Back to Journals » Cancer Management and Research » Volume 11
High preoperative serum prealbumin predicts long-term survival in resected esophageal squamous cell cancer
Authors Wei J, Jin M, Shao Y, Ning Z, Huang J
Received 30 April 2019
Accepted for publication 12 August 2019
Published 26 August 2019 Volume 2019:11 Pages 7997—8003
DOI https://doi.org/10.2147/CMAR.S214037
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Jun Wei,1 Ming Jin,2 Yingjie Shao,1 Zhonghua Ning,1 Jin Huang1
1Department of Radiation Oncology, The Third Affiliated Hospital of Soochow University, Changzhou 213003, People’s Republic of China; 2Department of Clinical Laboratory, The Third Affiliated Hospital of Soochow University, Changzhou 213003, People’s Republic of China
Correspondence: Zhonghua Ning; Jin Huang
Department of Radiation Oncology, The Third Affiliated Hospital of Soochow University, 185 Juqian Road, Changzhou 213003, People’s Republic of China
Tel +86 5 196 887 1132
Email [email protected]
[email protected]
Purpose: The current study aimed to explore the prognostic role of preoperative prealbumin in resectedesophageal squamous cell carcinoma (ESCC).
Methods: A total of 1374 resected ESCC patients were retrospectively reviewed. Serum for prealbumin analyses was taken within 1–3 days before the operation. Overall survival (OS) was determined using the Kaplan-Meier method; the univariate log-rank test and the multivariate Cox proportional hazard model were used to evaluate the prognostic role of prealbumin. A receiver operating characteristic (ROC) curve was plotted, and the area under the curve (AUC) was calculated to compare the prediction accuracy of prealbumin and albumin for OS.
Results: Finally, 532 patients were included in this study. The 5-year OS rate was favourable for the high prealbumin group versus the median and low prealbumin groups (58.1% vs 44.6% and 31.1%, respectively; P<0.001). Univariate and multivariate analyses identified serum prealbumin, T stage, N stage, differentiation and albumin as independent prognostic factors for OS. ROC curves indicated that prealbumin may be superior to albumin as a prognostic predictor in ESCC patients, but the difference between the two AUCs was not statistically significant (P=0.068).
Conclusion: Prealbumin is an independent prognostic factor and a prognostic indicator of postoperative outcomes in ESCC patients. Future prospective studies are warranted to confirm our results.
Keywords: oesophageal squamous cell carcinoma, prealbumin, albumin, prognosis, serum marker
Introduction
Oesophageal cancer is highly malignant and has a high incidence in China. More than 90% of oesophageal cancers in China are oesophageal squamous cell carcinomas (ESCCs), ranking 4th in mortality among all malignant tumours in China 2011.1 Despite the development of comprehensive treatment strategies, the long-term survival of oesophageal cancer patients after radical oesophagectomy remains poor.2,3 The TNM staging system, including the T, N and M classifications, is the most important prognostic factor for determining the treatment strategy and predicting treatment outcomes. However, it is becoming increasingly apparent that the prognostic role of TNM staging for oesophageal cancer is not satisfactory. Consequently, the identification of new biological markers that can predict long-term survival and determine the treatment strategy is needed in the treatment of ESCC.
Malnutrition is a common problem in cancer patients, accounting for 80% of patients with advanced cancer.4,5 The preoperative nutritional status is associated with the host immune status as a host defence ability. Malnutrition is the most common cause of immunodeficiency.6,7 Patients with a heavy tumour burden may be vulnerable to nutritional impairment and may have a high risk of postoperative recurrence. Serum prealbumin is an important serum protein that reflects a patient’s nutritional status, and its level can predict postoperative outcomes in non-small cell lung cancer.8,9 However, the relationship between preoperative serum prealbumin and survival in ESCC has not been fully elucidated.
We hypothesized that the preoperative nutritional status might also have a potent prognostic impact in ESCC. Therefore, this study aimed to evaluate the effect of preoperative serum prealbumin and other nutritional parameters, including preoperative serum albumin, on the prognosis of oesophageal cancer patients after curative resection.
