Back to Journals » Clinical Interventions in Aging » Volume 18
Higher Preoperative Red Blood Cell Distribution Width Increases the Risk of Myocardial Injury After Noncardiac Surgery in Advanced-Age Patients: A Retrospective Cohort Study
Authors Liu C, Zhang K, Zhang T, Sha X, Xu Y, Gu J, Tian Y, Liu Y, Cao J, Mi W, Li H
Received 10 October 2022
Accepted for publication 19 January 2023
Published 11 February 2023 Volume 2023:18 Pages 169—179
DOI https://doi.org/10.2147/CIA.S392778
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Chang Liu,1,2,* Kai Zhang,1,2,* Ting Zhang,1,* Xiaoling Sha,1 Yuhai Xu,3 Juanjuan Gu,1 Ye Tian,4 Yanhong Liu,1,2 Jiangbei Cao,1,2 Weidong Mi,1,2 Hao Li1,2
1Department of Anesthesiology, The First Medical Center, Chinese People’s Liberation Army (PLA) General Hospital, Beijing, People’s Republic of China; 2Medical School of Chinese PLA General Hospital, Beijing, People’s Republic of China; 3Anesthesiology Department, Air Force Medical Center, Chinese PLA General Hospital, Beijing, People’s Republic of China; 4Anesthesiology Department, The Sixth Medical Center, Chinese PLA General Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weidong Mi; Hao Li, Medical School of Chinese PLA, Department of Anesthesiology, The First Medical Center, Chinese PLA General Hospital, 28th Fuxing Road, Haidian District, Beijing, 100853, People’s Republic of China, Email [email protected]; [email protected]
Background: Myocardial injury after noncardiac surgery (MINS) has been associated with worse outcomes. The aim of our study was to investigate the relationship between higher red blood cell distribution width (RDW) and postoperative 30-day MINS among advanced-age patients.
Methods: This was a retrospective observational study including advanced-age patients (≥ 65 years old) who underwent noncardiac surgery between January 2017 and August 2019 at the First Medical Center of the Chinese People’s Liberation Army General Hospital. Patients were divided into two groups according to the cutoff value identified the lowest risk using Restricted Cubic Splines (RCS) model. The primary outcome was the incidence of MINS within 30 days after surgery. The relationship between RDW and MINS was investigated by univariable and multi-variable logistic regression analysis. Propensity score (PS) analysis, including propensity score matching (PSM) and inverse probability treatment weighting (IPTW), as well as subgroup analysis were also conducted to identify the effect of RDW.
Results: The result of the RCS analysis showed that the risk of MINS in advanced-age patients increases when the baseline RDW is > 12.8%. In the univariate analysis, baseline RDW > 12.8% was a risk factor for postoperative MINS [odds ratio (OR)=2.11; 95% confidence interval (CI): 1.83– 2.44; p< 0.001]. After adjustment for all possible components, there was also a high risk of MINS for patients with elevated RDW [Adjusted OR (aOR)=1.43; 95% CI: 1.27– 1.61; p< 0.001). The relationship remained after PS analysis (aOR=1.24; 95% CI: 1.04– 1.47; p=0.016 in PSM; aOR=1.23; 95% CI: 1.05– 1.44; p=0.012 in IPTW, respectively). Significant differences between two groups were established in the incidence rate of postoperative cardiac complications and mortality.
Conclusion: Elevated preoperative RDW is significantly associated with an increased risk of MINS within postoperative 30 days.
Keywords: myocardial injury after noncardiac surgery, red blood cell distribution width, advanced-age patients
Myocardial injury after noncardiac surgery (MINS) has recently been identified as a common, yet often unrecognized, complication during the perioperative period due to its asymptomatic nature.1 A minority of MINS cases are diagnosed by electrocardiogram (ECG) abnormalities and involve typical chest pain symptoms.2 These also deserve increasing concern because of their poor prognosis. Individuals with MINS have greater risks of myocardial infarction, other cardiac complications, and even long-term cardiovascular mortality than those without MINS.3 MINS was related to an increased risk of 30-day deaths in the VISION study, and MINS-induced mortality accounted for at least 1/6 of the deaths.4 Over the longer term, patients suffering from MINS have 1.48 times the risk of one-year mortality as those without MINS.5 Moreover, advanced-age patients (older than 65 years) undergoing noncardiac surgery are among those at highest risk of developing MINS and other cardiovascular complications due to the fragile functioning of multiple comorbid systems. Therefore, early prediction and reliable detection are crucial to enhancing the outcomes of this underappreciated complication for advanced-aged patients.
