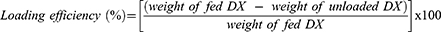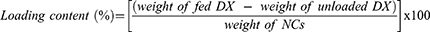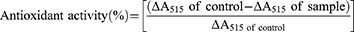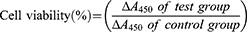Back to Journals » International Journal of Nanomedicine » Volume 18
Highly Water-Dispersed and Stable Deinoxanthin Nanocapsule for Effective Antioxidant and Anti-Inflammatory Activity
Authors Yu S, Kim S, Kim J, Kim JW, Kim SY , Yeom B, Kim H, Choi WI , Sung D
Received 20 January 2023
Accepted for publication 5 July 2023
Published 9 August 2023 Volume 2023:18 Pages 4555—4565
DOI https://doi.org/10.2147/IJN.S401808
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Sohyeon Yu,1,2 Sangwoo Kim,1,3 Jisu Kim,1,3 Ji-Woong Kim,4 Su Young Kim,4 Bongjun Yeom,2 Hyungjun Kim,5 Won II Choi,1 Daekyung Sung1
1Center for Bio-Healthcare Materials, Bio-Convergence Materials R&D Division, Korea Institute of Ceramic Engineering and Technology, Cheongju, 28160, Republic of Korea; 2Department of Chemical Engineering, Hanyang University, Seoul, 04763, Republic of Korea; 3Department of Chemical and Biomolecular Engineering, Yonsei University, Seoul, 03722, Republic of Korea; 4Materials Science Research Institute, LABIO Co., Ltd, Seoul, 08501, Republic of Korea; 5Department of Chemistry and Bioscience, Kumoh National Institute of Technology, Gyeongbuk, 39177, Republic of Korea
Correspondence: Won II Choi; Daekyung Sung, Center for Bio-Healthcare Materials, Bio-Convergence Materials R&D Division, Korea Institute of Ceramic Engineering and Technology, Cheongju, 28160, Republic of Korea, Tel +82-43-913-1513 ; +82-43-913-1511, Fax +82-43-913-1597, Email [email protected]; [email protected]
Introduction: Deinoxanthin (DX), a carotenoid, has excellent antioxidant and anti-inflammatory properties. However, owing to its lipophilicity, it is unfavorably dispersed in water and has low stability, limiting its application in cosmetics, food, and pharmaceuticals. Therefore, it is necessary to study nanoparticles to increase the loading capacity and stability of DX.
Methods: In this study, DX-loaded nanocapsules (DX@NCs) were prepared by nanoprecipitation by loading DX into nanocapsules. The size, polydispersity index, surface charge, and morphology of DX@NCs were confirmed through dynamic light scattering and transmission electron microscopy. The loading content and loading efficiency of DX in DX@NCs were analyzed using high-performance liquid chromatography. The antioxidant activity of DX@NCs was evaluated by DPPH assay and in vitro ROS. The biocompatibility of DX@NCs was evaluated using an in vitro MTT assay. In vitro NO analysis was performed to determine the effective anti-inflammatory efficacy of DX@NCs.
Results: DX@NCs exhibited increased stability and antioxidant efficacy owing to the improved water solubility of DX. The in situ and in vitro antioxidant activity of DX@NCs was higher than that of unloaded DX. In addition, it showed a strong anti-inflammatory effect by regulating the NO level in an in vitro cell model.
Conclusion: This study presents a nanocarrier to improve the water-soluble dispersion and stability of DX. These results demonstrate that DX@NC is a carrier with excellent stability and has a high potential for use in cosmetic and pharmaceutical applications owing to its antioxidant and anti-inflammatory effects.
