Back to Journals » Infection and Drug Resistance » Volume 16
Hepatitis C Virus Dynamic Transmission Models Among People Who Inject Drugs
Received 30 December 2022
Accepted for publication 14 February 2023
Published 20 February 2023 Volume 2023:16 Pages 1061—1068
DOI https://doi.org/10.2147/IDR.S403133
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Shiferaw Bekele Woyesa,1 Kellemuwa Desalegn Amente2
1School of Medical Laboratory Science, Jimma University, Jimma, Ethiopia; 2Jimma University Medical Center, Jimma University, Jimma, Ethiopia
Correspondence: Shiferaw Bekele Woyesa, Email [email protected]
Background: Transmission dynamic model is a concrete structure to describe and investigate the complex system of host–pathogen interactions. Hepatitis C virus (HCV) is a blood-borne virus that is transmitted from infectious to susceptible individuals when they come into contact with HCV-contaminated equipment. Injecting drug use is the most known transmission route, and about 80% of new HCV cases have been confirmed as having acquired HCV infection via drug injection.
Objective: The main objective of this review paper was to review the importance of HCV dynamic transmission model, that enables the readers to understand the mechanism how HCV is transmissible from infectious to susceptible hosts and the effective controlling strategies.
Methods: PubMed Central, Google Scholar, and Web of Science electronic databases have been used to search data by using key terms like “HCV transmission model among people who inject drug (PWID)”, HCV potential herd immunity”, and “basic reproductive number for HCV transmission in PWID.” Data from research findings other than English version have been excluded from being used, and the most recently published data have been considered to be included.
Conclusion: HCV belongs to the Hepacivirus genus within the Flaviviridae family. HCV infection is acquired when the susceptible individuals in populations come into contact with medical equipment such as shared syringes and needles, or swabs contaminated with infected blood. Construction of HCV transmission dynamic model is very significant in order to predict the duration and magnitude of its epidemic and to evaluate the potential impact of intervention. Comprehensive harm reduction and care/support service strategies are the best approach for intervention regarding HCV infection transmission among PWID.
Keywords: HCV transmission, HCV dynamic model, PWIDs
Introduction
Hepatitis C virus (HCV) belongs to the Hepacivirus genus within the Flaviviridae family that also includes yellow fever virus, West Nile virus, and dengue virus. HCV is an enveloped and positive-strand ribonucleic acid (RNA) virus.1
HCV is a blood-borne virus and is transmitted from infected person to non-infected person via exposure to infected blood, most commonly through intravenous drug use, blood transfusion, vertical transmissions or congenital (mother-to-child), organ transplant, or through sexual contact.2
HCV causes both acute and chronic hepatitis that may lead to lifelong severe illness like liver cirrhosis and cancer. According to 2019 report of the World Health Organization (WHO), about 58 million persons were chronically infected and living with HCV. Annually, about 400,000 deaths occur from chronic liver diseases caused by HCV infection, and thus HCV infection is a major public health problem.3 The risk of progression to different disease stage may vary for HCV infection. For instance, a guideline developed for HCV infection epidemiology, treatment, and prevention among PWID shows that for every 100 people infected with HCV, 75–80%, 60–70%, and 5–20% will develop chronic infections, chronic liver diseases, and cirrhosis, respectively, and 1–5% will die of cirrhosis or liver cancer.4
Symptoms of HCV can take decades to appear, and because of this about half of people with HCV do not know they are infected. However, the incubation period for HCV ranges from 2 weeks up to six months.5 The majority, about 80%, of HCV-infected individuals do not show any symptoms. The HCV is cleared spontaneously without any treatment from 30% of infected individuals within 6 months of infection, and the remaining develops chronic HCV infection.2,6 The Global Health Sector strategy on viral hepatitis set goals to be achieved by 2030 to eliminate HCV as a public threat by a 90% and 65% reduction in global incidence and mortality, respectively.7
Emerging and re-emerging infectious diseases are globally increasing, and their emergence and transmissibility cannot be followed by the traditional statistical models like linearity and independence of outcome.5 To forecast epidemics and develop strategies to mitigate infectious diseases transmissions, infectious diseases transmission dynamic models are essential.
