Back to Journals » Nature and Science of Sleep » Volume 14
High Rapid Eye Movement Sleep Apnea Hypopnea Index is Associated with Hypertension in Patients with Obstructive Sleep Apnea
Authors Wang L, Wei D, Zhang J, Cao J , Zhang X
Received 6 April 2022
Accepted for publication 29 June 2022
Published 6 July 2022 Volume 2022:14 Pages 1249—1258
DOI https://doi.org/10.2147/NSS.S369614
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Le Wang,1 Donghui Wei,1 Jing Zhang,1 Jie Cao,1 Xinxin Zhang2
1Department of Respiratory and Critical Care, Tianjin Medical University General Hospital, Tianjin, The People’s Republic of China; 2Department of Endocrinology and Metabolism, Tianjin Medical University General Hospital, Tianjin, The People’s Republic of China
Correspondence: Jing Zhang; Jie Cao, Department of Respiratory and Critical Care, Tianjin Medical University General Hospital, Tianjin, 300052, The People’s Republic of China, Tel +86 02260361612 ; Tel +86 02260361612, Email [email protected]; [email protected]
Purpose: Obstructive sleep apnea (OSA) plays an important role in the pathogenesis of hypertension. The aim of this cross-sectional study was to explore the clinical and polysomnographic characteristics of OSA patients with hypertension and to explore the gender differences in the relationship between rapid eye movement (REM) OSA and hypertension.
Patients and Methods: A total of 808 patients with OSA at a tertiary hospital were enrolled in this study, and OSA patients were divided into groups presenting with or without hypertension. The clinical and polysomnographic characteristics were compared between the groups. Multivariate binary logistic analysis was performed to assess the association between REM OSA and hypertension.
Results: After adjustment for potential confounders, the risk of hypertension in patients with OSA increased with severity categories of apnea hypopnea index during rapid eye movement sleep stage (REM AHI) (OR = 1.61 for REM AHI ≥ 58.87 events/h relative to REM AHI < 30.50 events/h, 95% CI 1.07– 2.42, P = 0.022). Consistent with this, when taken as a continuous variable, this association still remains significant (OR = 1.007, 95% CI 1.001– 1.014, P < 0.05). This effect was more pronounced in women patients, the OR for REM AHI ≥ 57.24 events/h relative to REM AHI < 30.36 events/h was 2.79 (95% CI, 1.16– 6.73; P = 0.022); however, there was no significant difference in male patients.
Conclusion: REM AHI was significantly and positively associated with hypertension in patients with OSA, and the effect was more pronounced in female patients.
Keywords: hypertension, obstructive sleep apnea, apnea hypopnea index during rapid eye movement sleep, risk factor
Introduction
Obstructive sleep apnea (OSA) is a common sleep-related breathing disorder characterized by repeated partial or complete collapse of the upper airway. Nearly one billion adults 30 to 69 years of age worldwide reportedly have OSA, and the number of affected individuals is highest in China, followed by the United States, Brazil, and India.1 OSA poses a threat to the cardiovascular system due to its adverse effects of intermittent hypoxemia and hypercapnia, sleep fragmentation, increased oxidative stress, excessive inflammatory response and frequent changes in intrathoracic pressure, leading to increased overall morbidity and mortality rates.2
Hypertension is one of the most prevalent cardiovascular diseases associated with OSA. It has been estimated that more than 50% of patients with OSA have underlying hypertension.3 Moreover, the prevalence of OSA is greater than 30% among patients with hypertension, and as high as 80% among patients with resistant hypertension.4,5 OSA is also associated with essential hypertension (mild OSA: OR = 1.184; moderate OSA: OR = 1.316; severe OSA: OR = 1.561; all: P<0.05).6 It is important to understand the pathophysiological link between OSA and hypertension, which can provide a theoretical basis for better prevention and treatment of hypertension in patients with OSA.
Repeated intermittent hypoxia and increased sympathetic activity are widely considered to be the major mechanisms by which OSA increases cardiovascular risk.7 It has been acknowledged that rapid eye movement (REM) sleep is associated with more frequent and prolonged apnea events, greater oxygen desaturation, and greater sympathetic activity than non-rapid eye movement (NREM) sleep, resulting in more frequent and greater fluctuations of blood pressure (BP).8 Some studies have suggested a significant association between REM-OSA and hypertension.9–12 Moreover, REM OSA occurs more often in women than in men due to the differences in airway structure and gender hormones.13,14 However, only few studies have focused on gender differences in the relationship between REM OSA and hypertension.