Keywords: deinoxanthin, nanoprecipitation, reactive oxygen species scavenging, antioxidant activity, anti-inflammation
Introduction
Carotenoids are one of the most commonly occurring fat-soluble, naturally colored bioactive substances and isoprenoid molecules that are synthesized by photosynthetic plants, fungi, and algae.1,2 Carotenoids are stable antioxidants that exhibit efficient scavenging activity for reactive oxygen species (ROS) that are derived from oxygen, namely the highly reactive forms of H2O2, hydroxyl radical (OH−) and superoxide radical (O2−);3–6 they can therefore eliminate ROS-causing and damaging biomolecules within cells that trigger harmful processes such as inflammation, and relieve excessive oxidative stress in the skin and body.7–9 The carotenoid deinoxanthin ((2R)-2,10-dihydroxy-30, 40-didehydro-10, 20-dihydro β,Ψ-caroten-4-one) (DX) is an ethanol obtained from the microorganism Deinococcus radiodurans.10,11 It is a red carotenoid that has been demonstrated to be twice as powerful an antioxidant as other colored carotenoids, such as beta-carotene, lutein, and zeaxanthin.12–14 This may be because of the structure of DX, comprising extended conjugated double bonds, conjugated keto groups, C-10 hydroxyl groups, and additional C-2 hydroxyl groups at the β-ring end, which differs from those of other carotenoids.15 Therefore, DX exhibits great potential as a functional medicine for treating various diseases mediated by oxidative stress.14,16 DX has been reported to protect against DNA damage and prevent the oxidation of proteins.17 In addition, it is an anti-inflammatory agent that lowers the content of inflammatory mediators, such as NO, that are produced in macrophages during inflammatory reactions, thereby providing a more effective anti-inflammatory effect than other carotenoids.2,18,19
However, although DX has antioxidant and anti-inflammatory properties, its extraction in large quantities is challenging. Therefore, it is difficult to use DX in cosmetics, food, and pharmaceutical products because it is not well dispersed in water owing to its lipophilicity.15,20 In addition, most carotenoids, including DX, similar to physiologically active substances, are vulnerable to external factors, such as heat, light, and oxygen.21,22 Furthermore, DX is mostly extracted with ethanol and has the disadvantage that it can be toxic to the skin, thereby hindering its use in biofields, such as pharmaceuticals.23 To overcome these shortcomings, several studies have been conducted to increase stability through nanoencapsulation, which supports fat-soluble carotenoids using amphiphilic polymers or solubilizers to solubilize them. However, among the existing encapsulations, nanoemulsions and nanoliposomes exhibit discoloration owing to their low storage stability, low loading capacity, and difficulty in size control.21,24 In our previous study, we increased bioavailability by dispersing the fat-soluble substance, idebenone, in water by encapsulating it within a solubilizer for increasing stability and solubility.25
Therefore, based on previous research, we developed the first DX-loaded nanocapsules (DX@NCs) in this study using nanoprecipitation after reacting DX dissolved in ethanol with an amphiphilic emulsifier, polyethylene glycol-40 hydrogenated castor oil (PEG-40 HCO) to improve the long-term stability, bioavailability and antioxidant properties of DX (Figure 1A). Through optimization, the NCs were formulated to have a high loading capacity of DX. Physicochemical properties, such as the size, polydispersity, surface charge, and morphology of the DX@NCs were analyzed using dynamic light scattering (DLS) and transmission electron microscopy (TEM). DX@NCs were highly stable for up to four weeks and showed increased in vitro antioxidant and anti-inflammatory efficacy. The antioxidant and anti-inflammatory properties of DX@NCs indicated that they can be used for treating various oxidative stress-related diseases (Figure 1B). The results of this study can pave the way to the commercialization of raw material business or capsule material in the fields of cosmetics, food, and pharmaceuticals.
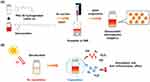 |
Figure 1 (A) Manufacturing scheme of DX@NCs. (B) Schematic of antioxidant, anti-inflammatory, and stability effects of DX@NCs. |
Materials and Methods
Polyethylene glycol-40 hydrogenated castor oil (PEG-40 HCO) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Deionized water (DIW) was purchased from HyClone (Logan, UT, USA). DPPH was purchased from Sigma-Aldrich. The murine fibroblast NIH 3T3 cells (ATCC, MD, USA) were used in the in vitro assays. For the in vitro cell culture, Dulbecco’s modified Eagle’s medium (DMEM), penicillin-streptomycin (PS), and fetal bovine serum (FBS) were obtained from Gibco (Grand Island, NY, USA). As reagents used for in vitro MTT assay, dimethyl sulfoxide-d6 (DMSO-d6, 99.8%) was purchased from Sigma-Aldrich, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Invitrogen (Carlsbad, CA, USA). For the in vitro ROS scavenging assay, H2O2 (30%) was purchased from Junsei Chemical Co. (Tokyo, Japan) and 2.7-dichlorodihydrofluorescein diacetate (H2DCFDA) was purchased from Invitrogen. LPS and Griess reagent used for the in vitro anti-inflammation assay were purchased from Sigma-Aldrich. All the solvents were used as received without further purification.
