Back to Journals » Journal of Blood Medicine » Volume 12
Heterogeneity in Hematological Parameters of High and Low Altitude Tibetan Populations
Authors Basak N, Norboo T, Mustak MS , Thangaraj K
Received 2 December 2020
Accepted for publication 16 March 2021
Published 17 May 2021 Volume 2021:12 Pages 287—298
DOI https://doi.org/10.2147/JBM.S294564
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Nipa Basak,1,2 Tsering Norboo,3 Mohammed S Mustak,4 Kumarasamy Thangaraj1,2,5
1CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India; 2Academy of Scientific and Innovative Research, Ghaziabad, India; 3Ladakh Institute of Prevention, Leh, India; 4Mangalore University, Mangalore, India; 5DBT-Centre for DNA Fingerprinting and Diagnostics, Hyderabad, India
Correspondence: Kumarasamy Thangaraj
CSIR-Centre for Cellular and Molecular Biology, Uppal Road, Hyderabad, 500 007, Telangana, India
Tel +91-40-27192634
Fax +91-40-27160591
Email [email protected]
Introduction: High altitude hypoxia is believed to be experienced at elevations of more than 2500 meters above sea level. Several studies have shed light on the biochemical aspects of high altitude acclimatization, where participants were sojourners to the high altitude from low altitude areas. However, information regarding the difference between the high altitude adapted Tibetans living at high altitude and their counterparts who reside at low altitude are lacking. To understand this, we have measured various hematological parameters in the Tibetan populations, who are residing in both high and low altitudes in India.
Methods: A total of 168 individuals (79 from high altitude (≥ 4500 meters) and 89 from low altitude (∼ 850 meters) were recruited for this study. Hematological parameters such as red blood cells (RBC) count, hematocrit (HCT), hemoglobin concentration (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) were measured from the individuals from high and low altitudes. Serum erythropoietin (EPO) was measured by ELISA. Statistical analyses were performed to compare data from both of the altitudes. Gender-wise comparison of data was reported. Correlation analysis was performed within relevant parameters.
Results: Highly significant differences (p < 0.0001) between high and low altitude Tibetans were detected in RBC count, HCT, Hb, MCHC in both males and females and in MCV in females. In the case of MCHC, however, age and BMI were potential confounders. Nominally significant differences (p < 0.05) were detected in MCV and MCH within males. No significant difference in serum EPO level was found between altitude groups, in any gender. No significant correlation was found between serum EPO with Hb as well as serum EPO with HCT.
Discussion: Our study explores significantly lower RBC count, HCT, Hb, MCH, MCHC and higher MCV in long-term Tibetan residents living at low altitude compared to their high altitude counterparts, which is likely due to the outcome of hematological adaptation to a relatively hyperoxic environment in low altitude areas.
Keywords: high altitude, low altitude, Tibetans, hematological parameters, erythropoietin
Introduction
High altitude is clinically defined as altitudes ≥2500 meters above sea level, where physiological changes start appearing in vulnerable people.1 The distinct environmental features of the high altitude area, that are, extreme cold and hypobaric hypoxia, solar radiation and aridity, make it difficult for sea-level residents to cope, at least for a few days after arrival until they acclimatize sufficiently. Often, travelers develop acute mountain sickness (AMS), high-altitude pulmonary edema (HAPE) or high-altitude cerebral edema (HACE); in contrast, native high altitude dwellers undergo adaptation and natural selection that give them survival benefits in the harsh environment.2 Tibetans, Andeans and Ethiopians are among the most studied native highlanders; among them, Tibetans are the oldest. Initial colonization of the high altitude Tibetan plateau dates back 25,000–30,000 years.1,3,4 Prolonged exposure allows the populations to experience physiological and genetic adaptations, as revealed by multiple studies.5–15 Compared to the Andeans, Tibetans have lower hemoglobin concentration at high altitude.16 The Tibetans have lower hemoglobin concentration and hematocrit compared to ethnically non-highlander, acclimatized Han migrants at high altitude.17,18
Several studies have looked at the genetic aspects of high altitude adaptation and physiological changes.5–15 However, studies on physiological aspects of high altitude acclimatization or adaptation performed to date have focused on individuals traveling to a high altitude from a low altitude or compared high altitude natives with non-native residents of high altitude areas to a large extent.11,17,19–22 Studies on ethnically high altitude adapted populations living at low altitude for a long period are also very limited.23,24 Adaptation is a slow process and might take thousands of years to integrate genetic changes, whereas acclimatization can happen in hours/days to combat the stress in an urgent manner. Therefore, if high altitude adapted populations reside at low altitude for several years, it is likely that they will experience the impact of this environmental shift as the partial pressure of oxygen is higher at low altitude compared to at high altitude.
