Back to Journals » OncoTargets and Therapy » Volume 14
Hodgkin Lymphoma as a Secondary Neoplasm During Therapy for Chronic Myeloid Leukaemia: Case Report and Review of the Literature
Authors Paczkowska E , Janowski M, Karpińska K, Ryłów M, Zdziarska B, Poncyljusz W , Machaliński B
Received 18 January 2021
Accepted for publication 6 March 2021
Published 12 April 2021 Volume 2021:14 Pages 2497—2503
DOI https://doi.org/10.2147/OTT.S300320
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alberto Bongiovanni
Edyta Paczkowska,1,2 Michał Janowski,2 Katarzyna Karpińska,3 Małgorzata Ryłów,2 Barbara Zdziarska,2 Wojciech Poncyljusz,4 Bogusław Machaliński1
1Department of General Pathology, Pomeranian Medical University, Szczecin, Poland; 2Department of Hematology with Bone Marrow Transplantation Unit, University Hospital No. 1 of Pomeranian Medical University, Szczecin, Poland; 3Department of Pathomorphology, Pomeranian Medical University, Szczecin, Poland; 4Department of Diagnostic Imaging and Interventional Radiology, Pomeranian Medical University, Szczecin, Poland
Correspondence: Edyta Paczkowska
Department of General Pathology, Pomeranian Medical University, Al. Powstańców Wlkp. 72, Szczecin, 70-111, Poland
Email [email protected]
Introduction: Incidences of chronic myeloid leukaemia (CML) after treatment of Hodgkin lymphoma (HL) are well described. Here, we report a case of secondary HL in a patient with CML treated with dasatinib as a third-line treatment.
Patient Information: A 64-year-old male was diagnosed with CML and initially treated with imatinib and then with nilotinib due to resistance. Finally, the patient experienced cardiovascular complications, and dasatinib was introduced. After 19 months of treatment, the patient experienced enlargement of lymph nodes that formed packs on the neck.
Interventions: Based on histopathological examination of the lymph nodes, a diagnosis of classical Hodgkin lymphoma – mixed cellularity was established. The patient was successfully treated with 4 cycles of AVD (adriamycin, vinblastine, dacarbazine) chemotherapy.
Outcomes: Complete metabolic remission of Hodgkin lymphoma is currently sustained, and the molecular response to dasatinib at a reduced dose of 50 mg daily corresponds with a deep molecular response.
Conclusion: In this report, we demonstrate the efficacy and safety of the combination of dasatinib and AVD regimens in coexisting CML and HL. This case report emphasizes the importance of insightful evaluation and differential diagnosis in cases of lymphadenopathy during CML treatment.
Keywords: chronic myeloid leukemia, Hodgkin lymphoma, dasatinib, lymphadenopathy, tyrosine kinase inhibitors
Introduction
Chronic myeloid leukaemia (CML) is a clonal proliferative disorder of haematopoietic stem cells that results in uncontrollable expansion of the myeloid lineage, mainly granulocytes. The underlying cytogenetic change in CML is reciprocal translocation of chromosomes 9 and 22. This aberration leads to creation of the oncogene BCR/ABL, which induces production of the constitutively active tyrosine kinase oncoprotein. This pathologic chimaeric protein activates multiple signalling pathways, leading to cell cycle disturbances and neoplastic transformation of stem cells. Clinical variants of CML consist of three phases: chronic, accelerated, and blast crisis. The introduction of the first tyrosine kinase inhibitor (TKI) imatinib to clinical practice was revolutionary. This targeted therapy achieved similar life expectancy in a group of CML patients and the general population.
Hodgkin lymphoma (HL) is a malignancy of B-cell origin that predominantly occurs in adolescents and young adults. The main clinical presentation of HL is painless lymphadenopathy, organomegaly, and reported general symptoms (fever, night sweats, and weight loss). Due to effective treatment of CML and improved long-term survival of CML patients, the probability of developing a secondary malignancy (SM) is increasing.1 The most common SMs that occur in CML patients are prostate, colorectal and lung cancer; non-Hodgkin’s lymphoma (NHL); melanoma; non-melanoma skin tumours; and breast cancer.2 The incidence of a CML and HL diagnosis in the same patient is very rare. Usually, in the sequence, CML is diagnosed after treatment of HL. Radiotherapy plays an important role in HL treatment and is considered one of the potential causes of CML development. More frequently, treatment of HL results in other myeloid malignancies, such as acute myeloid leukaemia and myelodysplastic syndromes.3 The reverse sequence of occurrence - HL development after treatment of CML - is extremely rare. To date, none of the large clinical trials investigating the impact of TKIs on the incidence of secondary malignancies have noticed HL. Moreover, only single case reports describing the occurrence of HL after CML treatment are available in the literature. We hereby report a CML patient who developed secondary HL presenting with unilateral cervical lymphadenopathy after five years of TKI treatment. This report can contribute to a better understanding of the incidence of secondary cancer during TKI treatment and furthermore provides a second case of HL occurrence during treatment with dasatinib (DA).