Production of DX (Deinoxanthin)
To produce standard DX, 1 L of wild-type Deinococcus radiodurans cells were grown for 48 h at 30 °C, and then harvested at 4000 rpm for 30 min. The pellet was washed twice with DIW, following which DX was extracted using 50 mL of methanol. DX extract was concentrated at 55 °C and 70 rpm using a rotary evaporator.11
Preparation of DX@NCs (DX-Loaded Nanocapsules)
DX@NCs were prepared by loading hydrophobic DX into amphiphilic PEG-40 HCO consisting of a hydrophilic carboxylic acid section and a hydrophobic castor oil section, followed by nanoprecipitation in DIW. First, 100 mg of PEG-40 HCO was added to 0, 1, 2, 4, and 8 mg of DX dissolved in 1 mL of ethanol and then loaded at each concentration at room temperature (25 °C) for 2 h under magnetic stirring. Second, DX or DX-containing PEG-40 HCO was added dropwise to 5 mL of DIW using a syringe pump (LEGATO100, KD Scientific, Korea) and stirred at 530 rpm. Subsequently, the ethanol solvent was totally removed via vacuum drying for 2 h to stabilize the freshly formed control (CTL) and DX@NCs (concentration range points: 1, 2, 4, and 8 wt%).
Characterization of DX@NCs (DX-Loaded Nanocapsules)
DX@NCs were evaluated to determine the hydrodynamic diameter, polydispersity index (PDI), and zeta potential using a Zetasizer (ELSZ-2000, Otsuka Electronics Co., Ltd., Tokyo, Japan). The morphology of the DX@NC 4 wt% was examined by TEM via analyzing 20 μL of sample on a copper grid with 200 mesh carbon film using a JEM-2100Plus HR (JEOL, Tokyo, Japan). Lastly, the unloaded DX was purified by ultrafiltration for 5 min at 1500 rpm using Amicon Ultra-15 centrifugal filters (molecular weight cutoff: 60 kDa). Unloaded DX from DX@NCs at 480 nm was analyzed with high-performance liquid chromatography (HPLC), and their loading efficiency and loading content were determined using the following equations:26,27
Stability of DX@NCs (DX-Loaded Nanocapsules)
The stability of DX@NCs in an aqueous solution (DIW) was analyzed using DLS for 4 weeks. The hydrodynamic diameter and PDI of the DX@NCs were evaluated at concentrations in the 1–8 wt% range after 0, 1, 2, 3, and 4 weeks at 25 °C. The re-dispersion stability of the DX@NCs was determined after 3 d of lyophilization and resuspension in DIW and PBS buffer at 37 °C and 100 rpm, at concentrations ranging from 0 to 4 wt%, without adding cryoprotectants. The discoloration of DX and DX@NCs (concentration: 1, 2, and 4 wt%) was measured visually as a function of the exposure time to light (sample exposure time: 0 h, 1 week, and 4 weeks).
In situ Antioxidant Activity of DX@NCs (DX-Loaded Nanocapsules)
The antioxidant activities of DX and DX@NCs were evaluated using the DPPH assay.28,29 First, DX and DX@NC were prepared as initial 1-week samples, and DX@NCs was also prepared as 4-week samples (concentration of DX@NCs: 4 wt%). Next, the DPPH solution was dissolved in ethanol in the dark at a concentration of 0.1 mM. Subsequently, 150 µL of each sample solution was reacted with 50 µL of the prepared DPPH solution. The control group was 150 µL of DIW added with 50 µL of DPPH solution, which had low radical scavenging activity. All reaction mixtures were stored at 25 °C for 24 h with minimal exposure to light, and the UV-vis absorbance of the mixtures was measured with a microplate reader (VICTOR X5, PerkinElmer, Singapore, Republic of Singapore) at a wavelength of 515 nm. The antioxidant activity values of DX@NCs were obtained using the following equation:30
In vitro Cytotoxicity of DX@NCs (DX-Loaded Nanocapsules)
To evaluate the cytotoxicity of DX@NCs, NIH 3T3 mouse embryonic fibroblast cells were seeded in DMEM supplemented with 10% FBS and 1% PS. NIH 3T3 cells were cultured into 96-well plates at a density of 10,000 cells per well and incubated at 37 °C for 1 d. Then, 10,000 cells were treated with different concentrations of DX@NCs (10–100 µg/mL) and incubated for 1 d at 37 °C.31–33 Subsequently, each well was replaced with fresh medium, a concentration of 1 mg/mL MTT solution was added, and then the culture was continued for 3 h. The medium was then removed, and the resulting purple formazan dye crystals were dissolved using DMSO-d6 for 30 min. The absorbance of the formazan produced by the viable cells was measured using a microplate reader (BioTek, Winooski, VT, USA) at a wavelength of 570 nm. Cell viability was determined using the following equation.
