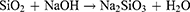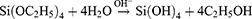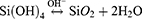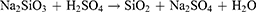Back to Journals » International Journal of Nanomedicine » Volume 17
How Advancing are Mesoporous Silica Nanoparticles? A Comprehensive Review of the Literature
Authors Porrang S, Davaran S, Rahemi N, Allahyari S, Mostafavi E
Received 11 December 2021
Accepted for publication 31 March 2022
Published 22 April 2022 Volume 2022:17 Pages 1803—1827
DOI https://doi.org/10.2147/IJN.S353349
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Sahar Porrang,1,2 Soodabeh Davaran,3,4 Nader Rahemi,1,2 Somaiyeh Allahyari,1,2 Ebrahim Mostafavi5,6
1Chemical Engineering Faculty, Sahand University of Technology, Tabriz, Iran; 2Environmental Engineering Research Centre, Sahand University of Technology, Tabriz, Iran; 3Department of Medical Nanotechnology, Faculty of Advanced Medical Science, Tabriz University of Medical Sciences, Tabriz, Iran; 4Research Centre for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences, Tabriz, Iran; 5Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, 94305, USA; 6Department of Medicine, Stanford University School of Medicine, Stanford, CA, 94305, USA
Correspondence: Ebrahim Mostafavi, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, 94305, USA, Email [email protected]; [email protected] Nader Rahemi, Sahand University of Technology, Tabriz, Iran, Tel +98-41-33459100, Email [email protected]
Abstract: The application of mesoporous silica nanoparticles (MSNs) is ubiquitous in various sciences. MSNs possess unique features, including the diversity in manufacturing by different synthesis methods and from different sources, structure controllability, pore design capabilities, pore size tunability, nanoparticle size distribution adjustment, and the ability to create diverse functional groups on their surface. These characteristics have led to various types of MSNs as a unique system for drug delivery. In this review, first, the synthesis of MSNs by different methods via using different sources were studied. Then, the parameters affecting their physicochemical properties and functionalization have been discussed. Finally, the last decade’s novel strategies, including surface functionalization, drug delivery, and cancer treatment, based on the MSNs in drug delivery and cancer therapy have been addressed.
Keywords: mesoporous silica nanoparticles, synthesis method, natural sources, synthetic sources, drug delivery, controlled release
Graphical Abstract:
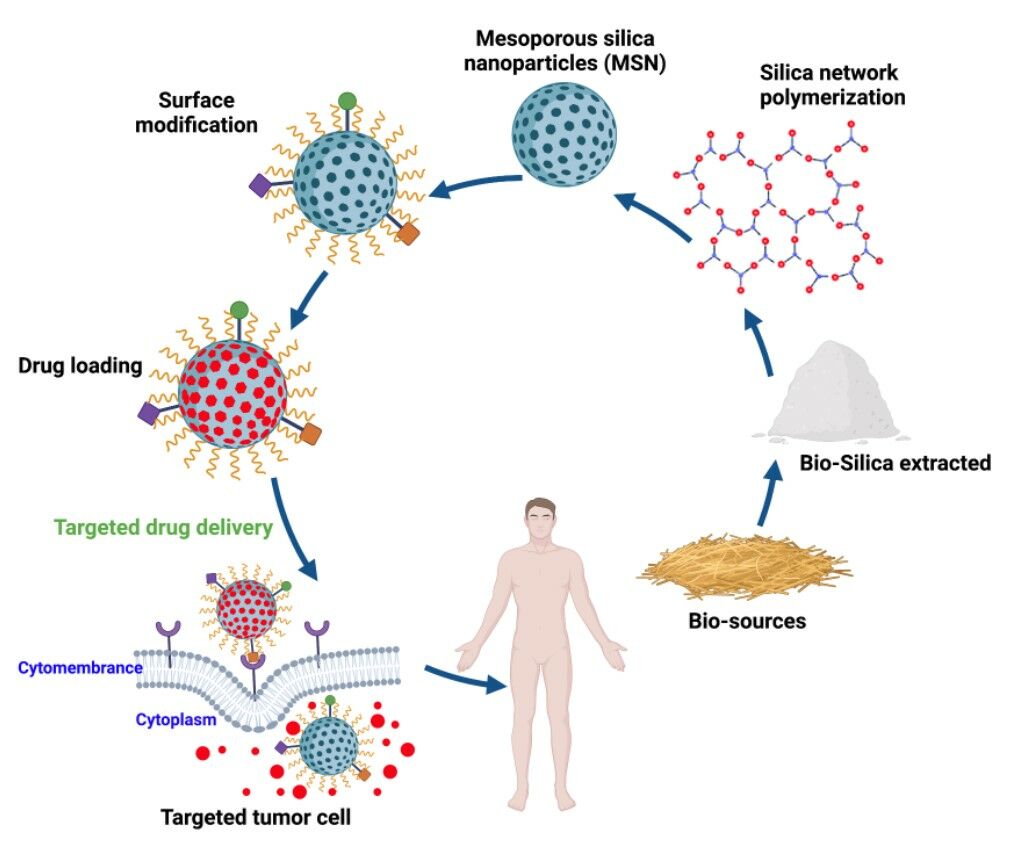
Introduction
In recent decades, implementing considerable investigations in the nanotechnology field caused effective outcomes in different subfields and even in daily life.1 The drug delivery field is a direct outcome of these recent achievements. Nanomaterials have many biological and medicinal applications in various fields such as drug delivery, tissue engineering, gene therapy, molecular imaging, etc.2–5 The utilization of nanomaterials in a new generation of medicines for cancer therapy is more efficient in comparison with conventional ones due to accessing targeting tissues, deep molecular targets, lower side effects, and controlled drug release. The US Food and Drug Administration (FDA), approved a new version of paclitaxel, which is loaded in albumin nanoparticles (AbraxaneTM) with fewer side effects and improved efficacy due to the more significant dose of the drug that can be administered and delivered.6 So, nano-sized drug carriers called nanocarriers in medical terminology, provide a wide range of practical applications for a targeted drug delivery process, such as: delivering poorly soluble, unstable, or systemically toxic drugs with extended blood half-lives and reduced side effects; Also, the nanocarriers can move smoothly along the vessels without any blocking. The drug’s accumulation increases by targeting agents at the surface of modified nanocarriers.7,8 Various advances in related sciences, such as polymer science, chemistry, engineering, biology, as well as mechanical and physics sciences, have all been able to influence the diverse types of nanoparticles and various carriers with unique characteristics and performance in medical sciences. Variety of polymers such as natural and synthetic, metal particles, lipids, etc., were used to manufacture nanoparticles for drug delivery. Diverse organic and inorganic nanostructures have already been investigated for this purpose. The first generation of drug delivery systems widely used for biomedical applications is organic materials.9 One of the most successful organic compounds were approved in this field are liposomes, which can be referred to as some of their commercialized types, including Doxil, as doxorubicin encapsulated in liposomes, AmBisome as Liposomal amphotericin B, and Liposomal morphine.10,11 Despite significant advances in organic drug delivery systems, some deficiencies exist, such as low drug loading capacity and low thermal chemical stability. Also, releasing a drug from organic nanocarriers due to the destruction and collapse of their structure is another disadvantage, while an ideal drug delivery system should have a controlled and stable release. Various advances in morphological control and the functionalization of inorganic nanoparticles, such as mesoporous silica nanoparticles (MSNs), provide new opportunities for the research and development of drug delivery systems.
Nowadays, among the various nanoparticles, MSNs are known as one of the most efficient delivery systems for delivering various drugs.12–14 Facile synthesis and modification methods, sustainability, and biocompatibility are unique properties, making them practical and accessible in drug delivery systems. Various types of MSNs with regular mesopores such as MCM-41, MCM-48, SBA-15, core-shell MSNs, and hollow MSNs have been used due to their unique properties in drug delivery. High surface area and pore volume allow high loading efficiency of the therapeutic agent into the MSNs. On the other hand, the drug release from the regular mesoporous structure increases the local concentration of the drug in the target area, which reduces the overall dose of the necessary drug and prevents acute or chronic side effects. Additionally, the surface of the MSNs is capable of being functionalized with a large variety of stimuli-responsive agents, targeting agents, polymers, biomolecules, photographic agents, caps for controlled drug release, and protective layer to prolonged drug circulation time.
Based on previous studies, silica nanoparticles can be synthesized from two general organic sources such as Tetra alkoxysilanes (Si(OR)4) and inorganic materials such as sodium silicate. Tetra alkoxysilanes have been selected as silica sources extensively. However, it is interesting to know researchers concluded that it was possible to obtain sodium silicate from bio-sources such as rice husk,15 wheat husk,16 coffee husk, barley grass,17 corn cob,18 sugar can bagasse,19 and palm oil.20
Here, MSNs’ synthesis methods from synthetic and natural sources were investigated, and their physicochemical properties were discussed. Then the last decade’s novel strategies based on MSNs for drug delivery and cancer treatments were reported.
Mesoporous Silica Nanoparticles
Silica nanoparticles have been used extensively in various sciences due to their structural characteristics, physicochemical properties, and surface modifiability. Scientists have studied the MSN’s synthesis from various sources and methods to enhance the properties of these nanoparticles. Based on previous studies, MSNs can be synthesized from two general sources of organic and inorganic materials. Alkoxysilanes, such as tetra alkoxysilanes, are organic materials that have been selected as synthetic silica sources widely in the research articles. It should be noted that the selection of the precursor type influences the physicochemical properties of nanoparticles. In such a way, smaller nanoparticles can be synthesized from shorter chain alkylsilane.21 As mentioned, other silica sources are inorganic materials, such as silicon tetrachloride,22,23 olivine,24,25 and sodium silicate solution.15,26,27 Sodium silicate as an economical and available precursor can be an excellent source for the synthesis of silica-based nanoparticles. However, industrial production of sodium silicate requires a great deal of energy. Because it is obtained by melting a mixture of high-quality sodium carbonate and quartz sand at a temperature of 1300 to 1600 °C,28,29 this method also produces a large volume of CO2 gas due to burning sodium carbonate and fuel needed to reach the desired temperature.30 Therefore, the synthesis of sodium silicate from silica-rich industrial by-products is under scrutiny.26 However, it is interesting to know that researchers concluded it was possible to obtain sodium silicate from natural sources such as materials that are considered agricultural waste or by-products such as rice husk, wheat husk, coffee husk, barley grass, corn cob, sugarcane bagasse, oil palm.15,26,27 These plants, due to the absorption of silica in the soil by their roots, can be as productive, economic, and accessible precursors of silica nanoparticles. So, a low-value substance can be converted into valuable MSNs. In the soil around the plants, silica is present in the form of silicic acid (Si (OH)4). Plants can absorb silicic acid from their roots. Through evaporation, the silicic acid is concentrated into SiO2.nH2O form, and therefore, a composite membrane of cellulose/silica is created.31 After silica extraction from biosources by different methods (the topic of discussion in the next section), it can be converted to sodium silicate solution (SSS) by reaction with sodium hydroxide solution.
The synthesized SSS can be condensed to form metasilicc acid as a silica polymerization initializer.
Table 1 contains the chemical composition of some Bio-sources ash.
 |
Table 1 Chemical Composition of Some Bio-Sources Ash |
Mesoporous Silica Nanoparticles’ Synthesis Mechanism from Synthetic Sources
For silica nanoparticles synthesis, various methods such as combustion flame, chemical vapor condensation, spray pyrolysis, microemulsion, high-energy ball milling, etc. are existed.32 Porous materials are known for their unique physicochemical properties and structural characteristics such as adjustable pore size, particle size and surface area, high pore volume, and surface modification ability that attracted the attention of many scientists. Because of these properties, mesoporous materials have various applications in the catalysis field, environmental applications, absorption and separation, biomedicine, etc.33 MSNs, as a member of this family, depending on their applications, have several synthesis methods. However, the basis of all these methods is hydrolysis and condensation of silica precursors. In 1968, for the first time, Stober et al synthesized monodispersed non-porous silica particles by the sol-gel method that involves hydrolysis of silica precursors in an alcohol and water solution using ammonia as a catalyst, that the size of them can be controlled in the range of a few nanometers to some microns. Michael et al investigated the degree of silica nanoparticle monodispersity, and they verified that the monodispersed silica particle formation by the Stober method consists of two main mechanisms, nucleation and then growth. Formulas for hydrolysis and condensation reactions of TEOS are as follows.
