Back to Journals » Journal of Inflammation Research » Volume 17
High Monocyte-to-Lymphocyte Ratio is Associated with Obstructive Sleep Apnea Hypopnea Syndrome
Authors Bao W , Gao J, Fang S , Zhang S, Wan Y
Received 20 December 2023
Accepted for publication 14 March 2024
Published 8 April 2024 Volume 2024:17 Pages 2137—2145
DOI https://doi.org/10.2147/JIR.S455559
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Wenyu Bao,* Junkang Gao,* Siyu Fang, Shiwei Zhang, Yufeng Wan
Department of Otolaryngology, Head and Neck Surgery, Chaohu Hospital Affiliated to Anhui Medical University, Hefei, 238000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yufeng Wan, Department of Otolaryngology, Head and Neck Surgery, Chaohu Hospital Affiliated to Anhui Medical University, Hefei, 238000, People’s Republic of China, Email [email protected]
Objective: This study aims to explore the correlation between serum monocyte-to-lymphocyte ratio (MLR) and other inflammatory parameters with the occurrence of obstructive sleep apnea-hypopnea syndrome (OSAHS) in patients.
Methods: This study included 310 patients who underwent polysomnography monitoring at our hospital between January 2021 and January 2023. Routine blood inflammatory parameters and polysomnography (PSG) results were also evaluated. The differences in inflammatory markers between the OSAHS and normal groups were compared, and OSAHS independent related factors were screened.
Results: The MLR of OSAHS group was significantly higher than that of control group, and the difference was statistically significant. Multivariate logistic regression analysis suggested that MLR is an independent risk factor for OSAHS.
Conclusion: High MLR was correlated with OSAHS. The diagnostic value of MLR was better than that of the other inflammatory parameters.
Keywords: obstructive sleep apnea hypopnea syndrome, inflammation, monocyte to lymphocyte ratio, polysomnography
Introduction
Obstructive sleep apnea-hypopnea syndrome (OSAHS) is a common sleep disorder characterized by partial or complete obstruction of the upper airway during sleep, leading to pauses or reductions in breathing. These respiratory symptoms cause oxygen deprivation and impaired carbon dioxide elimination, typically accompanied by snoring, disrupted sleep patterns, and daytime symptoms such as excessive sleepiness and impaired concentration.1
Epidemiological studies have shown that the global prevalence of sleep-disordered breathing (AHI ≥ 5 events/) in the population aged 30–69 years ranges from 711 million to 961 million,2 Among them, an estimated 272 million to 458 million people have moderate to severe sleep-disordered breathing (AHI≥15 events/h).3 The prevalence of OSA increases with age, male gender, and higher body mass index.4
OSAHS can lead to fragmented sleep and decreased oxygen saturation.5 Studies have shown that fragmented sleep can result in excessive daytime sleepiness, reduced quality of life6, and an increased risk of accidents.7 Additionally, it increases the risk of developing other diseases,8 including cardiovascular diseases,9 neurological diseases,10,11 metabolic diseases,12 cancer and reproductive disorders.13,14
OSAHS is currently recognized as an inflammatory disease. There is evidence to suggest that chronic intermittent hypoxia (IH) associated with OSAHS, sleep deprivation, and fragmentation can elevate levels of various markers such as inflammation, oxidative stress, procoagulant, and thrombotic activity.15 The gold standard for diagnosing OSAHS is a polysomnography (PSG) examination,16 which requires specialized facilities and overnight monitoring. Some researchers propose the use of biological and hematological parameters as biomarkers for early diagnosis and monitoring of OSAHS,17 however, these methods are often costly and require additional testing.
In recent years, the use of composite inflammatory parameters such as neutrophil-to-lymphocyte ratio (NLR) as specific biochemical markers for OSAHS has garnered increasing attention from researchers. NLR, MLR, systemic immune-inflammation index (SII), system inflammation response index (SIRI), and Pan-Immune-Inflammation Value (PIV) are biomarkers of endogenous stress and various disease risks,18–22 and these composite inflammatory parameters are easily accessible and convenient to calculate. Studies have shown that NLR is associated with the severity of OSAHS.23 Some research has investigated the relevance of SII and SIRI in the development of OSAHS24,25. However, there is currently no reported research on the relationship between MLR, PIV, and the occurrence of OSAHS, nor comparative studies of the association between different inflammatory parameters and the development of OSAHS.