In vitro Antioxidant Activity of DX@NCs (DX-Loaded Nanocapsules)
The intracellular antioxidant activity of DX@NCs was evaluated by seeding NIH 3T3 cells into a 96-well plate (10,000 cells/well) and culturing them for 1 d. In NIH 3T3 cells, ROS were generated via stimulation using the oxidative stress factor H2O2. Changes in ROS levels after treatment were also assessed. In advance, various concentrations of DX@NCs (10 ng/mL–1 μg/mL) and the positive controls DX, NC, and 50 μM of H2O2 were added to the cells and incubated for 8 h. H2O2 was not added to a negative control for comparison. After removing the remaining medium by washing using PBS, a 10-µM solution of the fluorescent ROS-indicator H2DCFDA was added to the cells and cultured in the dark for 90 min. The in vitro antioxidant activity of DX@NCs was determined by measuring the fluorescence intensity of dichlorofluorescein oxidized by ROS at excitation and emission wavelengths of 485 and 535 nm, respectively, with a microplate reader.30,34
In vitro Anti-Inflammation Activity of DX@NCs (DX-Loaded Nanocapsules)
To evaluate the intracellular anti-inflammatory activities of DX@NCs, RAW 264.7 cells were cultured into a 96-well plate at a density of 15,000 cells per well and incubated for 1 d. Inflammation was then induced in the cells by LPS, resulting in the production of NO in them. The changes in NO levels after sample treatment were evaluated. First, various concentrations (1–100 µg/mL) of DX@NCs and 20 ng/mL LPS were added to RAW 264.7 cells and then incubated for 1 d.35–38 A negative control, without LPS, was used for comparison. After 1 d, the supernatant was collected, put into a new 96-well plate, and Griess reagent was added to each well. The 96-well plate was then incubated for 10 min at 25 °C, following which the absorbance of the mixtures at a wavelength of 540 nm was measured using a microplate reader. The NO production level was calculated, and the in vitro anti-inflammatory activity of DX@NCs was analyzed.
Statistical Analysis
All experiments were performed in triplicates (n = 3). The resulting data are presented as mean ± standard deviation. Student’s t-test was used to compare differences between experimental groups. The statistical significance of all assessments was set at p < 0.05.
Results and Discussion
Preparation and Characterization of DX@NCs (DX-Loaded Nanocapsules)
DX is a carotenoid and a hydrophobic antioxidant.39 However, discoloration occurs owing to its low solubility in water and low stability, which limits its application in functional foods, cosmetics, and pharmaceuticals.20 Therefore, it was necessary for the developed DX nanocarriers to be capable of increasing their water solubility, stability, and antioxidant activity. In this study, an encapsulation method was used to prepare nanoparticles with different concentrations (1–8 wt%) of DX dissolved in ethanol using a PEG-40 HCO amphiphilic emulsifier (Table 1). Stable DX@NC nanocapsules with hydrophobic cores and hydrophilic shells were self-assembled in an aqueous solution by hydrophobic-lipophilic interactions. The physicochemical properties (hydrodynamic diameter, PDI, and zeta potential) of the DX@NCs were not affected when the DX content was increased from 1 to 8 wt%. The hydrodynamic diameter of 8 wt% DX@NCs was 16.7 ± 0.12 nm (Figure 2A). They were well dispersed, with a PDI value of 0.137 ± 0.02 and a narrow particle size distribution (Figure 2B). The surface charge of 8 wt% DX@NCs was negative, with an average zeta potential of approximately −3.3 ± 0.27 mV (Figure 2C). It was also observed that the surface charge of DX@NCs was constant within the range of −2.4 to −3.8 mV. The morphology of 4 wt% DX@NC was analyzed by TEM (Figure 2D). High-performance liquid chromatography (HPLC) demonstrated that the loading content and loading efficiency were 0.9991 and 99.91, respectively, for 1 wt% DX@NC; 1.998 and 99.9 for 2 wt% DX@NC; 3.999 and 99.97 for 4 wt% DX@NC; and 7.981 and 99.76 for 8 wt% DX@NC.
