Back to Journals » Infection and Drug Resistance » Volume 15
HIV-Associated Mycobacterium Avium Complex, Oral Candida, and SARS-CoV-2 Co-Infection: A Rare Case Report
Authors Ding X, Ma X, Xu Y, Xu L
Received 18 September 2022
Accepted for publication 10 November 2022
Published 2 December 2022 Volume 2022:15 Pages 7037—7042
DOI https://doi.org/10.2147/IDR.S390333
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xue Ding,1 Xiuxia Ma,2 Yanyan Xu,3 Liran Xu4
1Department of Medical Department, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, People’s Republic of China; 2Department of AIDS Clinical Research Center, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, People’s Republic of China; 3Department of General Practice, The Hospital of Zhengzhou First People’s Hospital, Zhengzhou, People’s Republic of China; 4Department of The First Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou, People’s Republic of China
Correspondence: Liran Xu, Department of The First Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou, People’s Republic of China, Email [email protected]
Background: Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread rapidly to become a global pandemic. Opportunistic infections (OIs) are common in patients with acquired immunodeficiency syndrome (AIDS). Mycobacterium avium complex (MAC) and oral candidiasis (OC) are frequently responsible for such infections. Here, we describe a patient with a recent history of COVID-19 who was also diagnosed with human immunodeficiency virus (HIV), MAC, and OC.
Case Presentation: The patient was a 23-year-old woman with a past medical history of HIV infection who was diagnosed with SARS-CoV-2 infection 6 days prior to her referral to hospital. Her chief complaints were chest distress and continuous fever with a background of a 5-month history of anemia and tuberculosis (TB). Chest X-ray showed bilateral parenchymal infiltrates suspicious for COVID-19. She was treated with oxygen, empiric antibacterial and antiretroviral therapy. Further workup showed MAC and OC infection. She was started on ethambutol, rifampin and antifungal treatment for influenzas and her symptoms resolved in 8 weeks. Follow-up chest computed tomography scanning showed that the lung lesions disappeared within a short period of time.
Conclusion: A thorough history and clinical examination are vital to arriving at the correct diagnosis or diagnoses. With the COVID-19 pandemic, clinicians caring for immunosuppressed patients need to remain vigilant of the simultaneous presence of OIs. This report highlights the importance of the treatment and prevention of OIs in HIV-infected persons, which may reduce adverse consequences after infection with SARS-CoV-2.
Keywords: coronavirus disease 2019, HIV, mycobacterium avium complex, oral candidiasis, case report
Background
An unprecedented public health emergency is unfolding worldwide with the coronavirus disease 2019 (COVID-19) pandemic.1 After COVID-19 emerged, concern was voiced regarding its impact on people living with HIV.2 Some of the factors that increase susceptibility to human immunodeficiency virus (HIV) are also relevant for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19.3,4 Certain comorbidities were stronger drivers of COVID-19 outcomes and were associated with an increased risk of death.5
Opportunistic infections (OIs) are a leading cause of mortality in patients with acquired immunodeficiency syndrome (AIDS). Worldwide, tuberculosis (TB) is the predominant cause of death from an infectious disease, causing more deaths than HIV/AIDS. However, the HIV pandemic has worsened the situation not only by leading to the resurgence of TB but also by suppressing the host immune system, which provides an opportunity for infection by non-TB mycobacteria (NTM).6
The most common NTM species that causes disseminated infections in patients with HIV/AIDS is Mycobacterium avium complex (MAC). HIV patients with disseminated MAC infection are at high risk of developing complications and have a higher mortality risk, thus necessitating more active clinical management.7 M. avium is one of the main causes of NTM infection-associated morbidity and mortality in HIV/AIDS patients.8 MAC infections have clinical symptoms similar to active TB infections and are therefore easily mistaken for TB.
Candidiasis are commensal fungi of the oral mucosa often found in immunocompromised patients and are potential pathogens that can cause an OI. In HIV-1 and TB co-infected patients, oral candidiasis (OC) is found with a prevalence of 35%, and in HIV-1 patients with OC, there is a 2.4 times higher odds of having TB.9
In this report, we retrospectively describe a 23-year-old woman who was diagnosed with HIV 17 years prior. This patient had concurrent SARS-CoV-2, MAC, and OC infections. We present the diagnosis and management of these infections that allowed the patient’s recovery.