Case Report
A 64-year-old male with a history of arterial hypertension was admitted to the Department of Haematology in February 2013 with symptoms of fatigue and weight loss. Physical examination revealed enlargement of the spleen (4 cm below the costal margin). Laboratory tests showed granulocytic hyperleukocytosis with excess basophils. Bone marrow FISH and cytogenetic examination revealed BCR-ABL1 rearrangement in 98% of cells. Quantitative real-time polymerase chain reaction (qRT-PCR) results for BCR-ABL expressed using the International Scale (IS) showed 43.2612% at the time of diagnosis. The patient carried the e14a2 transcript. A diagnosis of the chronic phase of CML was established, and treatment with imatinib (IM) 400 mg daily was started with good results. After three months of therapy, the patient achieved a complete haematologic response, with 9.5416% on the IS. Complete cytogenetic remission was noted in the 11th month of therapy (IS: 0.9441%). The patient did not achieve a major molecular response, with the lowest transcript level (IS: 0.2798%) in January 2015. In February 2016, transcript augmentation was observed to the level of IS 1.32%. Non-mutational IM resistance was diagnosed, and second-line therapy with nilotinib 800 mg daily was started. However, grade 3 severe non‐haematologic cardiovascular adverse events were observed during nilotinib treatment.4 The patient developed moderate peripheral arterial occlusive disease (PAOD) and required interventional treatment. He also developed ischaemic heart disease confirmed by coronary catheterization and underwent percutaneous coronary intervention with three stent implantations. A switch to an alternative TKI was decided because nilotinib was suspected to be a causal factor.4 The patient was also affected by risk factors for atherosclerosis, such as arterial hypertension and hypercholesterolemia. Nilotinib was reduced and then discontinued in February 2017.
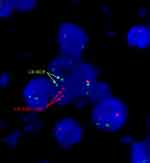
The IS level at the moment of nilotinib discontinuation corresponded with a deep molecular response: IS 0.0000%. Another second-generation TKI, DA, was introduced 4 years after diagnosis, and DA was well tolerated and efficient. The deep molecular response was sustained. In the meantime, the patient underwent percutaneous transluminal coronary angioplasty with implantation of a drug-eluting stent and percutaneous transluminal angioplasty of occluded arteries in the affected leg. In November 2018, almost 2 years after DA treatment initiation, the patient reported swelling of the neck on the left side and systemic symptoms, such as fever, night sweats and loss of appetite. Physical examination revealed enlarged lymph nodes forming packs on the left side of the neck and in the left supraclavicular fossa. There were no other palpable lymph nodes or hepatosplenomegaly. Ultrasonography examination revealed pathologic round-shaped, hypoechoic lymph nodes with no clear hilum along neck vessels (Figure 1). The presence of an abnormal vascular pattern of lymph nodes and a tendency to create packets was observed. PET-FDG (fluorodeoxyglucose)-images revealed chemical activity within left extensive cervical lymphadenopathy, which was well documented on coronal reconstruction (Figure 2). Laboratory tests revealed a WBC count of 14 G/l (25% neutrophils, 59% lymphocytes, 9% monocytes, 6% eosinophils, 1% basophils, and no presence of blasts), 13.2 g/dl haemoglobin (Hb), and 121 G/l platelets (PLTs). A peripheral blood smear showed no abnormal cells. BCR-ABL1 transcript levels corresponded with a deep molecular response (DMR). Histopathological analysis of lymph nodes with haematoxylin and eosin (HE) and immunohistochemical staining revealed that the lymph node architecture was partially preserved with an interfollicular growth pattern and a thin capsule. A mixed cellular infiltrate with lymphocytes, macrophages, and eosinophils was distributed among lymphoid follicles (Figure 3). Reed-Sternberg cells with large purple nucleoli were observed in this background (Figure 4). Malignant Hodgkin/Reed-Sternberg (HRS) cells were rather small and mononuclear, with CD30 positivity (Figure 5). PAX5 immunostaining revealed a weak reaction in the nuclei of HRS cells and a strong reaction in the nuclei of small benign B lymphocytes (Figure 6). FISH BCR-ABL1 analysis of lymph nodes showed a normal pattern of signals, and translocation t(9;22)(q34;q11) was not present (Figure 7). Finally, a diagnosis of classical Hodgkin lymphoma – mixed cellularity (Lugano II) was established. The patient received 4 cycles of AVD (adriamycin, vinblastine, dacarbazine; bleomycin was omitted due to patient age) chemotherapy5 and attained complete metabolic remission confirmed by PET-FDG. DA was continued during treatment at a reduced dose of 50 mg except on chemotherapy days.