Therefore, the objective of this cross-sectional study was to investigate the clinical and polysomnographic characteristics of OSA patients with hypertension, as well as to explore the gender differences in the relationship between REM OSA and hypertension.
Materials and Methods
Study Subjects
The medical records of a total of 808 patients diagnosed with OSA at the sleep center of Tianjin Medical University General Hospital from January 1, 2017, to December 31, 2020, were reviewed. Patients who fulfilled any of the following criteria were excluded: age <18 years; severe cardiopulmonary or other diseases that may lead to hypoxia; previous treatment for sleep-disordered breathing; central/mixed sleep apnea diagnosed by polysomnography (PSG); incomplete data; and total sleep time < 4h. The study protocol was approved by the Medical Ethics Committee of Tianjin Medical University General Hospital (IRB No.2020-WZ-091), and the procedures followed were in accordance with the principles of the Helsinki Declaration in 1995, as revised in 2013. The requirement for informed consent was waived because the patients’ information was extracted from electronic medical records at the sleep center, and the patients’ identities were maintained anonymous.
Covariates
Demographic and anthropometric data, including gender, age, smoking habit, alcohol consumption, medical history, family medical history, height, weight, neck circumference (NC), waist circumference (WC), systolic blood pressure (SBP), and diastolic blood pressure (DBP), were obtained from medical records. Body mass index (BMI) was calculated as weight divided by the square of the height (Kg/m2), waist-to-height ratio (WHtR) was calculated as waist circumference divided by height, and A Body Shape Index (ABSI) was calculated using the following formula: ABSI = WC/(BMI2/3*height1/2) (m11/6Kg−2/3).15
Polysomnography
Daytime sleepiness was assessed based on the Epworth sleepiness scale (ESS) score. Standard PSG (Alice 5 Diagnostic Sleep System; Philips Respironics, Bend, OR, USA) was performed at the sleep center of our hospital. Sixteen channels were used simultaneously to perform the following tests: electroencephalography, electrooculography, submental and leg electromyography, electrocardiography, airflow in the mouth and nose (thermistor, nasal pressure transducer), chest and abdominal respiratory efforts, blood oxygen saturation (pulse oximetry), snoring, and body position parameters. PSG data were automatically analyzed using a sleep analysis system and assessed by a professional polysomnographic technologist.
Definitions
Apnea was defined as cessation of airflow for at least 10s with continued effort or lack of effort to breathe. Hypopnea was defined as a reduction in airflow accompanied by electroencephalogram arousal and/or a ≥3% decrease in oxygen desaturation from the pre-event baseline. The apnea hypopnea index (AHI) was defined as the number of apnea and hypopnea events occurring per hour. An AHI score of < 5 was considered normal or simple snoring, score of 5 to 15 was considered mild OSA, score of 15 to 30 was considered moderate OSA, and score of >30 was considered severe OSA.16 Hypertension was defined as SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, self-reported history of hypertension, or use of antihypertensives.17
Statistical Analyses
The Kolmogorov–Smirnov test was performed to assess the normality of distribution. All continuous variables are presented as mean ± standard deviation (SD) or median with interquartile range (IQR), and the differences between the two groups for continuous variables were compared using Student’s t-test or the Mann–Whitney U-test. All categorical variables are presented as number (percentage); differences between these variables were analyzed using the chi-square test. The variables with statistical significance in univariate analysis were considered as potential risk factors. Then, multivariate binary stepwise logistic regression analysis was performed to select independent risk factors for hypertension in patients with OSA. Binary logistic regression analysis was used to assess the role of each risk factor in hypertension after adjusting for covariates in three models: model 1 was adjusted for age; model 2 was adjusted for model 1 plus current smoking and current alcohol consumption; and model 3 was adjusted for model 2 plus BMI, gender, and family history of hypertension. Risk factors were also separately categorized into tertiles, and the lowest tertile was used as the reference group in the binary logistic regression. The results are shown as odds ratios (ORs) and 95% confidence intervals (CIs); a two-tailed P-value <0.05 was considered statistically significant. SPSS 25.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and GraphPad Prism 8.0 (GraphPad, San Diego, CA, USA) was used to construct figures.
