Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Hepatocellular Carcinoma with Radiological Progression: Lenvatinib Plus PD-1 Inhibitor Combined with Microwave Ablation and Synchronous Transarterial Chemoembolization
Authors Shi Q, Huang P, Zhang Z, Zhang W, Liu L, Yan Z
Received 12 July 2023
Accepted for publication 21 September 2023
Published 21 October 2023 Volume 2023:10 Pages 1861—1871
DOI https://doi.org/10.2147/JHC.S426308
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Imam Waked
Qin Shi,1– 3,* Peng Huang,2,4,* Zihan Zhang,1– 3,* Wen Zhang,1– 3 Lingxiao Liu,1– 3 Zhiping Yan1– 3
1Department of Interventional Radiology, Zhongshan Hospital, Fudan University, Shanghai, 200032, People’s Republic of China; 2Shanghai Institution of Medical Imaging, Fudan University, Shanghai, 200032, People’s Republic of China; 3National Clinical Research Center for Interventional Medicine, Zhongshan Hospital, Fudan University, Shanghai, 200032, People’s Republic of China; 4Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, 200032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lingxiao Liu; Zhiping Yan, Email [email protected]; [email protected]
Purpose: To determine the clinical outcomes of lenvatinib plus PD-1 inhibitor combined with microwave ablation (MWA) and synchronous transarterial chemoembolization (TACE) in patients with progressive hepatocellular carcinoma (pHCC).
Materials and Methods: This retrospective study enrolled pHCC patients who underwent lenvatinib plus PD-1 inhibitor combined with MWA and TACE (LP-MT) or lenvatinib combined with MWA and TACE (L-MT) from January 2019 to December 2022. Treatment-related adverse events (AEs) were recorded during the follow-up. Progression-free survival (PFS) and overall survival (OS) were the primary outcomes. The prognostic analyses for survival were performed using Cox proportional hazard regression model.
Results: In total, 90 eligible patients with pHCC who received combination therapy were included in the study. Among them, 42 patients received LP-MT and 48 patients received L-MT. There were no significant differences in the baseline characteristics between the two groups. Patients who underwent lenvatinib plus PD-1 inhibitor combined with MWA and TACE had better PFS (median, 10.0 vs 7.4 months, P = 0.03) than those who underwent combination therapy without PD-1 inhibitor, although no significant difference was found in OS (median, 22.5 vs 20.0 months, P = 0.19) between the two groups. The disease control rate of LP-MT group was higher than that of L-MT group (88.1% vs 64.6%, P = 0.01), especially in patients with BCLC stage C (89.3% vs 70.0%, P = 0.03). Univariate and multivariate analyses indicated that treatment method and Child-Pugh class were independent prognostic factors for PFS. The AEs of LP-MT group were comparable and tolerable to those of L-MT group (Any grade, 78.6% vs 62.5%, P = 0.10; Grade 3, 23.8% vs 12.5%, P = 0.16).
Conclusion: Lenvatinib plus PD-1 inhibitor may be slightly superior to lenvatinib alone when combined with local interventional therapy for progressive HCC, especially in patients with BCLC stage C.
Keywords: hepatocellular carcinoma, radiological progression, microwave ablation, lenvatinib, PD-1 inhibitor
Introduction
Hepatocellular carcinoma (HCC) is a malignant tumor associated with high morbidity and mortality.1,2 Although the recommended first-line treatments such as surgical resection for patients with early-stage HCC have the potential to cure, most cases are not candidates.3 In addition, 70% of patients have tumor progression and less than 30% of patients can benefit from curative therapies.4 Transarterial chemoembolization (TACE) and microwave ablation (MWA) are the commonly local treatment for unresectable HCC.5 Previous studies have indicated that the combination of TACE and MWA has potential synergistic effects, including reduction of liver function damage and maximum tumor cells necrosis.6,7 However, TACE and MWA, all belongs to the local treatment, are unable to treat systemic tumors and prevent tumor recurrence and progression. The problem of whether local interventional therapies combined with other therapies can achieve better efficacy remains to be solved.