Red blood distribution width (RDW) is a widely available parameter from the routine component of the complete blood count test. It is calculated as a percentage that equals the standard deviation (SD) of red blood cell volume divided by the mean red blood cell volume (MCV). Over the years, RDW has been used exclusively to measure the extent of anisocytosis and distinguish different kinds of anemia.6 In addition to its diagnostic value, it has recently served as a risk and prognostic factor. Previous studies have shown that by affecting various physiological and pathological processes, such as nutrition, aging, and inflammatory status, RDW contributes to cardiovascular disease progression and even mortality7,8 in both cardiac patients and the general population.9 In Isik’s study, RDW at admission was an independent predictor of long-term major adverse cardiac events (MACEs).10 Gul et al also found that elevated RDW was associated with higher long-term cardiovascular mortality.11
Although RDW is easily available and valuable, few studies have concentrated on the relationship between RDW and MINS during the perioperative period and its application value. Therefore, our study intended to investigate the association between RDW and MINS within 30 days among advanced-age patients after noncardiac surgery.
Materials and Methods
Study Design and Population
This study is a retrospective single-center study comprising advanced-age patients (≥65 years old) who underwent noncardiac surgery in the First Medical Center of the Chinese People’s Liberation Army General Hospital (PLAGH) from January 2017 to August 2019. We excluded patients meeting the following criteria: American Society of Anesthesiologists (ASA) classification of V or above, short operation interval (undergoing more than one surgery within a week), surgery duration of less than 30 minutes and low-risk surgery. In addition, patients with renal insufficiency (with the need for dialysis treatment), severe anemia that required treatment (hemoglobin<90 g/L) or no preoperative RDW values were also excluded. To minimize bias and maximize accuracy, patients with more than 5% missing data were also excluded. All data were extracted from our computerized database, in which all patient medical records were found. The process of extraction was supported by Shanghai Lejiu Healthcare Technology Co., Ltd. The study was examined and approved by the Ethics Committee Board of the First Medical Center of Chinese PLA (No. S2019-311-02) and in line with the Declaration of Helsinki. Because this was a retrospective cohort study with a low risk for all participants, informed permission was not needed.
Data Collection
At enrollment, demographic, commodity, medication use, routine laboratory test and intraoperative data were collected. Covariates of interest included age, sex, body mass index (BMI); comorbidities: hypertension, diabetes mellitus, coronary heart disease, cerebrovascular disease, arrhythmia, myocardial infarction, peripheral artery disease, prior cardiac interventions (including percutaneous coronary intervention or coronary artery bypass graft in the past history in the admission record), chronic obstructive pulmonary disease (COPD); use of preoperative medications: β blockers, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blocker (ARB), calcium channel blockers (CCBs), antiplatelets, diuretics, statin, anticoagulants, fibrates; preoperative serological examination and index: hemoglobin, red blood cells (RBC) count, mean corpuscular volume (MCV), RDW, platelets (PLT), white blood cells (WBC), serum creatinine (SCr), albumin. Surgery-related variables included facility, American Society of Anesthesiologists (ASA) physical status class, intraoperative crystal and colloid input, estimated blood loss, urine output, blood transfusion, surgery duration, and duration of intraoperative hypotension (defined by a mean arterial pressure less than 65 mmHg intraoperatively).12 In addition, we classified surgeries into the following categories: general, urology, vascular, thoracic, neurosurgery, orthopedics, hepatobiliary and others (ENT, etc.). Baseline laboratory values were derived from the last measurement before surgery.