 |
Table 1 Formulation of Nanoparticles Referred to as DX@NCs 1–8 Wt Formed by Varying the Mass of DX |
Stability of DX@NCs (DX-Loaded Nanocapsules)
The long-term stability of DX@NC was evaluated by observing the changes in nanoparticle size and PDI under the following two conditions using DLS: (1) in an aqueous solution (DIW, 25 °C) and (2) redispersing in DIW and PBS after freeze-drying. The initial size (Figure 3A) and PDI (Figure 3B) of DX@NCs in DIW were maintained for 4 weeks, except for the 8 wt% solution. In addition, 4 wt% DX@NCs were successfully lyophilized. No change in size was observed (Figure 3C), and the PDI was measured to be <0.2 (Figure 3D). Therefore, the optimal loading capacity of DX@NCs was determined to be 4 wt%. Furthermore, it was easily resuspended in DIW and PBS, enabling easy use and storage. In particular, the stability of DX@NCs was preserved without the addition of cryoprotectants, such as glucose, sucrose, or trehalose, to improve dispersibility. These findings indicate that the DX@NCs we developed can serve as an effective platform for a stable drug delivery system, as it shows excellent stability under various conditions, including re-dispersion.
Prevention of Discoloration of DX@NCs (DX-Loaded Nanocapsules) by Light Exposure
The deterioration of carotenoids’ color and effectiveness is associated with structural damages induced by light (visible and ultraviolet) and oxygen. Deinoxanthin, a carotenoid, also discolors when its structure is changed by light. However, encapsulation of DX can block the sunlight from going through the particles, protecting the structure of DX from degradation. Therefore, DX@NC was developed to prevent discoloration. The discoloration was assessed visually by exposing DX@NCs (concentrations of 1, 2, and 4 wt%) and unencapsulated DX to light. DX and DX@NC initially had the same orange color (Figure 4A). However, the color of some of the unencapsulated DX faded in the first week, while all DX@NCs retained their initial color (Figure 4B). In the 4th week, the color of all the DX was lost, while all the DX@NCs retained their initial color (Figure 4C). These results show that the nanoparticles protected DX from discoloration by light exposure. The hydrophobic part of DX interacts with the hydrophobic group of PEG-40 HCO, an emulsifier having an HLB value of 12.5, forming nanocapsules that suppress the decomposition of DX by light.
 |
Figure 4 Confirmation of discoloration of DX@NCs and CTL. (A) Initial state of DX@NCs and CTL, (B) DX@NCs and CTL after 1 week, and (C) DX@NCs and CTL after 4 weeks. |
In situ Antioxidant Activity of DX@NCs (DX-Loaded Nanocapsules)
DX is a highly powerful antioxidant compared with other carotenoids.12,40 However, because of its lipophilic properties, it is challenging to advance its antioxidant activity in aqueous solutions, and it has disadvantages such as discoloration and poor antioxidant activity when exposed to light. Based on previous works, we optimized and developed DX@NCs to maximize the stability and activity of DX in water-soluble formations. First, using the DPPH radical scavenging assay, the antioxidant activity of DX@NCs was analyzed at the initial stage as well as the first and fourth weeks (Figure 5). DPPH, an organic nitrogen radical, has a visible ultraviolet absorption at 515 nm. When the DPPH radical solution reacts with the antioxidant, the radical scavenger gives the DPPH a hydrogen atom or electron; as a result, the absorbance is reduced at 515 nm and the color of the solution changes from purple to yellow. Ultraviolet–visible absorbance at a wavelength of 515 nm was measured to calculate the percentage of DPPH radicals scavenged. The antioxidant efficacy of unencapsulated DX was not as effective as that of conventional DX using DIW instead of organic solvent.11 However, the encapsulated DX@NCs showed more than 90% antioxidant efficacy. Moreover, compared with unencapsulated DX, DX@NCs showed little decrease in antioxidant potency over 4 weeks. However, for the same concentration of DX, the antioxidant level decreased dramatically to 13% after just one week. The stable antioxidant activity of DX@NCs is attributed to the fact that the nanoparticles maintain their antioxidant activity during encapsulation by increasing the DX stability. These results demonstrate that encapsulated DX has increased stability and stable radical scavenging ability compared with those of non-encapsulated DX exposed to the external environment. These reasons suggest that encapsulation of DX is important for its antioxidant potency.