Systemic therapies, such as tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs), are the standard treatment for advanced HCC.8,9 Lenvatinib is one of the most common TKIs that approved as the first-line treatment for advanced HCC.10 The REFLECT trial has indicated that lenvatinib is non-inferior to sorafenib in overall survival for HCC.11 According to the results of data analysis, lenvatinib make it possible to improve the survival benefits in patients with hepatitis B-related HCC. ICIs, including PD-1 and PD-L1 inhibitors, have shown promising anti-tumor activity for advanced HCC in some Phase I and Phase II trials.12,13 However, the Phase III trials of CheckMate 459 and KEYNOTE-240 all failed to confirm superiority of ICIs compared with standard of care.14,15 Since atezolizumab combined with bevacizumab resulted in better survival than sorafenib in the IMbrave150 trial, the combination therapy of anti-PD-(L)1 agent and anti-VEGF agent has been proved to be effective and safe for advanced HCC.16
Previous studies indicated that systemic therapy can improve the local tumor response and make it more sensitive to the local treatment.17,18 Interventional therapies, including TACE and MWA, result in necrosis of the local tumor tissue and release tumor-related antigens which may promote tumor-specific immune responses.19,20 Based on the synergistic mechanisms, this study aimed to evaluate the clinical outcomes of patients with progressive HCC (pHCC) who received lenvatinib plus PD-1 inhibitor combined with MWA-TACE versus lenvatinib combined with MWA-TACE.
Materials and Methods
Study Design
Institutional Review Boards of Zhongshan Hospital, Fudan University reviewed and approved this study. The pHCC was defined as growth of existing tumors (≥20%), new lesions and/or vascular invasion after first-line treatment, based on non-invasive criteria in accordance with the guidelines. Patients with pHCC who underwent combination therapy from January 2019 to December 2022 were screened. The decision-making for the combination therapy using lenvatinib plus PD-1 inhibitor combined with MWA-TACE or lenvatinib alone combined with MWA-TACE according to the guidelines, patients’ selection and physicians’ favor.
Inclusion criteria for this study were as follows: (a) clinically confirmed as HCC with radiological progression; (b) patients ≥18 years old, (c) Eastern Cooperative Oncology Group (ECOG) performance status 0–1, (d) Child-Pugh class A or B; (e) underwent more than one session of LP-MT or L-MT during the treatment period. Patients were excluded: (a) incomplete clinical medical records, (b) lost to follow-up, (c) participated in other clinical trials during the combination therapy, (d) received drugs less than 1 month.
Treatment
All patients underwent MWA and synchronous TACE. Briefly, the tumor number and blood supply were evaluated using hepatic angiography. Then, appropriate puncture point was selected to perform MWA using a water-cooled microwave system. Ultrasound imaging was used to monitor the ablation process and avoid damage to normal organs. After MWA, arteriography was performed again to assess the ablative results and residual tumor blood supply. Catherization of tumor-feeding arteries and standardized TACE were performed.
The multidisciplinary team recommended the treatment strategy of interventional therapy combined with systemic therapies for pHCC. Lenvatinib was administrated 3 days later after the first MWA-TACE. The recommended dosage of lenvatinib was 12 mg (≥60 kg) or 8 mg (<60 kg) once daily based on patient’s body weight. The reduction and discontinuation of lenvatinib were performed under the guidance of physicians. The used PD-1 inhibitors included Sintilimab, Camrelizumab and Pembrolizumab in the study. The administration was injected intravenously at a dose of 200 mg once every 3 weeks after the first MWA-TACE.
Clinical Outcomes
The primary outcomes were progression-free survival (PFS) and overall survival (OS). The overall tumor responses and safety were included as the secondary outcomes. PFS was defined as the time interval from the beginning of combination therapy to the radiological progression, death, or the final follow-up. Meanwhile, the duration of OS was calculated from the initiation of combination therapy to death or the final follow-up. The overall tumor responses, classified as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) were evaluated based on the mRECIST. The objective response rate (ORR) was defined as the percentage of the sum of CR and PR. The disease control rate (DCR) was expressed as the percentage of the sum of CR, PR, and SD.
The safety assessment of treatments mainly depended on the records of adverse events (AEs). AEs related to interventional therapy, lenvatinib and PD-1 inhibitor were recorded and assessed according to the National Cancer Institute Common Terminology Criteria, version 5.0.
