Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Hispanic Individuals are Underrepresented in Phase III Clinical Trials for Advanced Liver Cancer in the United States
Authors Monge C , Maldonado JA, McGlynn KA, Greten TF
Received 24 April 2023
Accepted for publication 5 July 2023
Published 27 July 2023 Volume 2023:10 Pages 1223—1235
DOI https://doi.org/10.2147/JHC.S412446
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Cecilia Monge,1 J Alberto Maldonado,1 Katherine A McGlynn,2 Tim F Greten1,3
1Gastrointestinal Malignancies Section, Thoracic and GI Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA; 2Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA; 3NCI CCR Liver Cancer Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Betheda, MD, USA
Correspondence: Tim F Greten, Thoracic and GI Malignancy Section, TGMB NIH/NCI/CCR, Building 10 Rm 2B28B, 9000, Rockville Pike, Bethesda, MD, 20892, USA, Tel +1 240 760 6114, Email [email protected]
Background: Hispanic individuals comprise the second-largest subpopulation after non-Hispanic White (NHW) individuals in the United States (US). We compared the relative contribution of Hispanic individuals to the ten most common causes of cancer-related deaths and studied enrollment of Hispanic patients in multinational phase III advanced liver cancer trials with the aim to investigate whether racial subpopulations are adequately represented in liver cancer trials.
Methods: Relative cancer incidence rates in Hispanic individuals, NHW individuals, non-Hispanic black (NHB) individuals, and Asian individuals were obtained from both the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results Program and the Center for Disease Control and Prevention (CDC), United States Cancer Statistics (USCS) database. Searching PubMed, Embase, and Web of Science, we identified phase III clinical trials studying advanced liver cancer in the last ten years and collected enrollment for each race and ethnicity. Incidence rates of liver cancer and enrollment rates in phase III trials were compared by race and ethnicity.
Results: The cancer type with the relatively highest contribution of Hispanic individuals was liver cancer. From 2015 to 2019, 15.1% of liver cancer cases occurred in Hispanic individuals compared to 12.5% in Asian individuals, 11% in NHB individuals, and 7.5% in NHW individuals. In the last ten years, Hispanic individuals made up 1.6% of patients and NHB individuals 1.3% of patients included in phase III multinational liver cancer trials, compared to 31% NHW individuals and 47% Asian individuals.
Conclusion: Hispanic individuals are disproportionately underrepresented in multinational phase III clinical trials for liver cancer despite having the highest relative incidence rates among the four major racial or ethnic groups in the US.
Keywords: liver cancer, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, health care disparities, cancer care disparities, Hispanic individuals, non-Hispanic black individuals
Introduction
According to the United States Census Bureau, the term “Hispanic origin” refers to people of Cuban, Mexican, Puerto Rican, South or Central American or other Spanish origin.1 This population has diverse genetic heritages and varying socio-demographic characteristics.2 Hispanic individuals comprise the second largest subpopulation in the United States (US) behind non-Hispanic white (NHW) individuals. The US Census Bureau reported that 63.6 million Hispanic individuals lived in the US in the year 2021, accounting for approximately 19% of the US population. This group experienced a growth rate of 23% between the years 2010 and 2020.3 Significant disparities in cancer care exist for Hispanic individuals when compared to NHW individuals for most cancer types.4,5
The term “liver cancer” refers to different types of cancers when used by cancer registries and in the context of clinical trials. Cancer registries use the term liver cancer to refer to primary cancers affecting the liver, mainly hepatocellular carcinoma and intrahepatic cholangiocarcinoma.6 This is also the terminology we use in this manuscript. Of note, clinical trials in biliary tract cancers include not only intrahepatic cholangiocarcinoma but also extrahepatic cholangiocarcinoma and gallbladder cancer and thus also non-liver cancers are included in those studies.
