Back to Journals » International Journal of General Medicine » Volume 14
Hepatitis B Virus-Related Glomerulonephritis with Positive and Negative Serum HBsAg: Different Clinicopathologic Characteristics of Two Clinical Subtypes
Authors Yu F, Li G, Hao W , Hu W
Received 29 April 2021
Accepted for publication 10 June 2021
Published 30 June 2021 Volume 2021:14 Pages 3069—3077
DOI https://doi.org/10.2147/IJGM.S318087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Feng Yu,1,* Guanglan Li,1,2,* Wenke Hao,1 Wenxue Hu1
1Department of Nephrology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangdong Provincial Geriatrics Institute, Guangzhou, 510080, People’s Republic of China; 2Shantou University Medical College, Shantou, 515041, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wenxue Hu
Department of Nephrology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangdong Provincial Geriatrics Institute, 106th, Zhongshan Road II, Guangzhou, 510080, People’s Republic of China
Tel +8620-83827812-70811
Email [email protected]
Introduction: The clinicopathologic characteristics of Hepatitis B virus-associated glomerulonephritis (HBV-GN) patients with different serum HBsAg are not well known. This study aims to investigate the characteristics and treatments between HBV-GN patients with positive and negative serum HBsAg.
Methods: A retrospective review of patients with renal biopsies in Guangdong Provincial People’s Hospital from 2005 to 2018 was performed. Clinicopathological data, treatments and remission of proteinuria were collected and compared between HBsAg+ and HBsAg- group.
Results: A total of 101 HBV-GN were recruited. Serum HBsAg+ and HBsAg- patients accounted for 62.4% and 37.6%, respectively. HBsAg+ group had poor kidney and liver functions. Pathological data showed the percentage of membranous nephropathy in HBsAg- group is significantly higher than that of HBsAg+ group (60.3% HBsAg+ vs 89.5% HBsAg-, P< 0.05). Chronic renal tubular/interstitial injury was more prevalent in HBsAg+ group (16.9% HBsAg+ vs 2.6% HBsAg-, P< 0.05). The deposition sites of immune complexes were significant different between the two groups. In addition, more HBsAg+ patients were given anti-HBV and less were given corticosteroid or immunosuppressants for treatment than that of HBsAg- patients. Percentages of clinical remission were increasing in both HBsAg+ and HBsAg- patients from 1, 3, 6 months to 1 year (18.75%, 45.2%, 67.8%, 82.4% vs 24.4%, 41.2%, 62.8%, 59.3%). The differences of remission betwen two groups were not significant (P> 0.05).
Conclusion: The clinicopathological characteristics and treatments of HBV-GN with serum HBsAg+ and HBsAg- were distinct, which indicated that the pathogenesis might be different and specific treatments were needed for HBV-GN patients with different serum HBsAg.
Keywords: hepatitis B, hepatitis B virus related glomerulonephritis, serum HBsAg
Introduction
Chronic HBV infection is present in 240 million people worldwide, representing 3.5% of the world population.1 And there are more than 350 million are chronic carriers of the virus. HBV infection has feature of territoriality and the prevalence was the highest Western Pacific regions (6.2%).2–4 China is classified as a highly endemic area of HBV infection with estimated 93 million carriers of HBsAg.5 HBV infection leads to a wide spectrum of liver disease ranging from acute (including fulminant hepatic failure) to chronic hepatitis, cirrhosis, and hepatocellular carcinoma.6 Moreover, kidney could be involved, which results in HBV-associated glomerulonephritis. Overall, renal disease may occur in 3–5% of patients with chronic HBV infection. The histologic manifestations of HBV-associated glomerulonephritis are diverse. Histologic patterns including membranous nephropathy (MN), membranoproliferative glomerulonephritis (MPGN), immunoglobulin (Ig) A nephropathy, and focal segmental glomerulosclerosis (FSGS) have been reported.7 Drugs for primary glomerulopathy such as corticosteroids, immunosuppressive agents, which might lead to replication of HBV, development of hepatitis and even hepatic failure. Therefore, it is important for doctors to make the diagnosis HBV-associated glomerulonephritis.
Occult HBV infection is defined as the presence of replication competent HBV DNA in the liver (ie episomal HBV covalently closed circular DNA) and/or HBV DNA in the blood (usually at levels <200IU/mL) of individuals who test negative for serum HBV surface antigen (HBsAg) with currently available assays.8 A small sample study reported that patients with HBsAg-negative were diagnosed as hepatitis B virus (HBV)-associated glomerulonephritis (HBV-GN) by featured with HBsAg/HBcAg (hepatitis B core antigen) positive in kidney tissues and extensive subendothelial electron dense deposits in the mesangium and subendothelial space.9 However, the characteristics of HBV-GN with negative HBsAg were not well-known. This retrospective study compared the clinicopathological features and treatments of HBV-GN with serum negative or positive HBsAg so that to investigated the heterogenicity HBV-GN and provide guidance for clinical doctors.