Follow-Up
The follow-up period concluded on May 31, 2023. Patients underwent regular evaluation at an interval of 6–8 weeks following the initiation treatment. Tumor response was assessed by contrast-enhanced CT or MRI. Some local therapies, such as MWA-TACE, TACE alone, radioactive seed implantation or transarterial infusion, would be performed when the residual and recurrent tumors were visible on CT or MRI.
During the follow-up, the laboratory tests including liver function tests, blood cell count and level of alpha-fetoprotein (AFP) were detected. The calculation formula of albumin-bilirubin (ALBI) score as follow: ALBI = 0.66 * log10[total bilirubin (μmol/L)] – 0.085 * [albumin (g/L)]. A value of −2.60 or less was defined as grade 1, a value between −2.60 and −1.39 was defined as grade 2 and a value greater than −1.39 was defined as grade 3. The duration of lenvatinib, the frequency of MWA-TACE and PD-1 inhibitor were recorded during the follow-up.
Statistical Analysis
Continuous variables were presented as mean ± standard deviation. The differences between the two groups were compared using Student’s t-test for continuous variables and Person χ2 and continuity correction for categorical variables. Survival curves were performed using the Kaplan–Meier method and differences in survival rates between the two groups were compared using the Log rank test. Univariate and multivariate analyses were performed to indicate the prognostic factors affecting survival using Cox proportional hazard regression model. Variables with a P value ≤0.10 in the univariate analysis were introduced into the multivariate analysis. Statistical analyses were performed using SPSS version 25.0. All analyses were two-tailed, and P value <0.05 indicated significant difference.
Results
Baseline Characteristics
In the study, a total of 115 patients with progressive HCC were enrolled. Among them, 25 patients were excluded, including 9 patients who had incomplete medical records, 10 patients who lost to follow-up, 3 patients who had participated in other clinical trials during the treatment and 3 patients who received drugs less than 1 month (Figure 1). Finally, 42 patients who underwent combination therapy of lenvatinib, PD-1 inhibitor, MWA and TACE (LP-MT group) and 48 patients who underwent combination therapy of lenvatinib, MWA and TACE (L-MT group).
 |
Figure 1 Patient flow chart. Abbbreviations: HCC, hepatocellular carcinoma; MWA, microwave ablation; TACE, transarterial chemoembolization. |
The detailed baseline characteristics of all patients enrolled are shown in Table 1. There was no significant difference between the two groups. The patients were diagnosed with progressive HCC according to the CT/MRI images. The average size of the maximal tumor was 5.5 ± 2.4 cm. Forty-four patients (48.9%) had portal vein tumor thrombus and twenty-seven patients (30.0%) had extrahepatic metastases. Nearly two-third of patients (64.4%) were at BCLC C stage, and most of patients (85.6%) had previous hepatitis B infections.
 |
Table 1 Baseline Characteristics of the Patients with pHCC |
The mean follow-up period was 19.3 months (range, 5.8–38.2 months) in the LP-MT group and 15.7 months (range, 7.0–31.5 months) in the L-MT group. In the LP-MT group, 42 patients underwent a total of 85 cycles MWA-TACE (median, 2) and 203 cycles of PD-1 inhibitor (median, 4). In the L-MT group, 48 patients underwent 90 cycles MWA-TACE (median, 2). The median duration of lenvatinib was 8.0 months in the LP-MT group and 6.0 months in the L-MT group, respectively.
Efficacy Outcomes
In the study population, patients who underwent lenvatinib plus PD-1 inhibitor combined with MWA and TACE had better survival benefits than those who received combination therapy without PD-1 inhibitor (Figure 2a). The median PFS was 10.0 months (95% confidence interval [CI], 6.6–13.4 months) in the LP-MT group and 7.4 months (95% CI, 4.6–10.2 months) in the L-MT group (P = 0.03). The median OS was 22.5 months (95% CI, 19.0–26.0 months) in the LP-MT group and 20.0 months (95% CI, 13.3–26.7 months) in the L-MT group (P = 0.19), which indicated no significant difference between the two groups. Notably, subgroup analysis of patients with BCLC stage C had similar trends in survival benefits (LP-MT vs L-MT; median PFS, 8.8 vs 5.8 months, P = 0.04; median OS, 20.0 vs 16.5. P = 0.70) (Figure 2b). There was no difference in PFS (LP-MT vs L-MT; median, 13.1 vs 9.0 months, P = 0.15) and OS (LP-MT vs L-MT; median, 28.5 vs 20.0 months, P = 0.16) between patients with BCLC stage B who received the two treatment schemes (Figure 2c).