To explain the silica nanoparticle formation, first, in the presence of OH, TEOS molecule hydrolysis and tending to produce silicic acid. In the following, the Si-O-Si complex was formed by silicic acid.34 In the aim of MSNs synthesis, the surfactants were used to form mesopores. Some cationic surfactants like Cetyltrimethylammonium bromide (CTAB) were chosen as cationic surfactants to form micelles.35 First, a solution containing silica precursors such as TEOS or TMOS, and surfactants had been prepared. Then followed by hydrolysis and condensation of silica precursors, these molecules condensed at the surface of surfactant micelles via siloxane bonds (Si-O-Si). After surfactant removal, the MSNs are formed. Thus far, various MSNs with uniform pore size and ordered pore structure were reported by using surfactants named structure-directing agents (SDAs).36,37 MCM-41, MCM-48, and SBA-15 are the most popular ordered MSNs used in drug delivery applications. These families of MSNs are known for their uniform pore size and long-range ordered pore structure. The existence of various surfactants or templates and complete knowledge of the factors involved in the sol-gel process have allowed scientists to design MSNs in different pore sizes, pore structures, and morphologies.38
Optimization of Mesoporous Silica Nanoparticles Characteristics
Manipulating some of the essential parameters in the synthesis of nanomaterials, can lead to significant changes in their physicochemical, surface and structural properties. The change in such basic parameters and the creation of new properties in silica nanoparticles was sometimes referred to as the new synthesis method and sometimes to the parametric investigation. In this review paper, we discuss examples of basic parameters (such as pH and temperature) that their corresponding changes can affect the MSNs’ properties.
Depending on the application of MSNs, specific targets for the physicochemical properties such as particle size, specific surface area, pore size, and pore volume will be defined. For example, in the bio-medicine application, the particle size of MSNs should be in the range of 50 to 300 nm to facile endocytosis by living animal and plant cells without any significant cytotoxicity. Alternatively, in drug delivery and cancer treatment applications, the nanocarrier‘s particle size must be below 100 nm to long circulation in the blood vessel and act as a passive drug delivery system based on the EPR effect. On the other hand, MSNs pore size can be tailored from 2 to 6 nm, so these features allow for the loading of different drug molecules.39 Moreover, nanoparticles with large pore volumes have high drug loading and high encapsulation efficiency. So, to achieve the desired properties of MSNs after selecting the synthesis method, which is mainly the sol-gel method, the desirable design can be achieved by changing the synthesis and reaction essential parameters.
The sol-gel method consists of two main stages of nucleation and growth or hydrolysis and condensation. The fast nucleation rate causes the formation of the smaller nanoparticles, and if the growth stage is faster or more extensive, the larger nanoparticles will be synthesized. So by manipulating these rates with reaction parameters, we can change the size of the nanoparticles. pH is one of the parameters that can change the nucleation and growth rates. Figure 1 represents the changes in charge density and silica condensation rate with pH.40 As illustrated in Figure 1, below the silica isoelectric point (IEP=2.0), a decrease in pH causes an increase in the charge density. However, above the isoelectric point, the charge density starts to increase up to pH near 7.5, so in this case, silica nanoparticles assemble electrostatically or interact by a hydrogen bond with the positive or neutral molecules like surfactants or polymers.40 It should be noted that the silica condensation rate increase parallelly with charge density up to pH equal to 7.5, and it is due to an increase in the amount of Si-O− in the silica condensation reaction. In the pH >7.5 area, charge density is constant, and silicates can only interact with cationic surfactants.41 However, in this area, due to the gradual silica instability, the silica condensation rate decreases. Therefore, the pH of the medium will play an essential role in the size tuning of the nanoparticles, and it can be changed by adding some alcohols, amines, inorganic bases inorganic salts. Qiao et al used different additives like TEA, DEA, and NH3 to change the reaction acidity to determine the effect of the initial pH value on the size of the nanoparticles. They believed that all chemicals could be used as additive agents which able to supply OH− in the sol-gel process medium. They concluded that by reducing the initial acidity from 10 to 6, the nanoparticle size increased from 30 to 85 nm.42 It should be noted that, pH changes during the condensation reaction can increase or decrease the particle size depending on the initial conditions of the system. MSNs size effect on cellular uptake was investigated by Lu et al to achieve MSNs with various sizes, changed the medium pH by ammonium hydroxide. They observed that when the pH is decreased from 11.52 to 10.86, the particle size decreases. In this range of the pH variation, by pH increasing, the silica condensation rate decreases due to the increase in the negatively charged silicates amount.43 Precursor concentration, catalyst, co-solvent, water amount, and reaction temperature are other parameters that affect particle size. In a parametric study by Dabbaghian et al, these parameters were investigated. The results indicated that an increase in reaction temperature causes a decrease in particle size due to the rising in nucleation rate. However, in contrast, in another study, increasing reaction temperature from 30 °C to 70 °C causes nanoparticles in a larger size (28.91 nm to 113.22 nm), and this behavior was explained by the promoting the hydrolysis and rate of condensation reaction in high temperature.44 Also, increasing the catalyst amount and silica precursor ratio to water can cause an increase in particle size, because they encourage hydrolysis and condensation reactions. Nevertheless, excessive increases in mentioned parameters will have a negative response due to the limitation in water amount and silica precursor hydrolysis. The ratio of co-solvent to water can have an essential effect on particle size. Increasing in co-solvent content causes more hydrolyzing of TEOS, so, as a result, larger particles are synthesized. However, a further increase in co-solvent content causes the reaction medium to be dilute. So, due to an increase in the reacting agents distance, the condensation rate declines. These phenomena interrupt the conversion of sol to the gel network.45 The reaction temperature also can affect the pore size of nanoparticles. Li et al studied the effect of mixture temperature on the pore size in the range of 80 to 180 °C. They observed damage on pore structure at 130 °C. While at 150 °C, the pores again show the ordered structure. However, the ordered structure is destroyed by the temperature increasing up to 180 °C.46 It is important to note that the synthesizing conditions should be provided in such a way as to avoid aggregation of nanoparticles. Uses diluted surfactant concentration systems are one way to overcome nanoparticles aggregation. However, by this method the product isolating could be challengable and production yield will be low.42 Another way to overcome the aggregation is adding additives such as triethanolamine (TEA),42 pluronic polymer f127,47,48 PEG,49 and the amino acid L-lysine50 as the protective agents to forming stable MSNs without aggregation. Another tunable feature of MSNs is their pore size, which can be tailored by some techniques. The first strategy is pore size controlling by surfactants. Variation along the surfactant chain results in nanoparticles of different pore sizes. MSNs with large pores can be synthesized using longer chain length, and in contrast, smaller pores MSNs can be fabricated by shorter chain length surfactants.51 Also, the researchers have shown that, with the use of some swelling agents such as N,Ndimethylhexadecylamine (DMHA), and 1,3,5 trimethylbenzene (TMB), pore-expanded MSNs can be synthesized.52–54
 |
Figure 1 Effects of pH value on the silica condensation rate, charge properties and charge density on the surface of the silica species. Notes: Adapted from Wu S-H, Mou C-Y, Lin H-P. Synthesis of mesoporous silica nanoparticles. Chem Soc Rev. 2013;42(9):3862–3875.40 |
Mesoporous Silica Nanoparticles Synthesis from Natural Sources
Several methods exist for the synthesis of silica nanoparticles based on bio-genic sources. In general, we can divide these methods into two categories. One is top-down methods in which the biosources are converted into smaller nano-sized particles. Another category is bottom-up approaches, in which the nanostructures are synthesized by stacking atoms onto each other. Considering this brief introduction, the following section presents some research studies in which the MSNs were synthesized by these two approaches.
Ball-milling is an illustrative example of top-down methods.1 Salavati et al synthesis of amorphous spherical silica nanoparticles by ball-milling rice husk ash biosource in 6 hours. The average particle size of the silica powders was around 70 nm, which decreases with increasing ball-milling time or mill rotational speed.55 Finally, the drug delivery capability of the synthesized nanoparticle was evaluated. Penicillin-G was loaded successfully, and 80% of the loaded drug could be released in about 60 hours. Nanoparticle synthesis with laser ablation is another instance of top-down system. San et al used the raw sugarcane bagasse sample directly as the laser ablation target, without any purification or cleaning procedure, to synthesize silica nanoparticles in a range of 38–190 nm.56 Another form of silica nanoparticle was synthesized by chemical treatments, which are a subset of bottom-up approaches. After purification of bio-sources from dirt, metal, and carbon components in these methods, silica nanoparticles were synthesized by sol-gel or precipitation methods.
In synthesizing silica nanoparticles from bio-sources, rice husk (RH) is usually the first choice of researchers due to ist high silica content (20–25%) in its dry weight. The first studies in this field are the synthesis of silica nanoparticles from direct calcination of rice husk in the presence of air. The composition of rice husk ash (RHA) derived from calcination of rice husk at 600 °C for 12 h has been shown in Table 2.57
 |
Table 2 Composition of RHA Derived from Calcination of Rice Husk at 600 °C for 12 h |
To achieve the high purity of silica, scientists have implemented water washing pretreatment to remove adhering soil, dust, and some metal cations.58 Wang et al showed that water pretreatment could effectively remove most minerals except for K and Ca in RH.59 So, this method is beneficial for obtaining amorphous and high-purity silica. However, due to some metallic residues and carbonaceous impurities, scientists used acid pretreatment before the RH calcination to thoroughly remove impurities. Hydrochloric acid has been one of the widely selected acids in these studies. Zemnukhova et al treated rice husk by 0.1 N HCl, followed by thermal treatment in two stages, initially at 400 °C and then at 700 °C, 99.9% purity amorphous silica with 297 m2/g specific surface area was synthesized.60 It should be noted that the presence of carbonaceous impurities is due to the incomplete combustion of organic components. So, for complete removal of the carbonaceous impurity, a high calcination temperature of about 700 °C is needed.59 Acids such as sulfuric acid,61,62 nitric acid,63 hydrofluoric acid,64 etc., have also been used as pretreatment agents. Amorphous silica with a 99.3% purity can be synthesized from 5% H2SO4 pretreatment and then calcination at 800 °C for 30 minutes.65
Organic acids such as acetic acid,66 oxalic acid,67 and citric acid68 were also used in some studies due to their low corrosivity, low cost, and environmental friendliness. The strong chelate effect was created between the carboxyl groups of the organic acid and metallic impurities so that the impurities removal could be facilitated.58 By organic acid treatment, 93–96.7% silica component was achieved. Although the silica purification percentage is less than the amount obtained by HCl, however, the sodium and potassium-based impurities were entirely removed by this method.69 Another category of silica purification from rice husk related to use of base or salt purification, but satisfactory results have not been achieved. Yalçin et al using 3% NaOH solution as a pretreatment solution and followed by calcination pretreated RH at 600 °C for 4 hours, gained silica with 39.8% purity. This result showed that NaOH treatment was inefficient in producing high purity RH silica.70
The morphology and particle size distribution adjustment were studied by researchers. Djangang et al synthesized silica nanoparticles by hot injection method in the presence of PEG as a capping agent and non-toxic chemical to control the nanoparticle nucleation. In this method, a solution of RHA and ethanol was made, then the mixture was added quickly to hot PEG that heated to 180 °C. Then the reaction mixture was held on 80 °C for 2 hours for nanoparticle nucleation. As a result, The PEG as a stabilizing agent prevented nanoparticles agglomeration. So well-defined silica nanoparticles with a size distribution between 65 to 70 nm were synthesized.71 Another method to synthesize biogenic silica nanoparticles is the precipitation method. It is based on the precipitation of silica nanoparticles in the presence of concentrated H2SO4. The relevant reaction was given below:
By this method, Thuadaij et al synthesized silica nanoparticles in a range of 50 nm with a high amount of surface area of about 656 m2 gr−1. In their study, as-synthesized nanosilica was introduced in cement paste to improve its strength.72 To upgrade silica nanoparticles’ properties, MSNs such as MCM-41, MCM-46, SBA-15, SBA-16, etc. were synthesized from biosources with the presence of surfactants in the reaction mixtures and were used as drug nanocarrier, catalyst supports, CO2 adsorbent, Phosphate Removal from Solutions, adsorption some hydrophobic air pollutions, etc. MCM-48 type MSNs was synthesized by Jang et al from rice husk as a silica precursor with use a mixture of surfactants PLE (polyoxyethylene lauryl ether) and CTAB (cetyltrimethylammonium bromide). The surface of as-synthesized nanoparticles was functionalized by ATPES to create amine groups for CO2 adsorption. The results suggest that MCM-48 synthesized from rice husk ash could be used for CO2 removal.57
During a morphological study of biogenic MSNs, Renuka et al synthesized MCM-41 and SBA-16 types of MSNs with determining the effect of the molar ratio of surfactant on the texture of MSNs. They proved that the mesoporous texture of silica is altered from MCM 41 to SBA-16 by a reduction in CTAB to SiO2 molar ratio.73 Chiarakorn et al improved hydrophobicity of biogenic MCM-41 by the silylation of small molecular silane on them. The biogenic MCM-41 synthesized from rice husk and rice husk ash (obtained from the gasification process) as silica precursors were silylated with two different functional silanes trimethylchlorosilane (TMCS) and phenyldimethylchlorosilane (PDMS) and thus have a better performance in the adsorption of hydrophobic air pollutants.74 Bio-silica extraction from other natural biosources is similar to the methods used to extract silica from rice husk. Shaikh et al succeeded in synthesizing bio-MSNs from wheat husk with a similar procedure. In their study, after an HCl pretreatment and then calcination of the treated wheat husk, the wheat husk ash was obtained. The uniform size MCM-41 nanoparticles with a particle size in a range of 300 to 500 nm were synthesized by wheat husk ash as silica precursors and CTAB as a template.75 In another study, amorphous silica was extracted from wheat husk ash by Javed et al. The distinction of this study with others was the use of potassium permanganate in the calcination process to increase oxygen concentration to complete burning biosource. Also, various calcination temperatures were investigated for synthesize high purity products. They showed that after calcination, the carbon percentage was lower when the samples were soaked in the KMnO4 solution. So at a lower temperature, the product with an excellent purity was synthesized.16 Corn cob ash is another silica biosource that has been considered in this field. Okoronkwo et al, after water pretreatment, followed by calcination of corn cob at 650 °C, produced corn cob ash, then synthesized silica nanoparticles with sol-gel method.76 Also, Mohanraj et al synthesized nano-silica in a range of 34 nm by the precipitation method. They investigated the polyvinyl alcohol effect as a dispersing agent, and they showed that polyvinyl alcohol has reduced nanoparticles size to 22 nm. They also showed that if the sodium hydroxide concentration for sodium silicate solution preparation be high, purer silica nanoparticles were produced.77 As summarized, Table 3 provides properties of several biogenic silica nanoparticles and their application in various fields.