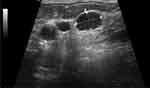 |
Figure 1 Ultrasound image of involved lymph nodes. |
 |
Figure 2 Fused positron emission tomography-fluorodeoxyglucose (PET-FDG) image before AVD treatment. |
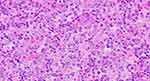 |
Figure 3 HE (magnification 400x). |
 |
Figure 4 HE (magnification 100x). |
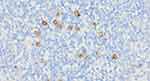 |
Figure 5 Immunohistochemical staining CD30 (magnification 400x). The stains included anti-CD20, CD3, CD30, CD15, PAX5, MUM-1, Ki-67. |
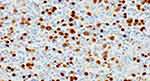 |
Figure 6 HE immunohistochemical staining (magnification 400x). The stains included anti-CD20, CD3, CD30, CD15, PAX5, MUM-1, Ki-67. |
In September 2019, the patient reported recurrent tinnitus. Magnetic resonance imaging of the brain revealed another 7x4 mm tumour suspected of being an acoustic neuroma in the internal acoustic canal. The patient is currently being monitored by a laryngologist and continues treatment with DA 50 mg daily with good results. To date, the HL is in remission.
Discussion
The patient presented was treated with three TKIs. Imatinib was switched to nilotinib due to non-mutational resistance. Treatment with the subsequent TKI was complicated by the occurrence of non-haematologic adverse events, such as cardiovascular complications. These were considered to be caused by nilotinib because the cardiovascular toxicity and unfavourable metabolic profile of nilotinib are well known.4 Next, we decided to start DA. Prior to this, the patient underwent cardiac evaluation, and no significant abnormalities were found on cardiac ultrasound. DA has a different cardiac toxicity profile than nilotinib. Rare cases of pulmonary arterial hypertension during DA treatment have been reported. In contrast, DA does not appear to accelerate atherosclerosis.4 The patient is currently undergoing long-term follow-up by the cardiologist and vascular surgeon. No deterioration was observed after the change in treatment.
Another problem that occurred in the presented patient was cervical lymphadenopathy, which caused oncological concern. Conditions that can cause lymphadenopathy in treated CML patients include extra-medullary development of blast crisis, secondary malignancy and lymphadenopathy associated with DA treatment.1,2,6 Regarding the response to the TKI, the patient maintained a DMR, which spoke against evolution to the blastic phase of CML. However, in the diagnostic process, cytogenetic analysis of the Philadelphia chromosome in the involved lymph nodes helped us to exclude extramedullary CML progression. The observed lymphocytosis in peripheral blood also raised the suspicion of dasatinib-related follicular hyperplasia.6 On the other hand, lymphadenopathy has been described in the course of treatment with DA, as well as characteristic lymphocytosis, which is usually correlated with a good response to treatment. Unfortunately, it is also correlated with the occurrence of pleural effusion in DA-treated patients.7 Dasatinib-related follicular hyperplasia is defined as a reversible, mild adverse reaction to DA treatment.8 This newly described entity is characterized by the occurrence of lymphadenopathy with follicular hyperplasia in histopathology analysis and spontaneous remission after interruption of DA. Ozawa et al6 reported 3 cases of patients with CML treated with DA who developed unexplained lymphadenopathy. In all cases, cervical or submandibular regions were involved, and constitutional symptoms were absent. The duration of DA treatment varied from 17 to 35 months. DA enhances the innate immune response by inducing NK cells and features antiviral activity, eg, it prevents replication of HIV-1.9 However, there are no data showing that it enhances the anti-cancer immune response. In our case, the patient experienced an additional neoplasm, vestibular schwannoma, which is a noncancerous and usually slow-growing tumour that develops on the vestibular nerve.
