Back to Journals » OncoTargets and Therapy » Volume 9
High-dose irradiation in combination with toll-like receptor 9 agonist CpG oligodeoxynucleotide 7909 downregulates PD-L1 expression via the NF-κB signaling pathway in non-small cell lung cancer cells
Authors Chen X, Zhang Q, Luo Y, Gao C, Zhuang X , Xu G, Qaio T
Received 6 July 2016
Accepted for publication 5 September 2016
Published 21 October 2016 Volume 2016:9 Pages 6511—6518
DOI https://doi.org/10.2147/OTT.S116629
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ingrid Espinoza
Xue Chen,1 Qi Zhang,1 Youjun Luo,1 Caixia Gao,1 Xibing Zhuang,1 Guoxiong Xu,2 Tiankui Qiao1
1Department of Oncology, Jinshan Hospital, Medical Center of Fudan University, 2Department of Center laboratory, Jinshan Hospital, Fudan University, Shanghai, People’s Republic of China
Objectives: Irradiation resistance appears as local recurrence and distant metastasis in advanced stages of non-small cell lung cancer (NSCLC). High-dose irradiation combined with immunotherapy improved overall survival and local control of NSCLC. This study explored the underlying molecular mechanism by which the effect of high-dose irradiation plus toll-like receptor 9 (TLR9) agonist CpG oligodeoxynucleotide (CpG ODN) 7909 on NSCLC.
Materials and methods: NSCLC H460 cells were exposed to constant high-dose irradiation (6.37 Gy) in irradiation (IR) group and the irradiation plus CpG group. Gene expression was assessed using quantitative reverse transcriptase-polymerase chain reaction and Western blot. Knockdown of nuclear factor kappa B (NF-κB) p65 expression was conducted using p65 siRNA.
Results: Expression of programmed death-ligand 1 (PD-L1) mRNA was significantly decreased in IR combined with CpG ODN 7909 group compared with the control or IR-alone groups (P<0.05). TLR9 expression was also obviously increased in the combination group compared with the control (P<0.05). Moreover, expression of NF-κB p65 was apparently reduced in the combination group compared with the control (P<0.05). However, expression of PD-L1 was significantly decreased after knockdown of p65 in IR group (P<0.05), but increased in the combination group (P<0.05) and slightly increased in CpG ODN-alone group (P<0.05), which was opposite to that without p65 knockdown group.
Conclusion: This study demonstrated that radiotherapy combined with CpG ODN 7909 was able to downregulate PD-L1 expression through inhibition via the NF-κB signaling pathway.
Keywords: CpG ODN, irradiation, immune escape, NF-κB, non-small cell lung cancer, PD-L1
Introduction
Lung cancer is one of the most common types of malignancy and the leading cause of cancer-related death in the world.1 Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer diagnosed globally, and most patients with NSCLC are diagnosed at the advanced stages of disease, in which radiotherapy is the primary treatment option,2,3 while chemotherapy offers little benefit to these patients. However, patients treated with irradiation, sooner or later, will develop resistance to radiotherapy which will lead to loss of local control and to tumor metastasis.4,5 Previous studies demonstrated that immune escape was the main problem, inducing radioresistance and distant metastasis in such patients.6,7 Thus, novel treatment strategies are urgently needed. Recent advancement in targeting therapy and immunotherapy offers a better strategy to control advanced NSCLC clinically.7,8 For example, one of the famous immunotherapy targeting molecules was the programmed death-ligand 1 (PD-L1), also known as CD274, which is a protein expressed in various human cancer cells, including NSCLC cells. PD-L1 plays a central role in suppression of the immune system and is critical for transduction inhibitory signal that contributes to immune escape.8,9 Previous studies showed that high-dose irradiation, eg, stereotactic body radiation therapy in combination with immunotherapy against PD-L1 could improve overall survival and local control of human cancer xenografts in nude mice.10 The high-dose irradiation in combination with anti-PD-L1 optimized anti-tumor immunity in NSCLC cells.11 Therefore, we speculated that an immune activator [the toll-like receptor 9 (TLR9) agonist CpG oligodeoxynucleotide (CpG ODN) 7909] in combination with radiotherapy could possess better anti-tumor activity and modulate PD-L1 expression. Indeed, in our previous studies, we demonstrated that CpG-ODN 7909 treatment was able to induce radiation sensitivity in a radiation-resistant lung adenocarcinoma cell line and that TLR9 expression was upregulated by CpG-ODN 7909 treatment,12 while CpG ODN 1826 in combination with radioresistant Lewis lung cancer cell vaccine significantly reduced PD-L1 expression in Lewis lung cancer mouse xenografts.13 In addition, one of the TLR pathways was able to activate nuclear factor kappa B (NF-κB) and its downstream genes.14,15 Therefore, in this study, we investigated PD-L1 expression using CpG ODN 7909 in combination with irradiation in NSCLC cells in vitro. We then assessed whether there is any association among TLR9, NF-κB, and PD-L1 to mediate NSCLC resistance to irradiation. Our study may provide insightful information regarding the combination of CpG ODN 7909 with irradiation therapy of NSCLC.