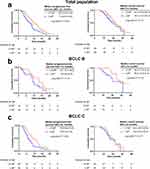 |
Figure 2 Kaplan–Meier curves of progression-free survival and overall survival in the total population (a), pHCC patients with BCLC B (b) and pHCC patients with BCLC C (c). |
The overall tumor response assessments based on the mRECIST are shown in Table 2. Two patients who received lenvatinib plus PD-1 inhibitor combined with MWA-TACE achieved systemic CR, including one with BCLC stage B and one with BCLC stage C. In the total population, the LP-MT group had better systemic DCR than L-MT group (88.1% vs 64.6%, P = 0.01). In the subgroup analysis, patients with BCLC stage C who received lenvatinib plus PD-1 inhibitor combined with MWA-TACE had higher DCR than those who received lenvatinib alone combined with MWA-TACE (89.3% vs 70.0%, P = 0.03), while no difference was found in DCR between the two groups (85.7% vs 55.6%, P = 0.10). In addition, there were no significant differences in ORR of total population and subgroups between the two groups.
 |
Table 2 Overall Tumor Response in the Total and Subgroups |
Prognostic Factor Analysis for Survival
The risk factors for PFS and OS assessed by univariate and multivariate analyses are shown in Table 3. In the univariate analyses, treatment option and Child-Pugh class were associated with PFS. The Child-Pugh class, ALBI grade, portal vein tumor thrombosis and ECOG performance status were associated with OS. On the basis of these findings, further multivariate analysis indicated that treatment option (LP-MT vs L-MT; hazard ratio [HR] = 0.53, 95% CI, 0.33–0.85, P = 0.01) and Child-Pugh class (B vs A; HR = 2.42, 95% CI, 1.29–4.54, P = 0.01) were identified as independent prognostic factors for PFS. The ALBI score (Grade 2 vs 1; HR = 3.57, 95% CI, 1.33–9.60, P = 0.01), portal vein tumor thrombosis (Yes vs No; HR =2.66, 95% CI, 1.05–6.71, P = 0.04) and ECOG performance status (1 vs 0; HR = 2.33, 95% CI, 1.01–5.38, P = 0.04) were identified as independent prognostic factors for OS.
 |
Table 3 Univariate and Multivariate Analysis of PFS and OS |
Safety Analysis
The treatment-related AEs of patients between the two groups are shown in Table 4. There was no significant difference in the total occurrence rate of AEs between the two groups (LP-MT vs L-MT; 78.6% vs 62.5%, P = 0.10). AEs related to MWA-TACE mainly included new ascites (11.9% vs 4.2%, P = 0.33) and segmental bile duct dilatation (2.4% vs 4.2%, P = 1.00) in the two groups. The AEs related to lenvatinib or PD-1 inhibitor were similar between the two groups, including hypertension, diarrhea, decreased appetite, hand-foot syndrome, reactive cutaneous capillary endothelial proliferation, proteinuria, elevated AST level, elevated ALT level, elevated TBIL level and leukocytopenia level. No treatment-related death or grade 4 AEs occurred in the study. And there was no significant difference in the occurrence rate of grade 3 AEs between the two groups (LP-MT vs L-MT; 23.8% vs 12.5%, P = 0.16). The AEs were transient and recovered within a short period after treatment.
 |
Table 4 Treatment-Related Adverse Events |
Discussion
HCC is a malignant tumor with high risk of recurrence and rapid progression.21 Although the technology of liver resection, local interventional therapies, such as TACE and ablation is sophisticated, the high risk of tumor recurrence and progression remains.22 Some studies indicated that TACE or ablation can cause tumor necrosis and promote more antigens release, which may activate antitumor immune response.23,24 However, some patients are still prone to relapse and progression after treatment. In addition, the existing distant tumors are hardly eliminated by local treatments alone. This leads to the exploration of local combined with systemic therapies for HCC.