Results
Clinical and Polysomnographic Characteristics of the Study Population
In this study, 416 OSA patients had hypertension and 392 did not. Compared with OSA patients without hypertension, those with hypertension were older (50.35 vs 43.90 years) and more obese (30.84 vs 29.07Kg/m2); had higher NC (42.40 vs 41.15 cm), WC (107.63 vs 101.70 cm), WHtR (0.63 vs 0.59), and ASBI score (0.084 vs 0.083 m11/6Kg−2/3); and had a higher proportion of family history of hypertension (73.3% vs 49.0%) (all P < 0.001). There was no significant difference in gender ratio, smoking habit, and current alcohol consumption between the groups (Table 1). As shown in Table 2, OSA patients with hypertension had higher ESS scores, AHI, AHI during rapid eye movement stage (REM AHI), AHI during non-rapid eye movement stage (NREM AHI), oxygen desaturation index (ODI), arousal index (ArI), apnea index (AI), hypopnea index (HI), mean hypopnea time (MHT), percentage of N1 sleep stage in total sleep time (N1%), proportion of cumulative sleep time with SpO2 below 90% in total sleep time (T90), and had lower mean oxygen saturation by finger pulse oximetry (MeanSpO2) and minimum oxygen saturation by finger pulse oximetry (MinSpO2) (all P < 0.05). However, there was no significant difference in the total sleep time (TST), longest apnea time (LAT), mean apnea time (MAT), longest hypopnea time (LHT), percentage of rapid eye movement sleep stage in total sleep time (REM%), percentage of N2 sleep stage in total sleep time (N2%), and percentage of N3 sleep stage in total sleep time (N3%).
 |
Table 1 Clinical Characteristics of the Study Population |
 |
Table 2 Polysomnographic Characteristics of the Study Population |
Risk Factors for Hypertension in Patients with OSA
The indicators with statistical significance in univariate analysis, such as AHI, ODI, and NREM AHI (shown in Tables 1 and 2), were included in the multivariate binary stepwise logistic regression analysis. As shown in Table 3 and Figure 1, the multivariate binary stepwise logistic regression analysis showed that age (OR = 1.057; 95% confidence interval [CI] 1.043–1.070, P < 0.001), WC (OR = 1.043; 95% CI 1.029–1.057, P < 0.001), family history of hypertension (OR = 3.604; 95% CI 2.598–5.000; P < 0.001), and REM AHI (OR = 1.007; 95% CI 1.000–1.014, P = 0.037) were positively associated with hypertension in patients with OSA. REM AHI was the only indicator in PSG related to hypertension in patients with OSA.
 |
Table 3 Multivariate Binary Stepwise Logistic Regression Analysis of Risk Factors for Hypertension in Patients with OSA |
Association of REM AHI with the Risk of Hypertension in Patients with OSA
Table 4 summarizes the results from binary logistic regression models estimating the risk of prevalent hypertension in patients with OSA based on REM AHI categories after adjusting for age, smoking, alcohol consumption, BMI, gender, and family history of hypertension. For the overall sample, after adjustment for potential confounders in model 3, the risk of hypertension increased with an increasing level of REM AHI (OR = 1.61 for tertile3 vs tertile1; 95% CI 1.07–2.42; P = 0.022 for trend). Consistent with this, when taken as a continuous variable, each SD increment of REM AHI was also significantly associated with the increased risk of hypertension in patients with OSA (OR = 1.007; 95% CI 1.001–1.014; P < 0.05). REM AHI was also positively related to the risk of hypertension among women cohort in model 3 whether as a categorical or continuous variable (all P < 0.05). However, in men cohort with OSA, no significant relationships were observed between increasing levels of REM AHI and the risk of hypertension in model 3 (all P > 0.05). Higher REM AHI categories were significantly associated with a higher risk of prevalent hypertension in the total cohort (P = 0.022; Figure 2A). In the subgroup with male cohort, higher REM AHI categories were associated with higher odds of hypertension but did not reach statistical significance (P = 0.181; Figure 2B). In contrast, the association between higher REM AHI categories and higher odds of prevalent hypertension in the subgroup with women cohort was highly significant (P = 0.022; Figure 2C).