These are also used to assess for changes in incidence rates of infectious diseases when there are changes in disease susceptibility and infectiousness in a population.9
Infectious diseases transmission models have been used to predict the magnitude and duration of infectious diseases outbreaks to estimate significant biological and clinical parameters and to evaluate the potential impact of interventions.10,11 The infectious diseases transmission model used is preferably defined by the question of interest and fitted to specific pathogens which give extraordinary powerful insights into the drivers of infectious diseases dynamicity.12 The model is also used for establishment of a framework to explore the impact and efficacy of lots of mitigation approaches.11
Methods
To develop this review paper, PubMed Central, Google Scholar, and Web of Science electronic databases have been searched to collect important data about HCV transmission dynamic model construction, calculated basic reproductive number with its interpretation, HCV disease transmission, and intervention mechanisms from internationally peer-reviewed published original articles, systematic reviews, and meta-analyses from international guidelines like the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC). Key terms like “HCV transmission model among PWID”, “HCV transmission model”, and “basic reproductive number for HCV transmission in PWID” have been used to search these published data. The searches are also restricted to English language articles and guidelines.
HCV Transmission Mechanism
HCV infection can be spread when susceptible individuals are in contact with surfaces, equipment, or objects that are contaminated with infected blood or blood products, as HCV is highly infectious.5 The HCV is capable to survive on equipment and dry surfaces for about 6 weeks, and people who inject drugs can get HCV infection from sharing and reusing HCV-contaminated needles and syringes, and from HCV-contaminated equipment used for drug preparation such as cotton, cooker, injection site, or swabs.13,14
Drug use injection (DUI) is the main mode of HCV transmission mechanism in developed countries, and about 60% of all HCV infection is due to sharing needles, syringes, and drugs.15 Injecting drug use is the most known transmission route for HCV, and about 80% new HCV cases were confirmed to have acquired HCV infection via drug injection.16 The World Health Organization (WHO) 2017 report revealed that the global prevalence of HCV infection among PWID was 67% in 77 countries and 80% in 12 countries. The report pointed out high prevalence of HCV infection in settings where PWID were criminalized and lacked access to harm reduction strategies.17,18
Infectious Diseases Transmission Dynamic Models
Transmission dynamic models are tools that help scientists or researchers understand the complex interaction between hosts and pathogens. These models are used to study how a system behaves and responds to interventions,11,19 for instance studying whether the prevalence of an infection increases or decreases in the absence of intervention, and also studying the fraction of population that needs to be immunized to prevent the pathogen from causing an epidemic.10
Infectious diseases transmission dynamic models are also used to better understand the system itself – for example, understanding whether the previous infection provides a protection from re- infection or not; or understanding and identifying the number of new cases that depend on the proportion of asymptomatic infectious individuals.12,19
There are different structures of infectious diseases transmission dynamic models. A “susceptible–infectious–recovered (SIR)” model is an example that contains three health states, namely susceptible, infectious, and recovered. A SIR model is selected when hosts becomes infectious soon after infection and retain at least partial immunity to re-infection on recovery.20 A “susceptible–exposed–infectious–recovered (SEIR)” model is another example of an infectious diseases transmission dynamic model that is appropriate for a pathogen with a period of latency.19,21
HCV Transmission Dynamic Model Among PWID
Constructing a transmission model for HCV is very important in order to understand the transmission dynamics and prevalence of HCV infection as well as to predict the trend of the disease and to search for a prevention and control mechanism.22
Basic susceptible–infected formulations with a variety of modeling approaches including stochastic individual-based and deterministic compartmental, HCV-mono-infection or HIV/HCV joint co-infection were used to examine transmission dynamics in a range of theoretical and real-world settings.13 Open populations are simulated in these models with injectors entering open initiation of injecting and exiting PWID population through permanent cessation or death.23
The compartment model is the most frequently used class of models by researchers to simulate the HCV epidemic among PWID.21 This model considers the transmission of HCV infection by classifying the PWID populations into compartments consequent to different HCV infectious processes: susceptible (S); infectious (I); recovered (R); susceptible (S), or the S-I-R-S model.
