Back to Journals » International Journal of Nanomedicine » Volume 18
How Advancing is Peripheral Nerve Regeneration Using Nanofiber Scaffolds? A Comprehensive Review of the Literature
Received 24 August 2023
Accepted for publication 2 November 2023
Published 15 November 2023 Volume 2023:18 Pages 6763—6779
DOI https://doi.org/10.2147/IJN.S436871
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Farooq A. Shiekh
Shaoyan Shi, Xuehai Ou, Deliang Cheng
Department of Hand Surgery, Honghui Hospital, Xi’an Jiaotong University, Xi’an Honghui Hospital North District, Xi’an, Shaanxi, 710000, People’s Republic of China
Correspondence: Deliang Cheng, Department of Hand Surgery, Honghui Hospital, Xi’an Jiaotong University, Xi’an Honghui Hospital North District, Xi’an, Shaanxi, 710000, People’s Republic of China, Tel +86 18740308729, Email [email protected]
Abstract: Peripheral nerve injuries present significant challenges in regenerative medicine, primarily due to inherent limitations in the body’s natural healing processes. In response to these challenges and with the aim of enhancing peripheral nerve regeneration, nanofiber scaffolds have emerged as a promising and advanced intervention. However, a deeper understanding of the underlying mechanistic foundations that drive the favorable contributions of nanofiber scaffolds to nerve regeneration is essential. In this comprehensive review, we make an exploration of the latent potential of nanofiber scaffolds in augmenting peripheral nerve regeneration. This exploration includes a detailed introduction to the fabrication methods of nanofibers, an analysis of the intricate interactions between these scaffolds and cellular entities, an examination of strategies related to the controlled release of bioactive agents, an assessment of the prospects for clinical translation, an exploration of emerging trends, and thorough considerations regarding biocompatibility and safety. By comprehensively elucidating the intricate structural attributes and multifaceted functional capacities inherent in nanofiber scaffolds, we aim to offer a prospective and effective strategy for the treatment of peripheral nerve injury.
Keywords: nanofiber, scaffolds, nanomedicine, nerve regeneration, tissue
Introduction
Peripheral nerve injury is one of the most challengeable issues in regenerative medicine, notably marked by a deficiency in the inherent processes of spontaneous healing and functional restoration.1,2 This innate incapacity for regeneration is derived from a variety of etiologies, ranging from traumatic incidents to surgical interventions and pathological anomalies.3 These disparate issues converge to disrupt the delicate equilibrium governing nerve tissue homeostasis, thereby engendering an impairment in the intricate process of repair and regeneration within the injured nerve.4,5 Importantly, peripheral nerve injuries transcend mere physiological disruption, setting in motion a cascade of incapacitating consequences that profoundly impair an individual’s quality of life. Furthermore, the pathological alterations often exert their influence on both sensory and motor functions, resulting in a constellation of associated symptoms.2,5,6 This multifaceted impact underscores the urgent need for comprehensive therapeutic interventions that not only restore the biological function of injured nerves but also mitigate the far-reaching consequences of these injuries on the holistic well-being of affected individuals.
Peripheral nerve injuries trigger a series of natural processes in the body aimed at recovery. First and foremost, an inflammatory response is initiated, involving immune cells like macrophages, which diligently work to clear away debris and damaged tissue from the injury site.7 Simultaneously, in the distal portion of the nerve, the axons – the lengthy projections responsible for transmitting signals – undergo degeneration, a natural response to the injury that may result in a loss of function in the affected area. During this phase, Wallerian degeneration takes place, involving the breakdown of the axon and its protective myelin sheath. Schwann cells, pivotal in peripheral nerve regeneration, multiply to clear debris and provide essential support for the subsequent axon regrowth.8,9 If the nerve injury is not too extensive and the nerve endings are in close proximity, axon regrowth becomes feasible, guided by the basal lamina tubes generated by Schwann cells. This leads to the vital phase of remodeling and reinnervation, wherein reestablishing connections between axons and their target tissues or organs is imperative. The accuracy of this reinnervation is critical for the restoration of proper function, making this a complex but essential process in peripheral nerve recovery.10 Recently, there has been a growing convergence between nanotechnology and tissue engineering, offering new approaches to address the significant challenges associated with peripheral nerve injuries.11–13 In this evolving landscape, a particularly promising avenue emerges—the utilization of nanofiber scaffolds. These scaffolds possess the potential to amplify the regenerative capacity of peripheral nerves.14 Modified with functional precision, nanofiber scaffolds are often created using advanced techniques such as electrospinning, resulting in a compelling biomimetic platform.15,16 This platform adeptly replicates the intricate architectural intricacies inherent in the natural extracellular matrix (ECM) enveloping nerve tissue.17 This orchestrated replication of biological processes fosters an environment uniquely favorable for three critical mechanisms: cellular attachment, robust cell growth, and the unhindered elongation of neurites.18 By beneficially mimicking the foundational cues of the native ECM, nanofiber scaffolds act as a guiding presence for cellular constituents, directing them towards coordinated growth and reconnection.19 Despite this, more studies need to be performed to elucidate the underlying mechanistic foundations that underpin the favorable contributions of nanofiber scaffolds in nerve regeneration.
Therefore, in this review, we aim to elucidate the considerable potential of nanofiber scaffolds as a transformative strategy for promotion of peripheral nerve regeneration. Through a comprehensive exploration of the intricate structural attributes and multifaceted functional capacities intrinsic to nanofiber scaffolds, we aim to provide a profound understanding of the current state-of-The-art advances, the accompanying challenges, and the potential frontiers within the advanced strategies for mitigating peripheral nerve injury.
How Advanced are Nanofiber Fabrication Strategies?
The functional manipulation of materials at the nanoscale has provided a new strategy for tissue engineering. Central to this transformative movement is the emergence of nanofiber scaffolds—an essential innovation with the capacity to traverse the gap between nerve injury and the reestablishment of operational capability.20 In this section, we introduce the complex approaches of nanofiber fabrication, discussing the methodologies, materials, and design principles that underlie their pivotal contribution to the advance of peripheral nerve regeneration.
Electrospinning as a Predominant Technique for Nanofiber Production
Among the various available techniques, electrospinning has risen to prominence as a dominant and flexible approach generating nanofibers.21,22 This process relies on a complex interaction of electric fields, polymer solutions, and intricate mechanisms that control the formation of fibers. By utilizing the electrostatic forces between a charged polymer solution and a collector, this method precisely orchestrates the ejection of extremely fine fibers, with diameters ranging from tiny nanometers to the scale of micrometers.17 This methodically controlled and reproducible course of action begets nanofiber scaffolds characterized by their discernible surface area-to-volume ratio, nanoscale porosity, and a biomimetic architecture that faithfully mirrors the organization of the ECM.21–23
The pursuit of electrospinning transcends mere fiber generation; it resides in its capacity for refined manipulation of fiber morphology.24 Parameters such as polymer concentration, solution viscosity, applied voltage, and collector design can be functionally modified, thus enabling precise control over attributes like fiber diameter, alignment, and even the integration of bioactive agents.25 This adaptability serves to facilitate critical processes such as cellular adhesion, migration, and the extension of neurites—a pivotal nexus for the accomplishment of successful nerve regeneration.24,25 The ability of electrospinning to produce nanofibers that mimic the natural microenvironment makes it an ideal choice for scaffolds that effectively guide the regenerative process of nerve tissue.
Diverse Materials and Compositions: Synthetic, Natural, and Hybrid
A pivotal attribute underscoring the efficacy of nanofiber scaffolds resides in their capacity for construction from an expansive assortment of materials, each distinguished by unique attributes that intricately shape their interactions with cells and tissues.26,27 The range of materials available spans a continuum, encompassing synthetic polymers on one end, natural biopolymers on the other, and even hybrid amalgamations that judiciously amalgamate the strengths of both realms.
