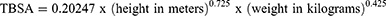Back to Journals » ClinicoEconomics and Outcomes Research » Volume 15
Holistic View of Autografting Patients by Percentage of Total Body Surface Area Burned: Medical Record Abstraction Integrated with Administrative Claims
Authors Hahn H, Yu TC, Teng CC, Tan H
Received 13 December 2022
Accepted for publication 23 February 2023
Published 8 April 2023 Volume 2023:15 Pages 251—267
DOI https://doi.org/10.2147/CEOR.S401003
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Helen Hahn,1 Tzy-Chyi Yu,1 Chia-Chen Teng,2 Hiangkiat Tan2
1Mallinckrodt Pharmaceuticals, Hampton, NJ, USA; 2Healthcore, Inc., Wilmington, DE, USA
Correspondence: Tzy-Chyi Yu, Mallinckrodt Pharmaceuticals, Shelbourne Building, 53 Frontage Road, Suite 300, Hampton, NJ, 08827, USA, Tel +1 908 238 6884, Email [email protected]
Aim: This retrospective observational study provides a holistic view of the clinical and economic characteristics of inpatient treatment of patients with thermal burns undergoing autografting, by integrating real-world data (RWD) from medical records from healthcare providers (HCPs) and administrative claims.
Methods: We identified eligible patients between July 1, 2010, and November 30, 2019, from the HealthCore Integrated Research Database® (HIRD®) and obtained their medical records from HCPs. We abstracted data from medical records to describe patient demographics and clinical characteristics and obtained costs of treatment from claims.
Results: Two hundred patients were stratified into cohorts based on the percentage of total body surface area (%TBSA) burned: minor (< 10%), moderate (10%– 24%), and major (≥ 25%). Data obtained from medical records and administrative claims were comparable to previous findings from administrative claims data. This privately insured study cohort predominantly consisted of White men. Diabetes mellitus and hypertension were frequently reported in a relatively young population. Key clinical characteristics that could influence burn treatment decisions and long-term outcomes, such as body mass index, size of autograft donor site, and mesh ratio, were frequently underdocumented in patients’ medical records.
Conclusion: Evidence generated from 2 orthogonal RWD sources confirmed that patients with larger %TBSA burned required more intensive care, thereby incurring higher costs. This study highlights considerable incompleteness in many critical fields in medical records, which limits the ability to generate broader insights. More comprehensive documentation of clinical characteristics and outcomes of autografts and donor sites in the operative and medical notes is critical to appropriately evaluate their impact on outcomes of burn treatments in future research using RWD.
Keywords: burn injury, healthcare resource utilization, treatment patterns, real-world data
Introduction
Burn injuries are a serious global public health concern. The Healthcare Cost and Utilization Project reported about 16.8 burn-related hospital stays per 100,000 population in the United States (US) alone in 2013.1 Most burn-related injuries occur at home and in the workplace.2 Fire and flames and scalding are the most common causes of thermal burns.3
Burn injuries may be classified as superficial, superficial partial-thickness, deep partial-thickness (DPT), full-thickness (FT), or fourth-degree burns, based on burn depth, which influences treatment during the acute injury phase, as well as the patient’s long-term functional and cosmetic outcomes.4,5 In the acute-injury phase (0 to 48 hours), the clinical severity of a burn can range from a superficial (first-degree) burn with red, dry, painful skin, to a FT (third-degree) burn with waxy-white to leathery dry skin and no pain sensation in the burned area.5
Patients with DPT and FT burns often require hospitalization for surgical excision and skin-grafting. Skin-grafting, such as autografts, allografts, xenografts, and other skin substitutes, is the most common procedure used in burn-related inpatient treatment.1 A recent retrospective analysis using administrative claims data from 2 large US private insurance health plan databases—HealthCore Integrated Research Database® (HIRD®) and IBM® MarketScan®—estimated that about a quarter of the patients admitted to hospitals with thermal burns underwent autografting, the gold standard in burn treatment,6 during their hospital stay.7 Burn treatments often require specialized care, surgery, prolonged hospitalization, and rehabilitation, which result in a substantial economic burden. The retrospective analysis revealed that the inpatient treatment of burns with autografting had an average daily total medical cost per patient of approximately $9000.7 The average total hospitalization cost of burn treatment with autografting was around $157,000, which accounted for 85% of the mean total all-cause cost of care in the first year of burn treatment. Healthcare costs and length of hospital stay increased with the increase in percentage of total body surface area (%TBSA) burned.