Postoperative hs‑TnT Measurements and Outcomes
In our hospital, patients with older age or poor preoperative state, undergoing major surgery were asked to carry out TNT measurements using the Roche Elecsys hs-cTnT assay on a Cobas c 801 analyzer (Roche Diagnostics, Mannheim, Germany). The peak hs-cTnT within postoperative 30 days were available in 51.4% patients (6192/12,035) since it is not routinely measured in clinical settings. MINS was defined as at least one postoperative high-sensitivity troponin T (hs-cTnT) of 20 to <65 ng/L with an absolute change of ≥5 ng/L or a high-sensitivity troponin T concentration ≥65 ng/L due to a presumed ischemic etiology irrespective of the presence or absence of clinical symptoms and electrocardiographic changes within the first 30 days after noncardiac surgery.13,14 Those patients who had not tested were considered negative for MINS.
The primary outcome of interest was the incidence of MINS. Secondary outcomes included postoperative cardiovascular and cerebrovascular complications: myocardial infarction, heart failure, arrhythmia, stroke, and angina. Death status of patients was achieved by follow-up visits. Follow-up data was collected through June 30, 2021. The state of individuals who lost to follow-up were confirmed by the Chinese Center for Disease Control and Prevention (CCDC) until the follow-up period to assess whether any of them had died.
Statistical Analysis
Variables with skewed distributions are reported as median (IQR) for nonnormally distributed variables and mean (SD) for normally distributed variables. The frequencies of categorical variables are indicated as percentages. For continuous variables, the variance test was used to compare baseline characteristics. For categorical variables, the chi-squared test or Fisher’s exact test was used. Dose-response relationship was examined by using restricted cubic spline (RCS) analysis and the threshold point of the risk of MINS was achieved. Univariable logistic regression analysis was conducted with the occurrence of MINS as the dependent variable. Those with statistically significant variations between groups in the univariate analysis, as well as characteristics considered important in clinical practice for the development of MINS, were among the independent variables evaluated. RDW was incorporated into univariate logistic regression in categorical variables. The presence of any interaction between RDW and other laboratory variables was tested by calculating the variance inflation factor (VIF), a VIF value exceeding 5 indicating multicollinearity. In the multivariate logistic regression analysis, variables of statistical significance and without multicollinearity in the univariate logistic regression analysis were selected as possible confounders. Four models adjusting for different variables were built to identify the independent risk influence of RDW. The findings of the regression are provided as odds ratios (ORs) with their 95% confidence intervals (CIs). We performed propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) analysis which belong to propensity score (PS) method to control for bias and enable the two groups comparable. Using the nearest neighbor method and a caliper of 0.2, a PSM control cohort with 1:1 match was generated from patients in different RDW groups. The IPTW was also built based on a propensity score to create a weighted cohort of patients with comparable baseline characteristics except for the exposure (RDW) through adjusting other potential confounders. The IPTW was developed using a binary logistic regression model to estimate each patient’s propensity score.15 A variable with a standardized mean difference (SMD) of more than 0.1 was considered unbalanced. Statistically significant effects were investigated using paired t-test and paired Wilcoxon rank-sum test. Matched univariate and multivariate analyses were also conducted by logistic regression. We also performed subgroup analysis according to age (advanced age or super-advanced age), sex (female or male), history of cardiovascular disease or without, ASA grade (1&2 or 3&4), surgery duration (more than or less than 3 hours) and anemia (defined as hemoglobin value below 130 g/L for men and 120 g/L for women), repeating the analysis and identifying the influence of RDW on the primary outcome.