Cancer research has led to the development of new treatments and prevention strategies, which have contributed to a decrease in the overall mortality of oncologic diseases.7,8 With the recent development in targeted therapies and immunotherapy as well as the availability of multiple new systemic treatment options for advanced liver cancer in the first- and second-line setting, it is of utmost importance that these advances and their effectiveness benefit the population as a whole.9 Novel treatment options should therefore be tested in all races affected and treated, and race and ethnicity must be clearly and uniformly reported in oncologic clinical trials. All ethnic and racial subgroups should have a fair relative representation according to the population demographics at the clinical trial site.
Prior studies have reported that Hispanic individuals experience higher incidence and mortality rates of liver cancer compared to non-Hispanic white individuals.10–12 It has also been reported that Hispanic individuals are underrepresented in clinical trials for several types of cancer, including breast, prostate, lung, and colorectal.2,13–16 In this study, we aimed to determine if Hispanic individuals are similarly underrepresented in liver cancer trials. We compared incidence rates of liver cancer and relative representation in multinational phase III trials for advanced liver cancer of each race and ethnicity group. Considering the current racial and ethnic makeup of the US population as well as the projected increasing number of Hispanic individuals over the next 50 years, health care disparities may continue to increase unless efforts are undertaken to increase the inclusion of Hispanic individuals in oncologic clinical trials.17
Materials and Methods
Incidence and Mortality Databases Used to Analyze Racial and Ethnic Groups
The age-adjusted incidence and mortality rates of the ten most common cancers in the US by racial and ethnic group were extracted from two databases: the National Cancer Institute, Surveillance, Epidemiology and End Results Program and the Center for Disease Control and Prevention United States Cancer Statistics (USCS).7,18 The incidence rate ratio was calculated by dividing the incidence rate among Hispanic individuals by the incidence rate in NHW individuals for each cancer.
Selection of Clinic Trials
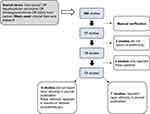
To identify publications of liver cancer trials, we conducted a database search covering the time frame of July 1, 2012 to July 31, 2022, using PubMed (National Library of Medicine), Embase (Elsevier), and Web of Science: Core Collection (Clarivate Analytics) for multinational phase III clinical oncology trials of liver cancer patients. The search terms used were “liver cancer OR hepatocellular carcinoma OR cholangiocarcinoma OR biliary tract cancer. The filters used were clinical trial and phase III trials. The initial search yielded 389 studies. Studies were excluded in a manual verification step if they were non-interventional or were Phase I II or IV, yielding 17 studies. All studies investigated treatments in patients with advanced disease. Two of those studies did not report race and ethnicity, and two other studies only reported proportion of Asian individuals, leaving 13 studies. Ethnic and racial characteristics of the patients in the 13 studies were extracted from publications associated with the clinical trials or from https://clinicaltrials.gov. In both publications and https://clinicaltrials.gov, ethnicity is reported as Hispanic/Latino, non-Hispanic (NH)/non-Latino, or unknown/not reported and race is reported as American Indian/Alaska Native (AI/AN), Native Hawaiian or Other Pacific Islander (NH/PI), Asian, non-Hispanic black, non-Hispanic white, more than one race, or unknown/not reported.
The percentage of Hispanic individuals enrolled in multinational phase III clinical trials of advanced or metastatic liver cancer from 2012 to 2022 was calculated. This percentage was compared with the percentage of Hispanic individuals diagnosed with liver cancer in the SEER database. The recent SEER data available are from 2019, therefore the incidence rates between 2012 and 2019 were used.
Statistical Analyses
The difference in incidence by race and ethnicity (D-IRE) was defined as the median absolute difference in race and ethnicity incidence between trial and corresponding cancer-specific SEER data; with a negative value indicating underrepresentation. The ratio of incidence by race and ethnicity (R-IRE) was defined as the median ratio of the trial and SEER incidence, with a value less than one indicating underrepresentation. The percent of enrollment of Hispanic, NHB, NHW and Asian individuals in advanced liver cancer studies from 2008 to 2018 was compared to the incidence of liver cancer in these populations during that timeframe.