Although data from administrative claims provide valuable informative trends of costs and healthcare resource utilization (HCRU) over time, such data lack detailed clinical information (eg, body mass index [BMI], size of autograft donor site) about patients. Such information could provide a more robust picture of inpatient burn treatment than administrative data alone. The objective of this study was to provide a holistic view of the clinical and economic characteristics of the inpatient care of patients with thermal burns undergoing autografting, by integrating data from 2 sources—inpatient medical records and administrative claims data.
Materials and Methods
Data Source and Study Design
In this retrospective observational study, we linked longitudinal pharmacy and medical claims data with medical records. The HIRD consists of de-identified patient-level longitudinal pharmacy and medical-claims data associated with large US commercial health plans (including Medicare Advantage) for more than 50 million individuals at the time of the study.7,8 Patients with thermal burns undergoing inpatient autografting between July 1, 2010, and November 30, 2019 were identified in the HIRD (Figure 1). The list of codes used to identify procedures, medications, and burn-related clinical characteristics can be found in Yu et al.7 The first observed hospitalization for thermal burns with autografting was regarded as the index event, and the admission date was designated as the index date. The preindex (baseline) period was defined as the 6-month period prior to and not inclusive of the index date. The follow-up period was the 6-month period after the index date.
 |
Figure 1 Timeline describing the study period. |
A list of patients and their healthcare providers (HCPs) at inpatient facilities on the index date was generated from the HIRD for eligible patients who met all the inclusion and exclusion criteria outlined in Figure 2. The study protocol, medical record abstraction methodology, and a standardized clinical data abstraction form were reviewed and approved by a central institutional review board (WCG Institutional Review Board (IRB), study number 1292435, granted on September 11, 2020). The acquisition and handling of study data complied with applicable state and federal privacy regulations, including the Health Insurance Portability and Accountability Act (HIPAA). Requests for patients’ full medical records, along with the institutional review board approval letter, were sent to multiple facilities. We aimed to abstract data on patient demographics, burn sites, %TBSA, autograft donor and recipient sites and sizes, treatment procedures, and medications, from a total of 200 medical records provided by HCPs.
We categorized patients according to %TBSA burned as minor (< 10%), moderate (10%–24%), and major (≥ 25%) %TBSA cohorts based on expert opinion. In the real world, there is a higher prevalence of patients in the minor (< 10%) %TBSA cohort.7,9 To gain insight into treatment patterns among patients with severe burns, we therefore prioritized and oversampled major/moderate %TBSA groups by obtaining as many records as possible. When patients with major/moderate %TBSA were exhausted, we used the medical records for patients with low %TBSA to achieve our target of 200 records.
Prior to data abstraction, the patients in our study cohorts were screened to confirm that they had a record of autografting procedures in their medical charts. For those patients whose %TBSA burned were not documented in their medical records, this was estimated by dividing area covered by autograft by TBSA. The TBSA was estimated using the formula:10
If data on the heights and weights of patients were unavailable, the TBSA was estimated based on age and sex.10 Data on the area covered by the autograft were missing for 3 patients, who were then included in the minor (< 10%) %TBSA cohort. Data abstraction was carried out between 30 days before and 60 days after the index date to account for the possibility of a staged autografting approach (ie, if the length of stay of index hospitalization was longer than 60 days, data were included until the discharge date).
Statistical Analyses
Patient demographics, comorbidities, and medication use during the baseline period and index burn-related clinical characteristics were summarized with descriptive statistics, such as frequency, mean, standard deviation (SD), and median. Univariate analyses were performed with regard to patient baseline characteristics, including demographics, clinical characteristics, and comorbidities, by %TBSA cohort. All baseline and index hospitalization measures were also described with univariate statistics by %TBSA cohort. In subgroup analyses by %TBSA cohorts, Chi-squared tests were used for categorical variables, Fisher exact tests were used for binary variables, and t-tests or Wilcoxon rank-sum tests were used for continuous variables to explore trends in the data. A Bonferroni-corrected alpha of 0.017 was used to account for post hoc multiple comparisons.
