stability and In Vitro Bioaccessibility. Pharmaceutics. 2023;15(3):859. doi:10.3390/pharmaceutics15030859
257. Uziel A, Gelfand A, Amsalem K, et al. Full-Spectrum Cannabis Extract Microdepots Support Controlled Release of Multiple Phytocannabinoids for Extended Therapeutic Effect. ACS Appl Mater Interfaces. 2020;12(21):23707–23716. doi:10.1021/acsami.0c04435
258. Grotenhermen F, Müller-Vahl K. The Therapeutic Potential of Cannabis and Cannabinoids. Dtsch Arztebl Int. 2012;109(29–30):495–501. doi:10.3238/arztebl.2012.0495
259. Weber J, Schley M, Casutt M, et al. Tetrahydrocannabinol (Delta 9-THC) Treatment in Chronic Central Neuropathic Pain and Fibromyalgia Patients: results of a Multicenter Survey. Anesthesiology Res Practice. 2009;2009:1–9. doi:10.1155/2009/827290
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