Materials and methods
Cell line and culture
Human NSCLC NCI-H460 cell line was purchased from The Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, People’s Republic of China) and cultured at 37°C in Roswell Park Memorial Institute-1640 medium (Corning Incorporated, Corning, NY, USA) supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified atmosphere of 5% CO2.
Reagents
CpG ODN 7909 was synthesized by Sangon Biotech (Shanghai, People’s Republic of China) and dissolved in sterilized phosphate buffered saline (PBS; Corning Incorporated) to make a stock solution at the concentration of 10 μg/mL as recommended by the manufacturer.
Experiment design
To determine the feasibility and efficacy of irradiation treatment combined with CpG ODN 7909, we designed our experiments for four groups as follows: the control group with the equal amounts of solvents; CpG ODN-alone group for constant stimulation of H460 cells with CpG ODN 7909 ten times; IR group with X-ray irradiation of H460 cells ten times; and IR plus CpG ODN group for initial stimulation of H460 cells with CpG ODN 7909 and followed by one cycle of radiotherapy. The treatment was repeated ten times.
Radiation treatment of H460 cells in vitro
H460 cells were inoculated into culture flasks and grown to reach approximately 70% confluency and then irradiated with 6.37 Gy X-rays using a Trilogy linear accelerator (Varian Medical Systems, California, CA, USA) at a rate of 2 Gy/min. After irradiation, the culture medium was refreshed immediately and the cells continued to grow for up to one or two days. When approximately 90% confluency was reached, the cells were subcultured and irradiated again with 6.37 Gy X-rays. The procedure was repeated ten times to obtain a stable H460 radioresistant cell line as described in a previous study.16
The IR plus CpG ODN group was first to have 10 μg/mL of CpG ODN 7909 administered to the culture flask. After 24 h, cells were subjected to irradiation treatment as described above. The experiments were repeated ten times.
Protein extraction and Western blot
H460 cells were seeded into culture flasks at a density of 1×106 cells per flask and grown for 24 h, and then treated with or without CpG ODN 7909 for 24 h and then with or without 6.37 Gy radiation. Twenty-four hours later, the cells were harvested for protein extraction using a sodium dodecyl sulfate (SDS) lysis buffer containing 1% phenylmethane sulfonyl fluoride (KeyGEN, Nanjing, People’s Republic of China). Protein samples containing 20 μg of total protein were separated in SDS-polyacrylamide gel electrophoresis gel and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked in 5% non-fat milk for at least 1 h and then incubated with a primary antibody overnight at 4°C. The primary antibodies included a monoclonal mouse anti-TLR9 (Abcam, Cambridge, MA, USA; at a dilution of 1:1000), anti-p65 (Cell Signaling Technology, Danvers, MA, USA; 1:1000), anti-PD-L1 (Abcam; 1:300), and anti-α-Tubulin and β-Actin antibodies (Proteintech, Wuhan, People’s Republic of China; both at 1:5000). On the next day, the membranes were washed with PBS-Tween 20 three times and then further incubated with goat anti-rabbit IgG horseradish peroxidase (Millipore; 1:5000) or goat anti-mouse IgG conjugated with horseradish peroxidase (Millipore; 1:5000) for 1 h at room temperature. After that, the membranes were subjected to incubation with enhanced chemiluminescence (Millipore) solution to detect protein bands. All target proteins were normalized against α-Tubulin or β-Actin expression and quantified using ImageJ Software (National Institute of Heath, Bethesda, MD, USA). Results were represented as mean ± standard deviation (SD) and the experiments were repeated three times.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
After treatments, cells were harvested for RNA isolation using the Axyprep Multisource Total RNA miniprep Kit (Axygen, Union City, CA, USA) according to the manufacturer’s instructions. These RNA samples were then reversely transcribed into cDNA using the PrimeScript RT Master Mix (TaKaRa, Dalian, People’s Republic of China) and the resulting cDNA samples underwent qPCR amplification of gene expression in Applied Biosystem 7300 (Applied Biosystems, Foster city, CA, USA) with a SYBR Premix Ex Taq (Tli RNaseH Plus; TaKaRa) according to the manufacturer’s protocols. The PCR condition was 95°C for 30 s, followed by 40 cycles of 95oC for 5 s, 60°C for 31 s. The gene primers were listed in Table 1. The cycle threshold (CT) values of the target gene levels were identified and levels of each mRNA were calculated as a ratio of normalized β-Actin level according to the ΔΔCT method. The experiments were performed in triplicate and repeated at least once.