Case Presentation
The patient was a 23-year-old woman who had been diagnosed with HIV 17 years prior and had since received standard antiretroviral therapy (ART). Notably, she had switched from first line to simplified dual regimen (abacavir-lamivudine-efavirenz to lamivudine-dolutegravir) due to adverse events. She was diagnosed with TB five months prior, when her sputum smear was positive for acid-fast bacilli and the sputum culture subsequently grew TB. She was treated with rifampin, isoniazid, ethambutol, and pyrazinamide. According to “Chinese Guidelines for HIV/AIDS Diagnosis and Treatment”,10 when she was diagnosed with SARS-CoV-2 infection, she was in the AIDS stage due to poor adherence to antiretroviral medications with an HIV load of <100 viral copies/mL and a CD4+ T-cell count of 7 cells/µL.
On hospital day 1, the patient had a positive PCR test for SARS-CoV-2 in a hospital screening. She was referred to a local hospital with constitutional symptoms, including intermittent fever with chills. She had no cough or expectoration but was diagnosed with anemia and TB 5 months prior. There were no retrievable previous trends to compare.
On hospital day 6, the patient was transferred to the Southern Hospital of Zhengzhou First People’s Hospital. She had fever and chills, her temperature was 39.0°C at admission, and her SPO2 was 100% (without oxygen inhalation). She had density shadows in both lungs but had no respiratory distress and had normal oxygen saturation. The patient was found to have normochromic anemia with a hemoglobin level of 85 g/L. Additional blood test results are presented in Table 1. TB and COVID-19 were managed with azithromycin, moxifloxacin, isoniazid, rifabutin, and ethambutol, and ART was continued with lamivudine and dolutegravir. Anemia was treated by transfusion with suspended red blood cells. On hospital day 8, chest computed tomography (CT) scanning revealed substantial pulmonary lesions (Figure 1).
 |
Table 1 Clinical Laboratory Results at Admission and Throughout the Clinical Course of Infection |
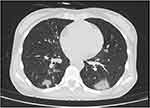 |
Figure 1 CT scanning revealed substantial pulmonary lesions. |
On hospital day 13, the patient reported fever (temperature of up to 38.8°C), but no cough, sputum cough, chest tightness, or shortness of breath. Laboratory tests results are presented in Table 1. A diagnosis of disseminated MAC infection was made by two sputum cultures positive for MAC. Co-infection is a high-risk factor for the progression of COVID-19 to severe and the patient was treated with BRII-196+BRII198 monoclonal antibody therapy. MAC was managed with moxifloxacin, azithromycin, rifabutin and ethambutol, sulfamethoxazole given orally this compound also helps to prevent pneumocystis pneumonia (PCP). Thymalfasin was given to improve immunity, low molecular weight heparin calcium was given as an anticoagulant therapy, and the other treatments described above were continued. On hospital day 16, the chest CT scan showed a thin ground-glass density shadow in both lungs, but it was slightly smaller than in previous scans (Figure 2). Laboratory tests showed C-reactive protein (CRP) of 87.6 mg/L. Azithromycin and moxifloxacin were added to the treatment protocol for anti-infection treatment, and amikacin was used as an anti-MAC treatment.
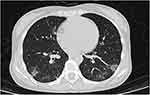 |
Figure 2 CT scan showed a thin ground-glass density shadow in both lungs, but it was slightly smaller than in previous scans. |
On hospital day 27, the patient had intermittent fever and pain in the left abdomen and the blood test results are presented in Table 1. Chest CT scanning was performed, which showed significant improvement of the pulmonary lesions (Figure 3). On hospital day 31, the patient received convalescent plasma therapy.
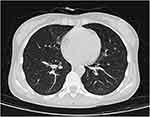 |
Figure 3 CT scanning showed significant improvement of the pulmonary lesions. |
During days 35–48 of hospitalization, the patient had stable signs and did not have cough, fever, chest tightness, or shortness of breath. The treatments described above were continued.
During days 49–54 of hospitalization, the patient had scattered leukoplakia on the oral mucosa and received fluconazole injection as an antifungal treatment and a sodium bicarbonate gargle. She received a nebulized inhalation of 2 mL of sterilized water and was administered a twice-daily injection of recombinant human interferon α. On hospital day 54, a throat swab was submitted for fungal culture and returned a positive result for Candida albicans infection. The above treatments were continued.
On days 65–67 of hospitalization, two sequential PCR tests for SARS-CoV-2 returned negative results. Three consecutive days of examination showed negative results. The patient was discharged in accordance with the “Diagnosis and Treatment Plan for New Coronavirus Infected Pneumonia (Trial Seventh Edition)”.11
Discussion
A thorough history-taking and examination, as well as the appropriate use of clinical tools, are crucial for identifying concomitant OIs in immunosuppressed patients.