Data on costs were adjusted to 2019 US dollars, given the most recent medical care price index information provided by the Bureau of Labor Statistics at the time of this study.11 Healthcare costs by %TBSA cohort were analyzed as continuous variables with mean, SD, and median. All analyses were conducted with Statistical Analysis System, version 9.3 (SAS Institute Inc., Cary, NC, USA). To protect the confidentiality of health-plan members and minimize the risk of re-identification, all data values for which the number of patients was in the range of 1 to 10 were reported as ≤ 10.
Results
Study Cohort
Using administrative claims data, 8049 patients hospitalized for thermal burns during the patient identification period (July 1, 2010, through November 30, 2019) were identified. Figure 2 shows the attrition of the 8049 patients to the prespecified target of 200 patients, whose hospital clinical records and associated administrative data were included in this study. Regarding %TBSA burned, we observed some differences between the data from administrative claims and medical records (Supplemental Table S1). %TBSA as documented in the medical records was more inclusive of different burn-depth categories. Therefore, patients were stratified based on %TBSA burned, as determined from their medical records, as follows:
90 patients with minor (< 10%) %TBSA
75 patients with moderate (10%–24%) %TBSA
35 patients with major (≥ 25%) %TBSA (Figure 2).
Of 200 medical records, 15 did not have %TBSA burned documented. Those missing %TBSA burned were estimated using the method described earlier in the Data Source and Study Design section.
Baseline Demographic and Clinical Characteristics
Nearly two-thirds of patients across the 3 %TBSA cohorts were male (Table 1). Of those in the moderate and major %TBSA cohorts, 65% and 74%, respectively, were White patients. The major %TBSA cohort (median age: 36 years) appeared to be younger than the minor (median age: 46 years) and moderate (median age: 46 years) %TBSA cohorts. Of the entire study cohort, 31% of the patients did not have their BMI reported in their medical records (Figure 3). Administrative claims data indicated that nearly two-thirds of patients across all cohorts had preferred provider organization (PPO) health insurance coverage.
 |
Table 1 Baseline Characteristics of %TBSA Cohorts Obtained From Medical Records and Administrative Claims |
 |
Figure 3 Extent of missing critical information in patient medical records. 1Reported at autografting operation level. |
The general comorbidity burden was low based on the Quan-Charlson Comorbidity Index across all cohorts.12 Chronic obstructive pulmonary disease (COPD) and anxiety were common comorbidities in the minor %TBSA cohort, reported in 18% and 17% of patients, respectively. Hypertension (> 23%) and diabetes mellitus (> 11%) were frequently reported comorbidities in the minor and moderate %TBSA cohorts. Opioid analgesics, antibiotics, and antidepressants were commonly used medications in the minor and moderate %TBSA cohorts. Thirty-one percent of patients in the minor and 37% of patients in the moderate %TBSA cohorts, respectively, were documented as current smokers. Smoking status at index was not documented in the medical records for 14% of the minor, 31% of the moderate, and 40% of the major %TBSA cohorts, respectively.
Burn-Related Clinical Characteristics
Medical records revealed that fire and flames were the most common cause of burn injuries in the moderate (75%) and major %TBSA (74%) cohorts, whereas the category of hot substance or object, caustic material, and steam (48%) was the most common cause of burn injuries in the minor %TBSA cohort (Table 2). Primary locations of burn sites, as noted in the medical records, were sensitive burn sites in the minor (58%), upper limb in the moderate (84%), and trunk in the major %TBSA cohorts. Fractures were also reported in 13% of patients in the minor (97%) %TBSA cohort. Fewer than 10 patients in each cohort were hospitalized in the 30 days leading up to the index date.
