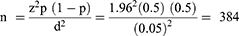Back to Journals » Infection and Drug Resistance » Volume 15
High Prevalence of Multi-Drug Resistance and Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae Among Hospitalized Patients Presumptive for Bacterial Infection at Debre Berhan Comprehensive Specialized Hospital, Ethiopia
Authors Sahle Z, Engidaye G, Shenkute D , Metaferia Y, Shibabaw A
Received 8 March 2022
Accepted for publication 18 May 2022
Published 25 May 2022 Volume 2022:15 Pages 2639—2656
DOI https://doi.org/10.2147/IDR.S363988
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Zenawork Sahle,1 Getabalew Engidaye,1 Demissew Shenkute,2 Yeshi Metaferia,3 Agumas Shibabaw3
1Department of Medical Laboratory Science, Debre Berhan Health Science College, Debre Berhan, Ethiopia; 2Department of Medical Laboratory Science, College of Health Sciences, Debre Berhan University, Debre Berhan, Ethiopia; 3Department of Medical Laboratory Science, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Correspondence: Zenawork Sahle, Department of Medical Laboratory Science, Debre Berhan Health Science College, PO.Box: 37, Debre Berhan, Ethiopia, Tel +251915536212, Fax +251116814296, Email [email protected]
Background: Multi-drug resistant Enterobacteriaceae (MDR-E), primarily extended-spectrum beta-lactamase producers (ESBLs), have emerged as a major public health concern. This study aimed to determine the prevalence of multi-drug resistance and extended-spectrum beta-lactamase-producing Enterobacteriaceae among hospitalized patients presumptive for bacterial infections at Debre Berhan Comprehensive Specialized Hospital, Ethiopia.
Methods: A hospital-based cross-sectional study was conducted from January to May 2021. A total of 384 hospitalized patients presumptive for bacterial infections were included in the study. Urine, wound, blood, stool, and sputum samples were collected and cultured on MacConkey agar, Cysteine Lactose Electrolyte Deficient medium, and Blood agar. Identification was done using a panel of biochemical tests. The antimicrobial susceptibility test was done by disc diffusion. Screening of ESBL production was done by using cefotaxime and ceftazidime and confirmed by the combination disk method per clinical laboratory standard institute guidelines. Data analysis was performed by Statistical Package for Social Sciences software version 25, and a P-value ≤ 0.05 was considered as statistically significant.
Results: Out of 384 study participants, a total of 164 Enterobacteriaceae were isolated. The overall multi-drug resistance rate (MDR) was 92.1%. The overall prevalence of ESBL-PE was 104 (63.4%). E. coli 50 (30.5%) and K. pneumoniae 24 (14.6%) were the predominant ESBL producers. The highest ESBL producers E. coli (13.4%) and K. pneumoniae (6.1%) were isolated from urine sample. History of antibiotic use for the last three months (P-value=0.01), admission in neonatal intensive care unit (P-value=0.02), history of hospital stays (P-value=0.01), and chronic disease (P-value=0.04) showed statistically significant association with ESBL-PE infection.
Conclusion: The prevalence of MDR-E and ESBL-PE was high. Therefore, strong infection prevention and control measures and careful selection of antibiotics are needed in the study area to block the transmission and infection in the healthcare setting.
Keywords: bacterial infection, extended-spectrum beta-lactamase Enterobacteriaceae, hospitalized patients, Debre Berhan, Ethiopia
Background
Enterobacteriaceae are a large family of gram-negative, facultative anaerobes and non-spore-forming Bacilli. The family includes enteric bacteria that are both natural and incidentally pathogenic, such as Escherichia spp., Klebsiella spp, Proteus spp, Enterobacter spp., Citrobacter spp., Providencia spp, Serratia spp, Salmonella spp, and Shigella spp.1
Antimicrobial resistance (AMR) is a major public health concern around the world. Higher mortality rates, extended hospitalization, medication failure, and health costs are linked to infections caused by resistant species. Infections caused by MDR bacteria have been declared an emerging public health epidemic by the World Health Organization (WHO).2
The widely used antibiotics for the treatment of infections caused by multi-drug resistant Enterobacteriaceae (MDR-E) are antibacterial agents of the beta-lactam category. However, these bacteria can hydrolyze certain beta-lactam antibiotics by the synthesis of beta-lactamase such as ESBLs and carbapenemase.3
The main mechanism of resistance to beta-lactam antibiotics in Enterobacteriaceae is the synthesis of beta-lactamases. Among the beta-lactamases, the production of ESBLs is the most common. These enzymes can break or hydrolyse many beta-lactam antibiotics.4
Extended-spectrum beta-lactamase (ESBLs)-producing pathogenic Enterobacteriaceae cause a serious antibiotic management problem, as the enzyme-encoding genes are easily transferred from one organism to the other via plasmids.5 Since ESBL-PE are also resistant to other antibiotic families, advanced procedures like surgeries, cancer treatment, and organ transplantation could become increasingly risky. Inability to treat these patients would increase morbidity and mortality. This problem is more pronounced in areas where there is no adequate infection control program, periodic surveillance, and multidrug-resistant bacteria detection laboratory facility.6
The effect of antibiotic resistance is not only on the individual patient but also on the entire health system. Infected patients require more complex therapy, take longer to recover, and are more likely to experience treatment failure and mortality. This will result in devastating financial, social, and psychological effects. Furthermore, AMR also imposes enormous financial burdens on society as a whole.7