Within the realm of synthetic polymers, exemplified by poly(lactic-co-glycolic acid) (PLGA), poly(caprolactone) (PCL), and polyethylene glycol (PEG), the advantages lie in their malleable mechanical properties, controlled degradation kinetics, and precision-controlled release of encapsulated agents.28,29 On the other hand, in the domain of natural biopolymers, including components like collagen, chitosan, and silk fibroin, a dual role becomes apparent.22 Alongside providing biocompatibility and bioactivity, they imbue scaffolds with subtle signals that closely align with the complex cellular surroundings.
The hybrid nanofiber compositions constitutes a convergence of the attributes from synthetic and natural domains. By functionally combining different materials, this approach creates scaffolds that combine the strong durability of synthetic polymers with the helpful biological signals from natural materials.30 The dynamic nature of these composite configurations harnesses the interactions among their constituent elements, yielding scaffolds that closely mimic the intricate architecture of native tissue. Consequently, these scaffolds metamorphose into conduits that potentiate heightened cellular responses, thereby fostering enhanced regenerative outcomes.
Tailoring Nanofiber Morphology, Porosity, and Mechanical Properties
The versatility of nanofiber scaffolds goes beyond material choice and encompasses factors that significantly influence how cells behave and how tissues integrate. Variables like shape, porosity, and mechanical properties collectively define the physical and structural features of these scaffolds, shaping their interaction with regenerating nerve tissue.
Morphology, encapsulated through facets like fiber diameter and alignment, assumes a guiding role in steering cellular responses by modulating critical factors such as cell adhesion, migration, and the outgrowth of neurites.15,26 Nanofibers designed to mimic the dimensions of natural ECM components provide structural cues that not only enhance cellular attachment but also provide guidance for regenerating axons to follow specific paths.31,32 The aspect of porosity, which shapes nutrient diffusion and cellular infiltration, is manipulated through factors including fiber density, alignment, and interconnectivity.33 The customized design of nanofiber scaffolds facilitates the exchange of essential nutrients and the removal of waste products, resulting in an environment that promotes cell nourishment and seamless tissue integration.34 The field of mechanical characteristics, including properties such as stiffness and elasticity, emerges as critical for maintaining scaffold durability and withstanding the physiological stresses applied.35 By carefully choosing polymer types, concentrations, and electrospinning conditions, researchers skillfully create nanofibers with mechanical properties that align with the biomechanical demands of the regenerating tissue.36
Advancement of Nanofiber-Cell Interactions
The intricate interaction between nanofiber scaffolds and cellular elements constitutes a fundamental aspect of the peripheral nerve regeneration process. This interplay coordinates a series of intricate events that meticulously oversee the healing process. The success of strategies focused on the application of nanofibers critically depends on their ability to closely integrate into the cellular microenvironment.37,38 This integration plays a crucial role, serving as a pivotal initiator for a cascade of regenerative responses. In this section, we explore the complexities that define the dynamic interplay between nanofibers and cellular components. This exploration involves a systematic breakdown of the fundamental mechanisms that dictate cellular responses (Table 1).17,39–43
 |
Table 1 Nanofiber Scaffolds in Peripheral Nerve Regeneration |
ECM Mimicry and Its Influence on Cellular Behaviour
Nanofiber scaffolds, engineered with a meticulous precision and a deliberate strategic purpose, manifest a promising strategy to mimic the intricate essence of the natural ECM.44 The biological matrix is a complex, multi-dimensional network that regulates a wide range of cellular activities. By replicating the architectural structure, composition, and mechanical cues inherent in cellular interactions within the ECM, nanofibers provide an intriguing entry point into the multifaceted realm of cellular entities.45
The pursuit of ECM emulation transcends the realm of mere visual resemblance. It includes the construction of a highly intricate biochemical and biomechanical environment, characterized by a coordination with the multifaceted complexities of cellular components. This intricate synergy culminates in the orchestration of intricately synchronized cellular responses, operating as a poised propulsion mechanism propelling the intricate regeneration process.46 This emulation, extending well beyond superficial mimicry, encompasses a methodical of a microenvironment that is modified to elicit a beneficial intercommunication of cellular activities. This intercommunication, in turn, contributes significantly to the progression of the regenerative process, marking a sophisticated interplay between engineered constructs and biological processes.
The functional arrangement of nanofibers, achieved through advanced methods like electrospinning, closely resembles the natural alignment seen in the original ECM parts.44 This imitation leads to a harmonious coordination that significantly influences a wide range of cellular behaviors, notably affecting important aspects like cell adhesion, movement, and specialization.47 The nanofiber scaffold, by functionally imitating the signals from the ECM, provides a strategic platform for cells to start their regenerative process. These signals, going beyond just helping cells stick to the scaffold, create an environment that not only supports but actively encourages cell growth.46 This multifaceted interplay underscores the intricate symbiosis between engineered nanoscale constructs and the orchestrated coordination of cellular responses, manifesting in the orchestration of regenerative approaches.
Cellular Adhesion, Migration, and Differentiation on Nanofiber Substrates
Molecular interactions play a pivotal role in facilitating cell adhesion to nanofiber scaffolds. These interactions are characterized by a series of key mechanisms. First, cells adhere to nanofibers through cell adhesion proteins, primarily integrins.17 Integrins are transmembrane receptors that act as mediators of cell adhesion by binding to specific motifs within the ECM proteins, creating a structural bridge between the cell’s cytoskeleton and the nanofiber scaffold.
Additionally, many nanofiber scaffolds are intentionally engineered to contain ECM-mimicking peptides, with the RGD (Arginine-Glycine-Aspartic Acid) sequence being a notable example. These peptides serve as recognition sites for integrins on the cell surface.48 Upon encountering these peptides on the nanofibers, integrins on the cell membrane readily bind to the RGD motif, facilitating firm adhesion.The binding of integrins to nanofibers then initiates the formation of focal adhesion complexes within the cell. These complexes consist of various signaling molecules and cytoskeletal proteins, allowing the cell to anchor itself securely to the nanofiber scaffold.
Comparatively, the interaction between cells and nanofiber scaffolds closely mimics natural ECM interactions in several ways. Firstly, nanofiber scaffolds are intentionally designed to replicate the structural and mechanical properties of the ECM.49 These structures often feature nanoscale topography and fiber alignment, resembling the natural ECM, thus promoting strong cell adhesion and migration.
Furthermore, nanofiber scaffolds incorporate ECM-derived peptides like RGD, emulating the biochemical cues provided by the ECM. Consequently, cells recognize and adhere to the scaffold in a manner similar to their interactions with natural ECM components.
Moreover, the engagement of integrins with ligands on nanofiber scaffolds initiates intracellular signaling pathways akin to those triggered by the natural ECM, including the focal adhesion kinase (FAK) pathway. These pathways have a profound influence on cell behavior, impacting proliferation, differentiation, and migration.50 This mimicry of natural ECM interactions by nanofiber scaffolds leads to enhanced cellular responses, thereby underscoring their significance as valuable tools in tissue engineering and regenerative medicine.
Cellular behavior is inherently intertwined with the intricate array of interactions transpiring at the interface between the cellular entities and the scaffold material.51 The surface attributes of nanofibers, spanning from their chemical composition to their surface roughness, exert a pivotal and definitive influence over the orchestration of these interactions.52 In this intricate interplay, one of the paramount junctures is cellular adhesion, a pivotal phase within the regenerative progression.52 This pivotal event is significantly shaped by the purposeful arrangement and presentation of molecular ligands and identifiable patterns that cells can recognize and engage with, establishing a strong and cohesive connection. The intentional design of nanofiber scaffolds, enriched with cues that functionally mimic the patterns found in the natural ECM, bestows an impressive ability for robust cellular adhesion.53,54
The Role of Nanofiber Topography in Neurite Guidance and Alignment
Central to the domain focused on the rejuvenation of peripheral nerves is an elemental mandate—guidance of regenerating axons. These axons, intricate extensions, bear the intricate responsibility of facilitating cellular communication among nerve cells. This orchestration, marked by its paramount significance, underpins the intricate web of nerve cell interactions.55 Nanofiber scaffolds introduce an exceptional substrate, distinguished by its capacity to guide neurites along predetermined trajectories.55,56 This attribute imparts to them a unique role in modulating the pathways for neural growth. The configuration of nanofibers, evocative of the inherent alignment observed in neural tissues, assumes the role of a discerning conductor, adeptly orchestrating the progression of neurite elongation.57 This alignment helps create a setting where neural connections can form and greatly contributes to building complex neural networks.