  | Table 1 Primer sequence of target gene and reference gene |
siRNA interference and transfection
To knock down p65 expression, we purchased p65-siRNA from GenePharma (Shanghai, People’s Republic of China) and the targeting sequences were 5′-CAGAUACAGACGAUCGUCATT-3′ (sense) and 5′-UGACGAUCGUCUGUAUCUGTT-3′ (antisense) and negative control siRNA sequences were 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAATT-3′ (antisense). For siRNA transfection, H460 cells were grown overnight to reach approximately 70% sub-confluence and transfected with 80 nM p65 siRNA or negative control siRNA using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) for 48 h according to the manufacturer’s instructions. The cells were then subjected to Western blot analysis of p65 expression. Results were represented as mean ± SD and the experiments were repeated three times.
Statistical analysis
The data were summarized as mean ± SD and statistically analyzed using SPSS software version 23.0 (SPSS, Chicago, IL, USA) and GraphPad Prism Software version 6.05 (GraphPad Software, La Jolla, CA, USA). The Wilcoxon rank-sum test was performed to determine the association between two different groups. A P value less than 0.05 was considered statistically significant.
Results
Effects of high-dose irradiation in combination with CpG ODN on regulation of gene expression in NSCLC H460 cells
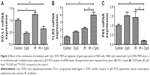
We explored the underlying molecular events in H460 cells after the treatments by first analyzing PD-L1 expression since PD-L1 was reported to be associated with radioresistance in tumor cells. Our data showed that PD-L1 expression was induced by irradiation treatment compared to the control cells (P<0.05; Figure 1A), whereas irradiation plus CpG ODN treatment (95% confidence interval [CI]: 0.38 to 0.92) downregulated PD-L1expression compared with the control group and the IR group (P<0.01; Figures 1A and 2A) (95% CI: 0.99 to 1.49). In the CpG ODN-alone group, PD-L1 expression was slightly reduced compared with the control group (P>0.05; Figures 1A and 2A). Moreover, TLR9 expression was induced in cells treated with CpG ODN or irradiation alone, and further upregulated in the combination group (95% CI: 2.97 to 4.96) compared to the controls (95% CI: 0.68 to 1.32; Figures 1B and 2B). In addition, expression of NF-κB p65 protein and mRNA level was significantly induced by irradiation treatment, but was dramatically downregulated by the combination treatment (95% CI: 0.09 to 0.27) compared to the controls (95% CI: 0.83 to 1.17; Figures 1C and 2C). Similar results were observed in PD-L1, TLR9, and p65 mRNA expression, respectively (Figure 2A–C).
Effects of NF-κB p65 knockdown on NSCLC cells
Next, to assess the efficacy of NF-κB p65 siRNA on knockdown of p65 expression in NSCLC cells, we performed siRNA transfection and Western blot assays. Our data showed that p65 siRNA was able to effectively silence p65 expression in tumor cells (Figure 3).