In sub-Saharan Africa, although estimations of the magnitude of the problem of antibacterial resistance are difficult and there is limited capacity for antibiotic resistance detection and surveillance, the existing reports showed a high antibiotic resistance rate to commonly used antibiotics. This condition is aggravated by lack of good hygiene, lack of safe water, civil conflicts, lack of strong infection control strategy and increasing numbers of immune-compromised people.8
In East Africa, gram-negative bacteria exhibited relatively high levels of resistance to antibiotics commonly used (50–100% to ampicillin and cotrimoxazole). They cause different infections including bloodstream infection (BSI), urinary tract infection (UTI), lower respiratory tract infections (LRTIs), and wound infection. Such infections are primarily treated with beta-lactam class of antibiotics such as penicillins, cephalosporins, monobactams, and carbapenems.9
Knowing the current status on the prevalence of MDR-E such as ESBL-PE infection is very important to understand their epidemiology and disease burden, to implement hospital infection control strategy and to prevent the spread of these bacteria. Although ESBL-PE causes significant and devastating public health problems, little is known about the prevalence of ESBL-PE infection in Ethiopia. Only a few studies have been conducted in Ethiopia regarding the prevalence of ESBL-PE infection.10,11 Furthermore, to the best of our knowledge, most microbiology laboratories in Ethiopia do not perform ESBLs detection tests both for diagnostic and for infection control or surveillance purpose. Hence, this study aimed to determine the prevalence of multi-drug-resistant Enterobacteriaceae, ESBL-PE and associated factors suspected for bacterial infection at Debre Berhan Comprehensive Specialized Hospital, Amhara Region, Ethiopia.
Materials and Methods
Study Design, Period and Setting
A hospital-based cross-sectional study was conducted from January 2021 to May 2021. The study was conducted at Debre Berhan Comprehensive Specialized Hospital (DBCSH), which is found in Debre Berhan town, North Shoa, Amhara Region, Ethiopia. Debre Berhan is located 130 Km far from the capital city of the country, Addis Ababa. According to the 2007 population and housing census of Ethiopia, the town has a total of 160,847 people. Debre Berhan is situated at an altitude of 2840 m above sea level with a mean annual temperature that ranges from 10 to 16°C. The weather condition of the town and surrounding areas is relatively cold, dry, and windy with two distinctive seasons: summer and winter.12 DBCSH was first established as a health facility in 1937 by Italians to serve their soldiers during the 2nd Italian attempt to colonize Ethiopia. It has 162 beds and provides services including paediatrics, emergency, surgical, medical gynaecology, psychiatry, ophthalmology, anti-retroviral treatment (ART) services, neonatal intensive care unit, microbiological laboratories, viral load, and other health care services.
Population
All patients who sought and got medical services at DBCSH were the source population, while all hospitalized patients who were suspected of bacterial infections for ≥48 hours during the study period were the study population. All hospitalized patients suspected of bacterial infection who were given informed consent or assent were included in the study.
Sample Size Determination and Sampling Technique
The sample size was determined using a single population proportion formula as follows:
Where: n = the minimum required sample size; z = Standard normal distribution value at 95% CI, which is 1.96; P = the prevalence, d = the margin of error taken as 5%.
All hospitalized patients who were clinically presumptive for different bacterial infections were included conveniently, and study participants who did not provide complete data and appropriate specimen (saliva and/or contaminated sputum), insufficient volume of all specimens, monitored for less than 48 hours, critically ill patients, and unable to give a specimen were excluded.
Data Collection
Sociodemographic and clinical data were collected using a structured questionnaire after obtaining informed consent or assent from their guardians/parents for each study participant. For participants who cannot read and write, the information sheet was read to them, and a witness was signed before data collection.
Clinical Specimen Collection and Processing
Blood samples: 10 mL of venous whole blood from adults, 5mL from the paediatrics and 2mL from neonates were collected aseptically. Then, blood samples were inoculated in tryptic soya broth (Oxoid, LTD) and incubated immediately at 35–37°C and inspected daily for the signs of bacterial growth until 7 days. Bottles that showed positive for growth were subcultured on blood agar (Sisco Research Laboratories Pvt. Ltd, Mumbai, India) and MacConkey agar (Oxoid, LTD). Those blood culture bottles which showed no growth were continuously monitored for the potential growth of pathogens until 7 days, and if there was no growth after 7 days, the blood culture was reported as negative. A minimum blood-to-broth ratio of 1:10 was maintained.11
Urine sample: About 10mL freshly voided midstream urine samples were collected with sterile, wide-mouthed, and leak-proof containers. A10µl (0.01mL) well-mixed urine sample was inoculated using calibrated wire loop into Cysteine Lactose Electrolyte Deficient medium (HiMedia, India) and sub-cultured into MacConkey agar and then incubated at 37°C for 24 hr under aerobic condition. The samples with significant bacteriuria (≥105 CFU/mL) were further processed, whereas specimens that produced <105CFU/mL were considered insignificant.