A two-sided P value <0.05 indicated statistical significance. Statistical analysis was conducted with R statistics (version 4.0.5, R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
A flowchart of participant selection is shown in Figure 1. A total of 12,035 patients were included in our study, and they were stratified according to the cutoff value from RCS (Figure 2): the RDW≤12.8% group (n=6383) and the RDW >12.8% group (n=5652). The patients’ mean age was 70.7 years, and men accounted for 55% of enrolled patients. In total, 859 patients (7.1%) developed postoperative MINS; among those, 310 (4.9%) patients in the RDW≤12.8% group, and 549 (9.7%) patients in the RDW >12.8% group had MINS. With a higher level of RDW, the majority of patients were older and had a lower BMI. Additionally, with increasing RDW, there was a higher incidence of existing comorbidities, such as hypertension, coronary heart disease, cerebrovascular disease, arrhythmia, myocardial infarction, COPD, and filled prescriptions for β-blockers, antiplatelets, diuretics, statins, anticoagulants, and fibrates. Although the median preoperative hemoglobin, albumin, RBC count, SCr and MCV were lower in the group with higher RDW, it had higher preoperative PLT, WBC, and SII. In terms of surgery type, the majority of the patients underwent hepatobiliary surgery (24.0%), orthopedic surgery (23.0%), or general surgery (21.2%). The differences in the demographic and other characteristics between patients with different levels of preoperative RDW are compared in Table 1.
 |
Table 1 Baseline Characteristics of Patients with or without MINS at the First Medical Center of the Chinese People’s Liberation Army General Hospital Before PS Analysis |
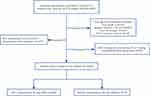 |
Figure 1 The flowchart of participants selection. |
 |
Figure 2 Association between levels of RDW and postoperative MINS using a Restricted Cubic Spline Regression Model. |
Univariate and Multivariate Analysis
As Table 2 demonstrates, the crude hazard ratio from the unadjusted analysis for postoperative MINS was 2.11 (95% confidence interval [CI], 1.83–2.44) in the RDW >12.8% group compared with the reference group of RDW ≤12.8% (P<0.001). Multivariable analysis after adjustment for significant variables, including demographic characteristics, comorbidities and laboratory parameters, still demonstrated that higher RDW serves as an independent risk factor for MINS. Hemoglobin, RBC count and MCV, all hematologic variables, showed multicollinearity with RDW, so they were not considered in multivariate models. In Model I, adjusting for demographic data, comorbidities and medication, the higher-RDW group had a significantly higher risk of MINS, with an adjusted OR (aOR) of 1.58 and 95% CI of 1.36–1.84 (p<0.001). The full model plus intraoperative information achieved a P value less than 0.001 and an aOR of 1.43 (95% CI: 1.27–1.61) between the two groups, indicating the independent risk effect of RDW on MINS in the full model. Postoperative complications as well as overall mortality rates were compared between two groups in Table 3. Overall, patients with higher RDW level were more at risk of later cardiovascular diseases and even mortality.
 |
Table 2 The Univariate and Multivariate Logistic Regression Analysis Exploring the Association of RDW with MINS in the Study |
 |
Table 3 Comparison of the Incidence of Postoperative Cardiovascular Events Among Patients with Different RDW Levels |
Comparison Between PSM and IPTW Groups
Matching variables were selected according to the SMD value in the baseline table. A total of 8020 patients remained after PS matching, 4010 in each group. Comparing the high-RDW PSM group with the PSM controls, all confounders were evenly distributed (SMD less than 0.1) and showed in Supplementary Table 1. The independent risk effect of higher RDW remained for MINS (aOR=1.24; 95% CI, 1.04–1.47; p=0.016). After IPTW, 4034 patients remained in the low-RDW group (≤12.8%), and 3969 patients remained in the high-RDW group (>12.8%). The same finding was obtained after the IPTW method (aOR=1.23;95% CI, 1.05–1.44; p=0.012). The distribution of adjusted variables under each method between the two groups is shown in Figure 3.
