Back to Journals » International Journal of General Medicine » Volume 17
High Ferritin and Low Total Iron-Binding Capacity in Plasma Predict All-Cause Mortality During the First 3 Years of Hemodialysis in Patients with End-Stage Chronic Kidney Disease
Authors Nguyen Thi Thu H, Nguyen Van H, Nguyen Minh T, Nguyen Trung K , Le Viet T
Received 23 October 2023
Accepted for publication 8 January 2024
Published 12 January 2024 Volume 2024:17 Pages 105—113
DOI https://doi.org/10.2147/IJGM.S446115
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ha Nguyen Thi Thu,1 Hung Nguyen Van,2 Tuan Nguyen Minh,3 Kien Nguyen Trung,4 Thang Le Viet1
1Department of Selection, Preparation and Treatment, Organ Transplant Center, Military Hospital 103, Vietnam Military Medical University, Ha Noi, Vietnam; 2Nephrology and Hemodialysis Department, Transport Hospital, Ha Noi, Vietnam; 3Nephrology, Urology and Hemodialysis Department, E Hospital, Ha Noi, Vietnam; 4Hematology and Blood Transfusion Center, Military Hospital 103, Vietnam Military Medical University, Ha Noi, Vietnam
Correspondence: Thang Le Viet, Military Hospital 103, Vietnam Military Medical University, 261 Phung Hung, Ha Dong, Ha Noi, Vietnam, Tel +84982249968, Email [email protected]
Aim: To determine all-cause mortality rate and the predictive value of plasma ferritin and total iron-binding capacity (TIBC) concentrations for mortality during the first 3 years of hemodialysis in patients with end-stage chronic renal disease (ESRD).
Methods: We conducted a study on 174 ESRD patients (estimated Glomerular Filtration Rate < 15 mL/min/1.73m2). The plasma TIBC level was quantified by the ELISA method in all patients at the time before hemodialysis. Based on TIBC concentration, patients were divided equally into 2 groups. Each group had 87 patients. Patients were initiated on hemodialysis, and patients who died from any cause during the first 3 years of hemodialysis were recorded.
Results: The all-cause mortality rate of ESRD patients in the first 3 years of maintenance hemodialysis was 22.9%. Plasma high hs-CRP, high ferritin, and low TIBC concentrations were independent factors associated with all-cause mortality in the patients. Plasma ferritin (cut-off value = 454.2 ng/L) and TIBC (cut-off value = 39.84 μmol/L) were predictors of all-cause mortality, AUC is: 0.772; 0.723, p < 0.001.
Conclusion: Plasma ferritin and TIBC were good predictors of all-cause mortality in ESRD patients during the first 3 years of hemodialysis.
Keywords: end-stage chronic renal disease, plasma TIBC, plasma ferritin, mortality prediction, the first 3 years of maintenance hemodialysis
Introduction
Chronic anemia is common in patients with chronic kidney disease (CKD). The incidence and severity of anemia increase as CKD progresses to more severe stages.1 The main cause of renal anemia is low erythropoietin (EPO) levels and/or reduced EPO receptor activity.2,3 Besides, iron deficiency is also one of the causes of anemia in CKD patients. Iron deficiency can occur due to low iron stores (absolute iron deficiency) or due to relative deficiency (functional iron deficiency).4 Serum ferritin and transferrin saturation (TSAT) are two indices used clinically to evaluate iron status in CKD patients, in which TSAT is calculated as free iron over TIBC.5,6
Ferritin binds iron as an iron complex, into which iron moves in and out, acting as an iron storage site.7 Cytoplasmic ferritin synthesis is stimulated by increased iron intake, which is decreased by iron deficiency. In maintenance hemodialysis patients, changes in serum ferritin are considered factors related to mortality, especially excessive iron supplementation therapy, which increases ferritin levels, leading to high mortality.8,9 Not only ferritin, low TIBC has been shown to be associated with iron deficiency, protein-energy wasting (PEW), inflammation, poor quality of life, and high mortality in maintenance hemodialysis (MHD) patients.10–12 Chronic kidney disease progresses silently, if not detected early or regularly monitored, the disease will progress to end-stage chronic renal disease (ESRD). Reality in Vietnam shows that many hospitalized patients have been diagnosed with ESRD, and are indicated to replace kidneys with maintenance hemodialysis; peritoneal dialysis or kidney transplant.13 These patients often have severe organ disorders, leading to poor treatment prognosis. Hemodialysis outcomes and mortality in the first 3 years of MHD have been proven to be related to many factors.14,15 In this study, we hypothesize that high plasma ferritin and low TIBC levels at the time patients are diagnosed with CKD stage 5 predict all-cause mortality in the first 3 years of MHD.