The disposition of nanofibers and the alignment of neurites extend beyond mere structural aspects. The surface attributes of nanofibers, including elements such as grooves, ridges, and patterns, emit discernible signals that proficiently guide neurites towards accurate trajectories.56,58 Analogous to navigational markers, these signals serve as beacons along the path, aiding neurites in synchronizing their orientation with the nanofiber arrangement. This harmonized alignment, modulated by an amalgamation of diverse surface cues, exerts the potency to adeptly channel developing axons toward their designated termini.
Nanofiber alignment serves as a physical guidance system for cell migration, as cells tend to align and move along the orientation of the fibers. This is particularly valuable in applications requiring precise directional cell migration, such as regenerative medicine. Aligned nanofibers enhance cell migration compared to random or disorganized fiber arrangements, allowing cells to move more efficiently along the fibers, which, in turn, accelerates tissue regeneration. Cell interaction with aligned nanofibers results in a polarized cytoskeletal arrangement, with actin fibers and microtubules aligning parallel to the nanofibers.59 This alignment is essential for guiding directional cell movement. The interaction of cells with aligned nanofibers activates specific signaling pathways and elicits unique cellular responses. Notably, the FAK pathway and the Rho GTPase family of proteins are engaged. FAK activation is critical for cell adhesion and migration, while Rho GTPases regulate cytoskeletal dynamics and cell motility.60,61 Aligned nanofibers also promote stronger cell adhesion through integrins and focal adhesion complexes, which are vital for maintaining stable cell movement and facilitating tissue regeneration.
Mechanotransduction pathways, particularly those involving the Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ), are influenced by the mechanical cues provided by aligned nanofibers.62,63 These pathways have a direct impact on cell proliferation, differentiation, and tissue growth. Beyond cell behavior, cell alignment on nanofibers has far-reaching implications for tissue regeneration and differentiation. In nerve tissue engineering, aligned nanofibers guide axon growth, leading to improved functional recovery.64 In summary, nanofiber alignment significantly influences cell behavior, thereby holding substantial promise in tissue engineering, regenerative medicine, and other fields by improving tissue regeneration, differentiation, and overall functional outcomes.
Recent Developments in Controlled Release Strategies
Nanofiber scaffolds stand out with several distinct advantages over other bioengineering scaffolds in peripheral nerve engineering. Firstly, their biomimetic topography closely mirrors the natural extracellular matrix, promoting cell adhesion and guided axon growth, ultimately leading to enhanced nerve regeneration. Additionally, nanofiber scaffolds offer the flexibility of adjustable mechanical and biodegradable properties, making them highly adaptable to specific applications and patient requirements. Their capacity for controlled drug delivery, releasing bioactive molecules like growth factors, further accelerates nerve regeneration. Moreover, their high biocompatibility minimizes the risk of adverse immune responses, ensuring successful integration with host tissues. Enhanced cellular infiltration, facilitated axon guidance, and extensive research backing their efficacy in peripheral nerve engineering underscore nanofiber scaffolds as the preferred choice for these applications (Table 2). In this section, we discuss and summarize the controlled release mechanisms of nanofiber scaffolds, unraveling the incorporation of growth factors, cytokines, and therapeutic agents within these scaffolds, along with their ensuing repercussions on the orchestration of regenerative outcomes.
 |
Table 2 Comparable Analysis of Nanofiber Strategies with Other Bioengineering Scaffolds in Peripheral Nerve Engineering |
Incorporation of Growth Factors, Cytokines, and Therapeutic Molecules
Growth factors and cytokines play significant roles in guiding various cell activities during nerve regeneration.65 The deliberate inclusion of growth factors and cytokines within nanofiber scaffolds significantly enhances the regenerative environment.66 These bioactive entities function as precise directives, proficiently governing cellular behavior. Utilizing a range of encapsulation methods, the process of incorporating these beneficial agents within nanofibers enables the scaffold to function as a controlled delivery system, providing these instructions with accuracy and long-lasting effect.21,23 This orchestrated release mechanism not only extends the duration of regulation but also optimally guides the regenerative process. For instance, Zhou et al introduces a novel approach involving the utilization of bone marrow stromal cells (BMSCs) and nerve growth factor (NGF) alongside an innovative cultivation regimen.66 BMSCs were procured and subsequently engrafted onto PCL nanofibrous nerve guide conduits (NGCs) that were augmented with NGF, the entire construct having been subjected to cultivation via a rotary cell culture system (RCCS) prior to implantation. Notably, outcomes stemming from the in vitro PC-12 cell investigations not only confirmed the sustained bioactivity of NGF but also unveiled a substantial augmentation in neurite elongation facilitated by PEG-diamine and bovine serum albumin.66 These findings underscored the feasibility of the proposed novel loading technique in preserving the bioactive attributes of growth factors while achieving controlled and prolonged release profiles under in vitro conditions. Concomitantly, the findings of the in vivo experimentation showed significant enhancements particularly upon combination of all supplementary constituents (Figure 1). These notable results indicated the pivotal roles played by NGF in conjunction with the RCCS approach, whereby NGCs seeded with BMSCs exhibited an elevated propensity for promoting the regeneration of peripheral nerves over protracted injury gaps.
 |
Figure 1 (A) Schematic depiction of the progressive methodology employed in the utilization of the PCL nerve conduit for subsequent in vitro and in vivo investigation. (B) Morphological images of the NGC captured with SEM. The white scale bars indicate 100 μm, and the black scale bar indicates 100 nm. (C). PC-12 cells culture for 14 21 and 28 days with the different treatments. The scale bar indicates 150 μm. Reprinted with permission from Zhou G, Chang W, Zhou X, et al. Nanofibrous nerve conduits with nerve growth factors and bone marrow stromal cells pre-cultured in bioreactors for peripheral nerve regeneration. ACS Appl Mater Interfaces. 2020;12(14):16168–16177. Copyright 2020, American Chemical Society.66 |
Noteworthy within this array are the growth factors, notably the NGF and the brain-derived neurotrophic factor (BDNF). These dynamic agents set in motion cascades of events that culminate in the stimulation of cell proliferation, elongation of neurites, and the maturation of tissues.21,66,67 Concurrently, cytokines, encompassing interleukins and transforming growth factor-beta (TGF-β) among others, undertake a substantial responsibility in harmonizing immune reactions and intricately reshaping the microenvironment.68 By incorporating these bioactive substances into nanofiber scaffolds, researchers possess the ability to manipulate cellular responses with high accuracy. This capacity further solidifies the scaffold’s role as a skilled regulator of regenerative processes.
Various Encapsulation and Release Mechanisms for Sustained Delivery
The success of controlled release strategies depends on detailed mechanisms that encapsulate therapeutic substances within the framework of nanofibers, leading to their gradual release over time.69 These encapsulation methods encompass a range from physically trapping agents to chemically binding them, each carrying distinct advantages in terms of regulated release patterns and maintaining agent stability.70 The intricate structures of nanofibers provide a confined environment for holding therapeutic substances, ensuring their protection and facilitating a gradual release over extended periods of time.71
The rate at which these substances are released can be customized to match the particular time needs of nerve regeneration. A variety of release methods, such as controlled diffusion, breakdown-triggered release, and sensitivity to environmental cues, provide researchers with the tools to precisely adjust the patterns of therapeutic agent release.72 This controlled release of bioactive agents enables the scaffold to serve as a time-based guide, releasing signals at exact intervals to foster the optimal cellular responses. In a pioneering study, Chang et al developed a natural, biodegradable, multichanneled scaffold for peripheral nerve regeneration.19 This scaffold was distinctive for its incorporation of aligned electrospun nanofibers and a neurotrophic gradient, denoted as MC/AN/NG. The scaffolds, formulated from gelatin-based materials, artfully emulated the fascicular arrangement characteristic of the natural nerve ECM. Furthermore, the multichanneled (MC) scaffolds, fortified through the cross-linking facilitated by microbial transglutaminase, displayed enduring mechanical robustness. Importantly, the controlled release pattern of two pivotal neurotrophic factors, including NGF and BDNF, was orchestrated in a temporally regulated manner.19 Under in vitro conditions, neural stem cells, upon differentiation, exhibited commendable extension of their neurites along the aligned nanofibers. Additionally, in a treated experimental cohort, augmented cell density was observed in regions of the gradient membrane characterized by heightened NGF concentration. Transitioning to an in vivo setting, utilizing a rabbit sciatic nerve transection model, the MC/AN/NG scaffold was demonstrated to yield superior nerve recovery outcomes. Remarkably, this scaffold was associated with reduced muscle atrophy, with outcomes akin to those observed in autograft treatments.19 By synergistically integrating a multitude of strategies devised to augment peripheral nerve regeneration, the MC/AN/NG scaffolds, operating as nerve guidance conduits, present promising therapeutic alternatives to conventional autologous nerve grafts (Figure 2).