Results
Liver Cancer Disproportionately Affects Hispanic Individuals
Liver cancer is in the top ten most common tumor types that cause the greatest number of deaths in the US. The overall age-adjusted incidence rate for all cancers from the year 2015–2019 was 444.5 per 100,000. While Hispanic individuals showed the second lowest overall incidence rate for all cancer types combined, they had the second highest incidence rate of liver cancer (behind NH AI/AN individuals) (Table 1 and Figure 1). The ratio of incidence rates of liver cancer between Hispanic individuals and NHW individuals was two, the highest of such ratios for all of the top ten cancer sites (Figure 2).
 |
Table 1 SEER 5-Year Age-Adjusted Incidence Rates for the ten Most Common Types of Cancer from 2015–2019 |
To confirm this observation using an independent database, we used data from the Center for Disease Control and Prevention (CDC), United States Cancer Statistics (USCS). This database includes cancer registry data from the CDC’s National Program of Cancer Registries (NPCR) and the NCI SEER program as well as mortality data from CDC’s National Center for Health Statistics. It includes cancer registries from forty-six states, the District of Columbia, Puerto Rico, U.S Virgin Islands and the US Pacific Island Jurisdictions. In the CDC USCS database, we found an age adjusted rate of 13.8 per 100, 000 of liver cancer in Hispanic individuals, a rate of 10.7 in NHB individuals and a rate of 7.3 in NHW individuals. The age adjusted frequency ratio for liver cancer in Hispanic individuals compared to NHW individuals was 1.9 supporting our finding that liver cancer is a type of cancer with a high proportion of Hispanic patients (Table S1).
To compare the incidence rates of the ten most common cancers in US subpopulations, we analyzed the incidence rate ratio (IRR) of Hispanic individuals vs NHW individuals by cancer type. Liver cancer resulted in the highest IRR in Hispanic individuals of 2 with NHB individuals having an IRR of 1.71. The closest IRR when compared to liver cancer in Hispanic individuals was in NHB individuals with prostate cancer that resulted in an IRR of 1.87 (Figure 2). Incidence rate ratios are used to compare two subpopulations according to their cancer incidence: with higher IRR indicating a higher proportion of cancer in that population.
Liver Cancer Incidence and Mortality Rates from 2000 to 2019 in the Three Largest Ethnic and Racial Subpopulations
Next, we asked whether the relative contribution of Hispanic individuals to liver cancer incidence changed over time in the past ten years. We studied the incidence rate and trend of liver cancer from 2000 to 2019 in the CDC-USCS database; in the three largest US subpopulations: Hispanic, NHB and NHW individuals. The incidence rate per 100,000 of liver cancer in Hispanic individuals was 9.8 in the year 2000, increasing to 13.2 in the year 2010 and had the same rate for the year 2019. Incidence rates increased in NHW individuals from 4.2 to 6.0 and 7.2 for the years 2000, 2010 and 2019; in NHB individuals the rates from the same time points were 6.3, 9.9 and 9.8, respectively (Figure 3A). The mortality rates per 100,000 for liver cancer for the years 2000, 2010 and 2019 in Hispanic individuals increased from 7.4, 8.7 to 9.0, respectively. NHB individuals showed an increasing mortality trend with rates of 6.2, 7.9 and 8.1 while NHW individuals had rates of 4.0, 5.1 and 5.9, respectively, for the three time points (Figure 3B). In summary, our data demonstrates that liver cancer predominantly affects Hispanic individuals in the United States.
Hispanic Individuals are Underrepresented in Phase III Clinical Trials
An age adjusted incidence of 9 per 100, 0000 with 60.6 million Hispanic individuals living in the United States suggests that close to 5691 Hispanic individuals were diagnosed with liver cancer in 2019 and 3710 died from the disease. This prompted us to evaluate how many Hispanic individuals were enrolled in clinical trials conducted in the US and worldwide aiming for FDA approval of drugs and building the rationale for the development of national treatment guidelines.