Model Description
- Susceptible compartment: This includes infectious-naïve PWID, or those achieving a sustained virological response (SVR) either through treatment or spontaneously, but do not develop immunity.
- Infectious compartment: This compartment includes PWID who are naïve to antiviral treatment, or PWID who do not clear their infection spontaneously including both initial infection (non-sustained virological response) and re-infection.
- Recovered compartment: This includes PWID who developed immunity through clearance of HCV infection either spontaneously due to natural immunity or through treatment.
- Susceptible: PWID who are treated by antiviral therapy but not achieving sustained virological response (non-SVR) due to treatment failure. These individuals can be susceptible to the same pathogen by re-infection.
The susceptible–exposed–infectious–recovered (S-E-I-R) model is used to give insights into the dynamics of HCV epidemics among the PWID population and is used to understand how public health interventions affect the trajectory of epidemics. The susceptible PWID enter this model in the S compartment, and they contract HCV infection when they come into contact with HCV-infectious individuals. Once the susceptible PWID become infected, they move to the infectious compartment. In the infectious compartment, infection in individuals who are exposed to HCV pathogen may remain latent or without signs and symptoms. The infection may be cleared spontaneously, without treatment, from the infected individual, who can become susceptible for re-infection or resistant to re-infection due to developing immunity. Those infected PWID can develop chronic HCV infection before treatment due to being treatment-naïve or after treatment due to treatment failure or non-sustained virological response (non-SVR). Those who have sustained virological response (SVR) after treatment can develop immunity and recover from the disease or due to cessation of injecting. Those individuals with non-SVR after viral treatment can have the probability to be susceptible to other genotypes of viral infection or become chronically infected and removed from the infectious compartment due to death. Other susceptible individuals may leave the susceptible compartment due to cessation of injecting or natural death because of other comorbidity.24–26 Some individuals might be also removed from infectious compartment after having SVR and being immune, because of cessation of injecting or natural death (Figure 1).
 |
Figure 1 Schematic description of HCV infection transmission model. |
The S-I-R-S model parameters include population dynamics (average new injector rate, average PWID leaving rate–cessation or death), infection dynamics (average HCV infection rate, average proportion of infection spontaneously cleared the infection resulting in immunity), and treatment dynamics (average treatment rate, average treatment duration, average proportion of cured infections due to treatment resulting in immunity and also average proportion of cured infections with sustained virological response (SVR)).
In closed populations (no births or deaths), the HCV transmission model and associated differential equations can be constructed and represented by the box-and-arrow diagram in Figure 1.
The basic reproductive number (R0) of HCV infection among PWID is the average number of HCV secondary infections arising from a typical HCV-infected individual in a population where everyone is susceptible to HCV infection. In the Figure 1, β stands for the rate of HCV transmission from infected individuals, and α stands for the rate of recovery from HCV infections. Thus, R0HCV infection is the ratio of the rate of HCV transmission from an infected individual and the rate of recovery from HCV infection, ie R0 = β/σ.
In the HCV transmission dynamic model indicated below, βI stands for the force of HCV infection, and βI is the product of the HCV transmission parameter and the prevalence of HCV-infectious individuals in the population, and φ stands for the rate of HCV disease progression (Figure 2).
 |
Figure 2 Susceptible–Exposed–Infectious–Recovered (S-E-I-R) HCV transmission dynamic model. |
The SEIR model is considered to be an appropriate transmission dynamic model for a pathogen with a period of latency between time of infection and time that an infectious individual becomes infectious to others.19 About 80% of HCV-infected individuals do not exhibit any signs and symptoms for 2 weeks to 6 months, so the SEIR model is an appropriate model for HCV transmission dynamic.27
S is the prevalence of PWID susceptible to HCV infections; E is the prevalence of HCV-exposed/latent PWID; I is the prevalence of infectious PWID; R is the number of recovered or immune PWID from HCV infections.14 Some literatures have established differential equations by associating parameters β, α, φ and variables S, E, I, and R as indicated below:
 , where φ is HCV disease's progression rate, and E is prevalence of HCV-exposed/latent individuals.
, where φ is HCV disease's progression rate, and E is prevalence of HCV-exposed/latent individuals. , where σ is recovery rate of individuals from HCV infection, and I is prevalence of HCV-infectious individuals.