According to Gustavo Zubieta-Calleja, high altitude residents experience “relative hyperoxia” at sea level, which is an apparent aggressive environment to the high altitude hypoxia-adapted people.25 This results in their experiencing lower ventilation, probably to combat “relative hyperoxia”. Zubieta-Calleja et al reported lower hematocrit after descent from high altitude to low altitude.26 Decreased hemoglobin has been reported in the Andean natives after arrival at sea level.27 Studies of people belonging to the same ethnic group but inhabiting high and low altitudes could be interesting as those are rare and could be insightful to understand the impact of the environmental shift. In this study, hence, we have included Tibetan people from high and low altitudes from India. Migration of ethnically Tibetan populations in India can be classified into two distinct categories: (1) migration of the Tibetans to Ladakh (sometimes described as “India’s little Tibet”) that started apparently during the 9th–10th centuries and continued for centuries thereafter (known as native Ladakhis also), and (2) migration of the Tibetans taking shelter in India during or after 1959 following subdual of the Tibetan uprising in 1959 by the People’s Liberation Army of China. The later migrants reside in various Tibetan settlements across India, including places at high altitude, Ladakh, and low altitude, Karnataka.28–31 Therefore, in our study, the high altitude group comes from different regions of Ladakh (altitude ≥4500 meters), and the low altitude group comes from Karnataka state (altitude ~850 meters). In the current study, we aimed to investigate how hematological parameters change in response to an extreme environmental shift (altitude) in the Tibetan population.
Materials and Methods
Participants and Study Design
Our study includes a total of 79 high altitude Tibetans from Samad Rakchan village and adjacent pastures (Norchen, Dipling, Kumlung), having an average elevation of 4500–4900 meters above sea level (barometric pressure <430 mmHg) in the Rupsho valley (part of the Changtang region of the Tibetan plateau, extending to Southeastern Ladakh) of the union territory of Ladakh, India. Another set of samples includes 89 low altitude Tibetans from a small town, Bylakuppe, having an average elevation of ~850 meters above sea level (barometric pressure ~690 mmHg), in Karnataka state, India, (Figure 1). People of non-Tibetan descent were excluded from our study.
 |
Figure 1 India map showing sampled area. The distance between Samad Rakchan and Bylakuppe is ~2320 km. Abbreviation: masl, meters above sea level. |
High altitude Tibetans in our study belong to semi-nomadic and pastoral communities and low altitude Tibetans are dependent on agriculture, handicraft and trade.32 In Ladakh, we organized health camps during July, 2017 and August, 2018 at the above-mentioned sites. In Karnataka, camps were organized during February−April, 2018. The current study was conducted in accordance with the Declaration of Helsinki. Informed written consent was obtained from all participants after explaining the purpose of the study. Consent was obtained from the father of a lone participant who was under the age of 18 years. The Institutional Ethical Committee (IEC) of the CSIR - Centre for Cellular and Molecular Biology, Hyderabad, the Institutional Review Board of the Ladakh Institute of Prevention and the District Ethical Committee, Leh, Ladakh approved the study.
About 3.0 mL of venous blood was collected in EDTA vacutainers (BD, New Jersey, USA) for the assessment of hematological parameters. About 3.0 mL of blood was also collected in vacutainer serum tubes (BD, New Jersey, USA) and were kept at room temperature for half an hour. Clots were removed by centrifuging at 2000 x g for 10 minutes. Clear serum was immediately transferred into clean polypropylene tubes, which were kept in dry ice temporarily and stored at −80 °C for further experiments.
Anthropometric and Hematological Data
Age and gender data were collected from all participants. Height and weight were measured. Body mass index (BMI) was calculated by dividing the weight in kilograms of the individuals by their height, in meters squared. Hematological data were acquired using ADVIA 2120i automated hematology analyzer (Siemens AG, Erlangen, Germany) for the low altitude participants. For high altitude participants, hematological data were obtained using manual techniques, where WBC and RBC were counted by microscopic examination of Leishman-stained blood smears within 3 days of sample collection. HCT was measured using Wintrobe hematocrit tubes.33 Hemoglobin was measured using Sahli’s method on the spot.34 RBC cellular indices were calculated accordingly.35 We analyzed WBC, RBC, HCT, MCV, MCH, MCHC and Hb parameters. A quantikine human erythropoietin kit from R & D Systems (Minneapolis, MN, USA) was used to measure serum EPO levels in high and low altitude Tibetans.