Sputum sample: after briefly instructing the patients to rinse their mouths with water, a sterile wide-mouth container was used to collect 2 mL purulent sputum. Sputum specimens with much watery saliva were excluded from being processed using microbiological procedures. The sputum was immediately smeared and examined for appropriateness for culturing. Specimens that had more than 25 polymorphonuclear leukocytes and less than 10 epithelial cells were inoculated into MacConkey agar and blood agar plate and incubated for 24 hr at 37°C.
Wound sample: purulent exudates, pus, and discharges were collected aseptically from the depth of the wound using a syringe or sterile cotton swab. The cotton swab was immersed in a tube of brain heart infusion transport medium. The brain-heart infusion culture was incubated for 24 hr and then sub-cultured onto MacConkey agar and blood agar plates and re incubated for 24 hr at 37°C.
Stool sample: Proper instruction was given to study participants about fresh stool sample collection. For each study participant, a sterile clean cup container was given to collect stool. A pea-sized stool was collected from each patient and inoculated onto MacConkey agar and incubated aerobically at 37°C for 18 to 24 hrs.11
Identification of Bacterial Isolates
Each culture plate was examined for the growth of Enterobacteriaceae. Lactose fermenters and non-lactose fermenters were characterized on MacConkey agar. Finally, pure colonies were taken for identification. Enterobacteriaceae was identified to species levels using triple sugar iron, indole, citrate, urea, lysine decarboxylase, H2S production, and motility.13
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing was performed using the Kirby-Bauer disk diffusion method as per Clinical Laboratory Standard Institute (CLSI) guidelines. Bacterial suspensions were prepared by suspending the freshly grown bacteria in 3–5 mL normal saline, and turbidity was adjusted to 0.5 McFarland standards. A sterile cotton swab was dipped and rotated several times and was pressed against the wall of the test tube. It was then swabbed over the entire surface of the Mueller Hinton agar (HiMedia, India). Antimicrobial susceptibility testing for all Enterobacteriaceae was performed using disk diffusion method against ampicillin (10μg), cefoxitin (30μg), gentamicin (10μg), ciprofloxacin (5μg), trimethoprim-sulfamethoxazole (1.25/23.75μg), imipenem (10μg), meropenem (10μg), amoxicillin-clavulanic acid (20/10 μg), cefotaxime (30 μg), ceftazidime (30μg), ceftriaxone (30μg), tetracycline (30μg), cefepime (30μg), and Chloramphenicol (30μg).
Screening of ESBLs
Enterobacteriaceae isolates which showed an inhibition zone size of ≤22 mm with ceftazidime (30 μg) and/or ≤27mm with cefotaxime (30 μg) considered as potential ESBL producers.14
Phenotypic Confirmation of ESBLs
A disk of ceftazidime (30 μg) and cefotaxime (30 μg) alone and their combination with Clavulanic acid (30 µg/10 µg) were placed on the MHA plate that was inoculated with a bacterial suspension of 0.5 McFarland turbidity standard and incubated overnight (18–24 hrs) at 37°C. Enterobacteriaceae that showed an increase in the inhibition zone diameter of ≥5mm for combination disks versus ceftazidime or cefotaxime disk alone were confirmed as ESBL producers.14
Data and Laboratory Quality Control
Standard operating procedures (SOP) were strictly followed verifying that media meet expiration date and quality control parameters. Culture media was prepared according to the manufacturer’s instruction, and the sterility test was performed before use. Visual inspections of crack freezing, bubbles, and contaminations in media were checked before use.
Quality control strain was used to check the quality of the medium. Each new lot was checked before use by testing the growth E. coli ATCC 25922 control strains. During ESBLs detection, ESBLs positive K. pneumoniae ATCC 700603 and ESBLs negative E. coli ATCC 25922 control strains were used. The clinical sample was collected following SOPs and immediately brought to the microbiology laboratory for bacteriological analysis. Culture results were recorded carefully before data entry, and the data were double-checked before analysis.
Data Analysis
Data were entered using Epi Data software version 3.1 and exported to SPSS 23 for analysis. The descriptive statistics such as frequency and percentage were presented with tables and graphs. Binary logistic regression was used to observe the association between dependent variables and independent variables. The variables that showed p-value less than or equal to 0.2 in bivariate logistic regression analysis were further selected for multivariate logistic regression analysis for final confirmation of statistical significance. P-value ≤0.05 with a 95% confidence interval was considered as statistically significant.
Ethical Consideration
The study was reviewed and approved by the Research and Ethics Review committee of College of Medicine and Health Sciences, Wollo University. A written permission letter was also obtained from the DBCSH. The purpose and procedures of the study were explained to the study participants and parents or guardians within the study period. Those patients and parents or guardians who gave informed consent were selected and enrolled in this study. The confidentiality of all study participants was maintained via study participant secret code and locking. For participants who cannot read and write, the information sheets were read to them, and a witness was signed that the process had been conducted appropriately. This research was carried out in accordance with the Helsinki Declaration.