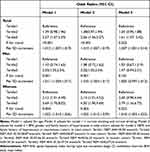 |
Table 4 Odds Ratio and 95% Confidence Intervals (CI) of Hypertension by Different Status of REM AHI in Patients with OSA |
Prevalence of Hypertension by Tertiles of REM AHI in Patients with OSA
The prevalence of hypertension by tertiles of REM AHI in the total cohort, male cohort, and female cohort are shown in Figure 3. The percentages of hypertension in the total cohort significantly increased in accordance with increasing tertiles of REM AHI (42.91% in tertile1, 52.35% in tertile2, and 59.92% in tertile3; P = 0.001). Moreover, the prevalence of hypertension increased in line with increasing tertiles of REM AHI in the male cohort, but did not achieve statistical significance (44.69% in tertile1, 48.65% in tertile2, and 56.67% in tertile3; P = 0.067). However, the prevalence of hypertension increased in line with increasing tertiles of REM AHI in the female cohort (40.00% in tertile1, 50.00% in tertile2, and 68.13% in tertile3; P = 0.007).
Discussion
It is generally acknowledged that hypertension is a multifactorial disease resulting from genetic and environmental factors.18 In this study, we found that REM AHI was significantly and positively associated with hypertension in patients with OSA after adjusting for potential confounding factors. Importantly, we found a graded relationship between increasing REM AHI severity categories and prevalent hypertension in the women of our sample. In contrast, there was no significant relationship between REM AHI severity categories and the risk of hypertension in men.
REM sleep, which was initially discovered in 1953, accounts for only 20% of total sleep time and is primarily concentrated in the latter half of the sleep period.19,20 Various mechanisms are likely to be involved in the link between REM OSA and hypertension. It has been reported that there were significant changes in autonomic nervous system and cardiopulmonary function during REM sleep, which may lead to serious cardiovascular consequences.21 Sympathetic activity was highest during REM sleep, resulting in a significant increase in blood pressure.22 Somers et al22 reported mean BP was 92 ± 4.5 mmHg when awake and reached peak levels of 116 ± 5 and 127 ± 7 mmHg during non-REM sleep and REM sleep in patients with OSA, respectively. Moreover, there was an increased tendency for upper airway collapse during REM sleep due to the decreased genioglossus muscle tone secondary to the inhibition of the hypoglossal nerve.23 The ventilation drive for hypoxia and hypercapnia was reduced significantly during REM sleep.24 These physiological phenomena could partly explain why obstructive apnea and hypopnea during REM sleep are longer in duration, are accompanied by greater oxygen desaturation, and lead to greater fluctuations in BP. These changes in hemodynamic and ventilation control during REM sleep could play a crucial role in the prevalence of hypertension.
The large, longitudinal, and community-based Wisconsin Sleep Cohort study involving 4385 sleep studies of 1451 individuals for 24 years established the significant and independent duration-association of REM AHI with prevalent hypertension and incident hypertension. The OR for REM AHI ≥15 relative to REM AHI <1 was 1.26 (95% CI, 1.01–1.57; P = 0.04) and as high as 3.38 (95% CI, 1.70–6.72; P < 0.001) in the subsample of subjects with non-REM AHI ≤5. A two-fold increase in REM AHI was associated with a 24% higher risk of hypertension (OR = 1.24; 95% CI, 1.08–1.41; P<0.005) for patients with non-REM AHI ≤5. In addition, according to the longitudinal analysis, compared with patients with REM AHI ≤5, those with REM AHI ≥15 had a 77% higher risk of hypertension development.23 Appleton et al11 also conducted a longitudinal study based on community-dwelling men, known as the Men Androgens Inflammation Lifestyle Environment and Stress study, and found that REM AHI ≥20 was associated with prevalent and recent-onset hypertension, even among those with AHI <10 and those with non-REM AHI <10. According to another longitudinal study based on the Wisconsin Sleep Cohort, REM AHI was also shown to be independently associated with the incidence of non-dipping BP.10 Consistent with this, in our hospital-based cross-sectional study, we found that high REM AHI levels were associated with prevalence of hypertension in patients with OSA. Compared with patients with REM AHI ≥58.87 events/h, those with REM AHI 30.50 events/h had an average 61% higher risk of hypertension. Every 10 additional apnea or hypopnea events per hour during REM sleep increased the risk of hypertension by an average of 7%. Additionally, we found that this effect was more pronounced in women patients, the OR for REM AHI ≥57.24 events/h relative to REM AHI <30.36 