, where σ is recovery rate of individuals from HCV infection, and I is prevalence of HCV-infectious individuals.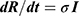 , where σ is recovery rate of individuals from HCV infection, and R is prevalence of recovered individuals.
, where σ is recovery rate of individuals from HCV infection, and R is prevalence of recovered individuals.
Literature Review for HCV Basic Reproductive Number
The basic reproduction number is the average number of secondary cases that would be generated by a primary case in a totally susceptible population. It depends on the transmission coefficient and the average duration of infectiousness of the host. It is used to provide the general measure of the potential for the transmission of an infection within a population.9 Naught, or zero, stands for the zeroth generation (patient zero) and refers to the first documented patient infected by a disease in an epidemic. R0 is calculated as a product of the number of contacts per unit time, transmission probability per contact, and duration of infectiousness, ie R0 = c × p × d.
To understand the various aspects of the HCV epidemic, a mathematical concept is very useful;23 however, the attempt to use the mathematical concept to understand HCV in terms of an interaction between the various actors like the host, the needle injection equipment, and others in an HCV outbreak is yet not well developed.
A HCV transmission model can be formulated by homogeneous mixing, in which an individual has an equal chance of contact with another in the population, and ignoring seasonal changes and space structure.28
R0 is always estimated retrospectively, and theoretical mathematical modeling or sero- epidemiological data are used for estimation.29
Different international research findings reported different basic reproductive values that show HCV transmission potential among different populations. For instance an epidemiological study conducted on data extracted from the Chinese Center for Disease Control and Prevention (China’s CDC) to understand the transmission and prevalence of HCV determined 1.6592 as an estimated reproductive number.28 Another study reported from the same country, China, on the available HCV epidemic data revealed that the incidence of HCV infection was rising continuously, which was indicated by the R0 = 4.0636 value, which is large.30 Again, another paper published data from Xiamen City, from China, conducted in six districts on modeling the transmissibility of HCV and determined the median R0 = 0. 41.20
In the case of modeling the spread of HCV among PWID, the study currently conducted in England to measure the potential transmission of HCV infection among PWID population determined a basic reproductive number that is greater than one, R0 = 2.9987, with a 0.5348 total endemic equilibrium fraction of infectious PWIDs and with a 0.275 total fraction of infectious needles.26
Interpretation of the Basic Reproductive Number (R0)
R0 is used to measure the transmission potential of infectious diseases; because of this it is considered as a central quantity in infectious diseases epidemiology. To study the dynamicity of infectious diseases, R0 is the most fundamental and often-used metric that is reported as a single numeric value or low–high range.29
R0or this basic reproductive number for infectious diseases is interpreted in a straightforward manner, even though the process of applying, calculating, and defining it is far from straightforward. The magnitude of the R0value is used to determine the potential size of an outbreak or epidemic of an infectious disease.29 It is also used to estimate the proportion of vaccination coverage to eliminate an infection from the population.24
If an infected individual averagely produces less than one new infected individual over the course of his/her infectious period, then R0< 1 and the infection cannot expand or grow. If each infected individual produces averagely more than one new infection, then R0> 1 and the disease can spread in the population. If R0 is equal to one (R0=1), the disease remains stable or endemic in the community, but will not cause an epidemic.29,31
Intervention Mechanisms
Potential Herd Immunity of HCV Through Infection
The epidemic threshold is the critical number of susceptible hosts required for an epidemic to occur. A minimum number of hosts is required for an infectious agent to survive. To prevent the transmission of infectious agents like HCV, reducing the number of susceptible hosts and preventing the infectious agent survival via vaccination is crucial. The infectious agent becomes extinct, and herd immunity is reached when the proportion of susceptible population is reduced below the threshold of susceptibility.8
Potential Herd Immunity Through Vaccination
The traditional vaccine development design strategies like live-attenuated and inactivated whole virus vaccine development have not been applied for HCV vaccine development due to the difficulty of growing the virus in viral culture media, mutation of the cultured strain, and the live-attenuated vaccine risk of causing HCV infection.32
The heterogeneous genetic diversity, immune selection, and the error-prone polymerase of the virus are challenging for vaccine development for HCV.32 Currently there are about eight HCV genotypes and about ninety sub-types. About 30% and 15% of the different HCV genotypes and sub-types within each genotype, respectively, are varying in nucleotide sequences.33 This is the fundamental challenge for HCV vaccine development globally. The other challenge for HCV vaccine development is the absence of an immuno-competent small laboratory animal model that is used to assist in determining whether the vaccination under study induces protective immunity or not.34
Treatment as Prevention (TasP)
Treating HCV-infected PWID is very important to prevent the transmission of HCV infection among PWID susceptible populations. Treatment as prevention of HCV transmission among PWID is very effective when PWID and their immediate injecting networks are treated together like “a treat-your-friends” strategy.