 |
Figure 3 SMD distribution of Propensity Score Analysis in patients with different RDW levels. |
Subgroup Analysis
Figure 4 shows the subgroup analysis based on age, sex, ASA grade, surgery duration, history of cardiovascular diseases, and anemia groups. After dividing all patients into advanced-age (65–75 years old) and super advanced-age (≥75 years old) groups, the hazard ratio of higher RDW was still significant [≥75 years: aOR (95% CI): 1.62 (1.232–2.141), p<0.001; <75 years: aOR (95% CI): 1.33 (1.095–1.618), p=0.004]. Higher RDW was only associated with postoperative MINS in male patients (aOR=1.605, 95% CI: 1.299–1.986, p<0.001), whereas it was not significant in female patients (aOR=1.194, 95% CI: 0.939–1.521, p=0.15). The odds ratio of higher RDW was significant in patients both with and without diabetes mellitus (aOR=1.342, 95% CI: 1.012-1.785, p=0.042; aOR=1.438, 95%CI: 1.187-1.746, p<0.001). The association between RDW and MINS was significant in patients with or without preexisting cardiovascular diseases (aOR=1.379, 95% CI: 1.035–1.842, p=0.029; aOR=1.426, 95% CI: 1.180–1.728, p<0.001). The odds ratio of higher RDW was significant in anemic patients (aOR=1.583, 95% CI: 1.242–2.03, p<0.001) but not in non anemic patients (aOR=1.181, 95% CI: 0.949–1.468, p=0.136). Increased risk of higher RDW was also observed among patients in the ASA grade 3 and 4 subgroup (aOR=1.357, 95% CI: 1.107–1.665, p=0.003) and the ASA grade 1 and 2 subgroup (aOR=1.324, 95% CI: 1.016–1.731, p=0.039). The association between RDW and MINS was significant for the long surgery duration group (aOR=1.279, 95% CI: 1.027–1.597, p=0.029) and short surgery duration group (less than 3 hours) (aOR=1.342, 95% CI: 1.059–1.703, p=0.015).
 |
Figure 4 Subgroup analysis of the association between RDW and MINS in different groups based on age, sex, history of diabetes mellitus, cardiovascular disease, anemia, ASA grade and surgery duration. |
Discussion
Our study investigated preoperative RDW level is associated with postoperative MINS within 30 days. The relationship remained after comprehensive adjustment for the possible components affecting the outcome. Propensity score analysis was applied, thus enabling the comparison of outcomes in the two groups with minimal biases. There was a consistent positive association between high RDW and the incidence rate of MINS in multiple subgroups of advanced-age surgical patients. This suggests that preoperative elevated RDW might be a signal of a further burden of cardiovascular complications, but it needs further prospective exploration.
In recent years, MINS has become a focus during the perioperative period due to its asymptomatic property but poor prognosis. It has an incidence ranging from 5% to 25%, according to different diagnostic assays.16,17 In our research, the overall MINS rate was 7.1%, which is consistent with the reported incidence. Due to its prevalence, many indicators and methods have been applied to predict the incidence of MINS. Among them, ECG has a low sensitivity and often induces missed diagnoses in clinical practice. High-sensitivity cardiac troponin (hs-cTnT), troponin T (TnT), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) have become recommendations for screening the risk of MINS in several studies,18,19 but not all patients undergo these measurements, as are seen as unwarranted procedures and increase medical expenses. However, our study provides a new biomarker for the early prediction and risk assessment of MINS. RDW is inexpensive and easily available from complete blood cells, which are measured for all inpatients. Compared with classical hematological indices such as hemoglobin, MCV, RBC count, RDW can be considered a comprehensive indicator, reflecting chronic inflammation, nutrition, anemia status and oxidative stress levels. Elevated RDW can also predict different kinds of cardiovascular diseases, acute kidney injury and even poor prognosis.20–22 Among patients with acute myocardial infarction (AMI), the baseline values and relative changes in RDW are higher. Lee et al23 conducted a study of 1596 consecutive patients with AMI and followed them up for one year, discovering that patients suffering major adverse cardiac events (MACEs) were more likely to have a greater degree of RDW (13.8% vs 13.3%). A dose–response relationship between RDW and MACEs was also observed in their research, in which patients with the highest quartile of RDW had 6.18 times the risk of those with the lowest quartile. Arbel et al24 found that RDW was a significant and independent predictor of 3-year MACE, and for every 1 unit increase in RDW, the risk of 3-year MACE was 1.12 times. In a study of 24,579 advanced-age patients after noncardiac surgery, Abdullah et al25 concluded that surgery patients with RDW>14.3% had an increased risk of short-term mortality. A relationship between preoperative RDW and long-term mortality has been described by Olafsson et al,26 where the highest RDW was found to be associated with a higher risk of long-term mortality in patients after noncardiac surgery. However, few studies on the relationship between RDW and MINS have been conducted. Our research explored their potential relationship and found that when RDW exceeded 12.8%, the risk of MINS greatly increased in our surgical cohort. This may suggest that RDW >12.8%, which is different from the reference range (10.5%-14.5%), may lead to adverse clinical outcomes. Therefore, our study investigated the value of this mostly ignored laboratory parameter for risk assessment and fills the gap in studies related to MINS. Our findings suggest the predictive value of the outcomes in future clinical applications.