events/h was 2.79 (95% CI, 1.16–6.73; P = 0.022); however, there was no significant difference in male patients. This may be related to the greater susceptibility of REM OSA in females.14 Females may be protected from disordered breathing during much of sleep, which can be attributed to increased genioglossal muscle tension and ventilatory response induced by estrogen and progesterone. However, this protective mechanism could be greatly weakened by the disturbance of muscle tension associated with REM sleep. Moreover, compared with males, the upper airway of females may be more prone to collapse in REM sleep because of its smaller size. Furthermore, medullary chemosensitivity to hypoxia and hypercapnia is reduced during REM sleep, limiting the stimulatory effect of estrogen and progesterone on breathing.12,14
Additionally, our findings may have important clinical implications for the duration of continuous positive airway pressure (CPAP) use that is needed to reduce the risk of hypertension and control BP. It has been reported that 4-h use of nightly CPAP therapy during 70% of all nights, which translates to an average CPAP use of 2.8 h every night, is considered adequate adherence to therapy, while the effects of CPAP therapy on hypertension were modest, even negative.25 Grimaldi et al26 reported that 3-h to 4-h use of CPAP therapy overnight would result in 75% and 60% of obstructive events left untreated during REM sleep, respectively. Moreover, it was shown that 7-h use of CPAP therapy would overlap with most REM sleep. These results may partly explain the negative or modest effects of CPAP therapy on BP control. A randomized controlled trial of patients with OSA showed that the hours of CPAP use were significantly correlated with the reduction of 24-h mean BP.27 A large meta-analysis suggested that regular CPAP therapy for at least 5 h per night could result in a reduction in both systolic BP and diastolic BP.28 Another randomized controlled trial based on patients with OSA demonstrated a significant reduction in incident hypertension in OSA patients adhering to an average of 6 h of regular CPAP therapy use per night.29 Further studies are needed to determine the effective CPAP therapy time required to control BP. Additionally, one study showed that up to 70% of sleep studies involving REM AHI ≥15 would have clinically classified cases as no OSA or mild OSA based on the current diagnostic criteria; however, this degree of disordered breathing during REM sleep is associated with an increased risk of hypertension.9 Additional research is needed to explore whether such patients need to be treated.
This study has several limitations. First, due to the cross-sectional nature of this study, we could not identify causality. In the future, we will conduct a prospective study about the development of hypertension in OSA patients.
Second, other potential confounding factors, such as dietary habits, labor intensity, economic status, and psychological conditions, were not included in our analysis because the data were extracted from the medical system. Finally, this was a single-center study based on the patients with OSA; therefore, it was restricted and could not be expanded to other groups.
Conclusions
Notwithstanding the limitations, this study presented REM AHI was significantly and positively associated with hypertension in patients with OSA. The effect was more pronounced in female patients. In contrast, no significant associations were observed between REM AHI and hypertension in men with OSA.
Data Sharing Statement
The data used during the current study are available from the corresponding author upon reasonable request.
Ethics Statement
The study protocol was approved by the Medical Ethics Committee of Tianjin Medical University General Hospital (IRB No.2020-WZ-091), and the procedures followed were in accordance with the principles of the Helsinki Declaration in 1995, as revised in 2013. The requirement for informed consent was waived because the patients’ information was extracted from electronic medical records at the sleep center, and the patients’ identities were maintained anonymous.
Acknowledgments
This work was supported by Grants from the Natural Science Foundation of China (No. 81970084). We would like to thank Editage (www.editage.cn) for English language editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors took part in drafting, writing, revising, and critically reviewing the article; reviewed and gave final approval for the version to be published; have agreed on the journal to which the article has been submitted; and agreed to take responsibility and be accountable for all aspects of the manuscript.
Disclosure
The authors declare that they have no competing interests.