A database search was performed using PubMed (National Library of Medicine), Embase (Elsevier), and Web of Science: Core Collection (Clarivate Analytics) for multinational phase III clinical oncology trials of patients with liver cancer covering the time from July 1, 2012, to July 31, 2022. The search terms used were “liver cancer OR hepatocellular carcinoma OR cholangiocarcinoma OR biliary tract cancer. The filters used were clinical trial and phase III trials. A total of 389 studies resulted, these were further scrutinized for multinational therapeutic trials for metastatic or advanced stage liver cancer with 17 studies remaining.19–35 All studies enrolled patients with advanced disease. Two of these studies did not report race or ethnicity and two more studies reported only the Asian patients accrued; of the remaining 13 studies, seven published the ethnicity or race characteristics of the participants in the respective journal and the six other studies we searched for the race and ethnicity characteristics of the patients on the website clinicaltrials.gov (Figure 4).
A total of 12,464 patients were enrolled in these seventeen trials. The proportion of patients enrolled who were Hispanic, NHB, or NHW individuals was reported in 8/17 (47%), 13/17 (76%), and 13/17 (76%) of studies, respectively. Among the thirteen studies that reported on race and ethnicity (for groups besides only Asian individuals), 2% (n=184) of participants were Hispanic individuals, 1.8% (n=158) were NHB individuals, 41.6% (n=3691) were NHW individuals, and 49.8% (n=4413) were Asian individuals. Study participants that were AI/AN individuals, NH/OPI individuals, or more than one race (MTR), together, represented less than 1% of the patients in the studies; while 6.5% (n=575) had unknown racial and ethnic characteristics (Table 2 and Figure 5).
 |
Table 2 Multinational Phase III Clinical Trials of Liver Cancer in the Last 10 Years by Race/Ethnicity of Trial Participants |
Hispanic individuals represented ~2% of patients enrolled in multinational studies of advanced liver cancer in the past ten years, yet the proportionate incidence of liver cancer in Hispanic individuals during the same time frame was approximately 15%.
We calculated the difference in incidence by race and ethnicity (D-IRE); Hispanic individuals had a median D-IRE of −31.3% and NHB individuals of −22.6% indicating severe underrepresentation of these groups in liver cancer trials; while NHW and Asian individuals were overrepresented with a median D-IRE of +13.3 and +8.3, respectively (Figure 6A). The ratio of incidence by race and ethnicity (R-IRE) in Hispanic individuals was 0.04, again indicating underrepresentation which was also seen in NHB individuals (R-IRE=0.05). NHW and Asian individuals had an R-IRE of 1.8 and 1.4, respectively (Figure 6B).
The accrual of Hispanic, NHB, NHW and Asian individuals from the year 2008–2018 was compared to the incidence of liver cancer in these populations according to CDC data. The population of Hispanic individuals presented with an increase in the incidence of liver cancer from 28.8% to 32.4% between 2008 and 2018 but their enrollment in liver cancer clinical trials remained relatively static and low. For multiple years in this time period the accrual of Hispanic individuals was 0%. Importantly, the comparison of accrual and incidence in NHB individuals also shows clear underrepresentation. Both NHW and Asian individuals present with a constant overrepresentation in liver cancer clinical trials compared to their incidence rates of liver cancer (Figure 6C).
Discussion
Hispanic individuals represent a large percentage of the US population and carry a disproportionate burden of liver cancer; this is not mirrored in Phase III multinational advanced liver cancer trials in the last ten years; 8 of which led to FDA approval and served as the scientific foundation for national treatment guidelines and recommendations in the US.