Patients and Methods
Patients
The study was conducted on 279 patients diagnosed with CKD stage 5 (eGFR < 15 mL/min/1.73m2) at Military Hospital 103 and Transport Hospital, Hanoi, Vietnam, from January 2016 to January 2020. So that previous treatment of chronic kidney disease does not affect the research results, we selected for study patients who met the following criteria: Age 18 or older. The patient was first diagnosed with ESRD. The patient had previously been diagnosed with chronic kidney disease but had not received any treatment. We excluded patients who are pregnant or breastfeeding. The patient does not have enough time for continuous hemodialysis for 3 years. Patients who had acute infections or cancer at the time of blood collection for tests were also excluded from this study. The remaining 174 patients with CKD stage 5 were treated with maintenance hemodialysis and were provided written informed consent prior to participation in our study. We collected all data on clinical characteristics and laboratory parameters at the baseline time of the study (The time when the patient is admitted to the hospital within 24–48 hours, has not received any treatment).
Fasting venous blood was taken to do a blood formula, and plasma was taken to quantify biochemical indicators, CRP-hs, iron, and ferritin. CRP-hs was estimated quantitatively using solid-phase ultrasensitive enzyme immunoassay. Plasma ferritin was quantified by the electrochemical immunoluminescence method. Plasma TIBC was determined by using a Diagnosis-Related Group (DRG) TIBC (bioactive) enzyme-linked immunosorbent assay (ELISA) kit, a solid phase ELISA, based on the principle of competitive binding. We divided all patients into 2 equal groups due to the plasma TIBC concentration: Tertile 1 (n = 87) with plasma TIBC concentration < 42.19 µmol/L and Tertile 2 (n=87) with plasma TIBC concentration ≥ 42.19 µmol/L.
Causes of chronic kidney disease such as chronic glomerulonephritis, chronic pyelonephritis, gout, lupus, and polycystic kidney disease were diagnosed according to the recommendations of KDOQI 2002.16 Diabetes mellitus was identified according to either a physician’s diagnosis, antidiabetic drug treatment, or 2 subsequent analyses demonstrating fasting blood glucose levels of >126 mg/dL or > 7.0 mmol/L. Hypertension was defined as the regular use of antihypertensive drugs for controlling blood pressure or at least 2 blood pressure measurements of ≥ 140/90 mm Hg. Anemia was diagnosed as hemoglobin < 130 g/L for males or < 120 g/L for females.5
The patients received hemodialysis three sessions per week using low-flux filter reuse (Polyflux 14L), each session lasting from 3.5 to 4.5 hours, aiming to achieve the goal of Kt/V ≥ 1.2, calculated according to the formula of Daugirdas.17 Patients were controlled blood glucose during the hemodialysis and treated for anemia, hypertension and other disorders as the Ministry of Health of Vietnam recommends. All patients were followed up for 36 months to determine all-cause mortality.
Statistical Analyses
All the normal distribution continuous data were represented by mean and standard deviation and were analyzed by Student’s t-test. All the skewed distributions were described by median (25 percentile – 75 percentile), analyzed by Mann Whitney U-test and Kruskal Wallis test. Categorical data were presented by the frequency with percentage and were analyzed using the Chi-square test. The receiver operating characteristic (ROC) curves with the area under the curve (AUC) were calculated to predict all-cause mortality of the patients after a 3-year follow-up. Multivariate adjusted regression analysis was performed to identify the predictor of all-cause mortality. Survival curves were assessed using the Kaplan–Meier analysis and evaluated using the Log rank test. Statistical analysis was performed using Statistical Package for Social Science (SPSS) version 20.0 (Chicago, IL, USA) with a p-value <0.05 was considered significant.