Results
Sociodemographic Characteristics of Study Participants
A total of 384 study participants were enrolled in the study. Among 384 study participants, 230 (59.9%) were female and 149 (38.8%) were children aged less than 15 years, with a mean age of 2.3 ±1.23. The majority of the study participants were rural dwellers, 240 (62.5%) (Table 1).
 |
Table 1 Sociodemographic Characteristics of Study Participants at Debre Berhan Comprehensive Specialized Hospital from January to May 2021 |
Clinical Profile of the Study Participants
Of the total 384 study participants, 106 (27.6%) had a previous history of antibiotic use for the last 3 months and 76 (19.8%) had a history of hospital stays (Table 2).
 |
Table 2 Clinical Profile of the Study Participants at Debre Berhan Comprehensive Specialized Hospital from January to May 2021 |
Distribution of Enterobacteriaceae Isolates and Antimicrobial Resistance Pattern
A total of 164 Enterobacteriaceae were isolated. Among these, the highest ESBL producers E coli (13.4%) and K. pneumoniae (6.1%) were isolated from urine sample (Table 3).
 |
Table 3 ESBL-PE Isolates Against Clinical Specimen Types at Debre Berhan Comprehensive Specialized Hospital from January to May 2021 |
In this study, fourteen antibiotics from different classes were used. The highest level of susceptibility was observed in meropenem (77.4%) followed by imipenem (70.4%). The highest level of resistance was observed to ampicillin (100%) followed by tetracycline (97.6%) (Table 4).
 |
Table 4 Antimicrobial Resistance Pattern of Enterobacteriaceae Isolates at Debre Berhan Comprehensive Specialized Hospital from January to May 2021 |
From the total 164 isolated Enterobacteriaceae, 151 (92.1%) were MDR (resistance to at least 3 antibiotics in a different class). The highest rate of MDR was seen in K. pneumoniae and E. coli, accounting for average resistance of 46 (97.9%) and 68 (91.9%), respectively (Table 5).
 |
Table 5 Multi-Drug Resistance Patterns of Enterobacteriaceae Isolates at Debre Berhan Comprehensive Specialized Hospital from January to May 2021 |
Magnitude of ESBL-PE
Of the total 164 Enterobacteriaceae isolates, 134 (81.7%) were suspected for ESBL production. Out of the 134 suspected Enterobacteriaceae isolates, ESBL production was confirmed in 104 (77.6%) using a combination disc test. The overall prevalence of ESBL-PE was 104 (63.4%) (Figure 1).
 |
Figure 1 Distribution of ESBL-PE at Debre Berhan Comprehensive Specialized Hospital from January to May 2021. |
Non-ESBL-producing Enterobacteriaceae were more sensitive to antibiotics than ESBL-PE. Ampicillin and tetracycline were (100%) resistant for ESBL-PE (Figure 2).
From the total 151 (92.1%) MDR-E isolates, 104 (68.9%) were ESBL producers. From the total of 68 (91.9%) multidrug resistant E. coli isolates, 50 (73.5%) were ESBL producers (Table 6).
 |
Table 6 Distribution of ESBL-PE and MDR Isolates at Debre Berhan Comprehensive Specialised Hospital from January to May 2021 |
Associated Risk Factors for ESBL-PE Infection
In a multivariate logistic regression model, history of hospital stays, the previous history of antibiotic use for the last three months, underlying diseases and admission in neonatal intensive care unit (NICU) ward showed statistical significance for ESBL-PE infection (Table 7).
 |
Table 7 Associated Risk Factors for ESBL-PE Infection Using Bivariate and Multivariate Analysis at Debre Berhan Comprehensive Specialized Hospital from January to May 2021 |
Discussion
ESBL-PE has become currently a global problem. ESBL dissemination compromises the activity of broad-spectrum antibiotics, creating major therapeutic difficulties with a significant impact on patient outcomes.
A total of 164 Enterobacteriaceae isolates were isolated that comprise, the predominant isolate, E. coli 45.1% followed by K. pneumoniae 28.7%. This finding was comparable with the study done in Bahir Dar, reported as E. coli (58.1%) and K. pneumoniae (23.3%),11 and in Northwest Ethiopia, reported as E. coli (61.2%) and K. pneumoniae (22.8%).15 Similar findings were also reported from other countries like in Sudan, E. coli (54.4%) and K. pneumoniae (29.5%),16 in Uganda, E. coli (53.9%) and K. pneumoniae (28.7%),17 and in Tanzania, E. coli (48.9%).18
Our study revealed that E. coli (45.1%) was the predominant pathogen among isolated Enterobacteriaceae. A similar finding was also reported in Uganda (44%).3 Like other studies, our study indicated that many types of infectious diseases caused by Enterobacteriaceae were predominantly by E. coli and K. pneumoniae.10,11,19
The overall prevalence of ESBL-PE was (63.4%) which is supported by various study done in Addis Ababa (58%),20 Bahir Dar (57.6%),21 Jimma (63.4%),22 Burkina Faso (58%),23 and India (62.7%).24
The prevalence of ESBL-PE in this study was lower compared to studies done in Addis Ababa (78%),10 Bahir Dar (85.5%)11 and Uganda (89%).3 This wide variation might be attributable to discrepancies in the study demographic, sample size, the patient’s isolation, and the type of antibiotics used. Furthermore, the variation might be due to the difference in local antibiotic prescribing habits and infection control programs in different health facilities.