Materials and Methods
Definition of Diseases
The diagnosis of HBV-GN was based on the advice established by the Beijing symposium: 1) serum HBV markers should be positive, 2) patients should have glomerulonephritis and be excluded from other secondary glomerular disease, and 3) renal biopsy is positive for one HBV antigen or more than one antigen can be detected in renal tissue sections. HBV-GN was diagnosed when criteria met for 1)+ 2)+ 3), or 2)+ 3).10
The estimated glomerular filtration rate (eGFR) was calculated use a Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.11
Study Population
A total of 101 patients underwent renal biopsies and were diagnosed with HBV-GN in Guangdong Provincial People’s Hospital from March 2005 to March 2018. This retrospective study involving human participants was approved by the Ethical Committee of Guangdong Provincial People’s Hospital. And this study was conducted in accordance with the Declaration of Helsinki.
Methods
All the renal biopsy specimens were prepared for regular staining of H&E, PAS and PASM-Masson. IgM, IgG (IgG1-4), C3, C4, C1q and fibrinogen were stained by immunofluorescence staining. The pathological were described by light microscopy. Fresh renal tissue specimens were also collected and prepared for examination of an electron microscope. Tissue immunofluorescence staining for HBV specific antigen, including HBeAg, HBcAg and HBeAb. The classification of renal pathology was in accordance with the glomerular pathological classification criteria established by the World Health Organization in 1995. The degree of renal tubular/interstitial injury was determined by semiquantitative method of renal tubular atrophy/interstitial fibrosis (Oxford Classification of IgA nephropathy).12 For each parameter, a score was attributed as follows: 0: <25% of the section affected; 1: 25–50% of the section affected; 2: >50% of the section affected. All cases were scored by Dr. Feng Yu and Guanglan Li, and reviewed by Dr. Wenxue Hu, who were extensively trained by an experienced nephropathologist.
Immunofluorescence Staining of HBeAg, HBcAg and HBeAb
Thin sections (3μm) of the renal biopsies were fixed with 4% paraformaldehyde (15 min), permeabilized with 0.5% Triton-X 100 (10 min) and blocked with 5% bovine serum albumin (BSA) for 1 h at room temperature. Samples were then incubated with the following primary antibodies diluted in 5% BSA at 4 °C over-night: anti-HBeAg antibody (1:60, Gene Tech, China), anti-HBcAg antibody (1:100, Abcam, UK) and anti-HBeAb antibody (1:60, Bioss, China). The secondary antibody green was Alexa Fluor 488 (1:500, Invitrogen) for 1 h. The sections were observed under a fluorescence microscope (Nikon 80i, Japan).
Evaluation of Therapeutic Outcome
Therapeutic outcomes were classified as complete remission (CR), partial remission (PR), and no remission (NR). CR of proteinuria was defined as a urinary protein excretion level of 0.5 g/24 h. Treatment failure (NR) was defined as persistence of urinary protein excretion that exceeded 50% of the baseline value or 3.5 g/24 h.
Statistical Analyses
Data were presented as means ± SDs or proportions as appropriate. Patients were divided into two groups (HBV-GN HBsAg+ group, HBV-GN HBsAg- group). Continuous variables were compared by the Student t test or Mann–Whitney U-test. Categorical variables were compared by χ2 test or Fisher exact test or Wilcoxon rank-sum test. All statistical calculations were processed by the SPSS 23.0 software (SPSS Inc., USA). A P value less than 0.05 was considered significant.
Results
Baseline and Clinical Characteristics of HBV-GN Patients
From March 2005 to March 2018, a total of 5224 patients who underwent renal biopsies were reviewed, 102 of which were diagnosed with HBV-GN (1.95%). 1 patient was excluded because of data missing. Totally, 101 patients with HBV-GN were recruited in this study. Serum HBsAg+ patients and HBsAg- patients accounted for 62.4% (63 cases) and 37.6% (38 cases), respectively. Clinical baselines, including age, sex, history of hypertension and diabetes are listed in Table 1. The differences of age, sex and history of diseases were not different.
 |
Table 1 The Clinical Characteristics of Hepatitis B Virus Related Glomerulonephritis Patients with Serum Positive or Negative HBsAg |
Hepatitis B sero-markers showed the prevalence rate of anti-HBc was significantly higher in HBsAg+ group than that of HBsAg- group (P<0.05). Poor kidney and liver function were found in HBsAg+ group. That is, the levels of serum creatinine (Scr), aspartate aminotransferase (AST), alanine aminotransferase (ALT) were much higher in HBsAg+ group when compared with HBsAg- group (P<0.05). Patients with positive HBsAg had lower levels of urinary protein creatinine ratio, serum glycerin trimyristate and blood complement 4 (C4) than those of patients with negative HBsAg. There were no significant differences in HBeAb, eGFR, BUN, serum albumin and urinary red blood cell counts (Table 1).