Results
The results in Table 1 show that age and plasma ferritin level in Tertile 1 was significantly higher compared with those of Tertile 2, p= 0.003 and 0.005. On the contrary, eGFR and plasma TIBC concentrations in Tertile 1 were significantly lower compared with those of Tertile 2, p= 0.004 and < 0.001. The all-cause mortality rate was 22.9%, of which Tertile 1 was significantly higher than Tertile 2, p < 0.001.
 |
Table 1 Clinical Characteristics and Laboratory Parameters of the Studied Patients |
The results in Table 2 show that old age, diabetic-cause of kidney failure, low hemoglobin and albumin levels, and increased hs-CRP were associated with mortality with p < 0.05.
 |
Table 2 Comparison of Clinical Characteristics and Laboratory Parameters of Death Group and Survival Group |
The multivariate logistic regression results in Table 3 show that plasma hs-CRP, ferritin, and TIBC were independent factors associated with all-cause mortality in maintenance hemodialysis patients during the first 3 years.
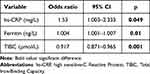 |
Table 3 Result of Multivariate Logistic Regression Analysis Showing Independent Factors Relating to All-Cause Mortality of Maintenance Hemodialysis Patients During 3 Years |
Plasma ferritin and TIBC were good predictors of all-cause mortality in maintenance hemodialysis patients during the first 3 years based on the ROC curve model in Figure 1
Figure 2 shows Kaplan–Meier analysis for all-causes mortality of 174 maintenance hemodialysis patients during the first 3 years, classified according to groups of plasma TIBC concentrations. Patients in the higher group of TIBC concentrations (blue line: T2) exhibited a significantly lower death rate compared to that of lower plasma TIBC concentrations (red line: T1) (Log rank test, p< 0.001).
 |
Figure 2 Kaplan–Meier analysis of all-causes mortality of maintenance hemodialysis patients, classified according to plasma TIBC concentrations in 2 Tertiles (36 months). |
Discussion
Ratio of All-Cause Mortality in Maintenance Hemodialysis Patients During the First 3 Years
Our study showed that the all-cause mortality rate in the first 3 years of hemodialysis was 22.9% (Table 1). The all-cause mortality rate in MHD patients and related factors are always a matter of concern, as evidenced by many recently published studies. Preciado et al studied 842 MHD patients, follow-up time (average 30.8 months), and mortality rate was 29.6% (249/842 patients). Multivariate logistic regression analysis showed that relative blood volume is an independent and significant predictor of mortality.18 Erdem et al conducted a study on 173 MHD patients, investigating the relationship between all-cause mortality and serum ferritin levels. Research results showed that, after 5 years of follow-up (average follow-up time is 38 months), 47% of patients died (81/173 patients).19 Liu et al reported an all-cause mortality rate in their study of 37.15% (188/506 MHD patients), with a median follow-up of 60 months.20 Thus, death is a fairly common event in MHD patients, with different rates depending on the follow-up time and patient characteristics studied. Another point in our study is that all 174 patients had their data taken before the first hemodialysis session and were fully monitored during the first 3 years of hemodialysis. Therefore, the all-cause mortality rate in our study is lower than in other studies.