The prevalence of ESBL-PE in our study was higher compared to studies reported in Adama (25%),13 Burkina Faso (42%),23 Bahir Dar (46%),21 Jimma (38%),25 and Nepal (34%).26 This might be anticipated due to the good infection control strategy of the countries, study participants, variation in drug management policies, and study methodology difference.
Our findings revealed that the highest ESBL production was observed in E. coli (30.5%) followed by K. pneumoniae (14.6%). The result was comparable to those reported in Arba Minch, E. coli (44%);27 in India, E. coli (44%);24 in Burkina Faso, K. pneumoniae (26%) (71); in Adama, K. pneumonia (11.5%).13
Our result was lower compared to findings from Tanzania, reported as E. coli (68%),28 and Burkina Faso, reported as E. coli (78%).23 However, our finding was higher compared to the study done in Gondar, reported as E. coli (16.2%),29 and Morocco, reported as E. coli (19.4%).30 The potential reason for the difference in the prevalence of ESBL-PE among Enterobacteriaceae could be variation in type and frequency of isolates, sample size, study participants and geographical location.
The recent study revealed that higher resistance to ampicillin (100%), followed by tetracycline (97.0%), sulfamethoxazole-trimethoprim (85%), ceftazidime (80.0%) and cefotaxime (79%). This could be due to the overuse of the drug for many years.
Our results were comparable to studies conducted in Bahir Dar, resistant to ampicillin (100%), followed by ceftazidime (96.6%) and cotrimoxazole (93.2%),11 Arab Minch, tetracycline (91.1%) and sulfamethoxazole-trimethoprim (93.84%),27 in Nepal, ceftazidime (83.2%) and cefotaxime (74.7%),26 in Iran, sulfamethoxazole-trimethoprim (94%) and ceftazidime (73%).31
However, it was higher compared to a study conducted in Debre Markos, reported as ampicillin (70.4%), sulfamethoxazole-trimethoprim (53.1%),32 Addis Ababa, sulfamethoxazole-trimethoprim (55.6%), cefotaxime (47%), ceftazidime (44.1%).19 The possible reason for this difference might be due to the indiscriminate use of antibiotics, patient condition, and empirical treatment.
E. coli isolates showed the highest resistance to ampicillin (100%) followed by sulfamethoxazole-trimethoprim (85%), ceftazidime (83.8%), and cefotaxime (83%). This is comparable to a study done in Addis Ababa, reported as sulfamethoxazole-trimethoprim (77%), cefotaxime (62.2%), cefepime (60.3%), and ceftazidime (60.8%),20 and in Saudi Arabia, reported as ampicillin (96.61%), cefotaxime (76.27%) and ceftazidime (81.36%)11
The second most predominant Enterobacteriaceae isolates in our study were K. pneumoniae which showed the highest level of resistance to ampicillin (100%), followed by tetracycline (93.6%), sulfamethoxazole-trimethoprim (83%), ceftazidime (78.7%). Our finding was comparable to study done in Gondar, reported as Ampicillin (100%), sulfamethoxazole-trimethoprim (72.2%),33 in Tanzania, reported as ampicillin (100%),34 in Arba Minch, reported as tetracycline (91.7%).27 The increased antibiotic resistance of Enterobacteriaceae may be due to antibiotic misuse and overuse, as well as poor infection prevention, control, personal and environmental hygiene. Furthermore, mainly due to the habit of empirical treatment, infrequent bacterial identification and absence of susceptibility testing, antimicrobial resistance among bacterial infections to the commonly used antibiotics become increasing that make clinicians left with very limited choices of drugs for the treatment of infection.
The overall rate of MDR-Enterobacteriaceae (MDR-E) was (92.1%), which is fairly similar to a study done in Gondar, reported as (93.5% and 87.4%),15,35 Bahir Dar, reported as (93.1%)36 and in Nepal, reported as (96.84%).26 However, this finding was higher compared to a study done in Dessie (74.6%).37
The possible reason for high MDR might be repeated, inappropriate, and incorrect use of antimicrobial agents in empirical treatment and poor infection control strategies. Furthermore, this inconsistency may be due to the selection of antibiotics from a different class, study period, specimen type and differences in the study population. However, the increased proportion of MDR seen in this study was considered worrying because only a few treatment options remain for infections. Therefore, implementing strong infection control strategies is required to reduce the MDR burden.