A longer course of COVID-19 has been reported in the setting of co-infection with HIV, particularly with low CD4 cell counts, low CD4+ levels and high levels of viral load influence the lethal progression of COVID-19.7,8,12 SARS-CoV-2 might damage lymphocytes, especially T lymphocytes, and the immune system was impaired during the period of disease.13 HIV-1 infection skewed the SARS-CoV-2 T cell response, HIV-1 mediated CD4+ T cell depletion associated with suboptimal T cell and humoral immune responses to SARS-CoV-2, and a decrease in the polyfunctional capacity of SARS CoV-2 specific CD4+ T cells was observed in COVID-19 patients.14 Our patient had multiple individual risk factors associated with prolonged viral shedding as well as a risk of severe SARS-CoV-2: advanced HIV with a low CD4 count. This maybe the reasons that over two months of intense treatment her CD4+ T cell and total lymphocyte counts remained very low, and her immune system did not improve.
The probability of an OI increases as the CD4+ T cell count declines, especially at counts below 200 cells/µL.15 This patient had switched from first line to simplified dual regimen (abacavir-lamivudine-efavirenz to lamivudine-dolutegravir) due to adverse event. She was in the AIDS stage due to poor adherence to antiretroviral medications and virological failure, with an HIV load of <100 viral copies/mL and a CD4+ T cell count maintained at <100 cells/µL, which may have contributed to the incidence of OIs. MAC are seen more commonly in patients with CD4+ T cell counts of <50 cells/µL.16 The risk of developing MAC infection increases in the presence of other concurrent infections such as TB.17 In AIDS patients, MAC infections often present clinically as disseminated MAC, with several weeks of early symptoms, such as prolonged fever, fatigue, weight loss, abdominal pain, diarrhea, and hepatosplenomegaly. MAC diagnoses are often delayed due to the nonspecific presentation of MAC pulmonary disease and radiological findings that overlap with other pulmonary diseases.18 Patients with risk factors and who meet the diagnostic criteria – which include clinical, radiological, and microbiological criteria - should be considered for treatment. In this case, disseminated MAC infection was diagnosed after two sputum MAC positive cultures over a period of two weeks, indicating that attention should be paid to the diagnosis. Oral candidiasis as a potential harbinger of T and B cell immunosuppression associated with viral infections and COVID-19 may be a risk factor for candidiasis.19 The practitioner should be aware of the importance of unexplained oral candidiasis associated with viral infections. PCP is a life-threatening opportunistic infection that can occur in immunodeficient individuals. The PCP mortality rate can reach 60% if the diagnosis is delayed.20 Early prevention reduces the incidence of PCP. In our case, PCP infection was prevented. This patient’s CD4+ T-cell count was below 200 cells/µL, and sulfamethoxazole was given orally to prevent PCP infection.
In terms of treatment, the use of monoclonal antibody therapy, ART, and broad-spectrum antibiotics is in line with recent literature for the treatment of COVID-19 in patients with impaired immune system functions. Neutralising monoclonal antibody therapies targeting SARS-CoV-2 accelerate reduction in viral loads and reduce the risk of disease progression for outpatient with mild COVID-19.14 Co-infection is a high-risk factor for the progression of COVID-19 to severe and the patient was treated with BRII196+BRII198 monoclonal antibody therapy. The only medication that has not been administered is steroids, which has an important reducing factor in lung injuries and was demonstrated to have better results regarding mortality rate in patients with moderate and severe COVID-19.21,22 However, at the time of the patient’s acquisition of severe immunosuppression and COVID-19, the main studies on the impact of steroids on COVID-19 mortality had not been published. Therefore, the patient did not receive steroids during hospitalization. Considering that our patient was diagnosed SARS-CoV-2 infection in a hospital screening, we speculated that in our patient, COVID-19 had existed for a long time before presentation.
Conclusion
There is a paucity of data on SARS-CoV-2 infection in people with HIV and low CD4 counts. Most studies have been retrospective cohort analyses of patients who are more likely to be virally suppressed and less likely to have CD4 counts of more than 200 cells per μL.9,23 In our cases illustrate that OIs is an important consideration in the presence of one in the setting of co-infection of advanced HIV disease and COVID-19. When treating immunocompromised individuals, some opportunistic infections, such as PCP, should be prevented in the presence of risk factors, these treatments may help reduce clinical symptoms and improve prognosis.
Ethics Statement
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. The institutional approval was not required for the publication of the case details.