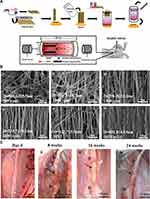 |
Figure 2 (A) Schematic representation of the process for constructing a nerve conduit comprising oriented nanofibers to facilitate directed axonal outgrowth. (B) Scanning electron microscopy (SEM) images of electrospun Gelatin/PEO nanofibers generated at various rotational speeds: (i) 500 rpm, (ii) 1000 rpm, (iii) 1500 rpm, and (iv) 2000 rpm. Additionally, SEM images of (v) Gelatin/PEO nanofibers after cross-linking via DHT treatment at 140 °C for 48 hours, and (vi) Gelatin/PEO nanofibers subjected to sequential cross-linking, first through DHT treatment and subsequently via immersion in a 30 U mTG solution at 37 °C for 2 hours (DHT/mTG). (C) Assessment of nerve regeneration in a rabbit sciatic nerve transection model: (i) Pre-operative state of the sciatic nerve. (ii−iv) Post-operative evaluation at 8, 16, and 24 weeks following the implantation of the MC/AN/NG nerve conduit, demonstrating conspicuous nerve repair. The arrowhead highlights the regenerated sciatic nerve. (P, proximal end; (D), distal end). Reprinted with permission from Chang YC, Chen MH, Liao SY, et al. Multichanneled nerve guidance conduit with spatial gradients of neurotrophic factors and oriented nanotopography for repairing the peripheral nervous system. ACS Appl Mater Interfaces. 2017;9(43):37623–37636. Copyright 2017, American Chemical Society.19 |
Impact on Neuronal Differentiation, Axonal Elongation, and Tissue Regeneration
The incorporation of controlled release strategies into nanofiber scaffolds orchestrates a harmonious interplay of regenerative processes. Growth factors and cytokines released from the scaffolds initiate a cascade of molecular interactions that govern cellular behaviors. Neuronal differentiation, essential for re-establishing functional neural pathways, is directed by the cues delivered through the scaffold’s controlled release mechanisms.67 Axonal elongation, a distinctive feature of nerve regeneration, is enhanced by the presence of growth factors that guide axonal outgrowth along the nanofiber pathways.29,73 For example, Yang et al reported a novel paradigm wherein a self-assembling peptide (SAP) nanofiber hydrogel, underpinned by the self-assembling Ac-(RADA)4-NH2 (RAD) backbone, has been strategically dual-functionalized.74 This dual-functionalization entails the integration of laminin-derived motif IKVAV (IKV) and a BDNF-mimetic peptide epitope RGIDKRHWNSQ (RGI). The hydrogel’s architectural design furnishes a three-dimensional (3D) milieu, thereby effectively accommodating Schwann cells (SCs) and neurites in their regenerative endeavor. These hydrogels, adept at emulating a 3D microenvironment akin to the native ECM, exert profound influence on SCs. Notably, the secretion of NGF, BDNF, and cholinergic neuronotrophic factor (CNTF) by SCs is significantly potentiated upon the dual-functionalized peptide hydrogels after 3 days. Upon implantation, the expressions of neurotrophin and myelin-associated genes within nerve grafts are markedly elevated in the SAP and Autograft groups, surpassing levels observed in the Hollow group. Morphometric analyses of regenerated nerves, electrophysiological assessments, innervated muscle weight evaluations, muscle fiber remodeling, and motor functional assessments collectively substantiate the profound axonal regeneration facilitated by RAD/IKV/RGI and RAD/IKV-GG-RGI hydrogels. Notably, the success extends to bridging a substantial 10-mm gap representative of sciatic nerve defects in rat models, thereby underscoring the hydrogels’ translational potential (Figure 3).
 |
Figure 3 (A) Sequences of biofunctionalized self-assembling peptides. (B) Representative circular dichroism (CD) spectra of RAD, RAD-IKV, RAD-RGI, RAD-IKV-GG-RGI, RAD/IKV, RAD/RGI, RAD/IKV-GG-RGI, and RAD/IKV/RGI Solutions. (C) Transverse sections of harvested nerve grafts stained with toluidine blue, alongside TEM of regenerated nerves. (D) Macroscopic visualizations of dissected gastrocnemius muscles at the 12th-week time point, accompanied by Masson’s trichrome staining of cross-sections from muscles affected by injury. Reproduced from Yang S, Wang C, Zhu J, et al. Self-assembling peptide hydrogels functionalized with LN- and BDNF- mimicking epitopes synergistically enhance peripheral nerve regeneration. Theranostics. 2020;10(18):8227–8249; under the terms of a Creative Commons Attribution 4.0 International License.74 |
Moreover, the impact of nanofiber scaffolds stretches beyond nerve regeneration to encompass the broader realm of tissue restoration. The signals imparted by controlled release strategies facilitate the maturation of tissues, the formation of new blood vessels (angiogenesis), and the recruitment of supporting cells.75 This convergence results in an environment that promotes the maturation of functional tissue. The synergistic interaction between nanofiber scaffolds and controlled release strategies accentuates their combined capacity to shape the regenerative landscape, offering a strategic path toward advancing peripheral nerve repair.76 The integration of controlled release strategies within nanofiber scaffolds elevates the outcome of peripheral nerve regeneration. Through the incorporation of growth factors, cytokines, and therapeutic compounds, coupled with the utilization of diverse encapsulation and release mechanisms, nanofiber scaffolds act as multifunctional platforms that coordinate the mechanisms of regenerative progression.
Current Trends in Nanofiber Scaffolds for Nerve Regeneration
The field of peripheral nerve regeneration is in a state of dynamic evolution, situated at the convergence of nanotechnology and regenerative medicine. Here, we unveil emerging trends and unexplored pathways with the potential to reshape nanofiber-based strategies for peripheral nerve repair.