Knockdown of p65 expression reduces level of PD-L1 mRNA in NSCLC cells
To investigate the association of PD-L1 and p65 expression, we first knocked down p65 expression in H460 cells and treated tumor cells for single therapy or a combination of irradiation and CpG ODN. We found that PD-L1 expression was significantly decreased in IR group (P<0.05; 95% CI: 0.17 to 0.71; Figure 4) when compared to the control group (95% CI: 0.73 to 1.27), but increased in IR plus CpG ODN group (P<0.05; 95% CI: 1.58 to 1.75; Figure 4) and slightly increased in CpG ODN-alone group (P<0.05; Figure 4), which was opposite to that without p65 knockdown (Figures 1A and 2A). This finding suggests that p65 may regulate PD-L1 expression to mediate radioresistance in NSCLC cells.
Discussion
Radiotherapy uses ionizing radiation to directly kill or eliminate tumor cells, which can be used as a single treatment option or as adjuvant or neoadjuvant therapy, like surgery or chemotherapy.17 Radiotherapy is also a useful treatment approach in treatment of advanced NSCLC to control NSCLC local recurrence and distant metastasis.5,17 As a primary treatment option, radiotherapy could achieve significant tumor control,18,19 but eventually, NSCLC develops radioresistance that impairs clinical outcome of patients.20 Radioresistant tumor cells are insensitive to radiation response any more and radioresistant tumor cells would be aggravated to metastasis.21 High-dose irradiation could improve this situation. Furthermore, increasing evidence indicated that immune evasion was another problem that impairs anti-tumor effect.22–24 The existence of a checkpoint molecule, especially PD-L1, weakened anticancer response.25,26 Since the discovery of PD-L1’s function, PD-L1 has attracted an increasing amount of attention due to its central role in the regulation of immune evasion.27,28 A variety of studies were focused on PD-L1 expression in different cancers and the effect of anti-PD-L1 with targeted drugs.8,26,27 Numerous attempts have been made in the past decades to exploit an effective strategy to control NSCLC with little or no success.2,17 However, few reports have paid attention to the available mechanism of regulating PD-L1 expression in radioresistant cells. Downregulation of PD-L1 expression in radioresistant cells could provide a promising form of anticancer therapy. In this study, we performed high-dose irradiation in combination with CpG ODN 7909 to assess PD-L1 expression in NSCLC cells. Our results showed that the PD-L1 expression was increased in irradiation group, whereas radiotherapy in combination with CpG ODN 7909 led to downregulation of PD-L1 expression in NSCLC cells. A previous study also indicated that radiotherapy could induce PD-L1 expression and it may, through IFN-γ, indirectly modulate PD-L1 expression.29 From these data, we found that radiotherapy may increase PD-L1 expression, but CpG ODN 7909 in combination with radiotherapy was able to effectively downregulate PD-L1 expression in H460 radiation-resistant cells (IR group and combination group). To study the possible mechanism of PD-L1 expression in radiation-resistant NSCLC cells, we continued to investigate the relative genes such as TLR9 and NF-κB subunit p65.
TLR9 is one of the TLR family members and located in the intracellular endosome, which is expressed in B lymphocytes and plasmacytoid dendritic cells.30 A previous study showed that TLR9 recognizes bacterial DNA, especially in unmethylated CpG motifs.31 Increasing evidence indicated that CpG ODN could stimulate immune response, and CpG ODN has been applied in cancer therapy.32,33 Recent studies from our and other groups revealed that CpG ODN in combination with radiotherapy could improve anti-tumor response both in vivo and in vitro.16,33,34 For example, CpG ODN107 in combination with 10 Gy irradiation suppressed tumor xenograft growth in a human glioma cell nude mice model. According to these data, we speculated that CpG ODN in combination with radiotherapy would regulate TLR9 expression in radiation-resistant tumor cells to enhance anti-tumor response. From this study, we observed that irradiation alone could elevate expression of TLR9 mRNA and protein. CpG ODN in combination with radiotherapy further induced TLR9 expression. This demonstrated that CpG ODN 7909 could regulate TLR9 expression in radioresistant cells. One of TLR pathways was to activate the NF-κB pathway to regulate immune response and radiation response.35 Therefore, we investigated the association of TLR9 and NF-κB with stimulation of CpG ODN 7909 in H460 cells.