 |
Figure 5 In situ antioxidant activity of DX@NCs. |
In vitro Cytotoxicity and Antioxidant Activity of DX@NCs (DX-Loaded Nanocapsules)
The viability of NIH 3T3 cells after treatment with DX@NCs was evaluated using an MTT assay. The cells were treated with DX samples with concentrations up to 100 μg/mL; the cell viability was 90% or higher at 24 h, indicating that the DX@NCs did not have a toxic effect on the cells. (Figure 6A). This indicates that DX@NCs are biocompatible at concentrations up to 100 μg/mL without causing harm. Furthermore, DX@NCs exhibited a considerably higher antioxidant activity than unencapsulated DX at a concentration of 1 μg/mL (Figure 6B). After treatment using H2O2, an oxidative stress agent, H2DCFDA fluorescence was analyzed to quantify the in vitro level of ROS. NIH 3T3 cells treated with H2O2 alone were normalized to 100% ROS levels. As expected, the H2O2-induced ROS levels in NIH 3T3 cells exposed to DX@NCs were significantly lower. In addition, as the sample treatment concentration increased from 10 ng/mL to 1 μg/mL, the ROS level of the DX@NCs reduced significantly to 17.96%. Therefore, both the in situ and in vitro results show that the DX@NCs exhibited considerably strong antioxidant activity.
In vitro Anti-Inflammatory Activity of DX@NCs (DX-Loaded Nanocapsules)
We measured the inhibitory effect of DX@NCs on LPS-induced NO production in RAW 264.7 cells, a mouse macrophage cell line. Each DX@NC concentration of 10, 50, 75, and 100 μg/mL and LPS (20 ng/mL) were simultaneously administered and compared with the LPS-treated group. The amount of NO was measured in the form of the NO2− content present in the cell culture medium using the Griess reagent. As a result, when treated with DX@NCs, the NO content decreased by 50% at 10 μg/mL and by over 85% at 100 µg/mL, unlike the group treated with LPS only. Thus, it was confirmed that DX@NCs suppressed the increase in NO concentration induced by LPS and had an anti-inflammatory effect (Figure 7).
Conclusion
In this study, we successfully developed DX@NCs through nanoprecipitation to improve the solubility and stability of DX, a fat-soluble substance. The DX@NCs were stable and re-dispersed well for sufficient durations without using cryoprotectants such as sucrose, trehalose, or glucose. This expands the storage and transfer possibilities of DX@NC in a variety of applications. In addition, in vitro models confirmed that the improved water solubility and photostability of DX@NCs could significantly enhance the antioxidant and anti-inflammatory properties of DX. Thus, DX@NCs prove to be a drug delivery platform with excellent stability, as well as antioxidant and anti-inflammatory properties. DX@NCs can be used as promising biomaterials in various cosmetic and pharmaceutical fields.
Abbreviations
ROS, reactive oxygen species; DX, deinoxanthin ((2R)-2,10-dihydroxy-30, 40-didehydro-10, 20-dihydro β,Ψ-caroten-4-one); DX@NCs, DX-loaded nanocapsules; DLS, dynam ic light scattering; DPPH, 2.2-diphenyl-1-picrylhydrazyl; LPS, lipopolysaccharides; PEG-40 HCO, Polyethylene glycol-40 hydrogenated castor oil; DIW, deionized water; DMEM, Dulbecco’s modified Eagle’s medium; PS, penicillin-streptomycin; FBS, fetal bovine serum; DMSO-d6, dimethyl sulfoxide-d6; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; H2DCFDA, 2.7-dichlorodihydrofluorescein diacetate; CTL, control; PDI, polydispersity index.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2021M3C1C3097647, 2021R1F1A1061145, 2021R1F1A1063492) and a grant from the Korea Institute of Ceramic Engineering and Technology (KICET) (1415181794).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26(6):459–516.
2. Ciccone MM, Cortese F, Gesualdo M, et al. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators Inflamm. 2013;2013:782137.
3. Shahmohammadi HR, Asgarani E, Terato H, et al. Protective Roles of Bacterioruberin and Intracellular KCl in the Resistance of Halobacterium salinarium against DNA-damaging Agents. J Radiat Res. 1998;39(4):251–262.