Materials and Methods
Study Population
A total of 414 patients who underwent polysomnography (PSG) and complete blood count at the Chao Lake Hospital, Anhui Medical University, from January 2021 to May 2023 were retrospectively analyzed. PSG and blood tests were performed within one week. Patients were divided into two groups based on their AHI scores: the control group (AHI < 5 events per hour) and the OSAHS group (AHI ≥ 5 events per hour). Inclusion criteria were: patients aged 18–80 years who were hospitalized for snoring and underwent PSG and complete blood count; patients who were conscious, able to communicate naturally, and had no severe neurological or psychiatric disorders. Exclusion criteria were: patients with hematological, gastrointestinal, or gynecological malignancies; chronic inflammatory diseases, infections, congestive heart failure, severe renal failure, liver failure, central sleep apnea, or cerebrovascular diseases; or those receiving treatment. A total of 104 patients with chronic inflammatory diseases (n=57), central sleep apnea (n=11), and those receiving CPAP therapy (n=36) were excluded, resulting in the inclusion of 310 patients, comprising 231 in the control group (105 males, 126 females) and 79 in the OSAHS group (42 males, 37 females). This study was approved by the Ethics Committee of our hospital.
Polysomnography
All patients underwent overnight examinations in the PSG examination room under the supervision of experienced technicians. Parameters including electroencephalography, electrooculography, chin electromyography, leg electromyography, electrocardiography, leg, chest and abdominal movements, nasal airflow (thermistor), pulse oximetry, and body position were recorded. According to the American Academy of Sleep Medicine (AASM) guidelines, the apnea-hypopnea index (AHI) was defined as the sum of the apnea index (AI) and hypopnea index (HI), with respiratory events defined as a decrease in airflow of more than 50% of baseline for at least 10 seconds, accompanied by an arousal or a decrease in oxygen saturation of at least 3%.2
General Information
The clinical data of all patients at admission were collected, including age, sex, height, weight, routine blood tests, and other parameters. BMI (weight/height ²), NLR (neutrophils/lymphocytes), PLR (platelets/lymphocytes), MLR (monocytes/lymphocytes), SII (neutrophils × platelets/lymphocytes), SIRI (neutrophils × monocytes/lymphocytes), PIV (neutrophils × platelets × monocytes/lymphocytes).
Statistical Analysis
Using SPSS 26.0 statistical software for analysis. For normally distributed quantitative data, the mean and standard deviation (Mean±SD) were used, while for non-normally distributed data, the median and quartiles were represented [M(Q25, Q75)]. Non-parametric Mann–Whitney U-test was used for between-group comparisons of non-normally distributed data, and independent sample t-test or one-way analysis of variance (ANOVA) was used for normally distributed data. Qualitative data were represented using frequency and percentage, and between-group comparisons were made using the chi-squared test. In general, inflammatory parameters were included in the single-factor logistic regression analysis, and those with P<0.2 were selected for inclusion in the multiple-factor logistic regression analysis to determine the independent factors related to OSAHS. The discriminative ability of the multiple-factor model was evaluated using the receiver operating characteristic (ROC) curve, and the calibration of the model was assessed using the Hosmer-Lemeshow test. Pearson correlation analysis was used to evaluate the correlation between each inflammatory parameter and AHI score. Using the SIRI, SII, PIV, MLR, NE, and MONO data of the patients, ROC curves were generated using SPSS 26.0 statistical software to analyze the predictive value of SIRI, SII, PIV, MLR, NE, and MONO for OSAHS. The optimal cut-off value for the ROC curve was chosen as the MLR value corresponding to the point where the sum of specificity and sensitivity was maximal. Differences with a P<0.05 were considered statistically significant.