Materials and methods
Patients
From January 2002 to December 2011, a total of 1374 consecutive patients with newly diagnosed, nondisseminated ESCC who underwent curative oesophagectomy at The Third Affiliated Hospital of Soochow University (Changzhou, China) were retrospectively reviewed. All patients who were conformed to be resectable ESCC without distant metastasis by clinical and experimental examination received radical surgical resection of a transthoracic enbloc esophagectomy with mediastinal and abdominal two-field lymphadenectomies. Mediastinal lymphadenectomies were performed to include subcarinal, left and right bronchial, lower posterior mediastinum, pulmonary ligament, and paraesophageal and thoracic duct nodes. Abdominal lymphadenectomies were performed to include the paracardial, lesser curvature, left gastric, common hepatic, celiac, and splenic nodes. The paratracheal and recurrent laryngeal nerve LNs were also dissected. Cervical lymphadenectomy was only performed in case of suspicious cervical lymphadenopathy. Patients who received neoadjuvant chemotherapy, radiation, adjuvant chemotherapy or radiation, with clinical signs of infection, rheumatism or other diseases that could affect the concentrations of serum prealbumin and albumin, and with incomplete clinical or pathologic information were excluded from this study. Twenty-four patients with incomplete experimental or clinical data, 12 patients with their sections no longer available for analysis, 688 patients who received neoadjuvant or adjuvant therapy, 15 patients who died during the perioperative period, 2 patients with chronic kidney disease, 37 patients with diabetes, 1 patient with abnormal thyroid function, 3 patients with abnormal hepatic function, 6 patients with metabolic or immune rheumatic diseases, and 56 patients lost to follow-up were excluded. Finally, 532 patients were identified and included in this study. This study was approved by our Institutional Review Board. All patients provided written informed consent, and that this study was conducted in accordance with the Declaration of Helsinki.
Biological and clinical data
Sera for prealbumin analyses were taken within 1–3 days prior to the operation. Prealbumin was analysed on an automatic biochemical analyser from Beckman Instruments (Brea, CA, USA). Other clinical data, including age, gender, tumour location, staging, pathology, and survival outcomes, were also collected.
Follow-up
All patients were followed up as described previously. The end of follow-up was July 31, 2014. The observation time in this study was the interval from the date of surgical resection to the date of death or the last follow-up. Surviving patients were censored on the day of last contact.
Statistical analysis
Optimal cutoff values for classifying preoperative serum prealbumin were determined using X-tile software (http://www.tissuearray.org/rimmlab).10 Survival was calculated using the Kaplan–Meier method, and the log-rank test was used to assess differences in survival between groups. In the multivariate analysis, a forward stepwise regression analysis was carried out with a Cox proportional hazards model. A receiver operating characteristic (ROC) curve was plotted, and the area under the curve (AUC) was calculated to verify the accuracy of prealbumin and albumin for overall survival (OS) prediction by the method of Delong et al11. All statistical analyses were performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL). A P-value less than 0.05 from the two-sided test was considered statistically significant.
Results
Determination of a threshold for serum prealbumin and albumin
The cutoff values for classifying preoperative serum prealbumin were 18.8 mg/dl and 24.7 mg/dl, respectively, in our series, as calculated by using X-tile software. All patients were divided into three groups according to the preoperative serum prealbumin value (low prealbumin group: less than 18.8 mg/dl, median prealbumin group: between 18.8 mg/dl and 24.7 mg/dl, and high prealbumin group: more than 24.7 mg/dl). Similarly, the cutoff value for classifying preoperative serum albumin was 3.65 mg/dl.