 |
Table 3 Several Biogenic Silica Nanoparticles and Their Applications |
Parametric Investigation of Mesoporous Silica Nanoparticles Synthesized from Natural Sources
Biosources, and elemental parameters in silica preparation, have critical roles in MSNs’ characterization. Calcination temperature, its duration, heating rate, combustion methods, and are type of pretreatment are effective parameters.
The investigation of calcination temperature and its duration effect on the nano-silica structure has shown that the calcination temperature and its duration rising increases the crystalline form of silica. Shen et al investigated the effects of calcination parameters on the nano-silica structural phase. They synthesized biosilica from direct calcination of rice husk at 600, 700, and 800 °C. The results showed that calcination at 600 °C causes amorphous silica nanoparticles formation. If the temperature increases to 700 °C, both amorphous and crystalline silica nanoparticles were created, and finally, at 800 °C, all of them were in the crystalline phase.58 Crystallization of silica is due to potassium and sodium impurities. Because they can induce the silica to melt. The melting of silica cause to decrease in silica surface area, increase particle size and this effect is due to impurities encapsulation. It should be noted that using a simple water pretreatment, the conversion of silica into a crystalline state is postponed. In a study, after rice husk water pretreatment, silica nanoparticles converted to the crystalline phase at 850 °C due to removing sodium and potassium impurities.59 With acid pretreatment, the purity of the product can reach 99.9%. In this condition, the temperature required for crystallization increases.60
The presence of potassium impurity and the calcination temperature can be effective in the formation of porous silica nanoparticles. The presence of potassium causes the clustering of primary silica nanoparticles, and as a result, a tunable porous structure appears. So, existence of a potassium nitrate solution as a potassium source in the calcination stage, can create porous silica nanoparticles.78
The combustion of biomass can be done by different combustion methods. Researches in this area has shown that for rice husks samples, their properties strongly depend on the combustion method used.79 Fernandes et al showed that the use of various methods of combustion could make changes in the specific surface area, the structure of silica, and the total carbon contents of the final ash. In this study, rice husk ash was synthesized without any pretreatment by three different combustion methods such as moving grate furnace, fluidized bed, and suspension/entrained combustion. The size of the nanoparticles in the suspension method was almost twice, and the results of BET analysis indicated that the surface area of SNs derived by these methods equals to 39.27, 11.35, and 26.74 m2gr−1, respectively. Also, the silica structure in RHA samples that combustion by fluidized bed and suspension methods was amorphous while the sample which was combusted by moving grate furnace was crystalline due to the presence of temperature gradient in the system. The TGA analysis showed that the carbon content in the fluidized bed was lower than others due to good combustion conditions.79 Table 4 presents the chemical composition of RHA with three combustion methods.
 |
Table 4 Chemical Composition Analyzed by XRF, Loss on Ignition, and Total Carbon of Three RHA Types (Wt%) |
Functionalization of Mesoporous Silica Nanoparticles
The presence of silanol groups on the surface of silica nanoparticles is one of their unique attributes making simple surface modification to create the desired functional groups on them. So MSNs can be functionalize with some functional silane precursors such as Vinyltrimethoxysilane (VTMO),80 Vinyltriethoxysilane (VTES),26,27 Aminopropyltriethoxysilane (APTES),81 and 3-mercaptopropyltriethoxysilane (MPTS)82 to create various type functional groups. The existence of MSNs with different types of functional groups has led to significant growth in various sciences such as catalysis, adsorption, separation, chromatography, chemical sensors, and bioscience. In drug delivery and cancer treatment, nanoparticle’s functionalization was improved to enhance the performance of the nanocarriers and equip them with active and passive targeting systems. Therefore, nanoparticle‘s functionalization is one of the most important steps in the nanocarrier‘s synthesis to approach ideal drug delivery systems. In general, MSNs’ surface functionalization is performed in two main ways: co-condensation and post-synthetic grafting methods. In the co-condensation method from the beginning of MSNs’ synthesis, alkoxysilanes with the desired functional group are in the mixture of reactions. So, by hydrolysis and condensation of silica precursor and alkoxysilanes, MSNs with the desired functional groups are synthesized. However, in the post-synthetic grafting method after MSNs’ synthesis, alkoxysilanes are interacted with silanol groups on the MSNs’ surface after or before template removal. Huh et al synthesized a series of functionalized MSNs by various functional groups such as amine, urea, vinyl, etc., by the co-condensation method. In this strategy, various organoalkoxysilanes were introduced in TEOS (silica precursor), CTAB (surfactant) sodium hydroxide (catalyzer). The organoalkoxysilanes included 3 -aminopropyltrimethoxysilane (APTMS), N-(2-aminoethyl)- 3-aminopropyltrimethoxy silane (AAPTMS), 3-[2-(2aminoethylamino)ethylamino]propyl trimethoxysilane (AEPTMS), ureidopropyltrimethoxysilane (UDPTMS), 3-isocyanatopropyl triethoxy-silane (ICPTES), 3-cyanopropyltriethoxysilane (CPTES), and allyltrimethoxysilane (ALTMS). They concluded that the particle morphology could be tuned in various shapes such as spheres, tubes, and rods by changing the precursor and its concentration.83 Saroj et al reported a pH-responsive drug delivery system based on grafting polyacrylic acid (PAA) on amine-functionalized MSN. In this research, after MCM-41 synthesis, the amine groups were introduced on nanoparticle surface by post-synthetic grafting method, which (3-Aminopropyl)triethoxysilane (APTES) used as amino silane. After that, PAA was covered on amino-functionalized MSN. Finally, the PAA-MSNs contained 20.19% grafted PAA as exhibited by thermogravimetric analysis (TGA).84 In drug delivery applications after MSNs functionalization, several components such as sensitive polymers, cell targeting agents, bio-molecules, etc., can be attached on the functionalized MSNs’ surface. Porrang et al synthesized MSNs from rice husk and introduced a novel method for their surface modification. In this approach, a Dielectric discharge barrier (DBD) plasma system was used as an accelerated method in uniform surface modification.27 By applying an electric field at atmospheric pressure in the presence of MSNs and alkoxysilanes, complex plasma species containing electrons, ions, neutral atoms, reactive species, metastable states, and UV radiation were created. These energetic species can transfer their momentum energy through direct collision with the material’s surface and act as a reaction initiator at atmospheric pressure and moderate temperature.85–88 In the recent study by Porrang et al, MSN’s surface was modified by triethoxyvinylsilane (TEVS) in a DBD plasma reactor, then the vinyl modified MSNs were functionalized by polyacrylic acid (PAA) and poly (N-isopropyl acrylamide) (PNIPAAm) to develop a dual stimuli-responsive doxorubicin (DOX) delivery system for breast cancer treatment.27 A schematic of MSN synthesis from rice husk and applied DBD plasma system with the occurred reactions are shown in Figure 2.
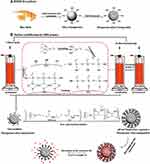 |
Figure 2 (A) The overall procedure of mesoporous silica nanoparticles synthesis from rice husk and (B) nanoparticles surface modification synthesis by DBD plasma modification with i) Direct and ii) direct hybrid modes to pH and Temperature-responsive drug delivery system synthesis. Note: Adapted from J Taiwan Inst Chem Eng, 123, Porrang S, Rahemi N, Davaran S, Mahdavi M, Hassanzadeh B, Gholipour AM. Direct surface modification of mesoporous silica nanoparticles by DBD plasma as a green approach to prepare dual-responsive drug delivery system. 47–58, copyright 2021, with permission from Elsevier.27 |
Nanocarriers Features in Drug Delivery and Cancer Therapy
Among the usual treatment modalities such as surgery, chemotherapy, and radiotherapy for cancer treatment, chemotherapy is still a top priority in clinical practice. Anticancer drugs still have an unsolvable problem due to their non-selective choosing capability, which could kill both normal and cancerous cells, thus increasing side effects and death risk. To minimize these drawbacks, a lower dose of drugs should be used, and their efficiency must increase. It seems that the side effects can be significantly reduced if drugs can be released at the target sites. This property can be created by encapsulating the drug into a smart nanocarrier.89 Nanocarriers can be defined as nanoparticles that are able to contain therapeutic agents. The therapeutic agents can place into the nanocarrier in various ways, such as dispersing in their matrix, encapsulating or covalently attached, and adsorbed to their surface.90–92 Some additive features of these nanoparticles include: extravasating across tumor vascular walls, penetration of the tumor interstitium, targetability with the use of surface receptors on cancer cells, and controlling the release of the anticancer drug locally.93,94 The development of nanocarriers has been influenced by nanotechnology including nanospheres, nanocapsules, liposomes, micelles, polymersomes, fullerenes, nanotubes, natural nanoparticles, and bio-nanoparticles.89,91,95–100 Also, the nanocarriers can protect the therapeutic agents from the biological milieu and decrease renal elimination. By chemical modification of their surface, they can be targetable and improve the bioavailability of the therapeutic agents. However, the solubility of water-insoluble drugs can be increased by them.101
Novel Drug Delivery Systems Based on Mesoporous Silica Nanoparticles for Controlled Drug Release
Stimuli-responsive drug delivery systems or nanocarriers, as shown in Figure 3 delivered therapeutic agents based on responses to some external or cancerous cell environmental stimuli such as temperature, pH, enzyme, redox, light, and magnetic field.