 |
Table 2 Index Burn-Related Clinical Characteristics Obtained From Medical Records by %TBSA Cohorts |
Index Hospitalization Characteristics and Healthcare Resource Utilization
Data from medical records indicated that larger %TBSA coincided with longer length of hospital stay (including intensive care), more autograft operations, and longer time period between index date and date of first autograft procedure (Table 3). Patients in the major %TBSA cohort spent a mean (SD) of 46.5 (25.16) days in the hospital and had 2.8 (1.92) autografting procedures; whereas patients in the minor and moderate %TBSA cohorts spent 10.6 (11.28) and 16.4 (9.35) days in the hospital, respectively, and most patients (100% in minor and 91% in moderate %TBSA cohorts) only had 1 autografting procedure.
 |
Table 3 Index Hospitalization and Treatment Characteristics Obtained From Medical Records by %TBSA Cohorts |
The time period between admission and first autograft procedure increased with increase in %TBSA: mean (SD) for the cohorts was 5.7 (8.03) days in the minor, 7.2 (4.87) days in the moderate, and 13.3 (10.8) days in the major %TBSA cohorts. Of the patients in the major %TBSA cohort, 77% spent a mean (SD) of 30.4 (20.75) days in the intensive care unit (ICU), and 51% required a ventilator. Fewer patients in the other 2 cohorts required intensive care and spent less time in the ICU. Across all cohorts, most patients received opioid and non-narcotic analgesics.
Nearly all patients underwent debridement during their inpatient stay (Table 3). In the major %TBSA cohort, 54% of autograft procedures were performed alongside additional skin graft procedures (eg, allograft and cultured epithelial autograft) during the surgery. Among the entire study cohort, autograft details, such as size of donor sites and mesh ratio, were frequently not documented in the patient charts, with 80% and 24% missing, respectively (Figure 3). Time to wound healing was also not documented in 43% of patient charts.
Patients with larger %TBSA also received more physical and occupational therapy. Medical records indicated that 69% of patients with minor %TBSA, 77% of patients with moderate %TBSA, and 83% of patients with major %TBSA burned received physical or occupational therapy during their index hospital stay. In the 6-month follow-up period, administrative claims data indicated that 80% of patients in the major %TBSA cohort received physical therapy, and 74% received occupational therapy in outpatient visits. In comparison, 54% and 59% of patients in the minor and moderate %TBSA cohorts, respectively, received outpatient physical therapy. Of the patients in the minor and moderate %TBSA cohorts, 37% and 47%, respectively, received outpatient occupational therapy (Supplemental Table S2).
All-Cause and Burn-Related Healthcare Costs from Claims Data
The mean total medical costs increased with increase in %TBSA burned (Figure 4). Administrative claims data revealed mean (SD) total costs of index hospitalization were $75,025 ($78,900) in the minor %TBSA cohort, $173,532 ($161,563) in the moderate %TBSA cohort, and $561,433 ($605,183) in the major %TBSA cohort, with burn-related inpatient treatment and pharmacy costs accounting for over 99% of these amounts in each cohort (Table 4).
 |
Table 4 Costs of Burn Treatment by %TBSA Cohort |
Discussion
This study confirms the previous finding by Yu et al7 that patients with larger %TBSA burned require more intensive care, thereby incurring higher costs. In the present analysis, the number of autograft procedures, length of hospital stay, number of physical and occupational therapy sessions, and overall treatment costs of burn injuries positively correlated with %TBSA burned. Further, among various %TBSA cohorts, the mean cost of treatment and mean length of hospital stay were comparable to those reported earlier.7
In the previous analysis of HCRU,7 Yu et al had only used data from administrative claims, and we expanded upon those findings in this study. Administrative claims data alone lacked detailed critical clinical information (eg, BMI, size of autograft donor site) on patients as they are primarily generated for billing and reimbursement purposes and may be subject to International Classification of Diseases-Ninth or Tenth Revision-Clinical Modification (ICD-9/10-CM) code availability and coding practices. This may result in inconsistencies and/or undercoded/missing data on several patient characteristics, such as patient BMI, which may often not directly relate to reimbursement, but are still important for providers to consider when making burn-treatment decisions. Therefore, medical records are important real-world data (RWD) sources to complement administrative claims data for observational studies because they often provide routine, HCP-collected clinical data, important patient characteristics, and detailed treatment patterns that are not always available and/or reliable from claims.13 As the objective of this study was to corroborate and bolster real-world evidence (RWE) to assess patient outcomes and inform treatment guidelines in burn care, it was essential to integrate data from medical records from HCPs and administrative claims to obtain comprehensive and complete documentation on patient characteristics, autografting and burn-related treatment patterns, accompanied by corresponding costs of treatment.