In order to completely eliminate HCV infection, an integrated health care system strategy involving multidisciplinary sectors that target the highest-risk groups like PWID is crucial in addition to antiviral treatment like direct-acting antiviral (DAA) medications.16
To prevent and reduce HCV transmission among PWID, it is important to use combination prevention strategies, for instance expansion of comprehensive harm reduction and care/support services to be facilitated by the community for PWID to get access.16 The World Health Organization (WHO) guidelines recommend safe and appropriate use of health care injections, safe handling and disposal of sharps and waste, and provision of comprehensive harm reduction services as primary prevention intervention of HCV infection among PWID.35
Treating HCV-infected PWID is an effective prevention mechanism as those who are cured of HCV infection do not transmit the virus. A very good approach to intervene in HCV infection transmission among PWID is to increase treatment rates such as testing and diagnosing more PWID, linking them to HCV care, and increasing treatment uptake, adherence, and rates of successful treatment.17 A comprehensive, evidence-based multi-professional harm reduction strategy (particularly concerning opioid substitution therapy and clean needles and syringes) and support/care services based in the community are essential to reduce HCV infection and transmission among PWID.28,35
Education about safety regarding injection is a complementary approach to prevent HCV infection and transmission among PWID. Routinely testing or screening people at risk for HCV infection has also been used to reduce the incidence of HCV by reducing risky behaviors and prevalence as those who become infected will be linked to treatment and care that reduces the transmission.36,37
Summary and Conclusion
Hepatitis C virus (HCV) belongs to the Hepacivirus genus within the Flaviviridae family. HCV infection is acquired when the susceptible individuals in populations come into contact with medical equipment (such as by sharing syringes and needles) or swabs contaminated with infected blood. About 60% of HCV infection transmission is via syringe and needle sharing. Thus, PWID are at high risk of acquiring HCV infection from medical equipment contaminated with HCV-infected blood. The HCV infection may remain latent for about 2 weeks up to 6 months without the individual showing signs and symptoms but being potential disease transmitters. Some of the HCV-infected individuals may establish chronic HCV infection, while in some the HCV infection may be cleared spontaneously. HCV-infected individuals can develop chronic infection due to being treatment-naïve or failure of treatment. Those who are treated with direct-acting antiviral (DAA) medications may also develop sustained virological response (SVR) and become immune, or may establish non-sustained virological response (non-SVR) and become chronic HCV-infected.
Construction of HCV transmission dynamic model is very significant in order to predict the duration and magnitude of its epidemic and to evaluate the potential impact of intervention. Different published data showed that the calculated basic reproductive number for HCV is greater than 1. A comprehensive harm reduction and care/support services strategy is the best approach to intervention regarding HCV infection transmission among PWID. Treating the infected individuals is also one mechanism of HCV infection prevention. HCV vaccine development is challenging due to the difficulty of growing the HCV on culture media as well as due to the mutation of the cultured HCV or heterogeneity of the virus.
Ethical Issue Concern
There was no any ethical issue concern about this paper as there were no any data collected or extracted from study subjects or their records.
Disclosure
The authors declare there are no potential conflicts of interest.
References
1. Wan H, Adams RL, Lindenbach BD, Pyle AM, James Ou J-H. The in vivo and in vitro architecture of the hepatitis C virus RNA genome uncovers functional RNA secondary and tertiary structures. J Virol. 2022;96(8). doi:10.1128/jvi.01946-21
2. Schillie S, Wester C, Osborne M, Wesolowski L; ABR. CDC Recommendations for hepatitis C screening among adults. J Chem Inf Model. 2020;53(9):1689–1699.