Nanofiber Surface Modification for Enhanced Cellular Interactions
The orchestration of interactions between nanofibers and cells can be further refined by exploring the nanofiber surface modification.77 Customizing the surface properties of the scaffold, including its charge and hydrophilicity, provides a versatile approach to enhance cellular adhesion, growth, and specialization.78 By introducing bioactive molecules, peptides, or growth factors to the nanofiber surface, a distinct environment of interactions is tailored, thus enhancing the results of the regeneration process.79
Through the integration of bioactive signals at the nanofiber surface, a platform is constructed that effectively communicates in the cellular language, triggering particular responses that harmonize with regenerating nerve tissue. For instance, aiming to synergize the distinctive physicochemical attributes of graphene oxide (GO) nanosheets with the topological configuration of aligned nanofibrous scaffolds, a novel approach was undertaken to coat GO nanosheets onto aminolyzed poly-l-lactide (PLLA) nanofibrous scaffolds.80 Microscopic examinations revealed that the introduction of GO nanosheets onto aligned PLLA nanofibers yielded a surface of increased roughness compared to that of both the aligned PLLA and aminolyzed PLLA nanofibrous scaffolds. More importantly, this GO-mediated coating did not disrupt the inherent alignment of the nanofibers. In-depth characterization unveiled the introduction of hydrophilic moieties such as NH2, COOH, and OH subsequent to aminolysis and the incorporation of GO nanosheets. This augmentation in hydrophilicity stands as a significant attribute. Furthermore, these scaffolds engendered a cytoskeletal orientation within SCs that aligned themselves with the nanofiber topography. Notably, this phenomenon outstripped the proliferative response evoked by aligned PLLA and aminolyzed PLLA nanofibrous scaffolds. In vitro, these surface modified scaffolds fostered the differentiation of PC12 cells and their consequent neurite extension. Of note, this extension was orchestrated along the alignment of the nanofibers, indicating the profound influence exerted by the scaffold’s topography (Figure 4). The intricate engineering of surface modifications serves as the foundation for scaffolds adept at directing cellular behavior with unmatched accuracy. As we uncover the complexity of nanofiber surface modification, the potential for enhanced cellular interactions emerges as a guiding force that shapes the course of peripheral nerve regeneration.
 |
Figure 4 (A) Schematic illustration of the fabrication of GO-modified PLLA nanofibrous scaffolds. (B) SEM captures of oriented PLLA nanofibrous scaffolds following aminolysis, illustrating sequential time intervals: (i) 0 minutes, (ii) 5 minutes, (iii) 10 minutes, and (iv) 15 minutes. (C) Immunohistochemical staining images of SCs following a 3-day culture on various nanofibrous scaffolds. Cytoplasmic components were labeled with rhodamine phalloidin (in red), while nuclei were counterstained with DAPI (in blue). (i) SCs cultured on PLLA nanofibrous scaffolds. (ii) SCs cultured on PLLA-NH2 nanofibrous scaffolds after aminolysis for 10 minutes. (iii) SCs cultured on PLLA-GO nanofibrous scaffolds coated with a 1.0 mg/mL GO solution. (iv) Proliferation of SCs during culture on different nanofibrous scaffolds for 1, 3, and 6 days. *p<0.05. Reprinted from Acta Biomater, 37, Zhang K, Zheng H, Liang S, Gao C. Aligned PLLA nanofibrous scaffolds coated with graphene oxide for promoting neural cell growth. 131–142, Copyright 2016, with permission from Elsevier.80 |
Combining Nanofibers with Other Advanced Therapies
The path of peripheral nerve regeneration is at a critical juncture where nanofiber scaffolds intersect with a variety of advanced therapeutic strategies. Stem cells, enriched with regenerative potential, and gene therapy, capable of mediating cellular behavior at the genetic level, emerge as potent collaborators capable of harmonizing in synergy with nanofiber scaffolds.81 Introducing stem cells into nanofiber matrices injects scaffolds with cellular vitality, amplifying the regenerative milieu and nurturing the integration of cells. For instance, Zhou et al introduced a strategy for integration of polypyrrole (PPy) within the matrix of PLA nanofibers using electrospinning technology.82 The outcome was the successful fabrication of a composite scaffold referred to as PLA/PPy, wherein PPy content was maintained at 15%. Importantly, this scaffold retained sustained conductivity while also featuring an aligned topographical configuration. The compatibility of the scaffold with human umbilical cord MSCs and SCs was well-established, substantiating its biocompatibility. Notably, cellular elongation exhibited a parallel orientation relative to the fiber direction, aligning seamlessly with the scaffold’s topographical features.82 These encouraging results indicate that the aligned PLA/PPy nanofibrous scaffold stands as a promising strategy for promoting peripheral nerve regeneration (Figure 5). In addition, gene therapy imparts precise molecular instructions to cells, guiding their actions towards augmented regeneration.17 The deliberate integration of nanofiber scaffolds with these pioneering therapies marks a transformative shift in the landscape of regeneration, reshaping the scaffold from a passive framework into an engaged orchestrator of cellular responses.
 |
Figure 5 (A) Schematic illustration of the electrospinning apparatus. (B) Morphology of the electrospun nanofibers containing PPy nanoparticles. (C) Immunocytochemical staining of SCs. (D) Distribution of SCs on the different nanofibrous scaffolds. Reproduced from Zhou JF, Wang YG, Cheng L, Wu Z, Sun XD, Peng J. Preparation of polypyrrole-embedded electrospun poly(lactic acid) nanofibrous scaffolds for nerve tissue engineering. Neural Regen Res. 2016;11(10):1644–1652; under the terms of a Creative Commons Attribution-NonCommercial-ShareAlike License (CC BY-NC-SA).82 |
Exploring 3D Printing and Additive Manufacturing for Customized Scaffolds
The scaffold fabrication is undergoing a resurgence with the introduction of 3D printing and additive manufacturing technologies.83 These advances mark a growing trend where scaffolds are functionally constructed to align with the complex shapes of nerve tissue and the distinct anatomical structures of individual patients. 3D printing provides the accuracy to construct scaffolds with controlled spatial architecture, porosity, and distribution of bioactive molecules.84 The combination of 3D printing with nanofiber fabrication methods opens up avenues for creating versatile scaffolds that leverage the advantages of both approaches.85 This collaboration not only enables the replication of native tissue microenvironments but also enhances the integration of therapeutic agents at specific locations within the scaffold.86 Prior studies have demonstrated the combination of 3D biomaterials with stem cells in addressing degenerative neurological disorders. The synergistic interplay between these biomaterials and stem cells has proven instrumental in exploring their potential for fostering the regeneration of neural tissues.87 With the ongoing advance of 3D printing and additive manufacturing techniques, the potential for tailor-made scaffolds that effortlessly accommodate the distinct requirements of individual patients becomes an intriguing avenue for future investigation.
Prospects and Challenges in Clinical Translation
The clinical promise linked to the use of nanofiber scaffolds in peripheral nerve regeneration is indeed significant. There have been several clinical applications and case studies that demonstrate the successful use of nanofiber scaffolds in peripheral nerve regeneration.15,16 In the case of sciatic nerve repair, researchers have utilized nanofiber scaffolds in animal models, resulting in substantial enhancements in nerve regeneration.21 These scaffolds have facilitated enhanced axonal growth, improved functional recovery, and a reduction in scarring when compared to traditional nerve grafts. Nanofiber scaffolds have also been applied to repair injuries to the trigeminal nerve, responsible for facial sensation.88 The studies conducted in this context have showed successful regeneration, diminished pain, and the restoration of sensory function. This makes nanofiber scaffolds a promising approach for facial nerve injuries. In the context of diabetic neuropathy, nanofiber scaffolds have been explored for promoting nerve regeneration.89 These studies have indicated their potential in alleviating symptoms like pain and numbness, while also enhancing sensory and motor functions in diabetic patients.
However, it is accompanied by a set of translational obstacles that require careful consideration. Nanofiber scaffolds offer an innovative method that has the potential to transform nerve repair strategies by creating a customized environment for cell interactions, enhancing axonal growth, and enabling the controlled release of bioactive substances.56,90 The integration of these attributes presents compelling possibilities for augmenting functional outcomes within the context of nerve regeneration.
The transition from laboratory-scale studies to clinical implementation demands addressing issues related to scalability, reproducibility, and regulatory considerations. The reproducibility of nanofiber fabrication processes, ensuring consistent scaffold properties, and establishing quality control measures are crucial to ensure reliable outcomes across patient cohorts. The regulatory landscape surrounding the use of nanomaterials in clinical settings necessitates rigorous safety assessments, biocompatibility evaluations, and long-term biodegradability studies.
Clinical translation also requires comprehensive understanding and management of immune responses elicited by nanofiber scaffolds. Immune reactions, although potentially beneficial for tissue repair, need to be controlled to prevent excessive inflammation or foreign body reactions that might hinder regeneration. Furthermore, the potential long-term stability and degradation profile of nanofiber scaffolds should be examined, considering their prolonged presence in the body during the regenerative process.