Data Analysis
GraphPad Prism trial version 8.4.3 (San Diego, CA) was utilized for statistical analysis. The distribution of the variables was checked using the D’Agostino-Pearson omnibus normality test. For comparison between the two groups, unpaired t-tests with Welch’s correction were performed where residuals followed Gaussian distribution and Mann–Whitney tests were performed where residuals did not follow Gaussian distribution. Spearman correlation coefficients were calculated to assess the correlation between parameters. Univariate and multiple linear regression analyses were performed in R (version 3.6.1). P-values of less than 0.05 were considered to be significant.
Results
Anthropometric and Hematological Parameters
Our study includes a total of 168 individuals, of whom 79 were from high altitude and 89 were from low altitude areas, with ages ranging from 24 to 80 years and 17 to 92 years, respectively. The median age was 58 years. The age distribution of participants is provided in Figures 2A and B. BMI of the participants was in the range 14.24–34.48 kg/m2 and median BMI was 23.82 kg/m2. Table 1 shows that high altitude participants had lower mean age and BMI compared to low altitude participants.
 |
Table 1 Description of Anthropometric Parameters of the Subjects (n=168); Data is Expressed in Mean ± SD Format |
 |
Figure 2 Age distribution among (A) high altitude and (B) low altitude participants. Abbreviations: HA, high altitude; LA, low altitude. |
Highly significant differences (p <0.0001) in RBC, HCT and hemoglobin concentration were detected between the high and low altitude Tibetans (Figures 3A–C). Mean values of RBC and HCT were higher in high altitude males and females compared to low altitude males and females, respectively. Mean values of RBC in males from high altitude and low altitude were 6.21 ± 0.74 and 4.45 ± 0.49 million cells/μL, respectively. In females, these were 5.4 ± 0.40 and 4.19 ± 0.40 million cells/μL, respectively. Mean values of HCT in high altitude males, low altitude males, high altitude females and low altitude females were 59.83 ± 6.89, 44.57 ± 4.56, 50.09 ± 3.6, 42.5 ± 4.06%, respectively. Hemoglobin concentration was significantly (p <0.0001) higher in high altitude individuals, irrespective of gender, compared to low altitude individuals. Mean hemoglobin concentrations in males from high and low altitudes were 20.01 ± 1.91 and 13.42 ± 1.75 g/dL, respectively. In females, these were 16.97 ± 1.74 and 12.56 ± 1.19 g/dL, respectively. MCV was significantly (p <0.05 in males and p <0.0001 in females) lower in both genders at high altitude compared to their low altitude counterparts, with mean values of 96.6 ± 8.06 and 100.5 ± 6.97 fL in males and 92.7 ± 6.17 and 101.5 ± 4.67 fL in females from high and low altitudes, respectively (Figure 3D). MCH showed a nominally significant difference (p <0.05) only in males, with mean values of 32.29 ± 2.51 and 30.27 ± 3.40 pg/cell in high altitude and low altitude males, respectively (Figure 3E). A significant difference was also detected in MCHC in individuals of both genders; however, age and gender were potential confounders (Figure 3F). Details of the tests performed and statistical parameters are given in Tables 2 and 3.
 |
Table 2 Description of Various Hematological Parameters in the Subjects; Data is Expressed in Mean ± SD Format |
Univariate linear regression analysis was performed separately with age, gender, altitude and BMI for each blood parameter. Out of these (age, gender, altitude and BMI), whichever showed significant association with the blood parameter, were considered for multiple linear regression analysis as potential confounding factors. Complete details of univariate and multiple linear regression analyses are provided in Supplementary Tables S1–S6. In multiple regression models, age and BMI did not show significant associations with most of the blood parameters (p >0.05) except MCHC and hemoglobin concentration. Both age and BMI showed a significant association with MCHC (p-values 0.0200 and 0.0232, respectively). However, only BMI (but not age) was significantly associated with hemoglobin concentration (p-value 0.0386) in the model and had a very small regression coefficient (0.0723), after correcting for confounding factors.