3. World Heath Organization. Updated Reccomendations on Simplified service delivery and diagnostics for hepatitis c infection; 2022. Available from: https://www.who.int/publications/i/item/9789240052697. Accessed
4. EMCDDA. Hepatitis C among drug users in Europe: epidemiology, treatment and prevention. Luxembourg: Publications Office of the European Union; 2016:104. Available from: http://www.emcdda.europa.eu/system/files/publications/2953/TDXD16002ENN_final_web.pdf%0Ahttp://www.emcdda.europa.eu/publications/insights/hepatitis-c-among-drug-users-in-europe_en%0Ahttp://www.emcdda.europa.eu/publications/insights/hepatitis-c-among-drug-.
5. Karimi SE, Bayani A, Higgs P, et al. Prevalence and high risk behaviours associated with HCV testing among people who inject drugs: a systematic review and meta-analysis. Subst Abuse Treat Prev Policy. 2020;15(1):1–16.
6. World Health Organization. Monitoring and Evaluation for Viral Hepatitis b and c. World Health Organization; 2016:40.
7. Sector GH. The Public Health Problem of HCV Infection; 2021.
8. Wendelbeo, AM, Garfe, C, Carabin, H. The benefits of transmission dynamics models in understanding emerging infectious diseases. Am J Med Sci. 2010;340(3) 181–186. doi:10.1097/MAJ.0b013e3181e937ca
9. Simon CM. The SIR dynamic model of infectious disease transmission and its analogy with chemical kinetics. PeerJ Phys Chem. 2020;2:e14. doi:10.7717/peerj-pchem.14
10. Peng L, Xie P, Tang Z, Liu F. Modeling and analyzing transmission of infectious diseases using generalized stochastic petri nets. Appl Sci. 2021;11(18):8400. doi:10.3390/app11188400
11. Chen D. Modeling the spread of infectious diseases: a review. Anal Model Spat Temporal Dyn Infect Dis. 2015;2015:19–42.
12. Buchwald AG, Adams J, Bortz DM, Carlton EJ. Infectious disease transmission models to predict, evaluate, and improve understanding of COVID-19 trajectory and interventions. Ann Am Thorac Soc. 2020;17(10):1204–1206. doi:10.1513/AnnalsATS.202005-501PS
13. Platt L, Stengel CM, Nkurunziza M, et al. Assessing risk of HIV and hepatitis C among people who inject drugs in East Africa: findings from a rapid assessment. J Viral Hepat. 2019;26(7):926–929. doi:10.1111/jvh.13088
14. Shah R, Boucheron P, Mandaliya K, et al. Hepatitis C virus infection in people who inject drugs in Africa. Lancet Infect Dis. 2020;20(3):282–283. doi:10.1016/S1473-3099(20)30049-9
15. Echevarria D, Gutfraind A, Boodram B, et al. Mathematical modeling of hepatitis C prevalence reduction with antiviral treatment scale- up in persons who inject drugs in metropolitan Chicago. PLoS One. 2015;10(8):1–14. doi:10.1371/journal.pone.0135901
16. Khalsa JH, Mathur P. Hepatitis c virus infection in persons who inject drugs in the Middle East and north Africa: intervention strategies. Viruses. 2021;13(7):1–10. doi:10.3390/v13071363
17. World Health Organization. Interim guidance for country validation of viral hepatitis elimination [internet]. Geneva: WHO; 2021:1–96. Available from: https://www.who.int/publications/i/item/9789240028395.
18. Shayan SJ, Nazari R, Kiwanuka F. Prevalence of HIV and HCV among injecting drug users in three selected WHO-EMRO countries: a meta-analysis. Harm Reduct J. 2021;18(1):1–13. doi:10.1186/s12954-021-00505-4
19. Abubakar I, Stagg HR, Cohen T, Rodrigues LC. Transmission-dynamic models of infectious diseases. In: Infectious Disease Epidemiology (Oxford Specialist Handbooks). OUP Oxford; 2016:1–23.