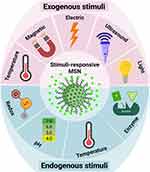 |
Figure 3 Stimuli-responsive drug delivery systems based on MSNs. Created with BioRender.com. |
The various types of these systems were fabricated and investigated by researchers. Due to higher metabolism and inflammation26,27,102 in tumor tissues, temperature-responsive systems were introduced. Poly (N-isopropyl acrylamide) (PNIPAAm),26,27 paraffin wax,103 and glycerophosphate104 are the most commonly used temperature-sensitive materials for temperature-responsive nanocarriers synthesis. The phase-transition temperatures of these materials are higher than the body temperature. Recent research has shown that hyperthermia may make some cancer cells more sensitive to radiation. The use of MSNs in this field is also expanding. Poudel et al fabricated a monodispersed mesoporous silica-coated silver-gold hollow nanoshell for remotely controllable chemo-photothermal therapy via phase-change molecule as gatekeepers with a size in a range of 115 nm.105 They prepared a silver-gold nanoshell (SGNS) within a hollow mesoporous silica shell with a thermoresponsive PCM lauric acid as a gatekeeper for prostate cancer therapy. Also, they chose 5-fluorouracil as a model drug. The system showed a controlled release behavior based on a thermosensitive strategy, activated via external heating. The temperature increasing melted the LA, which was immobilized in the pores as a gatekeeper. The nanocarrier had good pH and thermodynamic stability. The cellular analysis showed a good nanocarrier internalization by cancerous cells and an accepted drug release by applying low powered continuous-wave NIR laser with 808 nm wavelength. pH-responsive systems are another commonly used nanocarriers based on a high level of glycolysis in cancer cells, which produces lactic acid and carbon dioxide and decreases the pH of the cellular environment. While normal tissues have pH near 7.4, the tumor’s extracellular pH is expected to drop to 6.5 or less. Compared to the extracellular microenvironment, this abnormal pH gradient situation is even worse in intracellular organelles, such as endosomes (5.5) and lysosomes (below 5.5).106 This strategy can be applied in two ways: 1. by using polymers such as Polyacrylic acid (PAA) or materials such as calcium carbonate and aromatic amines107 that are sensitive to pH or 2. By sensitive linkers to pH such as imine, hydrazine, steric, acetal linkers.106 On the other hand, the high redox potential difference between intracellular and extracellular environments allows the design of nanocarriers for drug release, especially inside the cell.108 Glutathione is a potent antioxidant that protects important cellular components against reactions with reactive oxygen species such as free radicals and peroxides. Toxic compounds are usually combined with glutathione and are then removed from the body. However, the glutathione concentration in a tumor mass is drastically higher than the extracellular level of glutathione concentration in natural physiological environments and normal tissue. This difference is an important advantage in the drug delivery systems based on MSNs.109 Enzyme-responsive systems based on MSNs, are another category of drug delivery systems for controlled release. Due to the high metabolism of cancerous cells, some enzymes, such as matrix metalloproteinases (MMPs), have high composition and expression levels in tumor sites, which can be considered as biomarkers in nanomedicine applications.110,111 So MMP-sensitive MSNs can be produced to release the therapeutic agents at target sides.111 The magnetic field allows a targeted drug delivery system in the field of cancer treatment. In other words, when nanocarriers, equipped with a magnetic component and introduced in the body, they can be guided to the target side by applying a magnetic field. This field can be applied using an internally implanted permanent magnet or an external field.112–114 Also, if the nanocarriers are equipped with a magnetic system, they can be visualized by MRI,115 guided or held in place by applying a magnetic field, and can be heated to release the drug or creation the hyperthermia effect. Systems that are responsive to ultrasound are another type of controlled release system due to ultrasound’s power in destroying the nanocarrier’s structure and cause drug release. High-frequency ultrasound can non-invasively penetrate very deep into tissues and is well tolerated by the body116,117 and causes some thermal and mechanical effects, including pressure variation, acoustic fluid streaming, cavitation, and local hyperthermia. According to these effects, it can be used in different medical applications.118 Light-responsive systems are novel systems in drug delivery applications. One of the most promising light-sensitive strategies is photothermal therapy, in which the photothermal heating under NIR laser, causes to the trigger release of drug molecules and cancer cell’s death. Also, MSNs based on light-sensitive ability composed of a core-shell structure, which includes gold nanorods (AuNRs),107,119–123 graphene nanosheets,124 copper chalcogenides (Cu9S5),125 single-walled carbon nanotubes (SWNTs),126 selenium-coated tellurium nanoheterojunctions,127 and many others as core and mesoporous silica shell that encapsulated chemotherapeutic agents in their channels. Liu et al have reported light-responsive core-shell structures that have single-walled carbon nanotubes cores and DOX-loaded mesoporous silica shells. Finally, the nanocarriers were covered by polyethylene glycol (PEG) to improve solubility and prolong circulation time. By applying the NIR laser, the chemotherapeutic drug could be released successfully in the cancerous site and enhance cancer cell destruction by chemotherapeutics.126 The explained strategies are useful methods in stimuli-responsive drug delivery systems, in which several systems were fabricated based on them. However, there are still more promising methods for targeting cancerous cells called active targeting. By this method, the effects of passive targeting enhance to make the nanocarriers more specific to a target site. Binding targeting ligands to specific cancer cell receptors have created this ability, as shown in Figure 4.
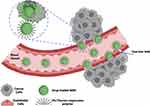 |
Figure 4 Active targeting strategies based on MSNs. Created with BioRender.com. |
If the nanocarriers are equipped with ligands that only bind to specific receptors on the cell surface, they can release the drug inside the cancerous cells. Antibodies, peptides, aptamers, Folate, and small or large molecules are examples of targeting ligands in this field. Tsai et al developed active targeting green dye-loaded MSN nanocarriers based on anti-HER2/neu monoclonal antibody (mAb).128 Monoclonal antibodies have the task of identifying target cells from specific surface receptors activated by oncogenes.129 As-synthesis nanocarriers were named Her-Dye@MSN, further were covered by PEG to increase circulation time. Therefore, their targeting ability and nanocarrier internalization toward HER2/neu overexpressing breast cancer cells were examined. The targeting capability of nanocarrier on NIH3T3 (mouse fibroblast cell), MCF-7 (breast cancer cell), and BT-474 (HER2/neu overexpressing breast cancer cell) cell lines was investigated by flow cytometry. As a result, the synthesized nanocarrier showed a high recognition activity against BT-474 cells after 3 hours of incubation.
Also, aptamers, have the unique potential in active targeting and diagnostic field.128,130,131 Aptamers are single-stranded synthetic sequences of RNA or DNA that fold into secondary and tertiary structures and bind to specific targets (antigens) with extremely high specificity and affinity. Xie et al investigated active targeting MSNs by the use of EpCAM aptamer. They modified MSNs by aptamer that can bond with the epithelial cell adhesion molecule (EpCAM). They chose DOX as a model drug and SW620 colon cancer cells as the model cells which have overexpressed EpCAM. As a result, they observed that the Ap-MSN-DOX binding to SW620 cell line was increasing compared to non-aptamer modified MSNs.132 Also, peptides were used in several studies as the active targeting agent based on the ligand-receptor strategy. They have some attractive alternatives compared to antibodies, such as their small size with higher stability, and lower immunogenicity.133 The peptides-based systems that penetrate the cell are among the most robust methods used to transfer drugs and therapeutic agents.134 Also, their high adaptability, and low cellular toxicity are characteristics of these systems. Several drug delivery systems have been developed by modifying nanocarriers surfaces with different peptides.135–137 Pan et al reported an active targeting drug delivery system by using TAT peptide conjugated onto the MSN surface.138 This system aimed to deliver DOX to the nucleus of cancerous cells. The cellular uptake of TAT modified MSNs, and unmodified MSNs was investigated by using confocal laser scanning microscopy (CLSM). Hela cells were chosen as model cancerous cells. Figure 5 illustrated MSNs-TAT nanocarriers with various sizes (25, 50, 67, and 105 nm), which incubated in Hela cells for 4, 8, and 24 hours. After 4 hours of incubation, few nanocarriers were observed in the nuclei of cells, as demonstrated by the green fluorescence from FITC lighting up the nuclei. Then after 8- and 24-hours incubation, the concentration of nanocarriers with 25 and 50 nm in size, were higher than others. First, there were very few nanoparticles in the nuclei after 4 h, regardless of the particle size. It was concluded that the MSNs-TAT with a size smaller than 50 nm can successfully penetrate nuclei after 24 hours.