References
1. Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi:10.1016/s2213-2600(19)30198-5
2. Mashaqi S, Gozal D. Obstructive sleep apnea and systemic hypertension: gut dysbiosis as the mediator? J Clin Sleep Med. 2019;15(10):1517–1527. doi:10.5664/jcsm.7990
3. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi:10.1056/nejm200005113421901
4. Muxfeldt ES, Margallo V, Costa LM, et al. Effects of continuous positive airway pressure treatment on clinic and ambulatory blood pressures in patients with obstructive sleep apnea and resistant hypertension: a randomized controlled trial. Hypertension. 2015;65(4):736–742. doi:10.1161/hypertensionaha.114.04852
5. Thunström E, Manhem K, Rosengren A, Peker Y. Blood pressure response to losartan and continuous positive airway pressure in hypertension and obstructive sleep apnea. Am J Respir Crit Care Med. 2016;193(3):310–320. doi:10.1164/rccm.201505-0998OC
6. Hou H, Zhao Y, Yu W, et al. Association of obstructive sleep apnea with hypertension: a systematic review and meta-analysis. J Glob Health. 2018;8(1):010405. doi:10.7189/jogh.08.010405
7. Kohler M, Stradling JR. CrossTalk proposal: most of the cardiovascular consequences of OSA are due to increased sympathetic activity. J Physiol. 2012;590(12):
8. Pirzada AR, BaHammam AS. Rapid eye movement predominant obstructive sleep apnoea: prognostic relevance and clinical approach. Curr Opin Pulm Med. 2021;27(6):514–522. doi:10.1097/mcp.0000000000000817
9. Mokhlesi B, Finn LA, Hagen EW, et al. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190(10):1158–1167. doi:10.1164/rccm.201406-1136OC
10. Mokhlesi B, Hagen EW, Finn LA, Hla KM, Carter JR, Peppard PE. Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin Sleep Cohort. Thorax. 2015;70(11):1062–1069. doi:10.1136/thoraxjnl-2015-207231
11. Appleton SL, Vakulin A, Martin SA, et al. Hypertension is associated with undiagnosed OSA during rapid eye movement sleep. Chest. 2016;150(3):495–505. doi:10.1016/j.chest.2016.03.010
12. Rishi AR, Rishi MA. Rapid eye movement related obstructive sleep apnea: where do we stand? Respir Investig. 2021;59(5):589–595. doi:10.1016/j.resinv.2021.06.006
13. Basoglu OK, Tasbakan MS. Gender differences in clinical and polysomnographic features of obstructive sleep apnea: a clinical study of 2827 patients. Sleep Breath. 2018;22(1):241–249. doi:10.1007/s11325-017-1482-9
14. Koo BB, Dostal J, Ioachimescu O, Budur K. The effects of gender and age on REM-related sleep-disordered breathing. Sleep Breath. 2008;12(3):259–264. doi:10.1007/s11325-007-0161-7
15. Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7(7):e39504. doi:10.1371/journal.pone.0039504
16. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of sleep medicine. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.2172
17. Guide TJCoCHP. Guidelines on Prevention and Treatment of Hypertension in China (2018 edition). Chin J Cardiovasc. 2019;24(1):24–56.
18. Olack B, Wabwire-Mangen F, Smeeth L, Montgomery JM, Kiwanuka N, Breiman RF. Risk factors of hypertension among adults aged 35-64 years living in an urban slum Nairobi, Kenya. BMC Public Health. 2015;15:1251. doi:10.1186/s12889-015-2610-8
19. Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118(3062):273–274. doi:10.1126/science.118.3062.273
20. Alzoubaidi M, Mokhlesi B. Obstructive sleep apnea during rapid eye movement sleep: clinical relevance and therapeutic implications. Curr Opin Pulm Med. 2016;22(6):545–554. doi:10.1097/mcp.0000000000000319
21. Mokhlesi B, Punjabi NM. “REM-related” obstructive sleep apnea: an epiphenomenon or a clinically important entity? Sleep. 2012;35(1):5–7. doi:10.5665/sleep.1570
22. Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. doi:10.1172/jci118235
23. Mokhlesi B, Carter JR. Growing evidence linking OSA during rapid eye movement sleep to systemic hypertension. Chest. 2016;150(3):475–477. doi:10.1016/j.chest.2016.03.047
24. Peppard PE, Ward NR, Morrell MJ. The impact of obesity on oxygen desaturation during sleep-disordered breathing. Am J Respir Crit Care Med. 2009;180(8):788–793. doi:10.1164/rccm.200905-0773OC
25. Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. doi:10.1016/j.smrv.2011.01.003
26. Grimaldi D, Beccuti G, Touma C, Van Cauter E, Mokhlesi B. Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care. 2014;37(2):355–363. doi:10.2337/dc13-0933
27. Martínez-García MA, Capote F, Campos-Rodríguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–2415. doi:10.1001/jama.2013.281250
28. Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167(8):757–764. doi:10.1001/archinte.167.8.757
29. Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–2168. doi:10.1001/jama.2012.4366
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