There were some differences in the %TBSA burned data between administrative claims and medical records. As mentioned earlier, claims are filed using codes, which, owing to code availability and coding practices, might represent a simplification of the complexities seen in clinical practice. The differences we observed in this study could, in part, be explained by the fact that the required diagnosis codes that accompany autografting for billing purposes used in administrative claims were third-degree burns or higher, whereas the %TBSA burned that was documented in medical records was more detailed and inclusive of varying burn-depth categories. In this study, patients were stratified into cohorts for analysis based on the %TBSA burned recorded in their medical charts by HCPs.
The complementary information we hoped to obtain from patient medical records was frequently not well documented. We found that even %TBSA burned, a critical piece of clinical information for burn treatment, was not documented in 15 out of the 200 medical records in our study. In the 200 records abstracted, incomplete documentation of key patient characteristics, such as BMI (31% undocumented), and autograft procedure details, such as size of autograft donor site (80% undocumented) and mesh ratio (24% undocumented), limited our ability to generate broader insights. BMI is a measure of obesity, which may present significant challenges to burn treatment management and wound healing.14 Metabolically and endocrinologically active adipose tissue, which is abundant in obese patients, releases proinflammatory mediators following severe burns.14 This release may lead to adverse events and obesity-related complications, thereby influencing the outcomes of acute and continuing burn care. Studies have shown that patients who have burn injuries and severe obesity have a longer overall length of hospital stay, as well as a higher mortality rate compared with normal-weight burn patients. Obesity is usually undercoded in administrative claims data, which emphasizes the need to obtain accurate documentation of BMI in patient medical records.15 Additionally, autograft harvesting creates a donor-site wound that is susceptible to fluid loss, infection, permanent scarring, and other morbidities.16–19 A higher skin graft mesh ratio allows a skin graft to stretch, and although this increases the area covered by the graft and improves adherence to convoluted wounds, it also increases healing times for the wound and causes a scar with a checkerboard appearance.20,21 The size of the burn wound excised/autografted is typically documented and used for reimbursement; however, it may not reflect the area of skin harvested (size of autograft donor site) to provide those autografts. The autograft donor area can vary greatly based on the mesh ratios chosen to stretch the skin to fit the excised area. Despite the importance of clinical information such as BMI, size of autograft donor site, and mesh ratio on inpatient burn care decision-making, a substantial number of medical records we obtained for this study had no documentation of such data. The burn community could establish a standard way to record the calculated area or %TBSA that was autografted and the autograft donor sites, as well as the mesh ratios for each area autografted. This approach might facilitate the documentation by building a module with those required data fields in electronic medical records systems. Additionally, notes on the healing progress (eg, percentage of wound re-epithelialized or still open) at discharge should be standardized. It is critical for providers to accurately record these key parameters in the patients’ medical records to provide a more robust picture of burn care.
Ideally, RWD from multiple sources should provide a more comprehensive view to generate RWE and, hence, better inform treatment decisions, as well as support the development of treatment guidelines and regulatory filings.22 Our study highlights that, in reality, the lack of consistent documentation on essential clinical characteristics of patients with burn injuries in their medical records limits our ability to better understand the relationship between burn treatment and patient outcomes, even after we integrated 2 important RWD sources of medical records and administrative claims. Similarly, a recent study examining the role of RWE in oncology product approvals by the US Food and Drug Administration found that incomplete data on important prognostic factors have rendered real-world studies and controlled clinical trials incomparable for certain cancer therapies.23 These findings emphasize the importance of comprehensive documentation of clinical characteristics and outcomes in the operative and medical notes for patients, which are critical to appropriately evaluate the impact of the treatment on outcomes in future research using RWD.
Our study, linking HCP-provided medical records on patients with administrative claims from the HIRD, had several limitations. Abstracted medical records data are only available for a subset of the patients identified in the administrative claims analysis; therefore, these data may not uniformly represent entire populations identified in the claims analysis. All patients included in the study were enrolled in commercial or Medicare Advantage health insurance plans in the US and satisfied all inclusion/exclusion criteria. The results may not be generalizable to patients with other types of health insurance (eg, Medicaid), those who are uninsured, or to those outside the US.