4. Hernández-Ortega M, Ortiz-Moreno A, Hernández-Navarro MD, Chamorro-Cevallos G, Dorantes-Alvarez L, Necoechea-Mondragón H. Antioxidant, antinociceptive, and anti-inflammatory effects of carotenoids extracted from dried pepper (Capsicum annuum L.). J Biomed Biotechnol. 2012;2012:524019.
5. Apak R, Gorinstein S, Böhm V, Schaich KM, Özyürek M, Güçlü K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl Chem. 2013;85(5):957–998.
6. Godic A, Poljšak B, Adamic M, Dahmane R. The role of antioxidants in skin cancer prevention and treatment. Oxid Med Cell Longev. 2014;2014:860479.
7. Kohchi C, Inagawa H, Nishizawa T, Soma G-I. ROS and innate immunity. Anticancer Res. 2009;29(3):817–821.
8. Na Y, Jeong S, Woo J, et al. Continuous synthesis of stable ferrocene nanoparticles using a self-aligned coaxial turbulent jet mixer. J Ind Eng Chem. 2021;97:434–440.
9. Thi PL, Lee Y, Tran DL, et al. In situ forming and reactive oxygen species-scavenging gelatin hydrogels for enhancing wound healing efficacy. Acta Biomater. 2020;103:142–152.
10. Lemee L, Peuchant E, Clerc M, Brunner M, Pfander H. Deinoxanthin: a new carotenoid isolated from Deinococcus radiodurans. Tetrahedron. 1997;53(3):919–926.
11. Jeong S-W, Kim J-H, Kim J-W, Kim CY, Kim SY, Choi YJ. Metabolic Engineering of Extremophilic Bacterium Deinococcus radiodurans for the Production of the Novel Carotenoid Deinoxanthin. Microorganisms. 2021;9(1):44.
12. H-F J. Insight into the Strong Antioxidant Activity of Deinoxanthin, a Unique Carotenoid in Deinococcus Radiodurans. Int J Mol Sci. 2010;11(11):4506–4510.
13. Slade D, Radman M. Oxidative Stress Resistance in Deinococcus radiodurans. Microbiol Mol Biol Rev. 2011;75:133–191.
14. Tian B, Xu Z, Sun Z, Lin J, Hua Y. Evaluation of the antioxidant effects of carotenoids from Deinococcus radiodurans through targeted mutagenesis, chemiluminescence, and DNA damage analyses. Biochim Biophys Acta Gen Subj. 2007;1770(6):902–911.
15. Kim W, Kim M, Hong M, Park W. Killing effect of deinoxanthins on cyanobloom-forming Microcystis aeruginosa: eco-friendly production and specific activity of deinoxanthins. Environ Res. 2021;200:111455.
16. Rodríguez JJG, Mirón AS, Camacho FG, et al. Causes of shear sensitivity of the toxic dinoflagellate Protoceratium reticulatum. Biotechnol Prog. 2009;25(3):792–800.
17. Farci D, Slavov C, Tramontano E, Piano D. The S-layer Protein DR_2577 Binds Deinoxanthin and under Desiccation Conditions Protects against UV-Radiation in Deinococcus radiodurans. Front Microbiol. 2016;7:155.
18. Fuller B, Smith D, Howerton A, Kern D. Anti-inflammatory effects of CoQ10 and colorless carotenoids. J Cosmet Dermatol. 2006;5(1):30–38.
19. Ucci M, Di Tomo P, Tritschler F, et al. Anti-inflammatory Role of Carotenoids in Endothelial Cells Derived from Umbilical Cord of Women Affected by Gestational Diabetes Mellitus. Oxid Med Cell Longev. 2019;2019:8184656.
20. Gargiulo G, Bradford S, Šimůnek J, Ustohal P, Vereecken H, Klumpp E. Transport and Deposition of Metabolically Active and Stationary Phase Deinococcus radiodurans in Unsaturated Porous Media. Environ Sci Technol. 2007;41(4):1265–1271.
21. Pereira Dos Santos P, de Aguiar Andrade L, Flôres SH, de Oliveira Rios A. Nanoencapsulation of carotenoids: a focus on different delivery systems and evaluation parameters. J Food Sci Technol. 2018;55(10):3851–3860.
22. Singh DK, Jagannathan R, Khandelwal P, Abraham PM, Poddar P. In situ synthesis and surface functionalization of gold nanoparticles with curcumin and their antioxidant properties: an experimental and density functional theory investigation. Nanoscale. 2013;5(5):1882–1893.