A perspective of long-term TKI therapy for patients with CML suggests the question of late effects of the treatment, including secondary therapy-related malignancies.8 However, the increasing age of patients influences the occurrence of other malignancies. The phenomenon of co-occurrence of two haematopoietic malignancies leads to consideration of various factors that may play a role in this setting, eg, genetic factors, microenvironmental factors and adverse effects of treatment. The association between the development of malignancies and TKI treatment has been assessed in several studies, and such a coincidence is well documented.10,11 However, the relationship between TKI treatment and neoplasms remains unclear. There are significant inconsistencies in the results of the studies that have been carried out. In a large cohort of 1445 patients, Verma et al demonstrated 66 cases of SM, mostly epithelial neoplasms, including non-melanoma skin cancers and cancers of the digestive tract.12 In a study conducted in 221 Polish CML patients treated with IM, an association between IM therapy and SM development was not found.13 In contrast, Kumar et al demonstrated a significant correlation between IM treatment and the occurrence of SM.14 Similar observations were made by Sasaki et al in a large group consisting of 13,276 patients.15 There are also other inconsistent reports on the risk of secondary malignancies in patients with CML treated with TKIs.16
A review of the available literature revealed several cases of NHL diagnosis in CML patients. Various lymphoma types, such as large diffuse large B-cell lymphoma, chronic lymphocytic leukaemia/small lymphocytic B-cell lymphoma (CLL/SLL-B), and follicular lymphoma,17–19 have been observed. In our case, Hodgkin’s lymphoma was diagnosed. To the best of our knowledge, none of the large observational studies include cases of HL. There is only one case report in the literature. Gajendra et al reported a single patient diagnosed with CML followed by HL ten years after TKI treatment initiation.20 In our patient, the interval between the CML and HL diagnosis was 6 years. Interestingly, both patients were initially treated with IM and subsequently switched to DA.
Concurrent treatment of two different neoplasms derived from distinct cell lineages is a clinical challenge. However, previous reports have indicated that ABVD treatment is feasible and well tolerated in association with IM.21 In the present case, the combination of DA and AVD chemotherapy was well tolerated. The patient continues DA treatment at a reduced dose because of a sustained DMR. Interestingly, DA is beginning to be used in the treatment of relapsed or refractory non-Hodgkin lymphomas with significant benefits. The potential mechanism of DA action against NHL cells concerns inhibition of a broad spectrum of oncogenic tyrosine kinase families, including BCR-ABL1, SRC, KIT, and PDGFR, which play a role in transmitting aberrant proliferation signals, for example, via activated B-cell receptors.22
Conclusions
The development of TKIs has allowed for a near normal life expectancy for patients with CML. However, there are cases of progression to the blastic phase as well as side effects of TKIs or finally the appearance of secondary cancers. Patients require molecular monitoring and clinical follow-up. In cases of lymphadenopathy raising oncological concern, it is necessary to perform extended diagnostics, preferably with histopathological verification of the lymph node, to establish the correct diagnosis. There are reported cases of secondary malignancies, including non-Hodgkin lymphomas, in patients on long-term TKI therapy. The case we describe of a patient who developed HL is a very rare clinical situation. Such patients can successfully be treated with appropriate chemotherapy and TKIs. Reporting cases of such patients will provide insight into the clinical scale of this phenomenon. On the other hand, studies at the molecular level will help to elucidate the pathophysiological relationship between these haemato-oncologic diseases.
Patient Consent Form
Written informed consent for publication of their details was obtained from patient. The institutional approval (local ethics committee) is not required to publish this case report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376(10):917–927. doi:10.1056/NEJMoa1609324
2. Miranda MB, Lauseker M, Kraus MP, et al. Secondary malignancies in chronic myeloid leukemia patients after imatinib-based treatment: long-term observation in CML Study IV. Leukemia. 2016;30(6):1255–1262. doi:10.1038/leu.2016.20
3. Millett R, Aggarwal A, Tabbara I, Nassereddine S. Chronic myeloid leukemia as secondary malignancy following the treatment of Hodgkin lymphoma: a case series. Anticancer Res. 2019;39(8):4333–4335. doi:10.21873/anticanres.13600. PMID: 31366526.
4. Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30(8):1648–1671. doi:10.1038/leu.2016.104
5. Gunther JR, Pinnix CC, Glober GR, et al. Partial omission of bleomycin for early-stage Hodgkin lymphoma patients treated with combined modality therapy: does incomplete ABVD lead to inferior outcomes? EJHaem. 2020;1(1):272–276. doi:10.1002/jha2.1
6. Ozawa MG, Ewalt MD, Gratzinger D. Dasatinib-related follicular hyperplasia: an underrecognized entity with characteristic morphology. Am J Surg Pathol. 2015;39(10):1363–1369. doi:10.1097/PAS.0000000000000488
7. Paydas S. Dasatinib, large granular lymphocytosis, and pleural effusion: useful or adverse effect? Crit Rev Oncol Hematol. 2014;89(2):242–247. doi:10.1016/j.critrevonc.2013.10.005
8. Fox LC, Cummins KD, Costello B, et al. The incidence and natural history of dasatinib complications in the treatment of chronic myeloid leukemia. Blood Adv. 2017;1(13):802–811. doi:10.1182/bloodadvances.2016003889
9. Climent N, Plana M. Immunomodulatory activity of tyrosine kinase inhibitors to elicit cytotoxicity against cancer and viral infection. Front Pharmacol. 2019;10:1232. doi:10.3389/fphar.2019.01232
10. Frederiksen H, Farkas DK, Christiansen CF, Hasselbalch HC, Sørensen HT. Chronic myeloproliferative neoplasms and subsequent cancer risk: a Danish Population-Based Cohort Study. Blood. 2011;118(25):6515–6520. doi:10.1182/blood-2011-04-348755
11. Rebora P, Czene K, Antolini L, et al. Are chronic myeloid leukemia patients more at risk for second malignancies? A Population-Based Study. Am J Epidemiol. 2010;172(9):1028–1033. doi:10.1093/aje/kwq262
12. Verma D, Kantarjian H, Strom SS, et al. Malignancies occurring during therapy with tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia (CML) and other hematologic malignancies. Blood. 2011;118(16):4353–4358. doi:10.1182/blood-2011-06-362889
13. Helbig G, Bober G, Seweryn M, et al. Occurrence of secondary malignancies in chronic myeloid leukemia during therapy with imatinib mesylate-single institution experience. Mediterr J Hematol Infect Dis. 2015;7(1):e2015003. doi:10.4084/mjhid.2015.003
14. Kumar V, Garg M, Chaudhary N, Chandra AB. An observational study on risk of secondary cancers in chronic myeloid leukemia patients in the TKI era in the United States. PeerJ. 2018;6:e4342. doi:10.7717/peerj.4342
15. Sasaki K, Kantarjian HM, O’Brien S, et al. Incidence of second malignancies in patients with chronic myeloid leukemia in the era of tyrosine kinase inhibitors. Int J Hematol. 2019;109(5):545–552. doi:10.1007/s12185-019-02620-2
16. Nakazato T, Iriyama N, Tokuhira M, et al. Incidence and outcome of second malignancies in patients with chronic myeloid leukemia during treatment with tyrosine kinase inhibitors. Med Oncol. 2018;35(7):99. doi:10.1007/s12032-018-1159-7
17. Cai Z, Liu S, Zi J, Ma J, Ge Z. A case of primary gastric diffuse large B-cell lymphoma occurring in chronic myeloid leukemia. Onco Targets Ther. 2019;12:5917–5923. doi:10.2147/OTT.S212838
18. Fujiwara SI, Shirato Y, Ikeda T, et al. Successful treatment of follicular lymphoma with second-generation tyrosine kinase inhibitors administered for coexisting chronic myeloid leukemia. Int J Hematol. 2018;107(6):712–715. doi:10.1007/s12185-017-2378
19. Moridaira K, Tamura J, Saitoh T, et al. Non-Hodgkin’s lymphoma following acute myeloid leukemia in complete remission. Acta Haematol. 1998;100(2):97–98. doi:10.1159/000040875
20. Gajendra S, Sharma A, Sharma R, et al. Hodgkin lymphoma in a case of chronic myeloid leukemia treated with tyrosine kinase inhibitors. Turk Patoloji Derg. 2019;35(1):74–78. doi:10.5146/tjpath.2016.01368
21. Ferrario A, Radaelli F, Goldaniga M, et al. ABVD associated with imatinib for coexisting chronic myeloid leukaemia and relapsed Hodgkin lymphoma. Leuk Res. 2010;34(10):e280–e281. doi:10.1016/j.leukres.2010.05.004
22. Umakanthan JM, Iqbal J, Batlevi CL, et al. Phase I/II study of dasatinib and exploratory genomic analysis in relapsed or refractory non-Hodgkin lymphoma. Br J Haematol. 2019;184(5):744–752. doi:10.1111/bjh.15702
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.