NF-κB was first described in 1986 and it has emerged as a ubiquitous factor involved in the regulation of many important processes, such as immune and inflammatory responses, apoptosis, and cell proliferation.36 NF-κB consists of five family members, ie, NF-κB2/P52, NF-κB1/P50, RelA/P65, c-Rel, and RelB. p65 is one of the most common and studied members, which is associated with modulating immune and irradiation responses.37 Therefore, we explored the diverse expression of p65 in radioresistant cells. The results showed that p65 expression was higher in the IR group compared with the control group, but p65 expression was significantly lower in the combination group compared with single IR group. According to this study and a previous study, we supposed that p65 may regulate PD-L1 expression. Thus, details between NF-κB p65 and PD-L1 were explored with transfection studies.
A better understanding of the mechanisms involved in NF-κB family members and PD-L1 may help guide the design of potent cancer therapies,38,39 thus, we used siRNA to knock down p65 expression in order to show its role in regulation of PD-L1expression. We found that the level of PD-L1 mRNA was reduced in p65 knocked down NSCLC cells, suggesting that p65 regulated PD-L1 expression. However, it was different from the previous study showing that p65 upregulated PD-L1 expression in ovarian cancer cells after chemotherapy.40 This discrepancy is unclear, but radiotherapy may have had a different effect on p65 and PD-L1 expression, and different cancers may also have different biological characters. Moreover, our current study used radioresistant NSCLC cells that were established after constant irradiation. The hallmarks of radioresistant cells were complex; for example, existence of cancer stem-like cells and overexpression of PD-L1 may impact their characters.41 Therefore, further study is needed to evaluate this combination therapy in treatment of advanced NSCLC patients.
However, our current data were just from one single cancer cell line, thus, this study is just proof-of-principle. In the literature, there are indeed publications showing data from one cell line.42–44 However, it is true that data generated from multiple cell lines will be more informative. Further investigation will use other cell lines to confirm our current data.
In summary, this study provided preliminary data on the molecular mechanism by which radiotherapy in combination with CpG ODN 7909 downregulated PD-L1 expression, which may be dependent on inhibition of NF-κB p65 signaling. Future study will further illustrate the whole picture of the combination of irradiation with CpG ODN 7909 in control of NSCLC.
Abbreviations
CpG ODN, CpG oligodeoxynucleotides; IR, irradiation; NF-κB, nuclear factor kappa B; PD-L1, programmed death-ligand 1; TLR9, toll-like receptor 9.
Acknowledgment
The authors thank the members of their laboratory for helpful technical support.
Disclosure
The authors report no conflicts of interest in this work.
References
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Schild SE, Vokes EE. Pathways to improving combined modality therapy for stage III non-small cell lung cancer. Ann Oncol. 2016;27(4):590–599. | ||
Larson SM, Carrasquillo JA, Cheung NV, Press OW. Radioimmunotherapy of human tumours. Nat Rev Cancer. 2015;15(6):347–360. | ||
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. | ||
Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14(8):561–584. | ||
Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21(4):687–692. | ||
Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–251. | ||
Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. | ||
Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695. | ||
He J, Hu Y, Hu M, Li B. Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Sci Rep. 2015;5:13110. | ||
Komiya T, Madan R. PD-L1 expression in small cell lung cancer. Eur J Cancer. 2015;51(13):1853–1855. | ||
Yan L, Xu G, Qiao T, Chen W, Yuan S, Li X. CpG-ODN 7909 increases radiation sensitivity of radiation-resistant human lung adenocarcinoma cell line by overexpression of Toll-like receptor 9. Cancer Biother Radiopharm. 2013;28(7):559–564. | ||
Zhuang XB, Xing N, Zhang Q, Yuan SJ, Chen W, Qiao TK. CpG Oligodeoxynucleotide1826 combined with radioresistant cancer cell vaccine confers significant antitumor effects. Neoplasma. 2015;62(6):905–914. | ||