Clinicopathological characteristics and survival outcomes
The clinicopathological characteristics of the 532 patients, of whom 388 (72.9%) were male and 144 (27.1%) were female, are presented in Table 1. The median age of the patients at surgery was 60 years (range, 38–85 years). All patients were staged according to the 7th edition of the International Union against Cancer/American Joint Committee on Cancer (UICC/AJCC) staging system for ESCC. The mean serum prealbumin level was 24.7±5.0 mg/dl. A total of 268 (50.3%) patients had high serum prealbumin levels in the overall population, and 54.5% and 46.9% of the patients were aged ≤60 and >60 years, respectively (P=0.213). By the end of follow-up, 271/532 patients (50.9%) had died. The overall 5-year survival rate for all patients was 49.7%. The 5-year OS rate was favourable for the high prealbumin group versus the median and low prealbumin groups (58.1% vs 44.6% and 31.1%, respectively; P<0.001).
 |
Table 1 Correlation of preoperative serum prealbumin with clinicopathological features in overall 532 patients with ESCC |
Association of serum prealbumin with the clinicopathological characteristics of ESCC patients
The preoperative serum prealbumin level correlated with the T stage of the tumour (P<0.001), BMI (P=0.003)and preoperative serum albumin (P<0.001). Preoperative serum prealbumin did not correlate with age, gender, tumour location, N stage or tumour differentiation.
Prognostic value of serum prealbumin
The univariate analysis identified serum prealbumin as a statistically significant predictive factor for OS (P<0.001; Table 2 and Figure 1). T stage, N stage, differentiation and albumin were also identified as independent predictive factors for OS by the log-rank test (Table 2 and Figure 1). The multivariate analysis for OS was performed to adjust for various prognostic factors. The following parameters were included in the Cox proportional hazards model: age (≤60 vs >60 years), gender (male vs female), T classification (T1-2 vs T3-4), N classification (N0 vs N1-3), pretreatment ALB (≤3.65 g/dl vs >3.65 g/dl) and pretreatment prealbumin (high vs low and median). Consistent with the univariate analysis, prealbumin was identified as an independent predictive factor for OS (P=0.013). T stage, N stage, differentiation and albumin were also identified as independent predictive factors for OS in the multivariate analysis. In contrast, patient age, gender and location were not independent predictive factors for OS (Table 3). To examine the influence of age, we also performed a multivariate analysis in 286 elderly ESCC patients aged more than 60 years and consistently identified serum prealbumin as a statistically significant predictive factor for OS in the elderly ESCC patients (Table 4). In 246 ESCC patients with age ≤60 years, multivariate analysis showed that serum prealbumin is still an independent prognostic factor for OS in the younger ESCC patients (Table 5).
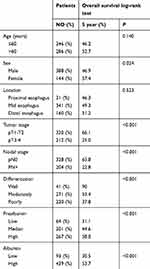 |
Table 2 Univariate analysis for OS in 532 ESCC patients who underwent curative resection without postoperative adjuvant radiotherapy or chemotherapy |
 |
Figure 1 Kaplan–Meier survival curve for ESCC patients with three different serum prealbumin levels (A) and for ESCC patients with two different serum albumin levels (B). |
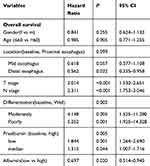 |
Table 3 Cox multivariate regression analyses for the influence of preoperative serum prealbumin on OS in overall 532 resected ESCC patients without postoperative adjuvant radiotherapy or chemotherapy |
ROC curve for the assessment of prognostic performance
The AUC was 0.605 (95% CI: 0.557 to 0.653) for prealbumin and 0.563 (95% CI: 0.514 to 0.611) for albumin, which indicated that prealbumin may be superior to albumin as a prognostic predictor in ESCC patients after curative resection, but the difference between the two AUCs was not statistically significant (P=0.068) (Figure 2).
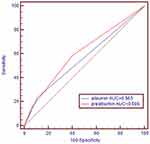 |
Figure 2 Receiver operating characteristic curve of serum albumin and albumin for 5-year overall survival. |
Discussion
Malnutrition is common in patients with cancer and is due to a variety of mechanisms involving the tumour, the host response to the tumour, and anticancer therapies.12,13 A previous study demonstrated that prealbumin showed the best concordance with the standard Detailed Nutritional Assessment (DNA) method and a good sensitivity/specificity profile compared with the Subjective Global Assessment (SGA) and the Prognostic Inflammatory and Nutritional Index score (PINI).14
Prealbumin is a carrier protein that transports thyroid hormones in the plasma and cerebrospinal fluid and transports retinol (vitamin A) in the plasma. Both serum prealbumin and albumin are valuable markers for assessing a patient’s nutritional status. However, because of its shorter half-life, the serum concentration of prealbumin more closely reflects recent changes in the nutritional status rather than the overall nutritional status. In this regard, serum prealbumin should be considered a sensitive nutritional biomarker for evaluating the preoperative nutritional status of cancer patients.