Additionally, we categorized our participants (up to 80 years, maximum age of high altitude participants) into three age categories, and checked the differences in hemoglobin concentrations between them. Details of BMI in these age categories are provided in Table 4. Hemoglobin concentration was significantly different in high altitude and low altitude participants, after categorization of age as well (Figures 4A–C; Table 5). For other parameters, age categorization could not be performed, as the number of individuals in each age category was very low or absent.
 |
Table 3 Description of Serum EPO and Hb in the Subjects; Data is Expressed in Mean ± SD Format |
 |
Table 4 Description of BMI Among Participants Belonging to Three Different Age Categories; Data is Expressed in Mean ± SD Format |
 |
Table 5 Description of Hemoglobin Concentration Among Participants Belonging to Three Different Age Categories; Data is Expressed in Mean ± SD Format |
Serum EPO
Serum EPO is one of the prominent outcomes of altitude-induced hypoxia that becomes elevated in people traveling to high altitudes. We checked serum EPO levels within high and low altitude Tibetan males and females. No significant difference was observed in either males or females, with mean values of 13.3 ± 11.61, 11.32 ± 7.77 mIU/mL in males and 13.21 ± 6.98 and 11.31 ± 7.63 mIU/mL in females from high and low altitudes, respectively (Figure 5; Table 3).
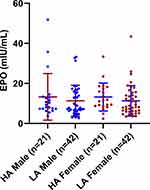 |
Figure 5 Distribution of serum erythropoietin (EPO) level among participants. Abbreviations: HA, high altitude; LA, low altitude. |
In an earlier study, serum EPO was shown to be correlated with HCT and hemoglobin concentration in children and young adults from Ladakh by Yanamandra et al.36 Since one group of our study participants is also from Ladakh, we checked the correlation of serum EPO with hemoglobin concentration and HCT of the participants. Both serum EPO and hemoglobin concentration data were available from 130 participants and both serum EPO and HCT data were available from 107 participants. Interestingly, none of these parameters showed any significant correlation (Spearman r = −0.03984, p-value = 0.6526 for serum EPO and Hb) with serum EPO in our study (Spearman r = 0.1562, p-value = 0.1081 for serum EPO and HCT).
Discussion
Change of hematological parameters has been shown to be associated with various pathological conditions like myocardial infarction, leukemia, coronary heart disease, cardiovascular disease and so on.37–40 Our study shows differences in hematological parameters between Tibetans residing at high and low altitudes for the first time, to the best of our knowledge. We observed significantly higher RBC, HCT and hemoglobin concentration and significantly lower MCV in high altitude males and females compared to their low altitude counterparts. Though BMI showed a significant association with hemoglobin concentration (p-value = 0.0386) after adjusting for age, gender, BMI and altitude, the coefficient of its association was too small (0.0723) to be considered a strong confounder, considering apparently small mean BMI differences (2.53 kg/m2) and apparently high mean hemoglobin concentration differences between high and low altitude male (6.59 g/dl) and female (4.41 g/dl) Tibetans. In our regression model for hemoglobin concentration (Hemoglobin concentration + Age + Gender + BMI + Altitude), the coefficient of altitude (low) was −5.5356 (p-value <2.25×10−7), which suggests that, on average, hemoglobin concentration is ~5.5 g/dl lower in low altitude individuals compared to high altitude individuals. Our observed values of hemoglobin concentration were a little higher than some studies conducted with high altitude Tibetans; however, it was reported that, at an elevation of more than 4500 meters, hemoglobin concentration in Tibetans also showed higher mean values.16,18,41–43 The hemoglobin concentration observed in our study for the low altitude Tibetans was on a par with another study with Tibetans at sea level.44 Since mean hemoglobin concentration was apparently high in our high altitude participants, we checked their chronic mountain sickness (CMS) scores, which had been recorded during the current health camp or a previous health camp organized in similar places. Data were available for the CMS scores from 41 male and 36 female participants. Among them, 12 males and 5 females had mild CMS, having scores of between 6 to 10 as per the Qinghai scoring system, resulting in a 22.07% CMS prevalence (unpublished data, Basak/Norboo et al).45 Earlier studies have reported a CMS prevalence of 13.73–28.7% in high altitude natives of the neighboring state, Himachal Pradesh.46,47 The Tibetans are known to have a lower prevalence of CMS compared to the Andeans.48–50 Though our participants had a comparatively higher prevalence of CMS, all of them had mild CMS. Though RBC, HCT and hemoglobin were significantly higher in the high altitude Tibetans, interestingly, serum EPO was only insignificantly higher in high altitude individuals; this might be because the normal concentration of serum erythropoietin has an apparently broad range: 3.3–16.6 mIU/mL.51 A similar observation was reported by Winslow et al, whereby HCT and hemoglobin were higher in Andean natives compared to Sherpas from Nepal living at the same altitude, yet serum erythropoietin was not significantly different.52 Moreover, serum EPO becomes elevated upon acute hypoxic exposure, when low altitude residents visit high altitude areas. Within a few days, it returns to almost the pre-exposure level. Both our high altitude and low altitude groups comprise long-term residents of the respective altitudes. Our observation of an almost similar level of serum EPO in both groups is consistent with that as well. A recent study showed that blood cell phenotypes are ancestry-dependent and selective pressure can give rise to different blood cell traits.53 Our study additionally shows that different exposure of the same population to different environments can also alter blood cell traits. These changes of hematological parameters are possibly part of the hematological adaptation mechanism, to cope with the “relative hyperoxia” that is evidently an aggressive environment to the high altitude hypoxia-adapted Tibetans when they migrate to low altitude.