Results and Analysis
General Data Comparison
Finally, 310 valid data points were included, including 147 males and 163 females, with an average age of 45 years. The OSAHS group was older, lower in height, heavier, and had a significantly increased BMI. MONO, LY, SIRI, PIV, NLR, and MLR were all higher than those in the control group (P<0.05) (Table 1).
 |
Table 1 Comparison of Clinical and Blood Routine Data Between OSAHS Group and Control Group |
Influencing Factors of OSAHS
Univariate Logistic Regression Analysis Was Used to Determine the Inflammatory Factors Associated with OSAHS
Taking P<0.2 as the standard, the inflammatory parameters with differences between the control group and the OSAHS group were included in univariate logistic regression analysis, and the related inflammatory factors affecting the occurrence of OSAHS were screened. The results showed that NE, MONO, SIRI, SII, PIV, NLR and MLR were risk factors for OSAHS, P<0.05 (Table 2).
 |
Table 2 Univariate and Multivariate Logistic Regression Analysis of OSAHS Influencing Factors |
The factor with statistical significance (P<0.2) in the single-factor logistic regression analysis was included in the multi-factor logistic regression analysis using the Forward: LR method to avoid the issue of multicollinearity. Neutrophil count, monocyte count, eosinophil count, SIRI, SII, PIV, NLR, and MLR were included in the multi-factor logistic regression analysis model to screen for independent inflammatory factors related to OSAHS. The results showed that MLR [OR=1.103, 95% CI (1.059–1.149), P<0.01] was an independent risk factor for OSAHS (Table 2).
Model Discrimination Test: The ROC curve for the prediction model [AUC=0.673, 95% CI (0.601–0.744), P<0.01] indicates a certain discriminative ability of the predictive model when AUC>0.65 (Figure 1A). Model calibration test: The calibration ability of the predictive model was evaluated using the Hosmer-Lemeshow test. The results showed that the Hosmer-Lemeshow Χ2=8.752, P=0.364 > 0.05, indicating that the difference between the model predicted values and the actual observed values was not statistically significant, suggesting that the predictive model has good calibration ability (Figure 1B).
MLR, SIRI and AHI Score Correlation
The Spearman correlation analysis results indicated that MLR and SIRI were positively correlated with AHI score (r=0.273; r=0.265, P<0.05), as shown in Table 3. As the MLR increased, the AHI score also increased, indicating a more severe condition of OSAHS in patients. MLR is positively correlated with the lowest oxygen saturation and mean oxygen saturation (r=−0.185; r=−0.155, P<0.05). A higher MLR is associated with more severe nocturnal hypoxia in patients (Table 4). The scatter plot (Figure 2) constructed based on the MLR and AHI of the patients showed a certain linear correlation between MLR and AHI.
 |
Table 3 Correlation Analysis Between MLR and Other Inflammatory Parameters and AHI Score |
 |
Table 4 MLR Correlated Analysis with the Lowest Blood Oxygen Saturation, Average Blood Oxygen Saturation, and AHI Score |
 |
Figure 2 Correlation Analysis of MLR and AHI. |
ROC Curve Analyzed the Predictive Value of MLR, SIRI and Other Parameters on OSAHS
The predictive value of inflammatory parameters such as MLR and SIRI (inflammatory parameters that are significant in single-factor logistic regression analysis) was evaluated using ROC curves. The results showed that when the MLR threshold was set at 0.248, it had the highest predictive efficacy for OSAHS, with a sensitivity of 34.2% and a specificity of 93.5%. The area under the ROC curve (AUC) was 0.673 [95% CI (0.601–0.744), P<0.01], indicating a certain predictive value for OSAHS (Figure 3), which was superior to other inflammatory parameters (Table 5).
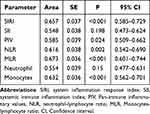 |
Table 5 Area Under the ROC Curve for Predicting Snoring Using Various Inflammatory Parameters |
 |
Figure 3 Analysis of ROC curve of MLR in OSAHS. |
Discussion
Our study found a significant correlation between high MLR and the prevalence of OSAHS. Moreover, we have demonstrated that MLR is an independent risk factor for OSAHS. MLR is likely to be an economical, objective, and simple blood biomarker that holds certain value for the early identification of high-risk OSAHS patients. Conducting PSG tests for these patients for further diagnosis can alleviate the economic burden on patients. Therefore, MLR may be an effective biomarker for predicting and evaluating OSAHS in clinical practice.