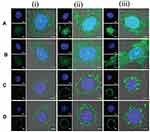 |
Figure 5 CLSM images of MSNs-TAT with diameters of (A) 25, (B) 50, (C) 67, and (D) 105 nm after incubation with Hela cells for (i) 4, (ii) 8, and (iii) 24 h. Scale bars: 5 μm. |
Last Decade’s Advances on Novel Dual/Multi-Responsive Drug Delivery Systems Based on Mesoporous Silica Nanoparticles
Nowadays, researchers have employed two or more of these stimuli-responsive strategies in combination, to achieve stimuli-responsive drug delivery systems with better performance. So, the dual/multi-responsive and multi-functionalized systems were fabricated. An et al introduced a glutathione-sensitive magnetic drug delivery system based on core-shell mesoporous silica nanocomposite (Fe3O4@mSiO2) as a carrier. The study showed that a simple silane coupling reaction could make a glutathione cleavable linker as a gatekeeper and grafted onto Fe3O4@mSiO2 to encapsulate anticancer drug DOX. With the absorption of these nanocomposites by objective cells via pinocytosis or phagocytosis, the “gate” is expected to be broken down owing to the high expression of GSH in the cancer cell, inducing the DOX release. So, the cytotoxicity to cancer cells will be much improved by the magnetic and FA targeting as well as the specific sensitive DOX release.139 Over time, MSN-based drug delivery systems became more complex and sensitive in detecting cancer cells. Shen et al fabricated a dual functionalized mesoporous silica nanoparticle to doxorubicin delivery to HER2-positive breast cancer cells. First, the MSNs were functionalized by benzimidazole (MSN-BM) as a pH-responsive agent. Then, β-cyclodextrin was introduced as a gatekeeper, and finally, HApt aptamer was conjugated by redox responsive s-s bond to the external surface of MSNs (MSN-BM/CD-HApt@DOX) as both a targeting agent (Her2) and antagonist.140 The schematic of MSN-BM/CD-HApt@DOX synthesis and the mechanism of targeting and apoptosis of cancerous cells have been illustrated in Figure 6. To evaluate the drug delivery potential and cell cytotoxicity, HER2-overexpressing SKBR3 and HER2-negative MCF7 were selected. MSN-BM/CD-HApt@DOX was more cytotoxic to HER2-positive SKBR3 cells with better uptake and stronger growth inhibition.140 In another study, Paris et al, by combining thermo- and ultrasound responsive strategies, developed a nanocarrier based on MSNs that was covered by PEG with a thermosensitive linker. By ultrasound applying, the local temperature has risen and caused the thermosensitive linker destroying.141 So, the PEG cover goes away, and further, the drug is released. In the field of dual-responsive systems, Porrang et al fabricated dual pH and temperature-responsive drug delivery system based on MSNs. In this study the silica source was rice husk as an economical, safe, and biocompatible bio-source. The acrylic acid and n-isopropylyacrylamide were used as pH, and temperature-responsive monomers were grafted on the vinyl modified MSNs by free-radical polymerization method. The nanocarrier’s LCST was adjusted at 38 °C by monomers ratio tuning in the reaction mixture. So, the drug release can be controlled in response to environmental stimuli such as temperature and pH. The cumulative drug release was about 75% in 24 at cancerous site conditions.26 In another study, the dual-responsive MSNs were fabricated by vinyl modification of MSNs surfaces by a dielectric barrier discharge plasma system. The as-synthesized nanocarriers had a uniform spherical shape without agglomeration with a particle size of about 162 nm. 98% of the loaded drug was released in cancerous site condition, which is 85% higher than released drug in normal condition. Moreover, the toxicity of the DOX-loaded nanocarrier was higher than free DOX. So, it can be helpful in reducing side effects in clinical cancer treatment.27 Zhang et al developed dendritic MSNs camouflaged with leukocyte/platelet hybrid membrane (LPHM@DLMSNs) for Triple-negative breast cancer (TNBC) combination treatment. A near-infrared (NIR) fluorescent dye IR780 and DOX were co-loaded into the LPHM@DLMSNs to prepare LPHM@DDI nanoparticles. The cells in the treatment group of LPHM@DDI NPs with laser irradiation exhibited the highest apoptosis rate, further verifying the synergistic anti-TNBC effect of photothermal therapy (PTT)/photodynamic therapy (PDT) and chemotherapy in vitro and also notably suppressed tumor growth and recurrence in TNBC mice through tumor ablation and antiangiogenesis.142 Chen et al reported dual redox and antibody-responsive drug delivery system based on MSNs. In this study, anti-carbonic anhydrase IX antibody (A-CAIX Ab) was bonded on the MSN surface by disulfide linkages. DOX was used as a chemotherapeutic agent. The in-vivo targeting studies on the 4T1 tumor-bearing mice showed DOX@MSNs-CAIX accumulation in tumor and induced more tumor cells apoptosis due to higher glutathione level CAIX positive cells in the model tumor.143 Tran et al reported multi-functionalized core-shell type of MSNs as cisplatin delivery system for bio-imaging, chemo-photothermal cancer therapy. The drug release strategy was based on pH, and near-infrared radiation (NIR) stimuli. EGFR antibody was conjugated on the MSNs surface as a targeting agent. As shown in Figure 7, magnetic MSNs were grafted with fluorescent conjugates to prepare FS3 nanoparticles. Cisplatin as a therapeutic agent was loaded into the nanoparticles and was coated with a polydopamine layer (FS3P/C). Then, two different strategies were implemented. i) Targeted, and ii) Photothermal cancer therapy.144 For targeted cancer therapy, as-synthesized FS3P/C was wrapped with graphene oxide (FS3P-G/C). Finally, it was conjugated with EGFR antibody ((FS3P-G-E/C) for targeted and dual stimuli (pH, NIR)- responsive controlled release. In the photothermal cancer therapy strategy, FS3P/C nanoparticle was coated by gold nanoparticles (FS3P-A/C). It was concluded that FS3P-A/C exhibited enhanced release rate under the same NIR irradiation and this kind of nanoparticle enabled high photothermal destruction of HeLa cells under NIR irradiation.144
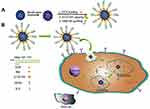 |
Figure 6 (A) Schematic illustration of MSN-BM/CD-HApt@DOX synthesis process and (B) the mechanism of targeting and cancer cells apoptosis. Note: Reproduced from Shen Y, Li M, Liu T, et al.A dual-functional HER2 aptamer-conjugated, pH-activated mesoporous silica nanocarrier-based drug delivery system provides in vitro synergistic cytotoxicity in HER2-positive breast cancer cells. Int J Nanomedicine. 2019;14:4029-4044. Originally published by and used with permission from Dove Medical Press Ltd.140 |
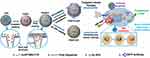 |
Figure 7 Schematic illustration of magnetic MSNs drug delivery systems preparation based on targeted and photothermal cancer therapy strategies. Abbreviations: APTMS-FITC, 3-AminoPropylTriMethoxySilane-Fluorescein IsoThioCyanate; F, Fe3O4 NPs; S, Silica nanoparticles (MSNs); P, Polydopamine (PDA); G, Graphene oxide; A, Au NPs; E, EGFR antibody; C, Cisplatin; GO, Graphene oxide. Note: Reproduced from Tran VA, Van Giau Vo KS, Lee S-W, An SSA, An SSA. Multimodal mesoporous silica nanocarriers for dual stimuli-responsive drug release and excellent photothermal ablation of cancer cells. Int J Nanomedicine. 2020;15:7667-7685. Originally published by and used with permission from Dove Medical Press Ltd.144 |
Several examples exist in the last decade, which were summarized in Table 5. The goal of most researches, which were reported in this area was to synthesize complex drug delivery systems for cancer treatment that have achieved extremely promising results. However, it is essential to note that to use these systems in clinical treatment, it will be necessary to scale up them. Therefore, future nanocarriers should have a simple synthesis method for successful scaling up with a high performance.
 |
Table 5 Some Examples of Drug Delivery Systems Based on MSNs |
The Future Perspectives
Mesoporous silica nanoparticles can be synthesized from various synthetic and natural precursors that can affect the properties of nanoparticles features. By hydrolysis and subsequent condensation of the silica precursors in the presence of templates, MSNs can be synthesized. To achieve high purity MSNs from biosources, parameters involved in synthesis, including the type of acid used, acid treatment duration, calcination temperature and duration, etc., were investigated. Due to the particular surface properties of MSNs, various controlled release drug delivery systems were synthesized. Here, last decade novel studies based on functionalized-MSNs as a nanocarrier for drug delivery were investigated. Despite considerable researches on MSNs and their potential for drug delivery and cancer treatment, MSNs are still not used in clinical applications. The uniform synthesis and surface modification of MSNs, the ability to scale up, the precursor’s availability, and of course, their cost are the most critical issues and challenges in the clinical applications. In this review paper, some articles were reviewed that have simplified the way for clinical applications by using new methods and novel innovative ideas in the synthesis and surface modification of MSNs. Recent researches were shown, agricultural wastes such as rice husk can be used as an available, non-toxic, and economical silica biosource which could be converted to sodium silicate to make MSNs. Moreover, using new technologies in surface modification, such as the DBD plasma system, the surface of MSNs can be modified regularly and uniformly. There is much work ahead of the scientific community to treat cancer by MSNs as a novel and effective method in the treatment protocol of patients. However, tremendously positive results encourage further exploration to scale-up functionalized MSNs for drug delivery and cancer treatment.
Acknowledgments
The authors gratefully acknowledge the Iran National Science Foundation for the financial support of the research under project number 97014149, as well as the Sahand University of Technology for complementary financial supports. E.M. would like to acknowledge the support from the National Institute of Biomedical Imaging and Bioengineering (5T32EB009035). Graphical abstract is created with BioRender.com.
Disclosure
The authors reports no conflicts of interest in this work.
References
1. Mostafavi E, Soltantabar P, Webster TJ. Nanotechnology and picotechnology: a new arena for translational medicine. In: Biomaterials in Translational Medicine. Elsevier; 2019:191–212.
2. Medina-Cruz D, Mostafavi E, Vernet-Crua A, et al. Green nanotechnology-based drug delivery systems for osteogenic disorders. Expert Opin Drug Deliv. 2020;17(3):341–356. doi:10.1080/17425247.2020.1727441
3. Mostafavi E, Medina-Cruz D, Kalantari K, Taymoori A, Soltantabar P, Webster TJ. Electroconductive nanobiomaterials for tissue engineering and regenerative medicine. Bioelectricity. 2020;2(2):120–149. doi:10.1089/bioe.2020.0021
4. Zare H, Ahmadi S, Ghasemi A, et al. Carbon nanotubes: smart drug/gene delivery carriers. Int J Nanomedicine. 2021;16:1681–1706. doi:10.2147/IJN.S299448
5. Mostafavi E, Zarepour A, Barabadi H, Zarrabi A, Truong LB, Medina-Cruz D. Antineoplastic activity of biogenic silver and gold nanoparticles to combat leukemia: beginning a new era in cancer theragnostic. Biotechnol Rep. 2022;34:e00714. doi:10.1016/j.btre.2022.e00714
6. Chenthamara D, Subramaniam S, Ramakrishnan SG, et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019;23(1):1–29. doi:10.1186/s40824-018-0153-7
7. Yoo J, Park C, Yi G, Lee D, Koo H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers. 2019;11(5):640. doi:10.3390/cancers11050640
8. Amani H, Mostafavi E, Alebouyeh MR, et al. Would colloidal gold nanocarriers present an effective diagnosis or treatment for ischemic stroke? Int J Nanomedicine. 2019;14:8013–8031. doi:10.2147/IJN.S210035
9. Virlan M, Miricescu D, Radulescu R, et al. Organic nanomaterials and their applications in the treatment of oral diseases. Molecules. 2016;21(2):207. doi:10.3390/molecules21020207
10. Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9(2):12. doi:10.3390/pharmaceutics9020012
11. Beltrán-Gracia E, López-Camacho A, Higuera-Ciapara I, Velázquez-Fernández JB, Vallejo-Cardona AA. Nanomedicine review: clinical developments in liposomal applications. Cancer Nanotechnol. 2019;10(1):1–40. doi:10.1186/s12645-019-0055-y
12. Jafari S, Derakhshankhah H, Alaei L, Fattahi A, Varnamkhasti BS, Saboury AA. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed Pharmacother. 2019;109:1100–1111. doi:10.1016/j.biopha.2018.10.167
13. Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system: a review. Int J Pharm Investig. 2015;5(3):124. doi:10.4103/2230-973X.160844
14. Castillo RR, Lozano D, Vallet-Regí M. Mesoporous silica nanoparticles as carriers for therapeutic biomolecules. Pharmaceutics. 2020;12(5):432. doi:10.3390/pharmaceutics12050432
15. Porrang S, Rahemi N, Davaran S, Mahdavi M, Hassanzadeh B. Preparation and in-vitro evaluation of mesoporous biogenic silica nanoparticles obtained from rice and wheat husk as a biocompatible carrier for anti-cancer drug delivery. Eur J Pharm Sci. 2021;105866. doi:10.1016/j.ejps.2021.105866
16. Naqvi J, Shah F, Mansha M. Extraction of amorphous silica from wheat husk by using KMnO4. J Faculty Eng Technol. 2011;18(1):39–46.
17. Kavaz D, Vaseashta A, Vaseashta A. Synthesizing nano silica nanoparticles from barley grain waste: effect of temperature on mechanical properties. Polish J Environ Stud. 2019;28(4):2513–2521. doi:10.15244/pjoes/91078
18. Okoronkwo E, Imoisili P, Olusunle S. Extraction and characterization of amorphous silica from corn cob ash by sol-gel-method. Chem Mater Res. 2013;3(4):68–72.
19. Sholeh M, Rochmadi R, Sulistyo H, Budhijanto B. Nanostructured silica from bagasse ash: the effect of synthesis temperature and pH on its properties. J Sol Gel Sci Technol. 2021;97(1):126–137. doi:10.1007/s10971-020-05416-7
20. Awal AA, Hussin MW. The effectiveness of palm oil fuel ash in preventing expansion due to alkali-silica reaction. Cement Concrete Composit. 1997;19(4):367–372. doi:10.1016/S0958-9465(97)00034-6
21. Heuer-Jungemann A, Feliu N, Bakaimi I, et al. The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chem Rev. 2019;119(8):4819–4880. doi:10.1021/acs.chemrev.8b00733
22. El Moussawi A, Endres T, Peukert S, et al. Multi-line SiO fluorescence imaging in the flame synthesis of silica nanoparticles from SiCl4. Combust Flame. 2021;224:260–272. doi:10.1016/j.combustflame.2020.12.020
23. Cheraghian G, Wistuba MP. Effect of Fumed silica nanoparticles on ultraviolet aging resistance of bitumen. Nanomaterials. 2021;11(2):454. doi:10.3390/nano11020454
24. Chen Y, Sepahvand S, Gauvin F, Schollbach K, Brouwers H, Yu Q. One-pot synthesis of monolithic silica-cellulose aerogel applying a sustainable sodium silicate precursor. Constr Build Mater. 2021;293:123289. doi:10.1016/j.conbuildmat.2021.123289
25. Stopic S, Dertmann C, Koiwa I, et al. Synthesis of nanosilica via olivine mineral carbonation under high pressure in an autoclave. Metals. 2019;9(6):708. doi:10.3390/met9060708
26. Porrang S, Rahemi N, Davaran S, Mahdavi M, Hassanzadeh B. Synthesis of temperature/pH dual-responsive mesoporous silica nanoparticles by surface modification and radical polymerization for anti-cancer drug delivery. Colloids Surf a Physicochem Eng Asp. 2021;623:126719. doi:10.1016/j.colsurfa.2021.126719
27. Porrang S, Rahemi N, Davaran S, Mahdavi M, Hassanzadeh B, Gholipour AM. Direct surface modification of mesoporous silica nanoparticles by DBD plasma as a green approach to prepare dual-responsive drug delivery system. J Taiwan Inst Chem Eng. 2021;123:47–58. doi:10.1016/j.jtice.2021.05.024
28. Alam Q, Hendrix Y, Thijs L, Lazaro A, Schollbach K, Brouwers H. Novel low temperature synthesis of sodium silicate and ordered mesoporous silica from incineration bottom ash. J Clean Prod. 2019;211:874–883. doi:10.1016/j.jclepro.2018.11.173
29. Lazaro A, Quercia G, Brouwers H, Geus J. Synthesis of a green nano-silica material using beneficiated waste dunites and its application in concrete. World J Nano Sci Eng. 2013;2013. doi:10.4236/wjnse.2013.33006
30. Tong KT, Vinai R, Soutsos MN. Use of Vietnamese rice husk ash for the production of sodium silicate as the activator for alkali-activated binders. J Clean Prod. 2018;201:272–286. doi:10.1016/j.jclepro.2018.08.025
31. Bryant R, Proctor A, Hawkridge M, et al. Genetic variation and association mapping of silica concentration in rice hulls using a germplasm collection. Genetica. 2011;139(11–12):1383–1398. doi:10.1007/s10709-012-9637-x
32. Patel B, Patel P. Synthesis and characterization of silica nano-particles by acid leaching technique. Res J Chem Sci. 2014;4:52–55.