20. Wang Y, Zhao Z, Wang M, et al. The transmissibility of hepatitis C virus: a modelling study in Xiamen City, China. Epidemiol Infect. 2020;148. doi:10.1017/S0950268820002885
21. Kondili LA, Andreoni M, Alberti A, et al. A mathematical model by route of transmission and fibrosis progression to estimate undiagnosed individuals with HCV in different Italian regions. BMC Infect Dis. 2022;22(1):1–15. doi:10.1186/s12879-022-07042-w
22. Gicquelais RE, Foxman B, Coyle J, Eisenberg MC. Hepatitis C transmission in young people who inject drugs: insights using a dynamic model informed by state public health surveillance. Epidemics. 2019;27(June2018):86–95. doi:10.1016/j.epidem.2019.02.003
23. Miller-Dickson MD, Meszaros VA, Almagro-Moreno S, Brandon Ogbunugafor C. Hepatitis C virus modelled as an indirectly transmitted infection highlights the centrality of injection drug equipment in disease dynamics. J R Soc Interface. 2019;16(158):20190334. doi:10.1098/rsif.2019.0334
24. Tatara E, Gutfraind A, Collier NT, et al. Modeling hepatitis C micro-elimination among people who inject drugs with direct-acting antivirals in metropolitan Chicago. PLoS One. 2022;17(3March):1–17. doi:10.1371/journal.pone.0264983
25. Cousien A, Tran VC, Deuffic-Burban S, Jauffret-Roustide M, Dhersin JS, Yazdanpanah Y. Dynamic modelling of hepatitis C virus transmission among people who inject drugs: a methodological review. J Viral Hepat. 2015;22(3):213–229. doi:10.1111/jvh.12337
26. Greenhalgh D, Al- Rashidi N. Modeling the spread of hepatitis C virus amongst people who inject drugs. Eng Rep. 2022;2021:1–30.
27. WHO. Hepatitis C 24; 2022:1–6.
28. Jia W, Weng J, Fang C, Li Y. A dynamic model and some strategies on how to prevent and control hepatitis c in mainland China. BMC Infect Dis. 2019;19(1):1–11. doi:10.1186/s12879-019-4311-x
29. Lim JS, Il CS, Ryu S, Il PS. Interpretation of the basic and effective reproduction number. J Prev Med Public Health. 2020;53(6):405–408. doi:10.3961/jpmph.20.288
30. Zhang S, Zhou Y. Dynamics and application of an epidemiological model for hepatitis C. Math Comput Model. 2012;56(1–2):36–42. doi:10.1016/j.mcm.2011.11.081
31. Foppa IM, Diekmann O, Heesterbeek JAP, Metz JAJ. The spread of infectious diseases in heterogeneous populations. In: A Historical Introduction to Mathematical Modeling of Infectious Diseases. Vol. 2021. Elsevier; 2017:157–194.
32. Bailey JR, Barnes E, Cox AL. Approaches, progress, and challenges to hepatitis C vaccine development. Gastroenterology. 2019;156(2):418–430. doi:10.1053/j.gastro.2018.08.060
33. Gastroenterology TL. Editorial the hunt for a vaccine for hepatitis C virus continues. Lancet Gastroenterol Hepatol. 2021;6(4):253. doi:10.1016/S2468-1253(21)00073-X
34. Cox AL. Virus vaccine; 2020:1–16.
35. Dillon JF, Lazarus JV, Razavi HA. Urgent action to fight hepatitis C in people who inject drugs in Europe. Hepatol Med Policy. 2016;1(1):1–10. doi:10.1186/s41124-016-0011-y
36. Walsh N, Verster A, Rodolph M, Akl EA. WHO guidance on the prevention of viral hepatitis B and C among people who inject drugs. Int J Drug Policy. 2014;25(3):363–371. doi:10.1016/j.drugpo.2014.01.009
37. Delile J-M, de Ledinghen V, Jauffret-Roustide M, et al. Hepatitis C virus prevention and care for drug injectors: the French approach. Hepatol Med Policy. 2018;3(1):1–9. doi:10.1186/s41124-018-0033-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