Some Related Factors Predict All-Cause Mortality in Maintenance Hemodialysis Patients During the First 3 Years
Ma L and Zhao S performed a meta-analysis of 23 studies with 86,915 MHD patients, showing that multiple markers and factors influence the risk of mortality and cardiac death in the patients.21 Risk factors highly consistent across studies were advanced age, low BMI, and high plasma CRP. In addition, there were other risk factors that most studies agree on: malnutrition, diabetes, patients with a history of cardiovascular disease, and iron overload. In our study, increased plasma hs-CRP, ferritin, and decreased TIBC were independent factors associated with all-cause mortality in ESRD patients undergoing the first 3 years of maintenance hemodialysis (Table 2). Plasma TIBC and ferritin had good predicting values for all-cause mortality in this group of patients, with AUC of 0.772; 0.723, p < 0.001 (Figure 1). At the time of the study, we had excluded all patients with signs of infection, so increased plasma hs-CRP in our patients with end-stage chronic kidney disease represents non-infectious chronic inflammatory reactions. Atherosclerosis is a common event in ESRD patients, and it often increases when the cause of CKD is diabetes. Atherosclerosis releases proinflammatory cytokines and other substances that cause inflammation and endothelial dysfunction, including hs-CRP. Therefore, increased plasma hs-CRP was found in a significant proportion of ESRD patients (20% to 65%) and had a predictive value for mortality in CKD patients with and without maintenance hemodialysis.21,22 Anemia in ESRD patients is related to many factors, including iron. Iron deficiency will lead to anemia because iron is a necessary red blood cell-forming ingredient. However, iron overload (indicated by increased plasma ferritin) is also a factor causing anemia in ESRD patients. Excess iron in the body acts as an oxidative stress agent, which can convert less reactive free radicals into more reactive hydroxyl radicals. Serum ferritin levels increase in an inflammatory environment. In CKD patients with inflammation, ferritin will increase more than in CKD patients without inflammation,23 causing more energy consumption and reduced body resistance, easily linked to death. In hemodialysis patients, a positive association with increased serum ferritin levels and mortality was also observed.24
Besides ferritin, plasma TIBC was also a good factor in predicting all-cause mortality in MHD patients during the first 3 years. In particular, we found that decreased plasma TIBC will be associated with increased mortality in these patients, p < 0.001 (Figure 2). In subjects who had been on maintenance hemodialysis for at least 8 weeks (mean hemodialysis duration was 31 ± 34 months), followed for 63 months, Bross et al found low serum TIBC concentrations related to mortality at the study time.11 Our findings may suggest that, in addition to hs-CRP and ferritin, plasma TIBC may help identify ESRD patients entering hemodialysis who are at risk of death during the first 3 years of hemodialysis, especially patients with plasma TIBC level < 39.84 µmol/L. Like other authors, the cause of death in our MHD patients is mainly related to cardiovascular events and infections including: coronary artery disease, stroke, sepsis. (data are not shown). These diseases often appear in patients with MIA (Malnutrition, Inflammation, Atherosclerosis) syndrome. It is clear that increased hs-CRP and ferritin increase inflammation, decreased TIBC also represents malnutrition in ESRD patients.11,12
In this study, we realize that there are some limitations as follows: Because we only selected patients who were diagnosed for the first time and have not been treated, the study sample size is small, so the results are uncertain. Research indicators are not abundant, most of them are commonly used clinical indicators.
Conclusion
The all-cause mortality rate of ESRD patients in the first 3 years of maintenance hemodialysis was 22.9%. Plasma high hs-CRP, high ferritin, and low TIBC concentrations were independent factors associated with all-cause mortality in the patients. Plasma ferritin and TIBC were good predictors of all-cause mortality.
Data Sharing Statement
The data supporting the findings of this study can be obtained from the corresponding author according to reasonable request, and the corresponding author/s can be directly contacted for further inquiry.
Ethical Disclosure
Animals did not participate in this research. All human research procedures followed the committee’s ethical standards for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. This study was approved by the Ethical Committee of Vietnam Military Medical University (No.2521/QĐ/HVQY).
Acknowledgments
In this study, we were strongly supported by our local hospital and university to complete our research.
Disclosure
We did not receive any funds to complete this study. The authors declare no conflict of interest, financial or otherwise.
References
1. Atkinson MA, Warady BA. Anemia in chronic kidney disease. Pediatr. Nephrol. 2018;33:227–238. doi:10.1007/s00467-017-3663-y
2. Shih HM, Wu CJ, Lin SL. Physiology and pathophysiology of renal erythropoietin-producing cells. J Formos Med Assoc. 2018;117(11):955–963. doi:10.1016/j.jfma.2018.03.017
3. Geng G, Liu J, Xu C, et al. Receptor-mediated mitophagy regulates EPO production and protects against renal anemia. Elife. 2021;10:e64480. doi:10.7554/eLife.64480
4. Gafter-Gvili A, Schechter A, Rozen-Zvi B. Iron deficiency anemia in chronic kidney disease. Acta Haematol. 2019;142:44–50. doi:10.1159/000496492