Abbreviations
ECM, extracellular matrix; PLGA, poly(lactic-co-glycolic acid); PCL, poly(caprolactone); PEG, polyethylene glycol; NGF, nerve growth factor; BMSCs, bone marrow stromal cells; NGCs, nerve guide conduits; RCCS, rotary cell culture system; BDNF, brain-derived neurotrophic factor; TGF-β, transforming growth factor-beta; MC, multichanneled; SAP, self-assembling peptide; 3D, three dimensional; CNTF, cholinergic neuronotrophic factor; SCs, Schwann cells; GO, graphene oxide; PLLA, poly-l-lactide; PPy, polypyrrole.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mahar M, Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci. 2018;19(6):323–337. doi:10.1038/s41583-018-0001-8
2. Pryce KD, Serafini RA, Ramakrishnan A, et al. Oxycodone withdrawal induces HDAC1/HDAC2-dependent transcriptional maladaptations in the reward pathway in a mouse model of peripheral nerve injury. Nat Neurosci. 2023;26(7):1229–1244. doi:10.1038/s41593-023-01350-3
3. Nagappan PG, Chen H, Wang DY. Neuroregeneration and plasticity: a review of the physiological mechanisms for achieving functional recovery postinjury. Mil Med Res. 2020;7(1):30. doi:10.1186/s40779-020-00259-3
4. Davies AJ, Kim HW, Gonzalez-Cano R, et al. Natural killer cells degenerate intact sensory afferents following nerve injury. Cell. 2019;176(4):716–28 e18. doi:10.1016/j.cell.2018.12.022
5. Ydens E, Amann L, Asselbergh B, et al. Profiling peripheral nerve macrophages reveals two macrophage subsets with distinct localization, transcriptome and response to injury. Nat Neurosci. 2020;23(5):676–689. doi:10.1038/s41593-020-0618-6
6. Welberg L. Commensals boost nerve regeneration. Nat Neurosci. 2023;26(2):175. doi:10.1038/s41593-023-01263-1
7. Jiang BC, Ding TY, Guo CY, et al. NFAT1 orchestrates spinal microglial transcription and promotes microglial proliferation via c-MYC contributing to nerve injury-induced neuropathic pain. Adv Sci. 2022;9(27):e2201300. doi:10.1002/advs.202201300
8. Li X, Zhang T, Li C, et al. Electrical stimulation accelerates Wallerian degeneration and promotes nerve regeneration after sciatic nerve injury. Glia. 2023;71(3):758–774. doi:10.1002/glia.24309
9. Al-Hadeethi Y, Nagarajan A, Hanuman S, et al. Schwann cell-matrix coated PCL-MWCNT multifunctional nanofibrous scaffolds for neural regeneration. RSC Adv. 2023;13(2):1392–1401. doi:10.1039/D2RA05368C
10. Rhode SC, Beier JP, Ruhl T. Adipose tissue stem cells in peripheral nerve regeneration-In vitro and in vivo. J Neurosci Res. 2021;99(2):545–560. doi:10.1002/jnr.24738
11. Jin F, Li T, Yuan T, et al. Physiologically self-regulated, fully implantable, battery-free system for peripheral nerve restoration. Adv Mater. 2021;33(48):e2104175. doi:10.1002/adma.202104175
12. Scheib J, Hoke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9(12):668–676. doi:10.1038/nrneurol.2013.227
13. Zhang M, An H, Gu Z, et al. Mimosa-inspired stimuli-responsive curling bioadhesive tape promotes peripheral nerve regeneration. Adv Mater. 2023;35:32.
14. Dong X, Yang Y, Bao Z, et al. Micro-nanofiber composite biomimetic conduits promote long-gap peripheral nerve regeneration in canine models. Bioact Mater. 2023;30:98–115. doi:10.1016/j.bioactmat.2023.06.015
15. Jia Y, Yang W, Zhang K, et al. Nanofiber arrangement regulates peripheral nerve regeneration through differential modulation of macrophage phenotypes. Acta Biomater. 2019;83:291–301. doi:10.1016/j.actbio.2018.10.040
16. Wang J, Cheng Y, Chen L, et al. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019;84:98–113. doi:10.1016/j.actbio.2018.11.032
17. Zheng C, Yang Z, Chen S, et al. Nanofibrous nerve guidance conduits decorated with decellularized matrix hydrogel facilitate peripheral nerve injury repair. Theranostics. 2021;11(6):2917–2931. doi:10.7150/thno.50825
18. Achenbach P, Hambeukers I, Pierling AL, et al. A novel in vitro assay for peripheral nerve-related cell migration that preserves both extracellular matrix-derived molecular cues and nanofiber-derived topography. J Neurosci Methods. 2021;361:109289. doi:10.1016/j.jneumeth.2021.109289
19. Chang YC, Chen MH, Liao SY, et al. Multichanneled nerve guidance conduit with spatial gradients of neurotrophic factors and oriented nanotopography for repairing the peripheral nervous system. ACS Appl Mater Interfaces. 2017;9(43):37623–37636. doi:10.1021/acsami.7b12567
20. Wang J, Xiong H, Zhu T, et al. Bioinspired multichannel nerve guidance conduit based on shape memory nanofibers for potential application in peripheral nerve repair. ACS Nano. 2020;14(10):12579–12595. doi:10.1021/acsnano.0c03570
21. Rao F, Wang Y, Zhang D, et al. Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats. Theranostics. 2020;10(4):1590–1603. doi:10.7150/thno.36272
22. Yen CM, Shen CC, Yang YC, et al. Novel electrospun poly(epsilon-caprolactone)/type I collagen nanofiber conduits for repair of peripheral nerve injury. Neural Regen Res. 2019;14(9):1617–1625. doi:10.4103/1673-5374.255997
23. Zhou ZF, Zhang F, Wang JG, et al. Electrospinning of PELA/PPY fibrous conduits: promoting peripheral nerve regeneration in rats by self-originated electrical stimulation. ACS Biomater Sci Eng. 2016;2(9):1572–1581. doi:10.1021/acsbiomaterials.6b00335
24. Gnavi S, Fornasari BE, Tonda-Turo C, et al. The influence of electrospun fibre size on Schwann cell behaviour and axonal outgrowth. Mater Sci Eng C Mater Biol Appl. 2015;48:620–631. doi:10.1016/j.msec.2014.12.055
25. Huang C, Ouyang Y, Niu H, et al. Nerve guidance conduits from aligned nanofibers: improvement of nerve regeneration through longitudinal nanogrooves on a fiber surface. ACS Appl Mater Interfaces. 2015;7(13):7189–7196. doi:10.1021/am509227t
26. Chen L, Song X, Yao Z, et al. Gelatin nanofiber-reinforced decellularized amniotic membrane promotes axon regeneration and functional recovery in the surgical treatment of peripheral nerve injury. Biomaterials. 2023;300:122207. doi:10.1016/j.biomaterials.2023.122207
27. Solomevich SO, Oranges CM, Kalbermatten DF, Schwendeman A, Madduri S. Natural polysaccharides and their derivatives as potential medical materials and drug delivery systems for the treatment of peripheral nerve injuries. Carbohydr Polym. 2023;315:120934. doi:10.1016/j.carbpol.2023.120934
28. Biazar E, Khorasani MT, Montazeri N, et al. Types of neural guides and using nanotechnology for peripheral nerve reconstruction. Int J Nanomedicine. 2010;5:839–852. doi:10.2147/IJN.S11883
29. Jin J, Limburg S, Joshi SK, et al. Peripheral nerve repair in rats using composite hydrogel-filled aligned nanofiber conduits with incorporated nerve growth factor. Tissue Eng Part A. 2013;19(19–20):2138–2146. doi:10.1089/ten.tea.2012.0575
30. Nune M, Krishnan UM, Sethuraman S. PLGA nanofibers blended with designer self-assembling peptides for peripheral neural regeneration. Mater Sci Eng C Mater Biol Appl. 2016;62:329–337. doi:10.1016/j.msec.2016.01.057
31. Debski T, Kijenska-Gawronska E, Zolocinska A, et al. Bioactive nanofiber-based conduits in a peripheral nerve gap management-an animal model study. Int J Mol Sci. 2021;22(11). doi:10.3390/ijms22115588
32. Du J, Liu J, Yao S, et al. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 2017;55:296–309. doi:10.1016/j.actbio.2017.04.010
33. Samadian H, Ehterami A, Sarrafzadeh A, et al. Sophisticated polycaprolactone/gelatin nanofibrous nerve guided conduit containing platelet-rich plasma and citicoline for peripheral nerve regeneration: in vitro and in vivo study. Int J Biol Macromol. 2020;150:380–388. doi:10.1016/j.ijbiomac.2020.02.102
34. Xie J, Macewan MR, Schwartz AG, Xia Y. Electrospun nanofibers for neural tissue engineering. Nanoscale. 2010;2(1):35–44. doi:10.1039/B9NR00243J