Kawai T, Akira S. Signaling to NF-κB by Toll-like receptors. Trends Mol Med. 2007;13(11):460–469. | ||
Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19(1):24–32. | ||
Xing N, Qiao T, Zhuang X, Yuan S, Zhang Q, Xu G. CpG oligodeoxyribonucleotide 7909 enhances radiosensitivity via downregulating Oct-4 expression in radioresistant lung cancer cells. Onco Targets Ther. 2015;8:1443–1449. | ||
Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16(13):e498–e509. | ||
Ngiow SF, McArthur GA, Smyth MJ. Radiotherapy complements immune checkpoint blockade. Cancer Cell. 2015;27(4):437–438. | ||
Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. | ||
Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–5468. | ||
Park JS, Jun HJ, Cho MJ, et al. Radiosensitivity enhancement by combined treatment of celecoxib and gefitinib on human lung cancer cells. Clin Cancer Res. 2006;12(16):4989–4999. | ||
Binder DC, Fu YX, Weichselbaum RR. Radiotherapy and immune checkpoint blockade: potential interactions and future directions. Trends Mol Med. 2015;21(8):463–465. | ||
Teng F, Kong L, Meng X, Yang J, Yu J. Radiotherapy combined with immune checkpoint blockade immunotherapy: achievements and challenges. Cancer Lett. 2015;365(1):23–29. | ||
Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11(1):24–37. | ||
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–12297. | ||
Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074. | ||
Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19(5):1021–1034. | ||
Zak KM, Kitel R, Przetocka S, et al. Structure of the complex of human programmed death 1, PD-1, and its ligand PD-L1. Structure. 2015;23(12):2341–2348. | ||
Abiko K, Matsumura N, Hamanishi J, et al. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112(9):1501–1509. | ||
Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Adv Drug Deliv Rev. 2008;60(7):795–804. | ||
Ju X, Zenke M, Hart DNJ, Clark GJ. CD300a/c regulate type I interferon and TNF-alpha secretion by human plasmacytoid dendritic cells stimulated with TLR7 and TLR9 ligands. Blood. 2008;112(4):1184–1194. | ||
Meng Y, Carpentier AF, Chen L, et al. Successful combination of local CpG-ODN and radiotherapy in malignant glioma. Int J Cancer. 2005;116(6):992–997. | ||
Li X, Liu D, Liu X, et al. CpG ODN107 potentiates radiosensitivity of human glioma cells via TLR9-mediated NF-κB activation and NO production. Tumor Biol. 2012;33(5):1607–1618. | ||
Wagner H. Interactions between bacterial CpG-DNA and TLR9 bridge innate and adaptive immunity. Curr Opin Microbiol. 2002;5(1):62–69. | ||
Li F, Sethi G. Targeting transcription factor NF-κB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805(2):167–180. | ||
Ravi R, Bedi A. NF-κB in cancer – a friend turned foe. Drug Resist Updat. 2004;7(1):53–67. | ||
Magné N, Toillon R, Bottero V, et al. NF-κB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett. 2006;231(2):158–168. | ||
Zanotto-Filho A, Braganhol E, Schröder R, et al. NFκB inhibitors induce cell death in glioblastomas. Biochem Pharmacol. 2011;81(3):412–424. | ||
Huang G. NF-kB plays a key role in inducing CD274 expression in human monocytes after lipopolysaccharide treatment. PLoS One. 2013;8(4):e61602. | ||
Peng J, Hamanishi J, Matsumura N, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75(23):5034–5045. | ||
Yun HS, Baek JH, Yim JH, et al. Radiotherapy diagnostic biomarkers in radioresistant human H460 lung cancer stem-like cells. Cancer Biol Ther. 2016;17(2):208–218. | ||
Fan M, Ahmed KM, Coleman MC, Spitz DR, Li JJ. Nuclear factor-κB and manganese superoxide dismutase mediate adaptive radioresistance in low-dose irradiated mouse skin epithelial cells. Cancer Res. 2007;67(7):3220–3228. | ||
Ezekwudo D, Shashidharamurthy R, Devineni D, Bozeman E, Palaniappan R, Selvaraj P. Inhibition of expression of anti-apoptotic protein Bcl-2 and induction of cell death in radioresistant human prostate adenocarcinoma cell line (PC-3) by methyl jasmonate. Cancer Lett. 2008;270(2):277–285. | ||
Bodzin AS, Wei Z, Hurtt R, Gu T, Doria C. Gefitinib resistance in HCC mahlavu cells: upregulation of CD133 expression, activation of IGF-1R signaling pathway, and enhancement of IGF-1R nuclear translocation. J Cell Physiol. 2012;227(7):2947–2952. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.