5. KDIGO clinical practice guidelines. Anemia. Kidney Int Supplement. 2012;2(4):281–335.
6. Mercadal L, Metzger M, Haymann JP, et al. A 3-marker index improves the identification of iron disorders in CKD anaemia. PLoS One. 2014;9(2):e84144. doi:10.1371/journal.pone.0084144
7. Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–3516. doi:10.1182/blood.V99.10.3505
8. Kuragano T, Matsumura O, Matsuda A, et al. Association between hemoglobin variability, serum ferritin levels, and adverse events/mortality in maintenance hemodialysis patients. Kidney Int. 2014;86:845–854. doi:10.1038/ki.2014.114
9. Zitt E, Sturm G, Kronenberg F, et al. Iron supplementation and mortality in incident dialysis patients: an observational study. PLoS One. 2014;9:e114144. doi:10.1371/journal.pone.0114144
10. Kalantar-Zadeh K, Kopple JD, Block G, et al. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–1263. doi:10.1053/ajkd.2001.29222
11. Bross R, Zitterkoph J, Pithia J, et al. Association of serum total iron-binding capacity and its changes over time with nutritional and clinical outcomes in hemodialysis patients. Am J Nephrol. 2009;29(6):571–581. doi:10.1159/000191470
12. Ikeda-Taniguchi M, Takahashi K, Shishido K, et al. Total iron binding capacity is a predictor for muscle loss in maintenance hemodialysis patients. Clin Exp Nephrol. 2022;26(6):583–592. doi:10.1007/s10157-022-02193-1
13. Borg R, Carlson N, Søndergaard J, et al. The growing challenge of chronic kidney disease: an overview of current knowledge. Int J Nephrol. 2023;2023:9609266. doi:10.1155/2023/9609266
14. Park JM, Lee JH, Jang HM, et al. Survival in patients on hemodialysis: effect of gender according to body mass index and creatinine. PLoS One. 2018;13(5):e0196550. doi:10.1371/journal.pone.0196550
15. Inagaki K, Tawada N, Takanashi M, et al. The association between body mass index and all-cause mortality in Japanese patients with incident hemodialysis. PLoS One. 2022;17(6):e0269849. doi:10.1371/journal.pone.0269849
16. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266.
17. Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993;4:1205–1213. doi:10.1681/ASN.V451205
18. Preciado P, Zhang H, Thijssen S, et al. All-cause mortality in relation to changes in relative blood volume during hemodialysis. Nephrol Dial Transplant. 2019;34(8):1401–1408. doi:10.1093/ndt/gfy286
19. Erdem E, Karatas A, Ecder T. The relationship between serum ferritin levels and 5-year all-cause mortality in hemodialysis patients. Blood Purif. 2022;51(1):55–61. doi:10.1159/000515639
20. Liu S, Wu Q, Zhang S, et al. Serum Galectin-3 levels and all-cause and cardiovascular mortality in maintenance hemodialysis patients: a prospective cohort study. BMC Nephrol. 2022;23(1):5. doi:10.1186/s12882-021-02636-z
21. Ma L, Zhao S. Risk factors for mortality in patients undergoing hemodialysis: a systematic review and meta-analysis. Int J Cardiol. 2017;238:151–158. doi:10.1016/j.ijcard.2017.02.095
22. Vidt DG. Inflammation in renal disease. Am J Cardiol. 2006;97(2A):20A–27A. doi:10.1016/j.amjcard.2005.11.012
23. Kang HT, Linton JA, Kwon SK, et al. Ferritin level is positively associated with chronic kidney disease in Korean men, based on the 2010–2012 Korean National Health and Nutrition Examination Survey. Int J Environ Res Public Health. 2016;13(11):1058. doi:10.3390/ijerph13111058
24. Hasuike Y, Nonoguchi H, Tokuyama M, et al. Serum ferritin predicts prognosis in hemodialysis patients: the Nishinomiya study. Clin Exp Nephrol. 2010;14:349–355. doi:10.1007/s10157-010-0288-x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