This study revealed that ESBL producers were high levels of resistance to third-generation cephalosporin, penicillin and sulfonamides: ceftazidime (99%), cefotaxime (97.1%), ceftriaxone (94.2%), ampicillin (100%), amoxicillin with clavulanic acid (84.6%), tetracycline (100%) sulfamethoxazole-trimethoprim (94.2%). This is supported by studies done in Nairobi, reported as sulfamethoxazole-trimethoprim (61.1%), ceftazidime (100%), ceftriaxone (100%),38 Nepal, reported as amoxicillin with clavulanic acid (100%)26 and Addis Ababa, reported as ampicillin (99.2%), ceftazidime (98.5%), ceftriaxone (98.5%), amoxicillin with clavulanic acid (98%) and sulfamethoxazole-trimethoprim (81%).19 The possible justification for co-resistance to non-beta lactam antibiotics in this study could be explained by the fact that gene codes for ESBL production are usually found on mobile genetic elements: that may also carry resistance genes for non-beta-lactam antibiotics.
Our study shows that ESBL-PE was predominantly found E. coli (13.4%) in urine and in the blood (8.5%) followed K. pneumoniae by in urine (6.1%) and in the blood (4.9%). This might be due to the larger number of urine and blood samples included in this study.
The current study revealed that antibiotic use in the last 3 months, presence of chronic underlying disease, history of hospital stays, and admission in neonatal intensive care unit ward showed statistically significant association with ESBL-PE infection.
Participants who used antibiotics in the previous three months were more likely to be infected with ESBL-PE than those who had not. This is supported by the studies done in Gondar,29 Tanzania,28 Cyprus,39 Algeria,40 and Iran.41
Another factor associated with ESBL-PE infection was the presence of chronic disease. This is similar to studies done in Arba Minch,27 Iran41 and Algeria.40 This might be because participants who have chronic diseases will have frequent contact with health professionals which may lead to ESBL infection.
The third factor that contributed to ESBL-PE infection was the history of hospital stays which was comparable to the study done in Iran,42 Mali43 and Arba Minch.27
The other factor that contributed to ESBL-PE infection was admission to the neonatal intensive care unit. This is comparable to studies done in Iran42 and Mali.43
The discrepancy of association with this finding could be explained by the difference in the clinical condition of the study participant, sample size, methodological difference, and study setting.
This study highlights the high magnitude of MDR-E and ESBL-PE in the study area among bacterial suspected patients that require careful selection of antibiotics following antimicrobial susceptibility test, strong infection prevention and control measures, and periodic surveillance of AMR in the study area. In addition, the finding of this study informs the need for routine screening of ESBL and especially for patients admitted to the intensive care unit and patients who had chronic diseases in the study site as well as nationwide for both diagnostic and infection control or surveillance purpose to limit the dissemination and infection caused by antimicrobial resistance strain.
Conclusions
In this study, the prevalence of MDR-E and ESBL-PE was high. The majority of ESBL-PE infections were found primarily in the urine specimen. E. coli and K. pneumoniae were the most frequent ESBL-PE. History of antibiotic use in the last 3 months, admission in NICU ward, history of hospital stays, and chronic disease showed a statistically significant association to ESBL-PE infection. Therefore, strong infection control measures should be needed to block the infection and the dissemination of antimicrobial-resistant bacteria in the study area.
Data Sharing Statement
All data that support the findings of the study are included.
Acknowledgment
We would like to acknowledge DBCSH staff, for their effort in the coordination and support of the full laboratory work. Our unforgettable acknowledgment goes to Wollo University and Debre Berhan Health Science College for their funding and supporting particularly the Department of Medical Laboratory Science. Finally, we would like to acknowledge the study participants.
Funding
This research work was supported by the College of Medicine and Health Sciences, Wollo University.
Disclosure
The authors declare that they have no competing interests.
References
1. Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae. Trend Mol Med. 2012;18(5):263–272. doi:10.1016/j.molmed.2012.03.003
2. World Health Organization. Antimicrobial resistance: global report on surveillance: World Health Organization; 2014. Available from: https://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf.
3. Andrew B, Kagirita A, Bazira J. Prevalence of extended-spectrum beta-lactamases-producing microorganisms in patients admitted at KRRH, Southwestern Uganda. Int J Microbiol. 2017;2017. doi:10.1155/2017/3183076
4. Mohamudha PR, Harish B, Parija S. AmpC beta lactamases among Gram negative clinical isolates from a tertiary hospital, South India. Braz J Microbiol. 2010;41(3):596–602. doi:10.1590/S1517-83822010000300009
5. Rawat D, Nair D. Extended-spectrum β-lactamases in gram negative bacteria. J Glob Infect Dis. 2010;2(3):263. doi:10.4103/0974-777X.68531
6. Schwaber MJ, Navon-Venezia S, Schwartz D, Carmeli Y. High levels of antimicrobial coresistance among extended-spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2005;49(5):2137. doi:10.1128/AAC.49.5.2137-2139.2005
7. Livermore D. Beta-lactamase-mediated resistance and opportunities for its control. J Antimicrob Chemother. 1998;41(suppl_4):25–41. doi:10.1093/jac/41.suppl_4.25
8. Kariuki S, Dougan G. Antibacterial resistance in sub-Saharan Africa: an underestimated emergency. Ann N Y Acad Sci. 2014;1323(1):43. doi:10.1111/nyas.12380
9. Ampaire L, Muhindo A, Orikiriza P, Mwanga-Amumpaire J, Boum Y, Bebell L. A review of antimicrobial resistance in East Africa. Afr J Lab Med. 2016;5(1):1–6. doi:10.4102/ajlm.v5i1.432
10. Legese MH, Weldearegay GM, Asrat D. Extended-spectrum beta-lactamase-and carbapenemase-producing Enterobacteriaceae among Ethiopian children. Infect Drug Resist. 2017;10:27. doi:10.2147/IDR.S127177
11. Moges F, Eshetie S, Abebe W, et al. High prevalence of extended-spectrum beta-lactamase-producing Gram-negative pathogens from patients attending Felege Hiwot Comprehensive Specialized Hospital, Bahir Dar, Amhara region. PLoS One. 2019;14(4):e0215177. doi:10.1371/journal.pone.0215177
12. Ethiopia FDRo. 2007 Population and housing census of Ethiopia. (Administrative Report). Central Statistical Authority Addis Ababa; 2012.