This study revealed for the first time the relationship between MLR, PIV, and the occurrence of OSAHS, comparing the correlation of different inflammatory composite parameters with the occurrence of OSAHS. The correlation between MLR and OSAHS was shown to be superior to inflammatory indicators such as NLR and SII. Comparative data indicated that the MLR level in the OSAHS group was significantly higher than in the control group. Both single-factor and multiple logistic regression analyses suggested that MLR is independently associated with the occurrence of OSAHS. Additionally, MLR was positively correlated with AHI scores (r=0.273, P<0.05), with an area under the ROC curve of 0.673, 95% CI (0.601–0.744), indicating a certain predictive value for OSAHS. Our research suggests that elderly patients with higher BMI, NLR, and SII levels are more likely to suffer from OSAHS, which is consistent with the findings of Senaratna et al4,23,24
Zahide’s study found that there was no statistically significant difference in the MLR between OSAHS patients and the normal population.26 However, in our study, we observed a significant correlation between MLR and the occurrence of OSAHS. This difference in findings may be attributed to variations in sample size and differences in the study populations. In comparison to Zahide’s study, our study had a larger sample size and used more sound statistical methods, which may render our results more reliable. Nonetheless, further validation through large-sample, multi-center studies is still necessary.
MLR has been demonstrated to be a novel hematological parameter in several medical fields, with elevated levels likely resulting from an increase in monocyte count and/or a decrease in lymphocyte count. MLR has been established as an independent predictor of severe Klebsiella pneumoniae infection risk,27 and has independent prognostic value in patients with laryngeal squamous cell carcinoma (LSCC)28, while also serving as a prognostic biomarker for risk stratification in lung cancer patients29. These studies collectively indicate that an elevated MLR level may indicate the initiation of an inflammatory process. Patients with OSAHS often experience IH, and monocytes play a crucial role in inflammatory recruitment and immune suppression following IH. During inflammation, monocytes increase and are recruited to inflamed sites, where they differentiate into end-stage cells and promote the renewal of dendritic cells and tissue macrophages.19 Research has demonstrated that monocytes in severe OSAHS patients exhibit higher NLRP3 activity than the control group, which is directly related to AHI and the index of hypoxemia. Monocytes in the context of IH increase NLRP1 signaling in a hypoxia-inducible factor-3α-dependent manner and/or in combination with plasma from OSA patients.30 The increased plasma norepinephrine levels among OSAHS patients lead to activation of the sympathetic nervous system and an increase in serum cortisol. Elevated cortisol levels subsequently result in a decrease in lymphocyte concentration.31 As a composite indicator of monocytes and lymphocytes, MLR can better reflect these changes in the disease process.
Our research has found that MLR is significantly associated with the occurrence of OSAHS, and it outperforms inflammation markers such as NLR, SII, and SIRI. Monocytes and differentiated macrophages are important components of the innate immune system, capable of regulating the secretion of inflammatory cytokines and tissue remodeling.32 Under the influence of IH caused by OSAHS, total monocytes in the bone marrow and peripheral blood significantly increase.33 Monocytes in OSAHS patients suppress NK cell activity by releasing TGF-β, demonstrating their ability to modulate NK cell activity through immunomodulation.34 MLR can reflect the inflammatory state within OSAHS patients through both monocytes and lymphocytes, providing valuable predictive insights for OSAHS. Additionally, MLR is easily obtainable through routine blood tests, with low testing costs and widespread practical value. The use of MLR for OSAHS prediction is scientifically sound, reasonable, and innovative.
Shortcomings of this study: 1) This was a single-center and retrospective study; therefore, we cannot establish a causal relationship between MLR and OSAHS. The sample size of cases is still limited, and further exploration of the relationship between various hematological indicators and inflammatory parameters with the occurrence and development of OSAHS requires larger sample sizes and multi-center studies. 2) All patients in this study were newly diagnosed with OSAHS. It is worth further investigating whether MLR and SIRI remain significantly associated with the condition for patients with a history of OSAHS who have received CPAP or surgical treatment. 3) Inflammatory markers were limited, only including relevant inflammatory parameters in routine blood tests, without incorporating CRP, interleukins, tumor necrosis factor, and other inflammatory parameters. Further research could involve capturing more inflammatory factors to clearly elucidate the relationship between inflammation and OSAHS.