The exact physiological reasons RDW is associated with cardiovascular diseases and mortality are not understood, but many mechanisms have been hypothesized to explain the link. First, inflammation is the leading mechanism responsible for abnormal RDW, according to previous related studies. Through impairment of iron metabolism and disruption of the erythropoietin response, inflammation may influence the maturation of erythrocytes. Immature erythrocytes enter the bloodstream, causing anisocytosis, which leads to a high RDW.27 Thus, a higher RDW might represent an uncontrolled long-term inflammatory reaction that also causes cardiomyocyte injury and fibrosis. The widening of RDW is also associated with the risk of atherosclerosis. Thrombosis might arise due to the atherosclerotic status, induced by inflammatory cytokines, which is a common mechanism of MINS.28 Moreover, an inverse relationship has been found between serum antioxidant concentrations and RDW.29 Through surgery and anesthesia stimulation, the higher extent of oxidative stress also aggravates the instability and further increases the risk of MINS. Second, increased RDW values might be related to poor nutritional states, such as deficiency of iron, folic acid or vitamin B12, and the influence of comorbidities. These factors play important roles in hematopoietic function.30 The presence of anisocytosis is usually indicative of a low oxygen capacity.21 Consequently, many peripheral tissues and cells (including cardiomyocytes) suffer from reduced oxygenation, whereas abnormal erythrocytes may also contribute to MINS.31 All of the above mechanisms are suspected to interact with each other, causing the elevation of RDW before surgery and ending with adverse outcomes. Given the relationship between RDW and MINS, doctors can stratify surgical patients into low-risk or high-risk postoperative MINS and develop targeted early prevention and intervention based on potential mechanisms. Several anti-inflammatory therapies and supplementary nutrition have also been recommended to conduct secondary cardiovascular prophylactic interventions to benefit postsurgical outcomes.32 Further prospective studies or randomized controlled trials on the link between RDW and adverse complications and the optimal interventions are warranted.
Several potential limitations of our study need to be considered. First, a retrospective single-center study with a small cohort cannot provide a high level of evidence and may have had selection biases. Prospective cohort studies or randomized controlled trials are needed to verify our conclusions. Second, information on the last 4-month blood transfusion therapy and cancer treatment, including radiotherapy and chemotherapy influencing hematopoietic function before surgery, was not available in our study. However, this would have an insignificant effect on our results because a minority of patients had the above treatments. Third, all known components that may affect outcomes were adjusted for, except for some nutritional factors, including iron, folate and vitamin B12, because they are not routinely measured in our hospital. This may lead to a bias of outcomes to a certain extent. Last, patients with previous renal insufficiency, severe anemia are more prone to be fragile and suffer from myocardial injury while these high-risk patients were excluded in our study. There might be an underestimate of the incidence of MINS.
In conclusion, this retrospective study demonstrated a robust association between preoperative RDW and postoperative MINS among advanced-age patients who underwent noncardiac surgery. By considering RDW a potential biomarker of inflammation and other poor conditions, doctors might stratify patients by RDW and assess the risk of adverse outcomes. Further studies should explore potential mechanisms by which RDW predicts MINS so as to guide preoperative optimization and improve patient prognosis.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
The Research Ethics Committee of PLAGH approved this study (approval reference no. S2019-311-02).