13. Mulisa G, Selassie L, Jarso G, et al. Prevalence of extended spectrum beta-lactamase producing Enterobacteriaceae: a cross sectional study at Adama Hospital, Adama, Ethiopia. J Emerg Infect Dis. 2016;1(1):1–6.
14. Wayne P. Clinical and Laboratory Standards Institute. In: Performance Standards for Antimicrobial Susceptibility Testing; 2011.
15. Eshetie S, Gelaw A, Ayelign B, Endris M, Moges F. Multidrug resistant and carbapenemase producing Enterobacteriaceae among patients with urinary tract infection at referral hospital, Northwest Ethiopia. Antimicrob Resist Infect Control. 2015;4(1):12. doi:10.1186/s13756-015-0054-7
16. Dahab R, Ibrahim AM, Altayb HN. Phenotypic and genotypic detection of carbapenemase enzymes producing gram-negative bacilli isolated from patients in Khartoum State. F1000Research. 2017;6(1656):1656. doi:10.12688/f1000research.12432.1
17. Kateregga JN, Kantume R, Atuhaire C, Lubowa MN, Ndukui JG. Phenotypic expression and prevalence of ESBL-producing Enterobacteriaceae in samples collected from patients in various wards of Mulago Hospital, Uganda. BMC Pharmacol Toxicol. 2015;16(1):1–6. doi:10.1186/s40360-015-0013-1
18. Kajeguka DC, Kabissi F, Kamugisha B, et al. Antimicrobial resistance patterns of phenotype extended spectrum beta-lactamase producing bacterial isolates in a referral hospital in northern Tanzania. Tanzan J Health Res. 2015;17(3). doi:10.4314/thrb.v17i3.2.
19. Tekele SG, Teklu DS, Tullu KD, Birru SK, Legese MH. Extended-spectrum beta-lactamase and AmpC beta-lactamases producing gram negative bacilli isolated from clinical specimens at International Clinical Laboratories, Addis Ababa, Ethiopia. PLoS One. 2020;15(11):e0241984. doi:10.1371/journal.pone.0241984
20. Teklu DS, Negeri AA, Legese MH, Bedada TL, Woldemariam HK, Tullu KD. Extended-spectrum beta-lactamase production and multi-drug resistance among Enterobacteriaceae isolated in Addis Ababa, Ethiopia. Antimicrob Resist Infect Control. 2019;8(1):1–12. doi:10.1186/s13756-019-0488-4
21. Abera B, Kibret M, Mulu W. Extended-spectrum beta (β)-lactamases and antibiogram in Enterobacteriaceae from clinical and drinking water sources from Bahir Dar City, Ethiopia. PLoS One. 2016;11(11):e0166519. doi:10.1371/journal.pone.0166519
22. Zeynudin A, Pritsch M, Schubert S, et al. Prevalence and antibiotic susceptibility pattern of CTX-M type extended-spectrum β-lactamases among clinical isolates of gram-negative bacilli in Jimma, Ethiopia. BMC Infect Dis. 2018;18(1):1–10. doi:10.1186/s12879-018-3436-7
23. Ouedraogo A-S, Sanou M, Kissou A, et al. High prevalence of extended-spectrum ß-lactamase producing Enterobacteriaceae among clinical isolates in Burkina Faso. BMC Infect Dis. 2016;16(1):1–9. doi:10.1186/s12879-016-1655-3
24. Babu R, Kumar A, Karim S, et al. Faecal carriage rate of extended-spectrum β-lactamase-producing Enterobacteriaceae in hospitalised patients and healthy asymptomatic individuals coming for health check-up. J Glob Antimicrob Res. 2016;6:150–153. doi:10.1016/j.jgar.2016.05.007
25. Siraj SM, Ali S, Wondafrash B. Extended-spectrum β-lactamase production in Klebsiella pneumoniae and Escherichia coli at Jimma University Specialized Hospital, south-west, Ethiopia. Mol Microbiol Res. 2015;5. doi:10.1186/s13756-019-0488-4
26. Nepal K, Pant ND, Neupane B, et al. Extended spectrum beta-lactamase and metallo beta-lactamase production among Escherichia coli and Klebsiella pneumoniae isolated from different clinical samples in a tertiary care hospital in Kathmandu, Nepal. Ann Clin Microbiol Antimicrob. 2017;16(1):1–7. doi:10.1186/s12941-017-0236-7
27. Aklilu A, Manilal A, Ameya G, Woldemariam M, Siraj M. Gastrointestinal tract colonization rate of extended-spectrum beta-lactamase-and carbapenemase-producing Enterobacteriaceae and associated factors among hospitalized patients in Arba Minch General Hospital, Arba Minch, Ethiopia. Infect Drug Resist. 2020;13:1517. doi:10.2147/IDR.S239092
28. Kibwana UO, Majigo M, Kamori D, Manyahi J. High fecal carriage of extended beta lactamase producing Enterobacteriaceae among adult patients admitted in referral hospitals in Dar es Salaam, Tanzania. BMC Infect Dis. 2020;20(1):1–7.