Data Sharing Statement
The datasets used and/or analyzed in this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Medical Ethics Committee of Chaohu Hospital, affiliated with Anhui Medical University (No:KYXM-202205-006), and conformed to the ethical standards for medical research involving human subjects, as laid out in the 1964 Declaration of Helsinki and its later amendments. All patients signed an informed consent form upon admission, granting permission for their medical data to be used in clinical research. All methods were performed in accordance with the applicable guidelines and regulations.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas, took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All the authors have read and approved the final manuscript.
Funding
This study was supported by the Chaohu Hospital, affiliated with the Anhui Medical University Young and Middle-aged Personnel Training Fund (No. Z202002).
Disclosure
The authors declare that they have no competing interests.
References
1. Patel SR. Obstructive sleep apnea. Ann Internal Med. 2019;171(11):Itc81–itc96. doi:10.7326/AITC201912030
2. Berry RB, Brooks R, Gamaldo C, et al. AASM scoring manual updates for 2017 (version 2.4). J Clin Sleep Med. 2017;13(5):665–666. doi:10.5664/jcsm.6576
3. Grote L. The global burden of sleep apnoea. Lancet Respir Med. 2019;7(8):645–647. doi:10.1016/S2213-2600(19)30226-7
4. Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi:10.1016/j.smrv.2016.07.002
5. Lim DC, Pack AI. Obstructive sleep apnea: update and future. Ann Rev Med. 2017;68(1):99–112. doi:10.1146/annurev-med-042915-102623
6. Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20(10):835–843.
7. Lin YC, Chen TY, Chien WC, et al. Stimulants associated with reduced risk of hospitalization for motor vehicle accident injury in patients with obstructive sleep apnea-a nationwide cohort study. BMC Pulm Med. 2020;20(1):28. doi:10.1186/s12890-019-1041-1
8. Lv R, Liu X, Zhang Y, et al. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Sig Transd Target Ther. 2023;8(1):218. doi:10.1038/s41392-023-01496-3
9. Redline S, Azarbarzin A, Peker Y. Obstructive sleep apnoea heterogeneity and cardiovascular disease. Nat Rev Cardiol. 2023;20(8):560–573. doi:10.1038/s41569-023-00846-6
10. Baillieul S, Dekkers M, Brill AK, et al. Sleep apnoea and ischaemic stroke: current knowledge and future directions. Lancet Neurol. 2022;21(1):78–88. doi:10.1016/S1474-4422(21)00321-5
11. Liguori C, Maestri M, Spanetta M, et al. Sleep-disordered breathing and the risk of Alzheimer’s disease. Sleep Med Rev. 2021;55:101375. doi:10.1016/j.smrv.2020.101375
12. Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323(14):1389–1400. doi:10.1001/jama.2020.3514
13. Justeau G, Bailly S, Gervès-Pinquié C, et al. Cancer risk in patients with sleep apnoea following adherent 5-year CPAP therapy. Europ resp J. 2022;59(4):2101935. doi:10.1183/13993003.01935-2021
14. Eisenberg E, Legro RS, Diamond MP, et al. Sleep habits of women with infertility. J Clin Endocrinol Metab. 2021;106(11):e4414–e4426. doi:10.1210/clinem/dgab474
15. Lurie A. Inflammation, oxidative stress, and procoagulant and thrombotic activity in adults with obstructive sleep apnea. Adv Cardiol. 2011;46:43–66.
16. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi:10.5664/jcsm.6506
17. Orrù G, Storari M, Scano A, Piras V, Taibi R, Viscuso D. Obstructive Sleep Apnea, oxidative stress, inflammation and endothelial dysfunction-An overview of predictive laboratory biomarkers. Eur Rev Med Pharmacol Sci. 2020;24(12):6939–6948. doi:10.26355/eurrev_202006_21685
18. Lin CW, Lin PW, Chiu LW, et al. Inflammatory biomarkers of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in 563 severe OSA patients before and after surgery. J Otolaryngol. 2023;52(1):49. doi:10.1186/s40463-023-00653-6
19. Cheng HR, Song JY, Zhang YN, et al. High monocyte-to-lymphocyte ratio is associated with stroke-associated pneumonia. Front Neurol. 2020;11:575809. doi:10.3389/fneur.2020.575809
20. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi:10.1158/1078-0432.CCR-14-0442
21. Dziedzic EA, Gąsior JS, Tuzimek A, et al. Investigation of the Associations of Novel Inflammatory Biomarkers-Systemic Inflammatory Index (SII) and Systemic Inflammatory Response Index (SIRI)-with the severity of coronary artery disease and acute coronary syndrome occurrence. Int J Mol Sci. 2022;23(17):9553. doi:10.3390/ijms23179553
22. Guven DC, Sahin TK, Erul E, Kilickap S, Gambichler T, Aksoy S. The association between the pan-immune-inflammation value and cancer prognosis: a systematic review and meta-analysis. Cancers. 2022;14(11):2675. doi:10.3390/cancers14112675
23. Oyama J, Nagatomo D, Yoshioka G, et al. The relationship between neutrophil to lymphocyte ratio, endothelial function, and severity in patients with obstructive sleep apnea. J Cardiol. 2016;67(3):295–302. doi:10.1016/j.jjcc.2015.06.005
24. Kim M, Cho SW, Won TB, Rhee CS, Kim JW. Associations between systemic inflammatory markers based on blood cells and polysomnographic factors in obstructive sleep apnea. Clin Exper Otorhinolaryngol. 2023;16(2):159–164. doi:10.21053/ceo.2022.01368
25. Pau MC, Zinellu A, Mangoni AA, et al. Evaluation of Inflammation and Oxidative Stress Markers in Patients with Obstructive Sleep Apnea (OSA). J Clin Med. 2023;12(12):3935. doi:10.3390/jcm12123935
26. Güneş ZY, Günaydın FM. The relationship between the systemic immune-inflammation index and obstructive sleep apnea. Sleep Breath. 2023;28:1–7.
27. Wang JL, Lu XY, Xu XH, et al. Predictive role of monocyte-to-lymphocyte ratio in patients with Klebsiella pneumonia infection: a single-center experience. Medicine. 2019;98(38):e17215.
28. Chen L, Zeng H, Yang J, et al. Survival and prognostic analysis of preoperative inflammatory markers in patients undergoing surgical resection for laryngeal squamous cell carcinoma. BMC Cancer. 2018;18(1):816. doi:10.1186/s12885-018-4730-x
29. Chen X, Wu J, Zhang F, Ying L, Chen Y. Prognostic significance of pre-operative monocyte-to-lymphocyte ratio in lung cancer patients undergoing radical surgery. Lab Med. 2018;49(2):e29–e39. doi:10.1093/labmed/lmx069
30. Díaz-García E, García-Tovar S, Alfaro E, et al. Inflammasome activation: a keystone of proinflammatory response in obstructive sleep apnea. Am J Respir Crit Care Med. 2022;205(11):1337–1348. doi:10.1164/rccm.202106-1445OC
31. Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97(1):19–22. doi:10.1161/01.CIR.97.1.19
32. Sercelik A, Besnili AF. Increased monocyte to high-density lipoprotein cholesterol ratio is associated with TIMI risk score in patients with ST-segment elevation myocardial infarction. Revista portuguesa de cardiologia. 2018;37(3):217–223. doi:10.1016/j.repc.2017.06.021
33. Alvarez-Martins I, Remédio L, Matias I, Diogo LN, Monteiro EC, Dias S. The impact of chronic intermittent hypoxia on hematopoiesis and the bone marrow microenvironment. Pflugers Archiv. 2016;468(5):919–932. doi:10.1007/s00424-016-1797-6
34. Hernández-Jiménez E, Cubillos-Zapata C, Toledano V, et al. Monocytes inhibit NK activity via TGF-β in patients with obstructive sleep apnoea. Europ Resp J. 2017;49(6):1602456. doi:10.1183/13993003.02456-2016
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