Hispanic individuals have the highest relative incidence of liver cancer among the three largest population subgroups in the US, which include Hispanic, NHB or NHW individuals. The age adjusted frequency ratio for liver cancer in Hispanic individuals compared to NHW individuals is the highest amongst the ten most common cancers in the US; demonstrating a notable difference in the incidence of liver cancer between these two subpopulations.36 Importantly, the population of Hispanic individuals in the US is on average younger than the population of NHW individuals; if Hispanic individuals were to present with the same age structure as NHW individuals, the incidence rate of liver cancer among Hispanic individuals would likely be even higher.37
The most common etiologies for liver cancer in the US are Hepatitis B (HBV) and Hepatitis C (HCV) viruses, heavy alcohol consumption, obesity and metabolic syndrome associated with nonalcoholic fatty liver disease (NAFLD).36,38,39 The population of Hispanic individuals has a higher prevalence of specific risk factors such as diabetes3,5, obesity and NAFLD40 compared to individuals of other races and ethnicities. A higher prevalence of such comorbidities is likely to contribute to the higher incidence rates observed in Hispanic individuals.3,5,41,42 Substantial variation in the prevalence of other cancer risk factors such as smoking, calorie dense and nutrient poor diets, and physical inactivity may also contribute to the disparities in incidence rates.4,42 The contribution of the underlying etiology to the racial disparities remains an important factor in targeting prevention and detection efforts.38,43,44
Incidence rates observed in US-born Hispanic individuals and foreign-born Hispanic individuals also differ from each other; with higher rates observed in US-born Hispanic individuals.3,45 The reason for this difference is likely multifactorial and is not yet fully understood. In general, the increase is attributed to cultural acculturation and increased rates of obesity, alcohol intake and smoking compared to foreign-born Hispanic individuals.3,4,46,47 However, accounting for the differences in risk factors is not sufficient alone to explain the differences for the higher incidence of liver cancer in US-born Hispanic individuals.4
Novel cancer therapies, such as immunotherapy, are rapidly emerging as highly effective but financially taxing in patients with advanced liver cancer. A recent retrospective cohort study reported significant disparities in early access to immunotherapy, with lower administration of this treatment to Hispanic individuals compared to NHW individuals. This difference in access to immunotherapy was more notable in non-academic cancer centers.48 The study of immunotherapy efficacy in Hispanic individuals is a pressing need and should be meticulously studied. The applicability of scientific research validity to individuals of all races and ethnicities requires adequate relative representation in multinational phase III studies of liver cancer which are aiming for FDA approval. Knowledge regarding the true efficacy of new therapeutic agents in real world populations is limited due to the underrepresentation of Hispanic and NHB individuals and over representation of NHW and Asian individuals relative to the US population.5 Potential drivers of underrepresentation are complex and may include narrow eligibility criteria, lack of access to participating centers, patient preference, social determinants of health, fear and/or mistrust of the health care system, as well as socioeconomic, language, and cultural barriers.5,13
Equitable representation and granular reporting of ethnicity and race in liver cancer trials is necessary to ensure that the results of these trials, which may result in FDA approval, are applicable to the whole population. Adequate ethnic and racial representation leads to equal access to new treatments that have proven efficacy in all subpopulations, especially in Hispanic individuals.13,49,50 It is fundamental for clinical trials to be designed with racial and ethnic equity considerations to avoid disproportionate analyses of a specific race or ethnicity. Within clinical trial design lies a social responsibility to both majority and minority groups within the population, ensuring valid efficacy conclusions for new therapeutic agents that will become part of the treatment guidelines for the population as a whole.51
This study describes a novel observation reporting the granularity of ethnic and racial representation of subpopulations in phase III advanced liver cancer clinical trials. The results have potential important clinical relevance and direct implication for health disparities and liver cancer research. Liver cancer treatments represent important financial toxicity for the patients and efficacy in all subpopulations must be ensured. Finally, we clearly delineate improvements that are much needed to decrease racial and ethnic disparities in the research and treatment of liver cancer specifically by highlighting the importance of adequate racial and ethnic reporting in the publication of clinical trials and the underrepresentation of Hispanic and NHB individuals in phase III advanced liver cancer studies in the last ten years.