It has previously been hypothesized that hemoglobin concentration might be modulated by environmental context.54 Our study reveals differences in various hematological parameters including hemoglobin concentration between the high and low altitude Tibetans, which fits the hypothesis well. Hemoglobin concentration has been shown to play an important role in reproductive fitness among Tibetan women, where lower hemoglobin favored reproductive fitness.55 Similarly, Tibetan men, having lower hemoglobin concentrations are known to show better exercise capacity at high altitude.41 It would be interesting to investigate those events separately in the low altitude Tibetans to check whether the trend is true since hemoglobin concentration was strikingly lower in the Tibetans from low altitude areas in our study.
John B. West discussed “Barcroft’s bold assertion” that assumes “All dwellers at high altitudes are persons of impaired physical and mental powers.” This is a provocative statement; nonetheless, it does provide scope to study native highlanders at low altitude and to assess their health status.56 Our study is a step in that direction, which would help to understand the physiological aspects better when native Tibetans are not present in their native high altitude environment. Simultaneously, these kinds of studies would help to understand the impact of environmental change, knowledge of which could be important for tourists and soldiers traveling to high altitude areas. It would be interesting to explore the molecular driving forces behind these kinds of differences. Epigenetic studies, important tools for exploring gene–environment interaction, could be fruitful in this context.
A limitation of our study is that we could not measure complete blood count (CBC) profiles of the high altitude and low altitude individuals using the same method because of geographical differences between the sampling sites, remote locations and limited resources (at high altitude). CBC profiles for the high altitude samples were obtained using manual techniques; while for the low altitude samples, these were obtained using an automated hematology analyzer. However, both of the methods are known to be well-correlated.33,57,58 Harris et al reported good correlation between ADVIA 2120 (previous version of ADVIA 2120i) and the manual method in an international, multicenter clinical trial. Values reported for WBC differentials measured by the ADVIA 2120 analyzer and the manual method in that study were close. The within-run precision of CBC on the platform was also very good.59 We used Sahli’s method, one of the most commonly used methods for hemoglobin estimation where resources are limited, for estimating hemoglobin concentration in remote high altitude areas. This method has been proven to be efficient, resulting in an absolute difference between two measurements of less than 1 g/dl. Another study reported that Sahli’s method provides lower values of hemoglobin concentration of 0.62gms/dl in capillary blood and 1.1gms/dl in venous blood compared to a hemiglobincyanide (HiCN)-based reference method. Our observed differences in mean hemoglobin concentration between high and low altitude male and female Tibetans were 6.59 g/dl and 4.41 g/dl, respectively, which are far above these values. Moreover, Sahli’s method was used to measure hemoglobin concentration in high altitude individuals, in whom we observed higher values than in low altitude individuals. On the other hand, technical specifications of ADVIA 2120i show that the precision of the instrument is good, with a coefficient of variation for MCV, Hb, RBC, WBC in the range of 0.78–2.7.60
Considering all these facts, it is highly unlikely that our observed differences in hematological parameters in high and low altitude individuals are due to artifacts. We needed to be a bit careful while selecting the parameters for analysis considering the gap between sample collection and hematological data collection. As the samples were collected from remote areas, due to logistic issues, hematological data collection (except hemoglobin measurement by Sahli’s method) of the samples could be done within 48–72 hours after sample collection. A recent meta-analysis and systematic review reports that Hb, WBC, RBC count, HCT, MCH, MCHC are quite stable within the time range in which we collected and measured them.61
Conclusion
In conclusion, our study reveals that prolonged residence of native Tibetans at a low altitude (over 50 years) results in significantly lower RBC count, HCT, Hb, MCH, MCHC and higher MCV compared to their high altitude counterparts, which is likely due to the outcome of hematological adaptation to the relatively hyperoxic environment in low altitude areas.