In recent years, many clinical trials have focused on the efficacy and safety of local (mainly including TACE) combined with systemic therapies for unresectable or advanced HCC.25–27 Xin et al28 reported that the triple therapy of lenvatinib, PD-1 inhibitor and TACE had the ability to achieve more favorable survival benefits than dual therapy of lenvatinib and PD-1 inhibitor in patients with unresectable HCC. The CHANCE001 trial also demonstrated that TACE plus PD-(L)1 blockades and molecular targeted treatments can improve survival benefits and tumor response versus TACE alone for Chinese patients with advanced HCC in real-world practice.29 In our study, the combination therapy with lenvatinib, PD-1 inhibitor, MWA and synchronous TACE had relatively longer PFS than the combination therapy with lenvatinib, MWA and synchronous TACE in patients with progressive HCC. These patients had a history of tumor progression, hence the median PFS was 10.0 months (95% CI, 6.6–13.4 months) when treated with LP-MT, relatively lower than other reported for local combined with systemic treatments. In addition, the systemic antitumor immune response could be strongly triggered due to the application of PD-1 inhibitor. This may further slow the time of tumor progression.
In addition, the present study indicated that patients who received LP-MT can achieve higher disease control rate than those who received L-MT (88.1% vs 64.6%, P = 0.01). This implied that the introduction of immunotherapy can enhance the efficacy of local combined with anti-angiogenesis targeted therapies. Anti-angiogenesis targeted drugs, such as sorafenib and lenvatinib, can reduce the tumor angiogenesis while reconstruct the normal structure of blood vessels.30 The activated effector T cells induced by PD-1 inhibitor can easily enter tumor tissue through vascular structure and kill tumor cells. In addition, MWA combined with TACE can induce more antigens release versus TACE or ablation monotherapy, while inhibit tumor growth to the maximum extent.7 Some studies have demonstrated that immune checkpoint inhibitors plus anti-angiogenesis therapy may be superior to anti-angiogenesis therapy alone for some HCC patients.16,31 These results reported were similar to our results.
In clinical practice, prognostic analysis including demographics and neoplastic characteristics have a profound impact on survival. In this study, the LP-MT treatment and better Child-Pugh class were identified as independent prognostic factors for longer PFS. Obviously, the combination therapy with lenvatinib, PD-1 inhibitor and MWA-TACE can effectively control tumor growth, and further improve progression-free survival. Better Child-Pugh class, means a well liver function reserve, affects the tolerance and duration of treatment. This may contribute to favorable efficacy of progressive HCC. In addition, higher ALBI grade, present portal vein tumor thrombosis and higher ECOG PS were associated with poor OS. The ALBI grade offers a convenient method for liver function evaluation in HCC that has been extensively tested in an international setting.32,33 The higher ALBI grade, the worse liver function reserve, similar to the Child-Pugh class. The difference of ALBI grade and Child-Pugh class in the prognostic analysis of survival may be mainly attributed to the vary in the number of people classified according to the both evaluation methods. Portal vein tumor thrombosis and ECOG PS, are the important risk factors, have been reported to be associated with OS in patients with HCC.2,34
In this study, the combination therapy in the treatment of HCC was manageable and acceptable, and no severe AEs even death occurred during the treatment. There was similar in the total occurrence rate of AEs between the two groups. The drug-related AEs recorded in this study were common and mild, consistent with those reported in some studies.35,36 These indicated that the occurrence rate of AEs had not been obviously affected by the introduction of PD-1 inhibitor. In addition, the frequency and interval of MWA-TACE procedure were strictly controlled according to the principle of on-demand. The AEs recorded in the perioperative period were little different between the two groups. Symptomatic treatment was given to maintain well-preserved liver function and continue to make patients benefit from systemic treatment.
There were still some limitations, although this study demonstrated the antitumor activity and tolerable safety of quadruple therapy with lenvatinib, PD-1 inhibitor, MWA and synchronous TACE in the progressive HCC. First, this study was a retrospective rather than a randomized study with a limited sample size that may lead to inevitable selection bias. These findings should be further confirmed in the large sample, prospective randomized controlled trials. Second, the study was based on a Chinese population with prevalent HBV-related cirrhosis, unlike foreign population commonly with hepatitis C infection and alcohol abuse. Both have various survival rates. Whether the quadruple therapy can be applied to HCC patients with other etiologies remains to be further studied. Finally, some unknown risk factors were not included and analyzed, which may lead to confounding bias.