This study has limitations. Because we were interested in clinical trials that were seeking FDA approval and therefore use of the potential treatment in the US, we used incidence rates and population data only from the US. But because the trials that we analyzed in this study were Phase III, there were none that had clinical trial sites only within the US, which is typical for phase III trials. This means that we used incidence and population data for individuals in the US and clinical trial enrollment for individuals globally. It would be interesting to utilize enrollment data only from the sites within the US, but such data was not available.
The NCI-funded SEER 18 database, used in this study captures approximately 25–28% of the US population and incident cancer cases nationwide; it collects and publishes cancer data from population-based cancer registries in 22 U.S geographic areas. Since the SEER database does not include tumor data for all geographical regions, it may underrepresent NHB and Hispanic individuals.38 Results of other investigators, indicate that the SEER population does resemble the US population regarding race and ethnicity, as well as specific determinants of health such as poverty and education.52 The CDC USCS database used as a comparative database in this study includes cancer registry data from the CDC’s National Program of Cancer Registries (NPCR) and the NCI SEER program as well as mortality data from CDC’s National Center for Health Statistics. The CDC supports central cancer registries in 46 States, the District of Columbia, Puerto Rico, the U.S Virgin Islands and the US Pacific Island Jurisdictions. This implies that the database being used for comparison in this study represents a larger part of the US population.
The primary limitation of this study is the number of trials not reporting race or ethnicity or reporting data on only Asian individuals as well as the inconsistent race and ethnicity groupings across studies and the associated bias. Some studies, although published in peer reviewed journals, did not show results in clinical trials.gov. Another limitation is the lack of uniform reporting of liver cancer and its subtypes in the literature as well as in the databases used in this study. We therefore had to group all subtypes of liver cancer together, which reduces the granularity of the results of our study.
The disproportionate burden of liver cancer in the population of Hispanic individuals in the US remains understudied and Hispanic individuals continue to be underrepresented in multinational Phase 3 clinical trials. Adequate representation of the growing population of Hispanic individuals as well as all racial and ethnic groups in clinical trials is essential to identify potential racial and ethnic differences, etiological heterogeneities, differences in cancer biology and pharmacokinetics and pharmacodynamics of new oncologic treatments. Underrepresentation of Hispanic individuals in clinical trials hinders the true efficacy and optimal use of new therapeutic agents for liver cancer since the unique characteristics of the hosts and tumors of patients of the major subpopulations are not accounted for. Mandatory, standard, and granular reporting of racial and ethnic characteristics should be considered in future multinational phase III clinical trials studying liver cancer as part of a continued effort to ensure equitable clinical trial enrollment. The regional geographic accrual of multinational phase III liver cancer studies seeking FDA approval should aim to include patients from geographic areas with similar etiologies of liver cancer and ideally a similar ethnic and racial makeup as the patients that suffer from liver cancer in the US. It is important to conduct trials in Hispanic individuals with liver cancer because of the increased burden they face. Continued efforts are needed to ensure equitable trial accrual and provide scientific results that apply to the population.
Ethics
The NIH IRB (IRB001710 NHSR Application) determined this project does not qualify as human subjects research, in that the activities do not involve human subjects as defined in the federal regulations. The activities only involve anonymous or otherwise de-identified information.
Acknowledgment
CM is a recipient of the Robert A. Winn Career Development Award Program. TFG was supported by the Intramural Research Program of the NIH, NCI (ZIA BC 011343 and ZIA BC 011870).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jensen E. Measuring racial and ethnic diversity for the 2020 census; 2023. Available from: https://www.census.gov/newsroom/blogs/random-samplings/2021/08/measuring-racial-ethnic-diversity-2020-census.html.
2. Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi:10.1155/2017/2819372
3. Miller KD, Ortiz AP, Pinheiro PS, et al. Cancer statistics for the US Hispanic/Latino population, 2021. CA Cancer J Clin. 2021;71(6):466–487. doi:10.3322/caac.21695
4. Stern MC, Fejerman L, Das R, et al. Variability in cancer risk and outcomes within US latinos by national origin and genetic ancestry. Curr Epidemiol Rep. 2016;3:181–190. doi:10.1007/s40471-016-0083-7
5. Giaquinto AN, Miller KD, Tossas KY, et al. Cancer statistics for African American/Black People 2022. CA Cancer J Clin. 2022;72(3):202–229. doi:10.3322/caac.21718
6. Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24(3):1073274817729245. doi:10.1177/1073274817729245
7. Surveillance, epidemiology, and end results program (SEER); 2017. Available from: https://seer.cancer.gov.
8. What’s new in liver cancer research?; 2023. Available from: https://www.cancer.org/cancer/liver-cancer/about/new-research.html.
9. Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ. 2020;371:m3544. doi:10.1136/bmj.m3544
10. Martinez Tyson D, Medina-Ramirez P, Flores AM, et al. Unpacking Hispanic ethnicity-cancer mortality differentials among Hispanic subgroups in the United States, 2004–2014. Front Public Health. 2018;6:219. doi:10.3389/fpubh.2018.00219
11. Sangaramoorthy M, Yang J, DeRouen MC, et al. Disparities in hepatocellular carcinoma incidence in California: an update. Cancer Epidemiol Biomarkers Prev. 2020;29(1):79–87. doi:10.1158/1055-9965.EPI-19-0560
12. Ren F, Zhang J, Gao Z, et al. Racial disparities in the survival time of patients with hepatocellular carcinoma and intrahepatic cholangiocarcinoma between Chinese patients and patients of other racial groups: a population-based study from 2004 to 2013. Oncol Lett. 2018;16(6):7102–7116. doi:10.3892/ol.2018.9550
13. Al Hadidi S, Mims M, Miller-Chism CN, et al. Participation of African American persons in clinical trials supporting U.S. food and drug administration approval of cancer drugs. Ann Intern Med. 2020;173(4):320–322. doi:10.7326/M20-0410
14. Stewart JH, Bertoni AG, Staten JL, et al. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Ann Surg Oncol. 2007;14(12):3328–3334. doi:10.1245/s10434-007-9500-y
15. Grette KV, White AL, Awad EK, et al. Not immune to inequity: minority under-representation in immunotherapy trials for breast and gynecologic cancers. Int J Gynecol Cancer. 2021;31(11):1403–1407. doi:10.1136/ijgc-2021-002557
16. Aldrighetti CM, Niemierko A, Van allen E, et al. Racial and ethnic disparities among participants in precision oncology clinical studies. JAMA Netw Open. 2021;4(11):e2133205. doi:10.1001/jamanetworkopen.2021.33205
17. Jaffee EM, Dang CV, Agus DB, et al. Future cancer research priorities in the USA: a Lancet Oncology Commission. Lancet Oncol. 2017;18(11):e653–e706. doi:10.1016/S1470-2045(17)30698-8
18. U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2023. Available from: https://www.cdc.gov/cancer/dataviz.
19. Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517–3524. doi:10.1200/JCO.2012.48.4410
20. Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31(28):3509–3516. doi:10.1200/JCO.2012.47.3009
21. Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–4075. doi:10.1200/JCO.2012.45.8372
22. Zhu AX, Rosmorduc O, Evans TR, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(6):559–566. doi:10.1200/JCO.2013.53.7746
23. Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–870. doi:10.1016/S1470-2045(15)00050-9
24. Cainap C, Qin S, Huang WT, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–179. doi:10.1200/JCO.2013.54.3298
25. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
26. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
27. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
28. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi:10.1016/S1470-2045(18)30937-9
29. Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807. doi:10.1016/S1470-2045(20)30157-1
30. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
31. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi:10.1200/JCO.19.01307
32. D-y O, He AR, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8):EVIDoa2200015.
33. Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi:10.1016/S1470-2045(21)00604-5
34. Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8):EVIDoa2100070. doi:10.1056/EVIDoa2100070
35. Kelley RK, Rimassa L, Cheng AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(8):995–1008. doi:10.1016/S1470-2045(22)00326-6
36. Flores YN, Datta GD, Yang L, et al. Disparities in hepatocellular carcinoma incidence, stage, and survival: a large population-based study. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1193–1199. doi:10.1158/1055-9965.EPI-20-1088
37. Parada H, Vu AH, Pinheiro PS, et al. Comparing age at cancer diagnosis between Hispanics and non-Hispanic whites in the United States. Cancer Epidemiol Biomarkers Prev. 2021;30(10):1904–1912. doi:10.1158/1055-9965.EPI-21-0389
38. Barzi A, Zhou K, Wang S, et al. Etiology and outcomes of hepatocellular carcinoma in an ethnically diverse population: the multiethnic cohort. Cancers. 2021;13(14):3476. doi:10.3390/cancers13143476
39. Petrick JL, Kelly SP, Altekruse SF, et al. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 2016;34(15):1787–1794. doi:10.1200/JCO.2015.64.7412
40. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. JAMA. 2020;324(12):1208–1210. doi:10.1001/jama.2020.14590
41. Bandi P, Minihan AK, Siegel RL, et al. Updated review of major cancer risk factors and screening test use in the United States in 2018 and 2019, with a focus on smoking cessation. Cancer Epidemiol Biomarkers Prev. 2021;30(7):1287–1299. doi:10.1158/1055-9965.EPI-20-1754
42. Lam C, Cronin K, Ballard R, et al. Differences in cancer survival among white and black cancer patients by presence of diabetes mellitus: estimations based on SEER -Medicare-linked data resource. Cancer Med. 2018;7(7):3434–3444. doi:10.1002/cam4.1554
43. Guo A, Pomenti S, Wattacheril J. Health disparities in screening, diagnosis, and treatment of hepatocellular carcinoma. Clin Liver Dis. 2021;17(5):353–358. doi:10.1002/cld.1057
44. Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122(11):1757–1765. doi:10.1002/cncr.29971
45. Endeshaw M, Hallowell BD, Razzaghi H, et al. Trends in liver cancer mortality in the United States: dual burden among foreign- and US-born persons. Cancer. 2019;125(5):726–734. doi:10.1002/cncr.31869
46. Pinheiro PS, Callahan KE, Stern MC, et al. Migration from Mexico to the United States: a high-speed cancer transition. Int J Cancer. 2018;142(3):477–488. doi:10.1002/ijc.31068
47. Pinheiro PS, Callahan KE, Boscoe FP, et al. cancer site-specific disparities in New York, including the 1945–1965 birth cohort’s impact on liver cancer patterns. Cancer Epidemiol Biomarkers Prev. 2018;27(8):917–927. doi:10.1158/1055-9965.EPI-18-0194
48. Ahn JC, Lauzon M, Luu M, et al. Racial and ethnic disparities in early treatment with immunotherapy for advanced HCC in the United States. Hepatology. 2022;76(6):1649–1659. doi:10.1002/hep.32527
49. Nazha B, Mishra M, Pentz R, et al. Enrollment of racial minorities in clinical trials: old problem assumes new urgency in the age of immunotherapy. Am Soc Clin Oncol Educ Book. 2019;39:3–10. doi:10.1200/EDBK_100021
50. Loree JM, Anand S, Dasari A, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019;5(10):e191870.
51. McDowell A. Why racial diversity in clinical trials is so important. Available from: https://www.antidote.me/blog/why-racial-diversity-in-clinical-trials-is-so-important.
52. Shebl FM, Capo-Ramos DE, Graubard BI, et al. Socioeconomic status and hepatocellular carcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1330–1335. doi:10.1158/1055-9965.EPI-12-0124
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.