Abstracted medical records were limited by what the HCPs documented in the patients’ medical charts and to what was supplied by the facilities even though we requested full medical records (eg, the HCP may have a “working copy” of a record that has pertinent information but not complete clinical data). Despite our best efforts to oversample patients with severe burn injuries, the final sample size in the major %TBSA group was small, somewhat limiting the statistical inference. The length of the follow-up period (6 months postindex date) required to estimate HCRU and treatment costs could potentially have excluded patients with severe complications who died during or right after initial autografting hospitalization, thus introducing survival bias into costs and HCRU examination.
Conclusion
This study, leveraging RWD from 2 independent sources, confirmed that length of hospital stay and overall cost of care positively correlated with %TBSA burned in patients who underwent inpatient burn care and autografting. In this privately insured burn patient cohort undergoing inpatient autografting, we found a predominance of White men, and prevalence of diabetes mellitus and hypertension in a relatively young population. There was considerable incompleteness in many critical fields in the medical records, which limited the ability to generate broader insights. In future studies investigating outcomes in patients with burns, researchers should be aware of these limitations, and consider combining data from multiple real-world sources. More detailed documentation of the clinical characteristics and outcomes (eg, size of autograft donor site, mesh ratio, failure to heal, healing times, etc.) of autografts and donor sites in the operative and medical notes are critical to facilitate the correct evaluation of their impact on the outcomes of burn treatments in future research using RWD.
Abbreviations
%TBSA, percentage of total body surface area; BMI, body mass index; COPD; chronic obstructive pulmonary disease; DPT, deep partial-thickness; FT, full-thickness; HCP, healthcare provider; HCRU, healthcare resource utilization; HIPAA, Health Insurance Portability and Accountability Act; HIRD®, HealthCore Integrated Research Database®; ICD-9/10-CM, International Classification of Diseases-Ninth or Tenth Revision-Clinical Modification; ICU, intensive care unit; IRB, institutional review board; PPO, provider preferred organization; RWD, real-world data; RWE, real-world evidence; SD, standard deviation; TBSA, total body surface area; US, United States.
Data Sharing Statement
Restrictions apply to the availability of the HIRD, and the data used in analysis are not available to the public.
Acknowledgments
Medical writing services were provided by Oishika Panda, PhD, of Oxford PharmaGenesis Inc., Newtown, PA, USA, and were funded by Mallinckrodt Pharmaceuticals. Portions of this manuscript have previously been presented at the following conferences: American Burn Association annual meeting; April 5–8, 2022, Las Vegas, NV; Southern Region Burn Conference; November 11–13, 2022, Baton Rouge, LA.
Author Contributions
All authors (HH, T-CY, C-CT, and HT) made a significant contribution to the conception, study design, and analysis and interpretation of data. All authors contributed to the drafting, revision, and critical review of the article. The authors have agreed on the submission of the article to ClinicoEconomics and Outcomes Research. The authors reviewed and agreed on the different versions of this article. They approved the final version for submission and any changes introduced at the proofing stage. All authors accept responsibility for the content of the article.
Funding
This work was conducted by researchers from HealthCore, Inc. and was funded by Mallinckrodt Pharmaceuticals. Medical writing services were provided by Oishika Panda, PhD, of Oxford PharmaGenesis Inc., and were funded by Mallinckrodt Pharmaceuticals.
Disclosure
HH and T-CY are employees of Mallinckrodt Pharmaceuticals, Hampton, NJ, USA. Authors C-CT and HT are employees of HealthCore, Inc., Wilmington, DE, USA, whose activities on research projects are funded by various pharmaceutical/biotechnology/medical device companies. The authors report no other conflicts of interest in this work.
References
1. McDermott KW, Weiss AJ, Elixhauser A. Burn-related hospital inpatient stays and emergency department visits, 2013: statistical Brief #217. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality; 2016.