Conclusion
In summary, this study indicated that the combination therapy with lenvatinib, PD-1 inhibitor, MWA and synchronous TACE had better progression-free survival and similar overall survival benefit for progressive HCC compared to the combination therapy with lenvatinib, MWA and synchronous TACE. In addition, the systemic therapies had an acceptable safety profile, with no occurrence of severe AEs. These findings demonstrated that lenvatinib plus PD-1 inhibitor may be superior to lenvatinib alone when combined with local interventional therapy for progressive HCC, especially in patients with BCLC stage C.
Data Sharing Statement
The data used in this study are available from the corresponding author on reasonable request.
Ethics Statement
The study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University. The study was conducted in accordance with the ethical principles laid down in the Declaration of Helsinki. Written informed consent was obtained from patients.
Funding
The work was supported by the National Health Commission Capacity Building and Continuing Education Center (No. GWJJ2022100303).
Disclosure
All authors declare no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Villanueva A, Longo DL. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
3. Guo Y, Ren Y, Chen L, et al. Transarterial chemoembolization combined with camrelizumab for recurrent hepatocellular carcinoma. BMC Cancer. 2022;22(1):270. doi:10.1186/s12885-022-09325-6
4. Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62(6):394–399. doi:10.3322/caac.21161
5. Zaitoun MMA, Elsayed SB, Zaitoun NA, et al. Combined therapy with conventional trans-arterial chemoembolization (cTACE) and microwave ablation (MWA) for hepatocellular carcinoma >3-<5 cm.. Int J Hyperthermia. 2021;38(1):248–256. doi:10.1080/02656736.2021.1887941
6. Shi Q, Wang F, Du N, et al. Microwave ablation combined with lipiodol-microsphere mixed or conventional transarterial chemoembolization for the treatment of colorectal liver metastases: a retrospective study. Clin Res Hepatol Gastroenterol. 2022;46(7):101986. doi:10.1016/j.clinre.2022.101986
7. Keshavarz P, Raman SS. Comparison of combined transarterial chemoembolization and ablations in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Abdom Radiol. 2022;47(3):1009–1023. doi:10.1007/s00261-021-03368-2
8. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
9. Bruix J, Chan SL, Galle PR, et al. Systemic treatment of hepatocellular carcinoma: an EASL position paper. J Hepatol. 2021;75(4):960–974. doi:10.1016/j.jhep.2021.07.004
10. Casadei-Gardini A, Rimini M, Kudo M, et al. Real Life Study of Lenvatinib Therapy for Hepatocellular Carcinoma: RELEVANT Study. Liver Cancer. 2022;11(6):527–539. doi:10.1159/000525145
11. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
12. Li Q, Han J, Yang Y, et al. PD-1/PD-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front Immunol. 2022;13:1070961. doi:10.3389/fimmu.2022.1070961
13. Sperandio RC, Pestana RC, Miyamura BV, et al. Hepatocellular Carcinoma Immunotherapy. Annu Rev Med. 2022;73(1):267–278. doi:10.1146/annurev-med-042220-021121
14. Yau T, Park J-W, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi:10.1016/S1470-2045(21)00604-5
15. Finn RS, Ryoo B-Y, Merle P, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: a Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38(3):193–202. doi:10.1200/JCO.19.01307
16. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
17. Singal AG, Kudo M, Bruix J. Breakthroughs in Hepatocellular Carcinoma Therapies. Clin Gastroenterol Hepatol. 2023;21(8):2135–2149. doi:10.1016/j.cgh.2023.01.039
18. Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(5):293–313. doi:10.1038/s41575-020-00395-0
19. Hatzidakis A, Müller L, Krokidis M, et al. Local and Regional Therapies for Hepatocellular Carcinoma and Future Combinations. Cancers. 2022;14(10):2469. doi:10.3390/cancers14102469