35. Entekhabi E, Haghbin Nazarpak M, Shafieian M, Mohammadi H, Firouzi M, Hassannejad Z. Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun polycaprolactone nanofibers for peripheral nerve regeneration. J Biomed Mater Res A. 2021;109(3):300–312. doi:10.1002/jbm.a.37023
36. Orkwis JA, Wolf AK, Shahid SM, Smith C, Esfandiari L, Harris GM. Development of a Piezoelectric PVDF-TrFE fibrous scaffold to guide cell adhesion, proliferation, and alignment. Macromol Biosci. 2020;20(9):e2000197. doi:10.1002/mabi.202000197
37. Chang B, Ma C, Liu X. Nanofibers regulate single bone marrow stem cell osteogenesis via FAK/RhoA/YAP1 pathway. ACS Appl Mater Interfaces. 2018;10(39):33022–33031. doi:10.1021/acsami.8b11449
38. Zhang RR, Chen SL, Cheng ZC, Shen YY, Yi S, Xu H. Characteristics of cytokines in the sciatic nerve stumps and DRGs after rat sciatic nerve crush injury. Mil Med Res. 2020;7(1):57. doi:10.1186/s40779-020-00286-0
39. Heidari M, Bahrami SH, Ranjbar-Mohammadi M, Milan PB. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater Sci Eng C Mater Biol Appl. 2019;103:109768. doi:10.1016/j.msec.2019.109768
40. Jang CH, Lee H, Kim M, Kim G. Effect of polycaprolactone/collagen/hUCS microfiber nerve conduit on facial nerve regeneration. Int J Biol Macromol. 2016;93(Pt B):1575–1582. doi:10.1016/j.ijbiomac.2016.04.031
41. Manto KM, Govindappa PK, Martinazzi B, et al. Erythropoietin-PLGA-PEG as a local treatment to promote functional recovery and neurovascular regeneration after peripheral nerve injury. J Nanobiotechnology. 2022;20(1):461. doi:10.1186/s12951-022-01666-5
42. Mohseni M, SA AR, Shirazi FH, Nemati NH. Preparation and characterization of self-electrical stimuli conductive gellan based nano scaffold for nerve regeneration containing chopped short spun nanofibers of PVDF/MCM41 and polyaniline/graphene nanoparticles: physical, mechanical and morphological studies. Int J Biol Macromol. 2021;167:881–893. doi:10.1016/j.ijbiomac.2020.11.045
43. Li D, Pan X, Sun B, et al. Nerve conduits constructed by electrospun P(LLA-CL) nanofibers and PLLA nanofiber yarns. J Mater Chem B. 2015;3(45):8823–8831. doi:10.1039/C5TB01402F
44. Li P, Ruan L, Jiang G, et al. Design of 3D polycaprolactone/epsilon-polylysine-modified chitosan fibrous scaffolds with incorporation of bioactive factors for accelerating wound healing. Acta Biomater. 2022;152:197–209. doi:10.1016/j.actbio.2022.08.075
45. Mammadov B, Mammadov R, Guler MO, Tekinay AB. Cooperative effect of heparan sulfate and laminin mimetic peptide nanofibers on the promotion of neurite outgrowth. Acta Biomater. 2012;8(6):2077–2086. doi:10.1016/j.actbio.2012.02.006
46. Wang X, Salick MR, Wang X, et al. Poly(epsilon-caprolactone) nanofibers with a self-induced nanohybrid shish-kebab structure mimicking collagen fibrils. Biomacromolecules. 2013;14(10):3557–3569. doi:10.1021/bm400928b
47. Xie J, Shen H, Yuan G, Lin K, Su J. The effects of alignment and diameter of electrospun fibers on the cellular behaviors and osteogenesis of BMSCs. Mater Sci Eng C Mater Biol Appl. 2021;120:111787. doi:10.1016/j.msec.2020.111787
48. Ahmadi Z, Yadav S, Kar AK, et al. An injectable self-assembling hydrogel based on RGD peptidomimetic beta-sheets as multifunctional biomaterials. Biomater Adv. 2022;133:112633. doi:10.1016/j.msec.2021.112633
49. Fan L, Li JL, Cai Z, Wang X. Creating biomimetic anisotropic architectures with co-aligned nanofibers and macrochannels by manipulating ice crystallization. ACS Nano. 2018;12(6):5780–5790. doi:10.1021/acsnano.8b01648
50. Cristofaro F, Gigli M, Bloise N, et al. Influence of the nanofiber chemistry and orientation of biodegradable poly(butylene succinate)-based scaffolds on osteoblast differentiation for bone tissue regeneration. Nanoscale. 2018;10(18):8689–8703. doi:10.1039/C8NR00677F
51. Sun Q, Pei F, Zhang M, et al. Curved nanofiber network induces cellular bridge formation to promote stem cell mechanotransduction. Adv Sci. 2023;10(3).
52. Zhang K, Bai X, Yuan Z, et al. Cellular nanofiber structure with secretory activity-promoting characteristics for multicellular spheroid formation and hair follicle regeneration. ACS Appl Mater Interfaces. 2020;12(7):7931–7941. doi:10.1021/acsami.9b21125
53. Arioz I, Erol O, Bakan G, et al. Biocompatible electroactive tetra(aniline)-conjugated peptide nanofibers for neural differentiation. ACS Appl Mater Interfaces. 2018;10(1):308–317. doi:10.1021/acsami.7b16509
54. Guo B, Ma PX. Conducting polymers for tissue engineering. Biomacromolecules. 2018;19(6):1764–1782. doi:10.1021/acs.biomac.8b00276
55. Sun W, Taylor CS, Zhang Y, et al. Cell guidance on peptide micropatterned silk fibroin scaffolds. J Colloid Interface Sci. 2021;603:380–390. doi:10.1016/j.jcis.2021.06.086
56. Cao H, Liu T, Chew SY. The application of nanofibrous scaffolds in neural tissue engineering. Adv Drug Deliv Rev. 2009;61(12):1055–1064. doi:10.1016/j.addr.2009.07.009
57. Mukhatyar VJ, Salmeron-Sanchez M, Rudra S, et al. Role of fibronectin in topographical guidance of neurite extension on electrospun fibers. Biomaterials. 2011;32(16):3958–3968. doi:10.1016/j.biomaterials.2011.02.015
58. Achenbach P, Hillerbrand L, Gerardo-Nava JL, et al. Function follows form: oriented substrate nanotopography overrides neurite-repulsive Schwann cell-astrocyte barrier formation in an in vitro model of glial scarring. Nano Lett. 2023;23(14):6337–6346. doi:10.1021/acs.nanolett.3c00873
59. Kuppan P, Sethuraman S, Krishnan UM. Interaction of human smooth muscle cells with nanofibrous scaffolds: effect of fiber orientation on cell adhesion, proliferation, and functional gene expression. J Biomed Mater Res A. 2015;103(7):2236–2250. doi:10.1002/jbm.a.35360
60. Rao SS, Nelson MT, Xue R, et al. Mimicking white matter tract topography using core-shell electrospun nanofibers to examine migration of malignant brain tumors. Biomaterials. 2013;34(21):5181–5190. doi:10.1016/j.biomaterials.2013.03.069
61. Meng J, Han Z, Kong H, et al. Electrospun aligned nanofibrous composite of MWCNT/polyurethane to enhance vascular endothelium cells proliferation and function. J Biomed Mater Res A. 2010;95(1):312–320. doi:10.1002/jbm.a.32845
62. Li Y, Guo F, Hao Y, et al. Helical nanofiber yarn enabling highly stretchable engineered microtissue. Proc Natl Acad Sci U S A. 2019;116(19):9245–9250. doi:10.1073/pnas.1821617116
63. Liu J, Wei Q, Man K, et al. Nanofibrous membrane promotes and sustains vascular endothelial barrier function. ACS Appl Bio Mater. 2023. doi:10.1021/acsabm.3c00668
64. Lam HJ, Patel S, Wang A, Chu J, Li S. In vitro regulation of neural differentiation and axon growth by growth factors and bioactive nanofibers. Tissue Eng Part A. 2010;16(8):2641–2648. doi:10.1089/ten.tea.2009.0414
65. Li R, Li DH, Zhang HY, Wang J, Li XK, Xiao J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol Sin. 2020;41(10):1289–1300. doi:10.1038/s41401-019-0338-1
66. Zhou G, Chang W, Zhou X, et al. Nanofibrous nerve conduits with nerve growth factors and bone marrow stromal cells pre-cultured in bioreactors for peripheral nerve regeneration. ACS Appl Mater Interfaces. 2020;12(14):16168–16177. doi:10.1021/acsami.0c04191
67. Lu Q, Zhang F, Cheng W, et al. Nerve guidance conduits with hierarchical anisotropic architecture for peripheral nerve regeneration. Adv Healthc Mater. 2021;10(14):e2100427. doi:10.1002/adhm.202100427
68. Ding Z, Jiang M, Qian J, et al. Role of transforming growth factor-beta in peripheral nerve regeneration. Neural Regen Res. 2024;19(2):380–386. doi:10.4103/1673-5374.377588
69. Jin B, Yu Y, Lou C, et al. Combining a density gradient of biomacromolecular nanoparticles with biological effectors in an electrospun fiber-based nerve guidance conduit to promote peripheral nerve repair. Adv Sci. 2023;10:4.