29. Bayleyegn B, Fisaha R, Kasew D. Fecal carriage of extended spectrum beta-lactamase producing Enterobacteriaceae among HIV infected children at the University of Gondar Comprehensive Specialized Hospital Gondar, Ethiopia. AIDS Res Ther. 2021;18(1):1–9. doi:10.1186/s12981-021-00347-x
30. Arhoune B, Oumokhtar B, Hmami F, et al. Rectal carriage of extended-spectrum β-lactamase-and carbapenemase-producing Enterobacteriaceae among hospitalised neonates in a neonatal intensive care unit in Fez, Morocco. J Glob Antimicrob Res. 2017;8:90–96. doi:10.1016/j.jgar.2016.11.004
31. Mansouri S, Abbasi S. Prevalence of multiple drug resistant clinical isolates of extended-spectrum beta-lactamase producing Enterobacteriaceae in Southeast Iran; 2010.
32. Mulu W, Abera B, Yimer M, Hailu T, Ayele H, Abate D. Bacterial agents and antibiotic resistance profiles of infections from different sites that occurred among patients at Debre Markos Referral Hospital, Ethiopia: a cross-sectional study. BMC research notes. 2017;10(1):1-9.
33. Moges F, Mengistu G, Genetu A. Multiple drug resistance in urinary pathogens at Gondar College of Medical science hospital, Ethiopia. East Afr Med J. 2002;79(8):415–420. doi:10.4314/eamj.v79i8.8827
34. Seni J, Sweya E, Mabewa A, Mshana SE, Gilyoma JM. Comparison of antimicrobial resistance patterns of ESBL and non ESBL bacterial isolates among patients with secondary peritonitis at Bugando Medical Centre, Mwanza–Tanzania. BMC Emerg Med. 2016;16(1):1–5. doi:10.1186/s12873-016-0106-1
35. Alemu A, Dagnew M, Alem M, Gizachew M. Uropathogenic bacterial isolates and their antimicrobial susceptibility patterns among HIV/AIDS patients attending Gondar University Specialized Hospital Gondar, Northwest Ethiopia; 2013.
36. Biadglegne F, Abera B. Antimicrobial resistance of bacterial isolates from urinary tract infections at Felege Hiwot Referral Hospital, Ethiopia. Ethiop J Health Dev. 2009;23(3):236–238.
37. Kibret M, Abera B. Antimicrobial susceptibility patterns of E. coli from clinical sources in northeast Ethiopia. Afr Health Sci. 2011;11(3):40–45. doi:10.4314/ahs.v11i3.70069
38. Magale H, Kassim I, Odera S, Omolo M, Jaoko W, Jolly P. Antibiotic susceptibility of organisms causing urinary tract infection in patients presenting at Kenyatta National Hospital, Nairobi. East Afr Med J. 2015;92(7):333–337.
39. Ruh E, Hoti K, Fekrat A, et al. Extended-spectrum β-lactamase, plasmid-mediated AmpC β-lactamase, fluoroquinolone resistance, and decreased susceptibility to carbapenems in Enterobacteriaceae: fecal carriage rates and associated risk factors in the community of Northern Cyprus. Antimicrob Resist Infect Cont. 2019;8(1):1–10.
40. Medboua-Benbalagh C, Touati A, Kermas R, et al. Fecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae strains is associated with worse outcome in patients hospitalized in the pediatric oncology unit of Beni-Messous hospital in Algiers, Algeria. Microbial Drug Res. 2017;23(6):757–763. doi:10.1089/mdr.2016.0153
41. Norouzi F, Hasanvand F, Nojoomi F. Risk factors associated with ESBL and CPE acquisition among pediatrics: a systematic review. Infect Epidemiol Microbiol. 2018;4(1):35–40.
42. Sharif MR, Soltani B, Moravveji A, Erami M, Soltani N. Prevalence and risk factors associated with extended spectrum beta lactamase producing Escherichia coli and Klebsiella pneumoniae isolates in hospitalized patients in Kashan (Iran). Electron Physician. 2016;8(3):2081. doi:10.19082/2081
43. Sangare SA, Maataoui N, Maiga AI, et al. Very high prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in bacteremic patients hospitalized in teaching hospitals in Bamako, Mali. PLoS One. 2017;12(2):e0172652. doi:10.1371/journal.pone.0172652
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.