2. World Health Organization. Burns; 2021. Available from: https://www.who.int/en/news-room/fact-sheets/detail/burns.
3. American Burn Association. Burn incidence fact sheet; 2016. Available from: https://ameriburn.org/who-we-are/media/burn-incidence-fact-sheet/.
4. Kagan RJ, Peck MD, Ahrenholz DH, et al. Surgical management of the burn wound and use of skin substitutes: an expert panel white paper. J Burn Care Res. 2013;34(2):e60–e79. doi:10.1097/BCR.0b013e31827039a6
5. Bittner EA, Shank E, Woodson L, Martyn JA. Acute and perioperative care of the burn-injured patient. Anesthesiology. 2015;122(2):448–464. doi:10.1097/ALN.0000000000000559
6. Girard D, Laverdet B, Buhe V, et al. Biotechnological management of skin burn injuries: challenges and perspectives in wound healing and sensory recovery. Tissue Eng Part B Rev. 2017;23(1):59–82. doi:10.1089/ten.teb.2016.0195
7. Yu TC, Zhang X, Smiell J, et al. Healthcare resource utilization, treatment patterns, and cost of care among patients with thermal burns and inpatient autografting in two large privately insured populations in the United States. Burns. 2020;46(4):825–835. doi:10.1016/j.burns.2019.10.019
8. Wasser T, Wu B, Ycas J, Tunceli O. Applying weighting methodologies to a commercial database to project US census demographic data. Am J Accountable Care. 2015;3(3):33–38.
9. American Burn Association. National burn repository; 2019. Available from: https://ameriburn.org/research/burn-dataset/.
10. Georgiev GZ. Body Surface Area Calculator. GIGAcalculator; 2021. Available from: https://www.gigacalculator.com/calculators/bsa-calculator.php.
11. Consumer Price Index. US bureau of labor statistics; 2019. Available from: https://www.bls.gov/cpi/tables/supplemental-files/home.htm.
12. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi:10.1097/01.mlr.0000182534.19832.83
13. Raman SR, Curtis LH, Temple R, et al. Leveraging electronic health records for clinical research. Am Heart J. 2018;202:13–19. doi:10.1016/j.ahj.2018.04.015
14. Tapking C, Houschyar KS, Rontoyanni VG, et al. The influence of obesity on treatment and outcome of severely burned patients. J Burn Care Res. 2019;40(6):996–1008. doi:10.1093/jbcr/irz115
15. Ammann EM, Kalsekar I, Yoo A, et al. Assessment of obesity prevalence and validity of obesity diagnoses coded in claims data for selected surgical populations: a retrospective, observational study. Medicine. 2019;98(29):e16438. doi:10.1097/MD.0000000000016438
16. Demirtas Y, Yagmur C, Soylemez F, Ozturk N, Demir A. Management of split-thickness skin graft donor site: a prospective clinical trial for comparison of five different dressing materials. Burns. 2010;36(7):999–1005. doi:10.1016/j.burns.2009.05.017
17. Brolmann FE, Eskes AM, Goslings JC, et al. Randomized clinical trial of donor-site wound dressings after split-skin grafting. Br J Surg. 2013;100(5):619–627. doi:10.1002/bjs.9045
18. Fearmonti RM. Efficacy of epidermal skin grafts over complex, chronic wounds in patients with multiple comorbidities. Wounds. 2016;28(7):226–232.
19. Asuku M, Yu TC, Yan Q, et al. Split-thickness skin graft donor-site morbidity: a systematic literature review. Burns. 2021;47(7):1525–1546. doi:10.1016/j.burns.2021.02.014
20. Braza ME, Fahrenkopf MP. Split-thickness skin grafts. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC; 2021.
21. Shimizu R, Kishi K. Skin graft. Plast Surg Int. 2012;2012:563493. doi:10.1155/2012/563493
22. US Food and Drug Administration. Real-world data (RWD) and real-world evidence (RWE) are playing an increasing role in health care decisions. Available from: https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence.
23. Arondekar B, Duh MS, Bhak RH, et al. Real-world evidence in support of oncology product registration: a systematic review of new drug application and biologics license application approvals from 2015-2020. Clin Cancer Res. 2021. doi:10.1158/1078-0432.CCR-21-2639
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.