33. Chen Y. Design, Synthesis, Multifunctionalization and Biomedical Applications of Multifunctional Mesoporous Silica-Based Drug Delivery Nanosystems. Springer; 2016.
34. Issa AA, Luyt AS. Kinetics of alkoxysilanes and organoalkoxysilanes polymerization: a review. Polymers. 2019;11(3):537. doi:10.3390/polym11030537
35. Hao N, Chen X, Jayawardana KW, Wu B, Sundhoro M, Yan M. Shape control of mesoporous silica nanomaterials templated with dual cationic surfactants and their antibacterial activities. Biomater Sci. 2016;4(1):87–91. doi:10.1039/C5BM00197H
36. Froyen T, Geysmans N, Vounckx U, Hardy A. Multilamellar mesoporous silica nanoparticles using a cationic co-surfactant dual-templating method. bioRxiv. 2021. doi:10.1101/2021.02.25.432869
37. Guo Q-Y, Yan X-Y, Zhang W, et al. Ordered mesoporous silica pyrolyzed from single-source self-assembled organic–inorganic giant surfactants. J Am Chem Soc. 2021;143:12935–12942. doi:10.1021/jacs.1c05356
38. Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24(12):1504–1534. doi:10.1002/adma.201104763
39. Li J, Shen S, Kong F, Jiang T, Tang C, Yin C. Effects of pore size on in vitro and in vivo anticancer efficacies of mesoporous silica nanoparticles. RSC Adv. 2018;8(43):24633–24640. doi:10.1039/C8RA03914C
40. Wu S-H, Mou C-Y, Lin H-P. Synthesis of mesoporous silica nanoparticles. Chem Soc Rev. 2013;42(9):3862–3875. doi:10.1039/c3cs35405a
41. Li P, Li T, Ishiguro M, Su Y. Comparison of same carbon chain length cationic and anionic surfactant adsorption on silica. Coll Interfaces. 2020;4(3):34. doi:10.3390/colloids4030034
42. Qiao Z-A, Zhang L, Guo M, Liu Y, Huo Q. Synthesis of mesoporous silica nanoparticles via controlled hydrolysis and condensation of silicon alkoxide. Chem Mater. 2009;21(16):3823–3829. doi:10.1021/cm901335k
43. Lu F, Wu SH, Hung Y, Mou CY. Size effect on cell uptake in well‐suspended, uniform mesoporous silica nanoparticles. Small. 2009;5(12):1408–1413. doi:10.1002/smll.200900005
44. Zainala NA, Shukor SRA, Wabb HAA, Razakb K. Study on the effect of synthesis parameters of silica nanoparticles entrapped with rifampicin. Chem Eng. 2013;32. doi:10.3303/CET1332375
45. Dabbaghian M, Babalou A, Hadi P, Jannatdoust E. A parametric study of the synthesis of silica nanoparticles via sol-gel precipitation method. Int J Nanosci Nanotechnol. 2010;6(2):104–113.
46. Li C, Qi N, Liu Z, Zhou B, Chen Z, Wang Z. Effect of synthesis temperature on the ordered pore structure in mesoporous silica studied by positron annihilation spectroscopy. Appl Surf Sci. 2016;363:445–450. doi:10.1016/j.apsusc.2015.12.055
47. Yildirim A, Demirel GB, Erdem R, Senturk B, Tekinay T, Bayindir M. Pluronic polymer capped biocompatible mesoporous silica nanocarriers. Chem Commun. 2013;49(84):9782–9784. doi:10.1039/c3cc45967e
48. Yu J, Qiu H, Yin S, Wang H, Li Y. Polymeric drug delivery system based on pluronics for cancer treatment. Molecules. 2021;26(12):3610. doi:10.3390/molecules26123610
49. Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi:10.1016/j.addr.2015.09.012
50. Yang C, Shi Z, Feng C, et al. An adjustable ph‐responsive drug delivery system based on self‐assembly polypeptide‐modified mesoporous silica. Macromol Biosci. 2020;20(6):2000034. doi:10.1002/mabi.202000034
51. Narayan R, Nayak UY, Raichur AM, Garg S. Mesoporous silica nanoparticles: a comprehensive review on synthesis and recent advances. Pharmaceutics. 2018;10(3):118. doi:10.3390/pharmaceutics10030118
52. Knežević NŽ, Durand J-O. Large pore mesoporous silica nanomaterials for application in delivery of biomolecules. Nanoscale. 2015;7(6):2199–2209. doi:10.1039/C4NR06114D
53. Möller K, Bein T. Talented mesoporous silica nanoparticles. Chem Mater. 2017;29(1):371–388. doi:10.1021/acs.chemmater.6b03629
54. Liu H-J, Xu P. Smart mesoporous silica nanoparticles for protein delivery. Nanomaterials. 2019;9(4):511. doi:10.3390/nano9040511
55. Salavati-Niasari M, Javidi J, Dadkhah M. Ball milling synthesis of silica nanoparticle from rice husk ash for drug delivery application. Comb Chem High Throughput Screen. 2013;16(6):458–462. doi:10.2174/1386207311316060006
56. San NO, Kurşungöz C, Tümtaş Y, Yaşa Ö, Ortac B, Tekinay T. Novel one-step synthesis of silica nanoparticles from sugarbeet bagasse by laser ablation and their effects on the growth of freshwater algae culture. Particuology. 2014;17:29–35. doi:10.1016/j.partic.2013.11.003
57. Jang HT, Park Y, Ko YS, Lee JY, Margandan B. Highly siliceous MCM-48 from rice husk ash for CO2 adsorption. Int J Greenhouse Gas Cont. 2009;3(5):545–549. doi:10.1016/j.ijggc.2009.02.008
58. Shen J, Liu X, Zhu S, Zhang H, Tan J. Effects of calcination parameters on the silica phase of original and leached rice husk ash. Mater Lett. 2011;65(8):1179–1183. doi:10.1016/j.matlet.2011.01.034
59. Wang W, Martin JC, Zhang N, Ma C, Han A, Sun L. Harvesting silica nanoparticles from rice husks. J Nanopart Res. 2011;13(12):6981–6990. doi:10.1007/s11051-011-0609-3
60. Zemnukhova L, Egorov A, Fedorishcheva G, Barinov N, Sokol’nitskaya T, Botsul A. Properties of amorphous silica produced from rice and oat processing waste. Inorgan Mater. 2006;42(1):24–29. doi:10.1134/S0020168506010067
61. Ali M, Haq EU, Karim MRA, et al. Effect of leaching with 5–6 N H2SO4 on thermal kinetics of rice husk during pure silica recovery. J Adv Res. 2016;7(1):47–51. doi:10.1016/j.jare.2015.01.007
62. Bakar RA, Yahya R, Gan SN. Production of high purity amorphous silica from rice husk. Procedia Chem. 2016;19:189–195. doi:10.1016/j.proche.2016.03.092
63. Hincapié-Rojas DF, Pineda-Gomez P, Rosales-Rivera A. Synthesis and characterisation of submicron silica particles from rice husk. Green Mater. 2018;6(1):15–22. doi:10.1680/jgrma.17.00019
64. Todkar BS, Deorukhkar OA, Deshmukh SM. Extraction of silica from rice husk. Int J Eng Res Dev. 2016;12(3):69–74.
65. Umeda J, Kondoh K, Michiura Y. Process parameters optimization in preparing high-purity amorphous silica originated from rice husks. Mater Transact. 2007;48(12):3095–3100. doi:10.2320/matertrans.MK200715
66. Chandrasekhar S, Pramada P, Praveen L. Effect of organic acid treatment on the properties of rice husk silica. J Mater Sci. 2005;40(24):6535–6544. doi:10.1007/s10853-005-1816-z
67. Oyawale, FA, Makinde OW, Ogundele KT. Effect of oxalic acid on rice husk. Int J Appl Sci Eng Res. 2012;1(5):663–668. doi:10.6088/ijaser.0020101067
68. Mahmud A, Megat-Yusoff P, Ahmad F, Farezzuan AA. Acid leaching as efficient chemical treatment for rice husk in production of amorphous silica nanoparticles. ARPN J Eng Appl Sci. 2016;11(22):13384.
69. Umeda J, Kondoh K. High-purification of amorphous silica originated from rice husks by combination of polysaccharide hydrolysis and metallic impurities removal. Ind Crops Prod. 2010;32(3):539–544. doi:10.1016/j.indcrop.2010.07.002
70. Yalcin N, Sevinc V. Studies on silica obtained from rice husk. Ceramics Int. 2001;27(2):219–224. doi:10.1016/S0272-8842(00)00068-7
71. Djangang C, Mlowe S, Njopwouo D, Revaprasadu N. One-step synthesis of silic a nanoparticles by thermolysis of rice husk ash using non toxic chemicals ethanol an d polyethylene glycol. J Appl Chem. 2015;4(4):1218–1226.
72. Thuadaij N, Nuntiya A. Synthesis and characterization of nanosilica from rice husk ash prepared by precipitation method. J Nat Sci Special Issue Nanotechnol. 2008;7(1):59–65.
73. Renuka N, Praveen A, Anas K. Influence of CTAB molar ratio in tuning the texture of rice husk silica into MCM 41 and SBA-16. Mater Lett. 2013;109:70–73. doi:10.1016/j.matlet.2013.07.074
74. Chiarakorn S, Areerob T, Grisdanurak N. Influence of functional silanes on hydrophobicity of MCM-41 synthesized from rice husk. Sci Technol Adv Mater. 2007;8(1–2):110. doi:10.1016/j.stam.2006.11.011
75. Shaikh IR, Shaikh AA. Utilization of wheat husk ash as silica source for the synthesis of MCM-41 type mesoporous silicates: a sustainable approach towards valorization of the agricultural waste stream. Res J Chem Sci. 2013;3(11):66–72.
76. Okoronkwo EA, Imoisili PE, Olubayode SA, Olusunle SO. Development of silica nanoparticle from corn cob ash. Adv Nanopart. 2016;5(02):135. doi:10.4236/anp.2016.52015
77. Mohanraj K, Kannan S, Barathan S, Sivakumar G. Preparation and characterization of nano SiO2 from corn cob ash by precipitation method. 2012.