70. Wang J, Liu Y, Lv M, et al. Regulation of nerve cells using conductive nanofibrous scaffolds for controlled release of Lycium barbarum polysaccharides and nerve growth factor. Regen Biomater. 2023;10:rbad038.
71. Fallah-Darrehchi M, Zahedi P, Safarian S, Ghaffari-Bohlouli P, Aeinehvand R. Conductive conduit based on electrospun poly (l-lactide-co-D, l-lactide) nanofibers containing 4-aminopyridine-loaded molecularly imprinted poly (methacrylic acid) nanoparticles used for peripheral nerve regeneration. Int J Biol Macromol. 2021;190:499–507. doi:10.1016/j.ijbiomac.2021.09.009
72. Bianchini M, Micera S, Redolfi Riva E. Recent advances in polymeric drug delivery systems for peripheral nerve regeneration. Pharmaceutics. 2023;15(2):640. doi:10.3390/pharmaceutics15020640
73. Kuihua Z, Chunyang W, Cunyi F, Xiumei M. Aligned SF/P(LLA-CL)-blended nanofibers encapsulating nerve growth factor for peripheral nerve regeneration. J Biomed Mater Res A. 2014;102(8):2680–2691. doi:10.1002/jbm.a.34922
74. Yang S, Wang C, Zhu J, et al. Self-assembling peptide hydrogels functionalized with LN- and BDNF- mimicking epitopes synergistically enhance peripheral nerve regeneration. Theranostics. 2020;10(18):8227–8249. doi:10.7150/thno.44276
75. Dong X, Liu S, Yang Y, et al. Aligned microfiber-induced macrophage polarization to guide Schwann-cell-enabled peripheral nerve regeneration. Biomaterials. 2021;272:120767. doi:10.1016/j.biomaterials.2021.120767
76. Fang Y, Wang C, Liu Z, et al. 3D printed conductive multiscale nerve guidance conduit with hierarchical fibers for peripheral nerve regeneration. Adv Sci. 2023;10:12.
77. Habibizadeh M, Nadri S, Fattahi A, et al. Surface modification of neurotrophin-3 loaded PCL/chitosan nanofiber/net by alginate hydrogel microlayer for enhanced biocompatibility in neural tissue engineering. J Biomed Mater Res A. 2021;109(11):2237–2254. doi:10.1002/jbm.a.37208
78. Lategan M, Kumar P, Choonara YE. Functionalizing nanofibrous platforms for neural tissue engineering applications. Drug Discov Today. 2022;27(5):1381–1403. doi:10.1016/j.drudis.2022.01.005
79. El-Seedi HR, Said NS, Yosri N, et al. Gelatin nanofibers: recent insights in synthesis, bio-medical applications and limitations. Heliyon. 2023;9(5):e16228. doi:10.1016/j.heliyon.2023.e16228
80. Zhang K, Zheng H, Liang S, Gao C. Aligned PLLA nanofibrous scaffolds coated with graphene oxide for promoting neural cell growth. Acta Biomater. 2016;37:131–142. doi:10.1016/j.actbio.2016.04.008
81. Hu F, Zhang X, Liu H, et al. Neuronally differentiated adipose-derived stem cells and aligned PHBV nanofiber nerve scaffolds promote sciatic nerve regeneration. Biochem Biophys Res Commun. 2017;489(2):171–178. doi:10.1016/j.bbrc.2017.05.119
82. Zhou JF, Wang YG, Cheng L, Wu Z, Sun XD, Peng J. Preparation of polypyrrole-embedded electrospun poly(lactic acid) nanofibrous scaffolds for nerve tissue engineering. Neural Regen Res. 2016;11(10):1644–1652. doi:10.4103/1673-5374.193245
83. Houshyar S, Pillai MM, Saha T, et al. Three-dimensional directional nerve guide conduits fabricated by dopamine-functionalized conductive carbon nanofibre-based nanocomposite ink printing. RSC Adv. 2020;10(66):40351–40364. doi:10.1039/D0RA06556K
84. Uz M, Buyukoz M, Sharma AD, Sakaguchi DS, Altinkaya SA, Mallapragada SK. Gelatin-based 3D conduits for transdifferentiation of mesenchymal stem cells into Schwann cell-like phenotypes. Acta Biomater. 2017;53:293–306. doi:10.1016/j.actbio.2017.02.018
85. Wu J, Xie L, Lin WZY, Chen Q. Biomimetic nanofibrous scaffolds for neural tissue engineering and drug development. Drug Discov Today. 2017;22(9):1375–1384. doi:10.1016/j.drudis.2017.03.007
86. Wang L, Wu Y, Hu T, Ma PX, Guo B. Aligned conductive core-shell biomimetic scaffolds based on nanofiber yarns/hydrogel for enhanced 3D neurite outgrowth alignment and elongation. Acta Biomater. 2019;96:175–187. doi:10.1016/j.actbio.2019.06.035
87. Ashraf R, Sofi HS, Beigh MA, Sheikh FA. Recent trends in peripheral nervous regeneration using 3D biomaterials. Tissue Cell. 2019;59:70–81. doi:10.1016/j.tice.2019.06.003
88. Sivolella S, Brunello G, Ferrarese N, et al. Nanostructured guidance for peripheral nerve injuries: a review with a perspective in the oral and maxillofacial area. Int J Mol Sci. 2014;15(2):3088–3117. doi:10.3390/ijms15023088
89. Meamar R, Chegini S, Varshosaz J, Aminorroaya A, Amini M, Siavosh M. Alleviating neuropathy of diabetic foot ulcer by co-delivery of venlafaxine and matrix metalloproteinase drug-loaded cellulose nanofiber sheets: production, in vitro characterization and clinical trial. Pharmacol Rep. 2021;73(3):806–819. doi:10.1007/s43440-021-00220-8
90. Alhosseini SN, Moztarzadeh F, Mozafari M, et al. Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int J Nanomedicine. 2012;7:25–34. doi:10.2147/IJN.S25376
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