Acknowledgments
This work was supported by a BSC-0118 (EpiHed) grant provided to KT from the Council of Scientific and Industrial Research (CSIR), Government of India (GoI). KT was also supported by a J.C. Bose fellowship from the Science and Engineering Research Board, Department of Science and Technology (DST), GoI. NB acknowledges DST for DST-INSPIRE fellowship and DST (GoI) and the British Council (UK) for awarding a short-term research internship under the Newton Bhabha PhD Placement Programme. We thank all the participants of our study. We also thank the officers of the Tibetan settlements in Karnataka for their support. We thank Nony P Wangchuk, Eashay Lamo, Ms Sherab Dolma, Tsering Motup, Tsering Palzes, Tsering Dolker, Mrs. Sonam Dolma, Jaison Sequeira and Achintya Basak for their help in collection and transportation of samples. We also thank Dr. Sheikh Nizamuddin and Dr. Nitin Tupperwar for their useful discussions and critical comments on the manuscript.
Disclosure
The authors declared no conflicts of interest for this work.
References
1. Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci U S A. 2007;104(Suppl 1):8655–8660. doi:10.1073/pnas.0701985104
2. Luks AM, Swenson ER, Bärtsch P. Acute high-altitude sickness. Eur Respir Rev. 2017;26(143):143. doi:10.1183/16000617.0096-2016
3. Aldenderfer MS. Moving up in the World: archaeologists seek to understand how and when people came to occupy the Andean and Tibetan plateaus. Am Sci. 2003;91(6):542–549. doi:10.1511/2003.6.542
4. Aldenderfer M. Peopling the Tibetan plateau: insights from archaeology. High Alt Med Biol. 2011;12(2):141–147. doi:10.1089/ham.2010.1094
5. Simonson TS, Yang Y, Huff CD, et al. Genetic evidence for high-altitude adaptation in Tibet. Science. 2010;329(5987):72–75. doi:10.1126/science.1189406
6. Yang J, Jin ZB, Chen J, et al. Genetic signatures of high-altitude adaptation in Tibetans. Proc Natl Acad Sci U S A. 2017;114(16):4189–4194. doi:10.1073/pnas.1617042114
7. Jeong C, Alkorta-Aranburu G, Basnyat B, et al. Admixture facilitates genetic adaptations to high altitude in Tibet. Nat Commun. 2014;5(1):3281. doi:10.1038/ncomms4281
8. Huerta-Sánchez E, Jin X, Bianba Z, et al. Altitude adaptation in Tibetans caused by introgression of Denisovan-like DNA. Nature. 2014;512(7513):194–197. doi:10.1038/nature13408
9. Zhuang J, Droma T, Sun S, et al. Hypoxic ventilatory responsiveness in Tibetan compared with Han residents of 3658 m. J Appl Physiol. 1993;74(1):303–311. doi:10.1152/jappl.1993.74.1.303
10. Bigham A, Bauchet M, Pinto D, et al. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet. 2010;6(9):e1001116. doi:10.1371/journal.pgen.1001116
11. Droma T, McCullough RG, McCullough RE, et al. Increased vital and total lung capacities in Tibetan compared to Han residents of Lhasa (3658 m). Am J Phys Anthropol. 1991;86(3):341–351. doi:10.1002/ajpa.1330860303
12. Moore LG. Measuring high-altitude adaptation. J Appl Physiol. 2017;123(5):1371–1385. doi:10.1152/japplphysiol.00321.2017
13. Brutsaert TD. Population genetic aspects and phenotypic plasticity of ventilatory responses in high altitude natives. Respir Physiol Neurobiol. 2007;158(2–3):151–160. doi:10.1016/j.resp.2007.03.004
14. Gassmann M, Muckenthaler MU. Adaptation of iron requirement to hypoxic conditions at high altitude. J Appl Physiol. 2015;119(12):1432–1440. doi:10.1152/japplphysiol.00248.2015