78. Wang W, Martin JC, Fan X, Han A, Luo Z, Sun L. Silica nanoparticles and frameworks from rice husk biomass. ACS Appl Mater Interfaces. 2012;4(2):977–981. doi:10.1021/am201619u
79. Fernandes IJ, Calheiro D, Kieling AG, et al. Characterization of rice husk ash produced using different biomass combustion techniques for energy. Fuel. 2016;165:351–359. doi:10.1016/j.fuel.2015.10.086
80. Sharma R, Sharma S, Dutta S, Zboril R, Gawande MB. Silica-nanosphere-based organic–inorganic hybrid nanomaterials: synthesis, functionalization and applications in catalysis. Green Chem. 2015;17(6):3207–3230. doi:10.1039/C5GC00381D
81. Fujiwara M, Shiokawa K, Sakakura I, Nakahara Y. Preparation of hierarchical architectures of silica particles with hollow structure and nanoparticle shells: a material for the high reflectivity of UV and visible light. Langmuir. 2010;26(9):6561–6567. doi:10.1021/la9043396
82. Deng T-S, Marlow F. Synthesis of monodisperse polystyrene@ vinyl-SiO2 core–shell particles and hollow SiO2 spheres. Chem Mater. 2012;24(3):536–542. doi:10.1021/cm203099m
83. Huh S, Wiench JW, Yoo J-C, Pruski M, Lin VS-Y. Organic functionalization and morphology control of mesoporous silicas via a co-condensation synthesis method. Chem Mater. 2003;15(22):4247–4256. doi:10.1021/cm0210041
84. Saroj S, Rajput SJ. Tailor-made pH-sensitive polyacrylic acid functionalized mesoporous silica nanoparticles for efficient and controlled delivery of anti-cancer drug Etoposide. Drug Dev Ind Pharm. 2018;44(7):1198–1211. doi:10.1080/03639045.2018.1438467
85. Ojah N, Saikia D, Gogoi D, et al. Surface modification of core-shell silk/PVA nanofibers by oxygen dielectric barrier discharge plasma: studies of physico-chemical properties and drug release behavior. Appl Surf Sci. 2019;475:219–229. doi:10.1016/j.apsusc.2018.12.270
86. Ojah N, Deka J, Haloi S, et al. Chitosan coated silk fibroin surface modified by atmospheric dielectric-barrier discharge (DBD) plasma: a mechanically robust drug release system. J Biomater Sci Polym Ed. 2019;30(13):1142–1160. doi:10.1080/09205063.2019.1622844
87. Das P, Ojah N, Kandimalla R, et al. Surface modification of electrospun PVA/chitosan nanofibers by dielectric barrier discharge plasma at atmospheric pressure and studies of their mechanical properties and biocompatibility. Int J Biol Macromol. 2018;114:1026–1032. doi:10.1016/j.ijbiomac.2018.03.115
88. Ojah N, Borah R, Ahmed GA, Mandal M, Choudhury AJ. Surface modification of electrospun silk/AMOX/PVA nanofibers by dielectric barrier discharge plasma: physiochemical properties, drug delivery and in-vitro biocompatibility. Prog Biomater. 2020;9(4):219–237. doi:10.1007/s40204-020-00144-1
89. Hossen S, Hossain MK, Basher M, Mia M, Rahman M, Uddin MJ. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res. 2019;15:1–18. doi:10.1016/j.jare.2018.06.005
90. Ud Din F, Aman W, Ullah I, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomedicine. 2017;12(7291):7291–7309.
91. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):1–33. doi:10.1186/s12951-017-0328-8
92. Chowdhury A, Kunjiappan S, Panneerselvam T, Somasundaram B, Bhattacharjee C. Nanotechnology and nanocarrier-based approaches on treatment of degenerative diseases. Int Nano Lett. 2017;7(2):91–122.
93. Moghimi S, Simberg D. Nanoparticle transport pathways into tumors. J Nanopart Res. 2018;20(6):1–4. doi:10.1007/s11051-018-4273-8
94. Vu MN, Rajasekhar P, Poole DP, et al. Rapid assessment of nanoparticle extravasation in a microfluidic tumor model. ACS Appl Nano Mater. 2019;2(4):1844–1856. doi:10.1021/acsanm.8b02056
95. Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Sign Transduct Target Ther. 2018;3(1):1–19. doi:10.1038/s41392-017-0004-3
96. Yang J, Jia C, Yang J. Designing nanoparticle-based drug delivery systems for precision medicine. Int J Med Sci. 2021;18(13):2943–2949. doi:10.7150/ijms.60874
97. Khalid K, Tan X, Mohd Zaid HF, et al. Advanced in developmental organic and inorganic nanomaterial: a review. Bioengineered. 2020;11(1):328–355. doi:10.1080/21655979.2020.1736240
98. Akbarzadeh A, Khalilov R, Mostafavi E, et al. Role of dendrimers in advanced drug delivery and biomedical applications: a review. Exp Oncol. 2018;40(3):178–183. doi:10.31768/2312-8852.2018.40(3):178-183
99. Zare H, Ahmadi S, Ghasemi A, et al. Carbon nanotubes: smart drug/gene delivery carriers. Int J Nanomedicine. 2021;16:1681. doi:10.2147/IJN.S299448
100. Mostafavi E, Medina-Cruz D, Vernet-Crua A, et al. Green nanomedicine: the path to the next generation of nanomaterials for diagnosing brain tumors and therapeutics? Expert Opin Drug Deliv. 2021;1–22. doi:10.1080/17425247.2021.1865306
101. De Villiers MM, Aramwit P, Kwon GS. Nanotechnology in Drug Delivery. Springer; 2011.
102. Ashrafizadeh M, Saebfar H, Gholami MH, et al. Doxorubicin-loaded graphene oxide nanocomposites in cancer medicine: stimuli-responsive carriers, co-delivery and suppressing resistance. Expert Opin Drug Deliv. 2022. doi:10.1080/17425247.2022.2041598
103. Yao P, Zou A, Tian Z, et al. Construction and characterization of a temperature-responsive nanocarrier for imidacloprid based on mesoporous silica nanoparticles. Colloids Surf B Biointerfaces. 2021;198:111464. doi:10.1016/j.colsurfb.2020.111464
104. Liu L, Ma H, Yu J, Fan Y. Fabrication of glycerophosphate-based nanochitin hydrogels for prolonged release under in vitro physiological conditions. Cellulose. 2021;28(8):4887–4897. doi:10.1007/s10570-021-03819-5
105. Poudel BK, Soe ZC, Ruttala HB, et al. In situ fabrication of mesoporous silica-coated silver-gold hollow nanoshell for remotely controllable chemo-photothermal therapy via phase-change molecule as gatekeepers. Int J Pharm. 2018;548(1):92–103. doi:10.1016/j.ijpharm.2018.06.056
106. Karimi M, Mirshekari H, Aliakbari M, Sahandi-Zangabad P, Hamblin MR. Smart mesoporous silica nanoparticles for controlled-release drug delivery. Nanotechnol Rev. 2016;5(2):195–207. doi:10.1515/ntrev-2015-0057
107. Zhang L, Li Y, Jimmy CY. Chemical modification of inorganic nanostructures for targeted and controlled drug delivery in cancer treatment. J Mater Chem B. 2014;2(5):452–470. doi:10.1039/C3TB21196G
108. Li R, Peng F, Cai J, Yang D, Zhang P. Redox dual-stimuli responsive drug delivery systems for improving tumor-targeting ability and reducing adverse side effects. Asian J Pharm Sci. 2020;15(3):311–325. doi:10.1016/j.ajps.2019.06.003
109. Kundu M, Sadhukhan P, Ghosh N, et al. In vivo therapeutic evaluation of a novel bis-lawsone derivative against tumor following delivery using mesoporous silica nanoparticle based redox-responsive drug delivery system. Mater Sci Eng C. 2021;126:112142. doi:10.1016/j.msec.2021.112142
110. Cabral-Pacheco GA, Garza-Veloz I, Ramirez-Acuña JM, et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci. 2020;21(24):9739. doi:10.3390/ijms21249739
111. Vaghasiya K, Ray E, Sharma A, Katare OP, Verma RK. Matrix metalloproteinase-responsive mesoporous silica nanoparticles cloaked with cleavable protein for “self-actuating” on-demand controlled drug delivery for cancer therapy. ACS App Bio Mater. 2020;3(8):4987–4999. doi:10.1021/acsabm.0c00497
112. Du M, Chen Y, Tu J, et al. Ultrasound responsive magnetic mesoporous silica nanoparticle-loaded microbubbles for efficient gene delivery. ACS Biomater Sci Eng. 2020;6(5):2904–2912. doi:10.1021/acsbiomaterials.0c00014
113. Wang Y, Wang L, Guo L, et al. Photo-responsive magnetic mesoporous silica nanocomposites for magnetic targeted cancer therapy. N J Chem. 2019;43(12):4908–4918. doi:10.1039/C8NJ06105J
114. Asadi N, Annabi N, Mostafavi E, et al. Synthesis, characterization and in vitro evaluation of magnetic nanoparticles modified with PCL–PEG–PCL for controlled delivery of 5FU. Artif Cells Nanomed Biotechnol. 2018;46(sup1):938–945. doi:10.1080/21691401.2018.1439839
115. Cheng C-A, Chen W, Zhang L, Wu HH, Zink JI. A responsive mesoporous silica nanoparticle platform for magnetic resonance imaging-guided high-intensity focused ultrasound-stimulated cargo delivery with controllable location, time, and dose. J Am Chem Soc. 2019;141(44):17670–17684. doi:10.1021/jacs.9b07591
116. Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991. doi:10.1038/nmat3776
117. Paris JL, Villaverde G, Cabañas MV, Manzano M, Vallet-Regí M. From proof-of-concept material to PEGylated and modularly targeted ultrasound-responsive mesoporous silica nanoparticles. J Mater Chem B. 2018;6(18):2785–2794. doi:10.1039/C8TB00444G
118. Wood AK, Sehgal CM. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med Biol. 2015;41(4):905–928. doi:10.1016/j.ultrasmedbio.2014.11.019
119. Chen Y, Chen H, Zhang S, et al. Multifunctional mesoporous nanoellipsoids for biological bimodal imaging and magnetically targeted delivery of anticancer drugs. Adv Funct Mater. 2011;21(2):270–278. doi:10.1002/adfm.201001495
120. Shen S, Tang H, Zhang X, et al. Targeting mesoporous silica-encapsulated gold nanorods for chemo-photothermal therapy with near-infrared radiation. Biomaterials. 2013;34(12):3150–3158. doi:10.1016/j.biomaterials.2013.01.051
121. Terentyuk G, Panfilova E, Khanadeev V, et al. Gold nanorods with a hematoporphyrin-loaded silica shell for dual-modality photodynamic and photothermal treatment of tumors in vivo. Nano Res. 2014;7(3):325–337. doi:10.1007/s12274-013-0398-3
122. Wu J, Bremner DH, Niu S, Li D, Tang R, Zhu L-M. Multifunctional A7R peptide-modified hollow mesoporous silica@ Ag2S nanotheranostics for photoacoustic/near-infrared fluorescence imaging-guided tumor-targeted chemo-photothermal therapy. J Biomed Nanotechnol. 2019;15(7):1415–1431. doi:10.1166/jbn.2019.2729
123. Ong C, Cha BG, Kim J. Mesoporous silica nanoparticles doped with gold nanoparticles for combined cancer immunotherapy and photothermal therapy. ACS App Bio Mater. 2019;2(8):3630–3638. doi:10.1021/acsabm.9b00483
124. Liu X, Wu X, Xing Y, et al. Reduced graphene oxide/mesoporous silica nanocarriers for pH-triggered drug release and photothermal therapy. ACS App Bio Mater. 2020;3(5):2577–2587. doi:10.1021/acsabm.9b01108
125. Song G, Wang Q, Wang Y, et al. A low‐toxic multifunctional nanoplatform based on Cu9S5@ mSiO2 core‐shell nanocomposites: combining photothermal‐and chemotherapies with infrared thermal imaging for cancer treatment. Adv Funct Mater. 2013;23(35):4281–4292. doi:10.1002/adfm.201203317
126. Liu J, Wang C, Wang X, et al. Mesoporous silica coated single‐walled carbon nanotubes as a multifunctional light‐responsive platform for cancer combination therapy. Adv Funct Mater. 2015;25(3):384–392. doi:10.1002/adfm.201403079
127. Chen S, Xing C, Huang D, et al. Eradication of tumor growth by delivering novel photothermal selenium-coated tellurium nanoheterojunctions. Sci Adv. 2020;6(15):eaay6825. doi:10.1126/sciadv.aay6825
128. Tsai C-P, Chen C-Y, Hung Y, Chang F-H, Mou C-Y. Monoclonal antibody-functionalized mesoporous silica nanoparticles (MSN) for selective targeting breast cancer cells. J Mater Chem. 2009;19(32):5737–5743. doi:10.1039/b905158a
129. Zahavi D, Weiner L. Monoclonal antibodies in cancer therapy. Antibodies. 2020;9(3):34. doi:10.3390/antib9030034
130. Irfan M, Khan RU, Qu F. Aptamers for personalized therapeutics. In: Aptamers for Medical Applications: From Diagnosis to Therapeutics. Springer; 2021:179.