Up to 85% of all patients with cancer develop clinical malnutrition, which negatively affects a patient’s response to therapy, increases the incidence of treatment-related side effects and can decrease survival.15 It has been reported that the serum prealbumin level can change early and reflect a patient’s nutritional status in several cancers, including oesophageal cancer.13,16–19 It is well accepted that nutrition is an important determinant of immune responses and that malnutrition is the most common cause of immunodeficiency.6,7,20,21 Malnutrition can impair the immune system, suppressing immune functions that are fundamental to host protection against tumour progression and metastasis.22 In this study, we showed that the preoperative level of serum prealbumin was closely associated with the infiltration depth of the tumour. The positivity rate of hypoalbuminemia increased when tumour invasion advanced. In addition, we found no relationship between the level of serum prealbumin and lymph node metastasis. Similar to our findings, previous studies have shown that the level of serum prealbumin was closely correlated with the depth of invasion but not with lymph node metastases.9 However, another study reported statistically significant associations with preoperative prealbumin levels for both lymph node metastases and the depth of tumour invasion.23 Overall, our results further support the conclusion that preoperative prealbumin levels are significantly associated with tumour progression.
The level of serum prealbumin was recently reemphasized by extending its clinical use to the severity of disease, disease progression, and prognosis.8,9,24 It has been reported that a low perioperative serum prealbumin level predicted early recurrence after curative pulmonary resection for non-small cell lung cancer and was associated with a poor prognosis.8 Similarly, Alifano et al demonstrated that prealbumin levels were independent prognostic markers in resected non-small cell lung cancer. To the best of our knowledge, no data have reported the prognostic value of serum prealbumin in ESCC patients. In this study, we showed that the serum prealbumin level was an independent prognostic indicator and that a low preoperative serum prealbumin level was associated with poor OS in resected ESCC patients without neoadjuvant or adjuvant therapy.
Previous studies have investigated the nutritional status as a possible prognostic factor in ESCC. In particular, the adverse effect of a low serum albumin level on OS has emerged from previously reported studies.25 Consistent with previous studies, we found that the serum albumin level remained as an independent predictor of OS. Moreover, our comparative assessment of the serum prealbumin level with that of albumin confirmed that the preoperative serum prealbumin level is superior to that of albumin for predicting long-term survival in patients with ESCC. However, the difference between prealbumin and albumin did not reach statistical significance.
This study has several potential limitations. The major limitation is that this study is a retrospective cohort study from a single institution. In addition, it should be noted that only squamous cell carcinoma was examined in this study, and the results from this study may not be suitable for oesophageal adenocarcinoma. Despite the limited scope of this retrospective cohort study, we suggest that ESCC patients with a low preoperative serum prealbumin level may need to receive perioperative immunonutrition therapy to improve postoperative survival. Prospective studies evaluating the role of nutritional modulation to improve long-term outcomes are required.
In conclusion, the results of the present study suggest that the preoperative serum prealbumin level could serve as a prognostic biomarker in patients with ESCC after curative resection. We suggest that preoperative nutritional parameters, as indicated by serum prealbumin and albumin levels, should be included in the prognosis prediction for resected ESCC patients.