15. West JB. Other Recent High-Altitude Studies. In: High Life. New York, NY: Springer; 1998:364–400.
16. Beall CM, Brittenham GM, Strohl KP, et al. Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am J Phys Anthropol. 1998;106(3):385–400. doi:10.1002/(SICI)1096-8644(199807)106:3<385::AID-AJPA10>3.0.CO;2-X
17. Garruto RM, Chin CT, Weitz CA, Liu JC, Liu RL, He X. Hematological differences during growth among Tibetans and Han Chinese born and raised at high altitude in Qinghai, China. Am J Phys Anthropol. 2003;122(2):171–183. doi:10.1002/ajpa.10283
18. Wu T, Wang X, Wei C, et al. Hemoglobin levels in Qinghai-Tibet: different effects of gender for Tibetans vs. Han. J Appl Physiol. 2005;98(2):598–604. doi:10.1152/japplphysiol.01034.2002
19. Basu M, Malhotra AS, Pal K, et al. Erythropoietin levels in lowlanders and high-altitude natives at 3450 m. Aviat Space Environ Med. 2007;78(10):963–967. doi:10.3357/ASEM.2085.2007
20. Brothers MD, Wilber RL, Byrnes WC. Physical fitness and hematological changes during acclimatization to moderate altitude: a retrospective study. High Alt Med Biol. 2007;8(3):213–224. doi:10.1089/ham.2007.8308
21. Akunov A, Sydykov A, Toktash T, Doolotova A, Sarybaev A. Hemoglobin changes after long-term intermittent work at high altitude. Front Physiol. 2018;9:1552. doi:10.3389/fphys.2018.01552
22. Moore LG, Young D, McCullough RE, Droma T, Zamudio S. Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am J Hum Biol. 2001;13(5):635–644. doi:10.1002/ajhb.1102
23. Petousi N, Robbins PA. Human adaptation to the hypoxia of high altitude: the Tibetan paradigm from the pregenomic to the postgenomic era. J Appl Physiol. 2014;116(7):875–884. doi:10.1152/japplphysiol.00605.2013
24. Gelfi C, De Palma S, Ripamonti M, et al. New aspects of altitude adaptation in Tibetans: a proteomic approach. FASEB J. 2004;18(3):612–614. doi:10.1096/fj.03-1077fje
25. Zubieta-Calleja G. Human Adaptation to High Altitude and to Sea Level: Acid-Base Equilibrium, Ventilation and Circulation in Chronic Hypoxia.
26. Zubieta-Calleja GR, Paulev PE, Zubieta-Calleja L, Zubieta-Castillo G. Altitude adaptation through hematocrit changes. J Physiol Pharmacol. 2007;58 Suppl 5(Suppl Pt 2):811–818.
27. McKenzie DC, Goodman LS, Nath C, et al. Cardiovascular adaptations in Andean natives after 6 wk of exposure to sea level. J Appl Physiol. 1991;70(6):2650–2655. doi:10.1152/jappl.1991.70.6.2650
28. Prakash LTO. Tibetan refugees in India: the case of Bylakuppe in Karnataka. Indian J Soc Dev. 2011;11(2):503–515.
29. Jian C. The Tibetan Rebellion of 1959 and China’s changing relations with India and the Soviet Union. J Cold War Stud. 2006;8(3):54–101. doi:10.1162/jcws.2006.8.3.54
30. Petech L. The Kingdom of Ladakh: C. 950-1842 A.D. Rome: Istituto italiano per il Medio ed Estremo Oriente; 1977.
31. Goldstein MC. Tibet, China, and the United States: Reflections on the Tibet Question. Washington, D.C.: The Atlantic Council of the United States; 1995.
32. Tamang R, Chaubey G, Nandan A, et al. Reconstructing the demographic history of the Himalayan and adjoining populations. Hum Genet. 2018;137(2):129–139. doi:10.1007/s00439-018-1867-2
33. Mondal H, Budh DP. Hematocrit; 2020 July 10. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2020 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542276/.
34. van Lerberghe W, Keegels G, Cornelis G, Ancona C, Mangelschots E, van Balen H. Haemoglobin measurement: the reliability of some simple techniques for use in a primary health care setting. Bull World Health Organ. 1983;61(6):957–965.
35. Maakaron JE, Taher AT, Conrad ME. Anemia Workup; 2019. Avialable from: https://emedicine.medscape.com/article/198475-workup#c8.
36. Yanamandra U, Senee H, Yanamadra S, et al. Erythropoietin and ferritin response in native highlanders aged 4–19 years from the Leh-Ladakh region of India. Br J Haematol. 2019;184(2):263–268. doi:10.1111/bjh.15553
37. Lee G, Choi S, Kim K, et al. Association of hemoglobin concentration and its change with cardiovascular and all-cause mortality. J Am Heart Assoc. 2018;7(3).
38. Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14(1):18–24. doi:10.1200/JCO.1996.14.1.18
39. Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45(10):1638–1643. doi:10.1016/j.jacc.2005.02.054
40. Ensrud K, Grimm RH
41. Simonson TS, Wei G, Wagner HE, et al. Low haemoglobin concentration in Tibetan males is associated with greater high-altitude exercise capacity. J Physiol. 2015;593(14):3207–3218. doi:10.1113/JP270518
42. Wagner PD, Simonson TS, Wei G, et al. Sea-level haemoglobin concentration is associated with greater exercise capacity in Tibetan males at 4200 m. Exp Physiol. 2015;100(11):1256–1262. doi:10.1113/EP085036
43. Beall CM, Goldstein MC. Hemoglobin concentration of pastoral nomads permanently resident at 4850–5450 meters in Tibet. Am J Phys Anthropol. 1987;73(4):433–438. doi:10.1002/ajpa.1330730404
44. Petousi N, Croft QP, Cavalleri GL, et al. Tibetans living at sea level have a hyporesponsive hypoxia-inducible factor system and blunted physiological responses to hypoxia. J Appl Physiol. 2014;116(7):893–904. doi:10.1152/japplphysiol.00535.2013
45. León-Velarde F, Maggiorini M, Reeves JT, et al. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol. 2005;6(2):147–157. doi:10.1089/ham.2005.6.147
46. Sahota IS, Panwar NS. Prevalence of chronic mountain sickness in high altitude districts of Himachal Pradesh. Indian J Occup Environ Med. 2013;17(3):94–100. doi:10.4103/0019-5278.130839
47. Negi PC, Asotra S, Marwah R, et al. Epidemiological study of chronic mountain sickness in natives of Spiti Valley in the Greater Himalayas. High Alt Med Biol. 2013;14(3):220–229. doi:10.1089/ham.2012.1127
48. Villafuerte FC, Corante N. Chronic mountain sickness: clinical aspects, etiology, management, and treatment. High Alt Med Biol. 2016;17(2):61–69. doi:10.1089/ham.2016.0031
49. Monge C, León-Velarde F, Arregui A. Increasing prevalence of excessive erythrocytosis with age among healthy high-altitude miners. N Engl J Med. 1989;321(18):1271.
50. Hancco I, Bailly S, Baillieul S, et al. Excessive erythrocytosis and chronic mountain sickness in dwellers of the highest city in the world. Front Physiol. 2020;11:773. doi:10.3389/fphys.2020.00773
51. Ahmed P, Chaudhry QU, Satti TM, Raza S, Mahmood SK. Erythrocytosis following allogeneic hemopoietic SCT in three cases of aplastic anemia. Bone Marrow Transplant. 2011;46(8):1163–1165. doi:10.1038/bmt.2010.265
52. Winslow RM, Chapman KW, Gibson CC, et al. Different hematologic responses to hypoxia in Sherpas and Quechua Indians. J Appl Physiol. 1989;66(4):1561–1569. doi:10.1152/jappl.1989.66.4.1561
53. Chen MH, Raffield LM, Mousas A, et al. Trans-ethnic and ancestry-specific blood-cell genetics in 746,667 individuals from 5 global populations. Cell. 2020;182(5):1198–1213.e1114. doi:10.1016/j.cell.2020.06.045
54. Beall CM, Cavalleri GL, Deng L, et al. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci U S A. 2010;107(25):11459–11464. doi:10.1073/pnas.1002443107
55. Cho JI, Basnyat B, Jeong C, et al. Ethnically Tibetan women in Nepal with low hemoglobin concentration have better reproductive outcomes. Evol Med Public Health. 2017;2017(1):82–96. doi:10.1093/emph/eox008
56. West JB. Barcroft’s bold assertion: all dwellers at high altitudes are persons of impaired physical and mental powers. J Physiol. 2016;594(5):1127–1134. doi:10.1113/JP270284
57. Mokhtari M, Najafi S. Evaluation of the correlation of automated and manual results of complete blood count in oncologic patients. Comp Clin Path. 2016;25(6):1151–1154. doi:10.1007/s00580-016-2319-9
58. Ike SO, Nubila T, Ukaejiofo EO, Nubila IN, Shu EN, Ezema I. Comparison of haematological parameters determined by the Sysmex KX - 2IN automated haematology analyzer and the manual counts. BMC Clin Pathol. 2010;10(1):3. doi:10.1186/1472-6890-10-3
59. Harris N, Jou JM, Devoto G, et al. Performance evaluation of the ADVIA 2120 hematology analyzer: an international multicenter clinical trial. Lab Hematol. 2005;11(1):62–70. doi:10.1532/LH96.04064
60. ADVIA 2120i Hematology System with Autoslide. Erlangen: Siemens Healthineers; 2021. Available from: https://www.siemens-healthineers.com/en-us/hematology/systems/advia-2120-hematology-system-with-autoslide.
61. Wu DW, Li YM, Wang F. How Long can we Store Blood Samples: A Systematic Review and Meta-Analysis. EBioMedicine. 2017;24:277–285.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