131. Phan QA, Truong LB, Medina-Cruz D, Dincer C, Mostafavi E. CRISPR/Cas-powered nanobiosensors for diagnostics. Biosens Bioelectron. 2021;197:113732. doi:10.1016/j.bios.2021.113732
132. Xie X, Li F, Zhang H, et al. EpCAM aptamer-functionalized mesoporous silica nanoparticles for efficient colon cancer cell-targeted drug delivery. Eur J Pharm Sci. 2016;83:28–35. doi:10.1016/j.ejps.2015.12.014
133. Ghosh D, Peng X, Leal J, Mohanty RP. Peptides as drug delivery vehicles across biological barriers. J Pharma Investig. 2018;48(1):89–111. doi:10.1007/s40005-017-0374-0
134. Ruoslahti E. Tumor penetrating peptides for improved drug delivery. Adv Drug Deliv Rev. 2017;110:3–12. doi:10.1016/j.addr.2016.03.008
135. Dong W, Wen J, Li Y, Wang C, Sun S, Shang D. Targeted antimicrobial peptide delivery in vivo to tumor with near infrared photoactivated mesoporous silica nanoparticles. Int J Pharm. 2020;588:119767. doi:10.1016/j.ijpharm.2020.119767
136. Ding Z, Wang D, Shi W, et al. In vivo targeting of liver cancer with tissue-and nuclei-specific mesoporous silica nanoparticle-based nanocarriers in mice. Int J Nanomedicine. 2020;15:8383. doi:10.2147/IJN.S272495
137. Zhang C, Pu K. Molecular and nanoengineering approaches towards activatable cancer immunotherapy. Chem Soc Rev. 2020;49(13):4234–4253. doi:10.1039/C9CS00773C
138. Pan L, He Q, Liu J, et al. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J Am Chem Soc. 2012;134(13):5722–5725. doi:10.1021/ja211035w
139. An N, Lin H, Yang C, et al. Gated magnetic mesoporous silica nanoparticles for intracellular enzyme-triggered drug delivery. Mater Sci Eng C. 2016;69:292–300. doi:10.1016/j.msec.2016.06.086
140. Shen Y, Li M, Liu T, et al. A dual-functional HER2 aptamer-conjugated, pH-activated mesoporous silica nanocarrier-based drug delivery system provides in vitro synergistic cytotoxicity in HER2-positive breast cancer cells. Int J Nanomedicine. 2019;14:4029-4044. doi:10.2147/IJN.S201688
141. Paris JL, Manzano M, Cabañas MV, Vallet-Regí M. Mesoporous silica nanoparticles engineered for ultrasound-induced uptake by cancer cells. Nanoscale. 2018;10(14):6402–6408. doi:10.1039/C8NR00693H
142. Zhang T, Liu H, Li L, et al. Leukocyte/platelet hybrid membrane-camouflaged dendritic large pore mesoporous silica nanoparticles co-loaded with photo/chemotherapeutic agents for triple negative breast cancer combination treatment. Bioactive Mater. 2021;6(11):3865–3878. doi:10.1016/j.bioactmat.2021.04.004
143. AbouAitah K, Hassan HA, Swiderska-Sroda A, et al. Targeted nano-drug delivery of colchicine against colon cancer cells by means of mesoporous silica nanoparticles. Cancers. 2020;12(1):144. doi:10.3390/cancers12010144
144. Tran VA, Van Giau Vo KS, Lee S-W, An SSA, An SSA. Multimodal mesoporous silica nanocarriers for dual stimuli-responsive drug release and excellent photothermal ablation of cancer cells. Int J Nanomedicine. 2020;15:7667-7685. doi:10.2147/IJN.S254344
145. Terzioğlu P, Yucel S. Synthesis of magnesium silicate from wheat husk ash: effects of parameters on structural and surface properties. BioResources. 2012;7(4):5435–5447. doi:10.15376/biores.7.4.5435-5447
146. Mhilu CF. Analysis of energy characteristics of rice and coffee husks blends. ISRN Chem Eng. 2014;2014:1–6. doi:10.1155/2014/196103
147. Pa FC, Chik A, Bari MF. Palm ash as an alternative source for silica production. EDP Sci. 2016;2016:01062.
148. Cordeiro GC, Toledo Filho RD, Fairbairn EM, Tavares L, Oliveira CH. Influence of mechanical grinding on the pozzolanic activity of residual sugarcane bagasse ash. Adv Appl Ceramics. 2004;731–740. doi:10.1179/1743676111Y.0000000050
149. Liu N, Huo K, McDowell MT, Zhao J, Cui Y. Rice husks as a sustainable source of nanostructured silicon for high performance Li-ion battery anodes. Sci Rep. 2013;3:1919. doi:10.1038/srep01919
150. Zhang Z, He W, Zheng J, Wang G, Ji J. Rice husk ash-derived silica nanofluids: synthesis and stability study. Nanoscale Res Lett. 2016;11(1):502. doi:10.1186/s11671-016-1726-9
151. Siriworarat K, Deerattrakul V, Dittanet P, Kongkachuichay P. Production of methanol from carbon dioxide using palladium-copper-zinc loaded on MCM-41: comparison of catalysts synthesized from flame spray pyrolysis and sol-gel method using silica source from rice husk ash. J Clean Prod. 2017;142:1234–1243. doi:10.1016/j.jclepro.2016.07.099
152. Seliem MK, Komarneni S, Khadra MRA. Phosphate removal from solution by composite of MCM-41 silica with rice husk: kinetic and equilibrium studies. Micropor Mesopor Mater. 2016;224:51–57. doi:10.1016/j.micromeso.2015.11.011
153. Shaikh IR, Shaikh AA. Utilization of wheat husk ash as silica source for the synthesis of MCM-41 type mesoporous silicates: a sustainable approach towards valorization of the agricultural waste stream. Res J Chem Sci ISSN. 2013;2231:606X.
154. Utama PS, Yamsaensung R, Sangwichien C. Silica gel derived from palm oil mill fly ash. Songklanakarin J Sci Technol. 2018;40(1). doi:10.14456/sjst-psu.2018.27
155. Ma’mani L, Nikzad S, Kheiri-Manjili H, et al. Curcumin-loaded guanidine functionalized PEGylated I3ad mesoporous silica nanoparticles KIT-6: practical strategy for the breast cancer therapy. Eur J Med Chem. 2014;83:646–654. doi:10.1016/j.ejmech.2014.06.069
156. Liu R, Zhang H, Zhang F, Wang X, Liu X, Zhang Y. Polydopamine doped reduced graphene oxide/mesoporous silica nanosheets for chemo-photothermal and enhanced photothermal therapy. Mater Sci Eng C. 2019;96:138–145. doi:10.1016/j.msec.2018.10.093
157. Hanafi-Bojd MY, Jaafari MR, Ramezanian N, et al. Surface functionalized mesoporous silica nanoparticles as an effective carrier for epirubicin delivery to cancer cells. Eur J Pharm Biopharm. 2015;89:248–258. doi:10.1016/j.ejpb.2014.12.009
158. Mladenović M, Morgan I, Ilić N, et al. Ph-responsive release of ruthenium metallotherapeutics from mesoporous silica-based nanocarriers. Pharmaceutics. 2021;13(4):460. doi:10.3390/pharmaceutics13040460
159. Pourjavadi A, Tehrani ZM. Mesoporous silica nanoparticles with bilayer coating of poly (acrylic acid-co-itaconic acid) and human serum albumin (HSA): a pH-sensitive carrier for gemcitabine delivery. Mater Sci Eng C. 2016;61:782–790. doi:10.1016/j.msec.2015.12.096
160. Lohiya G, Katti DS. Carboxylated chitosan-mediated improved efficacy of mesoporous silica nanoparticle-based targeted drug delivery system for breast cancer therapy. Carbohydr Polym. 2022;277:118822. doi:10.1016/j.carbpol.2021.118822
161. Shakeran Z, Keyhanfar M, Varshosaz J, Sutherland DS. Biodegradable nanocarriers based on chitosan-modified mesoporous silica nanoparticles for delivery of methotrexate for application in breast cancer treatment. Mater Sci Eng C. 2021;118:111526. doi:10.1016/j.msec.2020.111526
162. Pourjavadi A, Tehrani ZM, Jokar S. Chitosan based supramolecular polypseudorotaxane as a pH-responsive polymer and their hybridization with mesoporous silica-coated magnetic graphene oxide for triggered anticancer drug delivery. Polymer. 2015;76:52–61. doi:10.1016/j.polymer.2015.08.050
163. Mozafarinia M, Karimi S, Farrokhnia M, Esfandiari J. In vitro breast cancer targeting using Trastuzumab-conjugated mesoporous silica nanoparticles: towards the new strategy for decreasing size and high drug loading capacity for drug delivery purposes in MSN synthesis. Micropor Mesopor Mater. 2021;316:110950. doi:10.1016/j.micromeso.2021.110950
164. Mohseni M, Gilani K, Mortazavi SA. Preparation and characterization of rifampin loaded mesoporous silica nanoparticles as a potential system for pulmonary drug delivery. IJPR. 2015;14(1):27.
165. de Lima HH, Kupfer VL, Moisés MP, et al. Bionanocomposites based on mesoporous silica and alginate for enhanced drug delivery. Carbohydr Polym. 2018;196:126–134. doi:10.1016/j.carbpol.2018.04.107
166. Zhang Q, Guo J, Zhang X, Zhao Y, Cao L, Sun L. Redox-and enzyme-responsive fluorescent porous silica nanocarriers for drug delivery. Sens Actuators B Chem. 2018;276:370–377. doi:10.1016/j.snb.2018.08.118
167. Xu X, Lü S, Gao C, et al. Self-fluorescent and stimuli-responsive mesoporous silica nanoparticles using a double-role curcumin gatekeeper for drug delivery. Chem Eng J. 2016;300:185–192. doi:10.1016/j.cej.2016.04.087
168. Adhikari C, Mishra A, Nayak D, Chakraborty A. Drug delivery system composed of mesoporous silica and hollow mesoporous silica nanospheres for chemotherapeutic drug delivery. J Drug Deliv Sci Technol. 2018;45:303–314. doi:10.1016/j.jddst.2018.03.020
169. Zeleňák V, Halamová D, Almáši M, Žid L, Zeleňáková A, Kapusta O. Ordered cubic nanoporous silica support MCM-48 for delivery of poorly soluble drug indomethacin. Appl Surf Sci. 2018;443:525–534. doi:10.1016/j.apsusc.2018.02.260
170. Jadhav SA, Brunella V, Scalarone D, Berlier G. Poly (NIPAM-co-MPS)-grafted multimodal porous silica nanoparticles as reverse thermoresponsive drug delivery system. Asian J Pharm Sci. 2017;12(3):279–284. doi:10.1016/j.ajps.2017.02.002
171. Qiu L, Zhao Y, Li B, Wang Z, Cao L, Sun L. Triple-stimuli (protease/redox/pH) sensitive porous silica nanocarriers for drug delivery. Sens Actuators B Chem. 2017;240:1066–1074. doi:10.1016/j.snb.2016.09.083
172. Wang Y, Han N, Zhao Q, et al. Redox-responsive mesoporous silica as carriers for controlled drug delivery: a comparative study based on silica and PEG gatekeepers. Eur J Pharm Sci. 2015;72:12–20. doi:10.1016/j.ejps.2015.02.008
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.