Acknowledgment
This work was partly supported by funding from the National Natural Science Foundation of China (81803036), the Natural Science Foundation of Jiangsu Province (BK20180186), the Sanitation Bureau Guidance Project of Changzhou (WZ201303) and the Jiangsu Provincial Health Bureau of Medical Research (H201350).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi:10.3978/j.issn.1000-9604.2015.01.06
2. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi:10.1056/NEJMra035010
3. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi:10.1016/S0140-6736(12)60643-6
4. Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–871. doi:10.1038/nrc927
5. Dell DD. Cachexia in patients with advanced cancer. Clin J Oncol Nurs. 2002;6:235–238. doi:10.1188/02.CJON.235-238
6. Marcos A, Nova E, Montero A. Changes in the immune system are conditioned by nutrition. Eur J Clin Nutr. 2003;57(Suppl 1):S66–S69. doi:10.1038/sj.ejcn.1601819
7. Chandra RK, Kumari S. Nutrition and immunity: an overview. J Nutr. 1994;124:1433S–1435S. doi:10.1093/jn/124.suppl_8.1433S
8. Kawai H, Ota H. Low perioperative serum prealbumin predicts early recurrence after curative pulmonary resection for non-small-cell lung cancer. World J Surg. 2012;36:2853–2857. doi:10.1007/s00268-012-1766-y
9. Alifano M, Mansuet-Lupo A, Lococo F, et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS One. 2014;9:e106914. doi:10.1371/journal.pone.0106914
10. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi:10.1158/1078-0432.CCR-04-0713
11. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845.
12. von Meyenfeldt M. Cancer-associated malnutrition: an introduction. Eur J Oncol Nurs. 2005;9(Suppl 2):S35–S38. doi:10.1016/j.ejon.2005.09.001
13. Pan P, Tao G, Sun X. Subjective global assessment and prealbumin levels of esophageal cancer patients undergoing concurrent chemoradiotherapy. Nutr Hosp. 2015;31:2167–2173. doi:10.3305/nh.2015.31.5.8596
14. Devoto G, Gallo F, Marchello C, et al. Prealbumin serum concentrations as a useful tool in the assessment of malnutrition in hospitalized patients. Clin Chem. 2006;52:2281–2285. doi:10.1373/clinchem.2006.080366
15. Davies M. Nutritional screening and assessment in cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9(Suppl 2):S64–S73. doi:10.1016/j.ejon.2005.09.005
16. Fujii T, Yajima R, Takada T, et al. Serum albumin and prealbumin do not predict recurrence in patients with breast cancer. Anticancer Res. 2014;34:3775–3779.
17. Milano G, Cooper EH, Goligher JC, Giles GR, Neville AM. Serum prealbumin, retinol-binding protein, transferrin, and albumin levels in patients with large bowel cancer. J Natl Cancer Inst. 1978;61:687–691.
18. Liu L, Wang J, Liu B, et al. Serum levels of variants of transthyretin down-regulation in cholangiocarcinoma. J Cell Biochem. 2008;104:745–755. doi:10.1002/jcb.21661
19. Liu L, Liu J, Dai S, et al. Reduced transthyretin expression in sera of lung cancer. Cancer Sci. 2007;98:1617–1624. doi:10.1111/j.1349-7006.2007.00576.x
20. Chandra RK. Nutrition is an important determinant of immunity in old age. Prog Clin Biol Res. 1990;326:321–334.
21. Lesourd B. Protein undernutrition as the major cause of decreased immune function in the elderly: clinical and functional implications. Nutr Rev. 1995;53:
22. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–226. doi:10.1097/MCO.0b013e32832a7902
23. Fujii T, Sutoh T, Morita H, et al. Serum albumin is superior to prealbumin for predicting short-term recurrence in patients with operable colorectal cancer. Nutr Cancer. 2012;64:1169–1173. doi:10.1080/01635581.2012.718034
24. Geisler JP, Linnemeier GC, Thomas AJ, Manahan KJ. Nutritional assessment using prealbumin as an objective criterion to determine whom should not undergo primary radical cytoreductive surgery for ovarian cancer. Gynecol Oncol. 2007;106:128–131. doi:10.1016/j.ygyno.2007.03.008
25. Wang CY, Hsieh MJ, Chiu YC, et al. Higher serum C-reactive protein concentration and hypoalbuminemia are poor prognostic indicators in patients with esophageal cancer undergoing radiotherapy. Radiother Oncol. 2009;92:270–275. doi:10.1016/j.radonc.2009.01.002
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