Acknowledgments
We thank Prof. Lan Sun and Prof. Wei Wei of Shanghai Lejiu Healthcare Technology for their technical assistance in data extraction and assistance.
Author Contributions
All authors made a significant contribution to the conception, study design, acquisition of data, analysis and interpretation of data; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the National Key Research and Development Program of China (2018YFC2001900) and Beijing Nova Program (Z211100002121171). The authors declared no competing financial interests.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Meershoek AJA, Leunissen TC, van Waes JAR, et al. Reticulated platelets as predictor of myocardial injury and 30 day mortality after non-cardiac surgery. Eur J Vasc Endovasc Surg. 2020;59(2):309–318. doi:10.1016/j.ejvs.2019.06.027
2. Turan A, Cohen B, Rivas E, et al. Association between postoperative haemoglobin and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Br J Anaesth. 2021;126(1):94–101. doi:10.1016/j.bja.2020.08.056
3. Pereira-Macedo J, Rocha-Neves JP, Dias-Neto MF, Andrade JPV. Prognostic effect of troponin elevation in patients undergoing carotid endarterectomy with regional anesthesia - A prospective study. Int J Surg. 2019;71:66–71. doi:10.1016/j.ijsu.2019.09.015
4. Spence J, LeManach Y, Chan MTV, et al.; Vascular Events in Noncardiac Surgery Patients Cohort Evaluation Study I. Association between complications and death within 30 days after noncardiac surgery. CMAJ. 2019;191(30):E830–E7. doi:10.1503/cmaj.190221
5. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221–1232. doi:10.1161/CIRCULATIONAHA.117.030114
6. Ebina T, Tochihara S, Okazaki M, et al. Impact of red blood cell distribution width and mean platelet volume in patients with ST-segment elevation myocardial infarction. Heart Vessels. 2022;37(3):392–399. doi:10.1007/s00380-021-01936-6
7. Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52(2):86–105. doi:10.3109/10408363.2014.992064
8. Dabbah S, Hammerman H, Markiewicz W, Aronson D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol. 2010;105(3):312–317. doi:10.1016/j.amjcard.2009.09.027
9. Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009;169(6):588–594. doi:10.1001/archinternmed.2009.55
10. Isik T, Kurt M, Tanboga IH, et al. The impact of admission red cell distribution width on long-term cardiovascular events after primary percutaneous intervention: a four-year prospective study. Cardiol J. 2016;23(3):281–288. doi:10.5603/CJ.a2015.0080
11. Gul M, Uyarel H, Ergelen M, et al. The relationship between red blood cell distribution width and the clinical outcomes in non-ST elevation myocardial infarction and unstable angina pectoris. Coron Artery Dis. 2012;23(5):330–336. doi:10.1097/MCA.0b013e3283564986
12. Vasivej T, Sathirapanya P, Kongkamol C. Incidence and risk factors of perioperative stroke in noncardiac, and nonaortic and its major branches surgery. J Stroke Cerebrovasc Dis. 2016;25(5):1172–1176. doi:10.1016/j.jstrokecerebrovasdis.2016.01.051
13. Ruetzler K, Smilowitz NR, Berger JS, et al. Diagnosis and management of patients with myocardial injury after noncardiac surgery: a scientific statement from the American Heart Association. Circulation. 2021;144(19):e287–e305. doi:10.1161/CIR.0000000000001024
14. Devereaux PJ, Biccard BM, Sigamani A, et al.; Writing Committee for the VSI. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642–1651. doi:10.1001/jama.2017.4360
15. Bhanudeep S, Rameshkumar R, Chidambaram M, Selvan T, Mahadevan S. Prospective inverse probability of treatment-weighting analysis of the clinical outcome of red blood cell transfusion practice in critically ill children. Indian J Pediatr. 2021;88(10):985–990. doi:10.1007/s12098-021-03740-6