20. Zhang L, Wang J, Jiang J, et al. CTLA-4 Blockade Suppresses Progression of Residual Tumors and Improves Survival After Insufficient Radiofrequency Ablation in a Subcutaneous Murine Hepatoma Model. Cardiovasc Intervent Radiol. 2020;43(9):1353–1361. doi:10.1007/s00270-020-02505-6
21. Ma X-L, Shen M-N, Hu B, et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis. J Hematol Oncol. 2019;12(1):37. doi:10.1186/s13045-019-0724-7
22. Kang TW, Lim HK, Cha DI. Aggressive tumor recurrence after radiofrequency ablation for hepatocellular carcinoma. Clin Mol Hepatol. 2017;23(1):95–101. doi:10.3350/cmh.2017.0006
23. Han J-W, Yoon S-K. Immune Responses Following Locoregional Treatment for Hepatocellular Carcinoma: possible Roles of Adjuvant Immunotherapy. Pharmaceutics. 2021;13(9):1387. doi:10.3390/pharmaceutics13091387
24. Duan X, Wang M, Han X, et al. Combined use of microwave ablation and cell immunotherapy induces nonspecific immunity of hepatocellular carcinoma model mice. Cell Cycle. 2020;19(24):3595–3607. doi:10.1080/15384101.2020.1853942
25. Shi Q, Zhou X, Zhang Z, et al. Microwave ablation and synchronous transarterial chemoembolization combined with PD-1 inhibitor in patients with hepatocellular carcinoma following tyrosine kinase inhibitor intolerance. Front Immunol. 2022;13:1097625. doi:10.3389/fimmu.2022.1097625
26. Yin L, Liu K-C, Lv W-F, et al. Comparing the effectiveness and safety of Sorafenib plus TACE with Apatinib plus TACE for treating patients with unresectable hepatocellular carcinoma: a multicentre propensity score matching study. Cancer Imaging. 2023;23(1):52. doi:10.1186/s40644-023-00574-7
27. Yuan Y, He W, Yang Z, et al. TACE-HAIC combined with targeted therapy and immunotherapy versus TACE alone for hepatocellular carcinoma with portal vein tumour thrombus: a propensity score matching study. Int J Surg. 2023;109(5):1222–1230. doi:10.1097/JS9.0000000000000256
28. Xin Y, Zhang X, Liu N, et al. Efficacy and safety of lenvatinib plus PD-1 inhibitor with or without transarterial chemoembolization in unresectable hepatocellular carcinoma. Hepatol Int. 2023;17(3):753–764. doi:10.1007/s12072-023-10502-3
29. Zhu H-D, Li H-L, Huang M-S, et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8(1):58. doi:10.1038/s41392-022-01235-0
30. Stefanini B, Ielasi L, Chen R, et al. TKIs in combination with immunotherapy for hepatocellular carcinoma. Expert Rev Anticancer Ther. 2023;23(3):279–291. doi:10.1080/14737140.2023.2181162
31. Li S-Q, Yang Y, Ye L-S. Angiogenesis and immune checkpoint dual blockade: opportunities and challenges for hepatocellular carcinoma therapy. World J Gastroenterol. 2022;28(42):6034–6044. doi:10.3748/wjg.v28.i42.6034
32. Bannangkoon K, Hongsakul K, Tubtawee T. Validation of the ALBI-TAE model and comparison of seven scoring systems for predicting survival outcome in patients with intermediate-stage hepatocellular carcinoma undergoing chemoembolization. Cancer Imaging. 2023;23(1):51. doi:10.1186/s40644-023-00575-6
33. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
34. Fu Y, Peng W, Zhang W, et al. Induction therapy with hepatic arterial infusion chemotherapy enhances the efficacy of lenvatinib and pd1 inhibitors in treating hepatocellular carcinoma patients with portal vein tumor thrombosis. J Gastroenterol. 2023;58(4):413–424. doi:10.1007/s00535-023-01976-x
35. Jácome AA, Castro ACG, Vasconcelos JPS, et al. Efficacy and Safety Associated With Immune Checkpoint Inhibitors in Unresectable Hepatocellular Carcinoma: a Meta-analysis. JAMA Netw Open. 2021;4(12):e2136128. doi:10.1001/jamanetworkopen.2021.36128
36. Wang Y, Jiang M, Zhu J, et al. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed Pharmacother. 2020;132:110797. doi:10.1016/j.biopha.2020.110797
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