23. Jeong S-W, Kang CK, Choi YJ. Metabolic Engineering of Deinococcus radiodurans for the Production of Phytoene. J Microbiol Biotechnol. 2018;28(10):1691–1699.
24. Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci. 2018;13(4):288–303.
25. Yang H, Yu S, Kim J, et al. Facile Solvent-Free Preparation of Antioxidant Idebenone-Loaded Nanoparticles for Efficient Wound Healing. Pharmaceutics. 2022;14(3):521.
26. Adamec F, Farci D, Bina D, et al. Photophysics of deinoxanthin, the keto-carotenoid bound to the main S-layer unit of Deinococcus radiodurans. Photochem Photobiol Sci. 2020;19(4):495–503.
27. Na Y, Lee JS, Woo J, et al. Reactive oxygen species (ROS)-responsive ferrocene-polymer-based nanoparticles for controlled release of drugs. J Mater Chem B. 2020;8(9):1906–1913.
28. Cheng J, Zhang Z, Zheng Z, et al. Antioxidative and Hepatoprotective Activities of Deinoxanthin-Rich Extract from Deinococcus radiodurans R1 against Carbon Tetrachloride-Induced Liver Injury in Mice. Trop J Pharm Res. 2014;13(4):581–586.
29. Tian B, Sun Z, Shen S, et al. Effects of carotenoids from Deinococcus radiodurans on protein oxidation. Lett Appl Microbiol. 2009;49(6):689–694.
30. Na Y, Woo J, Choi WI, Lee JH, Hong J, Sung D. α-Tocopherol-loaded reactive oxygen species-scavenging ferrocene nanocapsules with high antioxidant efficacy for wound healing. Int J Pharm. 2021;596:120205.
31. Naji T, Ahmadi R, Iranshahi M. The Apoptotic Effects of Deinoxanthin on Human Breast Cancer Cells. Istanbul, Turkey; 2021.
32. Choi Y-J, Hur J-M, Lim S, Jo M, Kim DH, Choi J-I. Induction of apoptosis by deinoxanthin in human cancer cells. Anticancer Res. 2014;34(4):1829–1835.
33. Na Y, Woo J, Choi WI, Sung D. Novel carboxylated ferrocene polymer nanocapsule with high reactive oxygen species sensitivity and on-demand drug release for effective cancer therapy. Colloids Surf B Biointerfaces. 2021;200:111566.
34. Oh H, Lee JS, Sung D, Lim J-M, Choi WI. Potential Antioxidant and Wound Healing Effect of Nano-Liposol with High Loading Amount of Astaxanthin. Int J Nanomedicine. 2020;15:9231–9240.
35. Lin H-Y, Juan S-H, Shen S-C, Hsu F-L, Chen Y-C. Inhibition of lipopolysaccharide-induced nitric oxide production by flavonoids in RAW264.7 macrophages involves heme oxygenase-1. Biochem Pharmacol. 2003;66(9):1821–1832.
36. Han S, Sung K-H, Yim D, et al. The effect of linarin on lps-lnduced cytokine production and nitric oxide inhibition in murine macrophages cell line RAW264.7. Arch Pharml Res. 2002;25(2):170–177.
37. Lee E-J, Shin S-Y, Kim J-K, Woo E-R, Kim Y-M. Anti-inflammatory Effects of Amentoflavone on Modulation of Signal Pathways in LPS-stimulated RAW264.7 Cells. Bull Korean Chem Soc. 2012;33(9):2878–2882.
38. Au RY, Al-Talib TK, Au AY, Phan PV, Frondoza CG. Avocado soybean unsaponifiables (ASU) suppress TNF-alpha, IL-1beta, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages. Osteoarthritis Cartilage. 2007;15(11):1249–1255.
39. Raddadi N, Cherif A, Daffonchio D, Neifar M, Fava F. Biotechnological applications of extremophiles, extremozymes and extremolytes. Appl Microbiol Biotechnol. 2015;99(19):7907–7913.
40. Maqbool I, Sudharsan M, Kanimozhi G, Alrashood ST, Khan HA, Prasad NR. Crude Cell-Free Extract From Deinococcus radiodurans Exhibit Anticancer Activity by Inducing Apoptosis in Triple-Negative Breast Cancer Cells. Front Cell Dev Biol. 2020;8:707.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.