16. Park J, Kwon JH, Lee SH, et al. Prognosis of myocardial injury after. non-cardiac surgery in adults aged younger than 45 years. Circ J. 2021;85(11):2081–2088. doi:10.1253/circj.CJ-21-0106
17. Duchnowski P, Hryniewiecki T, Kusmierczyk M, Szymanski P. Anisocytosis predicts. postoperative renal replacement therapy in patients undergoing heart valve surgery. Cardiol J. 2020;27(4):362–367. doi:10.5603/CJ.a2019.0020
18. Vascular events In noncardiac Surgery patIents cOhort evaluatioN Writing Group oboTVeInSpceI. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564–578. doi:10.1097/ALN.0000000000000113
19. Kopec M, Duma A, Helwani MA, et al. Improving prediction of postoperative myocardial infarction with high-sensitivity cardiac troponin T and NT-proBNP. Anesth Analg. 2017;124(2):398–405. doi:10.1213/ANE.0000000000001736
20. Shen JT, Xu M, Wu Y, et al. Association of pre-operative troponin levels with major adverse cardiac events and mortality after noncardiac surgery: a systematic review and meta-analysis. Eur J Anaesthesiol. 2018;35(11):815–824. doi:10.1097/EJA.0000000000000868
21. Parizadeh SM, Jafarzadeh-Esfehani R, Bahreyni A, et al. The diagnostic and prognostic value of red cell distribution width in cardiovascular disease; current status and prospective. Biofactors. 2019;45(4):507–516. doi:10.1002/biof.1518
22. Abdullah HR, Sim YE, Sim YT, et al. Preoperative red cell distribution width and 30-day mortality in older patients undergoing non-cardiac surgery: a retrospective cohort observational study. Sci Rep. 2018;8(1):6226. doi:10.1038/s41598-018-24556-z
23. Lee JH, Yang DH, Jang SY, et al. Incremental predictive value of red cell distribution width for 12-month clinical outcome after acute myocardial infarction. Clin Cardiol. 2013;36(6):336–341. doi:10.1002/clc.22114
24. Felker GM, Allen LA, Pocock SJ, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM program and the Duke Databank. J Am Coll Cardiol. 2007;50(1):40–47. doi:10.1016/j.jacc.2007.02.067
25. Arbel Y, Birati EY, Finkelstein A, et al. Red blood cell distribution width and 3-year outcome in patients undergoing cardiac catheterization. J Thromb Thrombolysis. 2014;37(4):469–474. doi:10.1007/s11239-013-0964-2
26. Olafsson HB, Sigurdarson GA, Christopher KB, Karason S, Sigurdsson GH, Sigurdsson MI. A retrospective cohort study on the association between elevated preoperative red cell distribution width and all-cause mortality after noncardiac surgery. Br J Anaesth. 2020;124(6):718–725. doi:10.1016/j.bja.2020.02.009
27. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. doi:10.1056/NEJMra041809
28. Talarico M, Manicardi M, Vitolo M, et al. Red cell distribution width and patient outcome in cardiovascular disease: a “real-world” analysis. J Cardiovasc Dev Dis. 2021;8(10). doi:10.3390/jcdd8100120
29. Semba RD, Patel KV, Ferrucci L, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women’s Health and Aging Study I. Clin Nutr. 2010;29(5):600–604. doi:10.1016/j.clnu.2010.03.001
30. Tekce H, Kin Tekce B, Aktas G, Tanrisev M, Sit M. The evaluation of red cell distribution width in chronic hemodialysis patients. Int J Nephrol. 2014;2014:754370. doi:10.1155/2014/754370
31. Lippi G, Turcato G, Cervellin G, Sanchis-Gomar F. Red blood cell distribution width in heart failure: a narrative review. World J Cardiol. 2018;10(2):6–14. doi:10.4330/wjc.v10.i2.6
32. Devereaux PJ, Sessler D, Lalu M. Myocardial injury after noncardiac surgery. Can J Anaesth. 2022;69(5):561–567. doi:10.1007/s12630-022-02220-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