Funding
This work was supported by Henan Province COVID-19 Traditional Chinese Medicine Scientific Research Special Project (2022ZYFY02), the Nation Natural Science Foundation of China(U1904153), Science and Technology Research Project of Henan Province (222102310570) and Henan Province Special Project of Traditional Chinese Medicine Scientific research(2019AZB006,2019JDZX2096).
Disclosure
The authors declare that there are no competing interests in this work.
References
1. Fauci AS, Lane HC, Redfield RR. Covid-19 - navigating the uncharted. N Engl J Med. 2020;382(13):1268–1269. doi:10.1056/NEJMe2002387
2. Ballester-Arnal R, Gil-Llario MD. [The Virus that Changed Spain: impact of COVID-19 on People with HIV]. El Virus que cambió España: impacto del COVID-19 en las personas con VIH. AIDS Behav. 2020;24(8):2253–2257. Spanish. doi:10.1007/s10461-020-02877-3
3. Belaunzaran-Zamudio PF, Caro-Vega Y, Giganti MJ, et al. Frequency of non-communicable diseases in people 50 years of age and older receiving HIV care in Latin America. PLoS One. 2020;15(6):e0233965. doi:10.1371/journal.pone.0233965
4. Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17(4):118–123.
5. Johnston R. The first 6 months of HIV-SARS-CoV-2 coinfection: outcomes for 6947 individuals. Curr Opin HIV AIDS. 2021;16(1):54–62. doi:10.1097/coh.0000000000000654
6. Kali A, Charles MP, Noyal MJ, Sivaraman U, Kumar S, Easow JM. Prevalence of Candida co-infection in patients with pulmonary tuberculosis. Australas Med J. 2013;6(8):387–391. doi:10.4066/amj.2013.1709
7. Wang M, Luo L, Bu H, Xia H. One case of coronavirus disease 2019 (COVID-19) in a patient co-infected by HIV with a low CD4(+) T-cell count. Int J Infect Dis. 2020;96:148–150. doi:10.1016/j.ijid.2020.04.060
8. Huang J, Xie N, Hu X, et al. Epidemiological, virological and serological features of coronavirus disease 2019 (COVID-19) cases in people living with human immunodeficiency Virus in Wuhan: a Population-based cohort study. Clin Infect Dis. 2021;73(7):e2086–e2094. doi:10.1093/cid/ciaa1186
9. Vizcarra P, Pérez-Elías MJ, Quereda C, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;7(8):e554–e564. doi:10.1016/s2352-3018(20)30164-8
10. Hepatitis C Research Group CSoIDCCfDCaP. Chinese guidelines for HIV/AIDS diagnosis and treatment. Chin J AIDS STD. 2021;27(11):1182–1201. doi:10.13419/j.cnki.aids.2021.11.02
11. China GOoNHCotPsRo. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). China Med. 2020;15(06):801–805.
12. Ambrosioni J, Blanco JL, Reyes-Urueña JM, et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV. 2021;8(5):e294–e305. doi:10.1016/s2352-3018(21)00070-9
13. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi:10.1093/cid/ciaa248
14. Riou C, Stek C, Daroowala R, et al.; HIATUS consortium. Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2022;22(5):622–635. doi:10.1016/s1473-3099(21)00751-9
15. Buchacz K, Lau B, Jing Y, et al. Incidence of AIDS-defining opportunistic infections in a multicohort analysis of HIV-infected persons in the United States and Canada, 2000–2010. J Infect Dis. 2016;214(6):862–872. doi:10.1093/infdis/jiw085
16. Palella FJ
17. Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(Rr–4):1–207.
18. Daley CL, Winthrop KL. Mycobacterium avium complex: addressing gaps in diagnosis and management. J Infect Dis. 2020;222(Suppl 4):S199–S211. doi:10.1093/infdis/jiaa354
19. Katz J. Prevalence of candidiasis and oral candidiasis in COVID-19 patients: a cross-sectional pilot study from the patients’ registry in a large health center. Quintessence Int. 2021;52(8):714–718. doi:10.3290/j.qi.b1491959
20. Gilroy SA, Bennett NJ. Pneumocystis pneumonia. Semin Respir Crit Care Med. 2011;32(6):775–782. doi:10.1055/s-0031-1295725
21. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi:10.1056/NEJMoa2021436
22. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect −19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–1316. doi:10.1001/jama.2020.17021
23. Yousaf M, Hameed M, Alsoub H, Khatib M, Jamal W, Ahmad M. COVID-19: prolonged viral shedding in an HIV patient with literature review of risk factors for prolonged viral shedding and its implications for isolation strategies. Clin Case Rep. 2021;9(3):1397–1401. doi:10.1002/ccr3.3786
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