Pathological Characteristics of Serum HBsAg+ and HBsAg- Group
The biopsy core is assessed by light microscopy and immunofluorescence (Figure 1). The range of diagnoses of HBV-GN patients with serum HBsAg+ are membranous nephropathy (MN, accounting for 60.3%), mesangioproliferative glomerulonephritis (MPGN, 15.9%), mesangial proliferative glomerulonephritis (MsPGN, 11.1%). Other pathological types included IgA nephropathy (IgAN, 4 cases), crescentic glomerulonephritis (1 case), endocapillary proliferative glomerulonephritis (EnPGN, 1 case) and diabetic nephropathy (2 cases). Patients with serum HBsAg- showed MN were the majority (89.5%) pathological manifestation. Other pathological types were MPGN (1 case), MsPGN (2 cases) and IgAN (1 case). These data showed the percentage of MN in HBsAg- group was significant higher than that of HBsAg+ group, but other pathological types including MPGN, MsPGN, IgAN and crescentic glomerulonephritis were lower in patients with negative HBsAg (P<0.05) (Table 2).
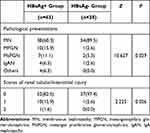 |
Table 2 Pathological Presentations and Renal Tubular/Interstitial Injury of Hepatitis B Virus Related Glomerulonephritis Patients with Positive or Negative Serum HBsAg |
The chronic renal tubular/interstitial injury was also estimated among these patients. Among HBV-GN patients with serum HBsAg+, 11 patients had tubular atrophy/interstitial fibrosis: 10 (15.9%) cases with a score of 1 (moderate injury) and 1 case (1.6%) with a score of 2 (severe injury). Only 1 case (2.6%) was with a score of 1 among HBV-GN patients with serum HBsAg-. These data proved chronic renal tubular/interstitial injury was more prevalent in serum HBsAg+ HBV-GN group. The degree and proportion of chronic renal tubular/interstitial injury were present in Table 2.
Deposition Sites of Immune Complexes
Deposition sites of immune complexes by immunofluorescence were compared between HBsAg- group and HBsAg+ group (Figure 2). Data showed that immune complexes more frequently deposited in mesangial regions, subendothelial regions and glomerular basement membrane of patients with serum HBsAg-. In contrast, immune complexes were much often present in subepithelial regions of patients with serum HBsAg+ (Table 3).
 |
Table 3 Deposition Sites of Immune Complexes of Hepatitis B Virus Related Glomerulonephritis Patients with Serum Positive or Negative HBsAg |
Treatment Protocols and Therapeutic Outcomes
There were four types of drugs involved in this retrospective study, which were angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers (ACEI/ARB), anti-HBV drugs, corticosteroid and immunosuppressants. The protocols were including ACEI/ARB alone (Protocol 1), anti-HBV with or without ACEI/ARB (Protocol 2), corticosteroid with or without anti-HBV/ACEI/ARB (Protocol 3), immunosuppressants with or without anti-HBV/ACEI/ARB/corticosteroid (Protocol 4).
Results showed that more serum HBsAg+ patients were given anti-HBV (90.4% vs 73.7%, P<0.05) for treatment than that of serum HBsAg- patients. Among serum HBsAg+ patients using antivirus drugs, 28 (44.4%) cases were given lamivudine and 29 (46%) cases were given entecavir. 10 case (26.3%) were given lamivudine and 18 (47.4%) cases were given entecavir among patients with serum HBsAg-.
On the contrary, much more HBsAg- patients were given corticosteroid (55.3% vs 28.6%, P<0.01) and immunosuppressants (57.9% vs 17.5%, P<0.01). Of serum HBsAg+ patients using immunosuppressants, 6 cases (9.5%) were given tacrolimus, 1 case (1.6%) were given cyclosporine A (CysA), 3 cases (4.8%) were given mycophenolate mofetil (MMF) and 1 case (1.6%) was given cyclophosphamide (CTX). Twenty-two cases were given immunosuppressants among patients with serum HBsAg-: 13 (34.2%) tacrolimus, 3 (7.9%) CysA, 5 (13.2%) MMF and 1 (2.6%) azathioprine. According to the therapeutic protocols, more HBsAg+ patients were treated with protocol 2 (58.8% vs 23.7%, P<0.01) and more HBsAg- patients were treated with protocol 4 (57.9% vs 15.9%, P<0.01) (Table 4).
 |
Table 4 Drugs and Treatment Protocols for Hepatitis B Virus Related Glomerulonephritis |
The percentages of clinical remission (complete remission plus partial remission) were increasing in both HBsAg- and HBsAg+ patients from 1, 3, 6 months to 1 year. There was a tendency in better outcome of HBsAg+ group at the time of 3 months (45.2% vs 41.2%, P>0.05), 6 months (67.8% vs 62.8%, P>0.05) and 1 year (82.4% vs 59.3%, P>0.05), but the differences were not different (Table 5).
 |
Table 5 Evaluation of Treatment Outcomes for Hepatitis B Virus Related Glomerulonephritis |
Discussion
In this study, we analyzed the clinical and histopathologic characteristics of HBV-GN patients with positive or negative serum HBsAg. Poor kidney and liver function were found in HBsAg+ group. Pathological investigation showed that MN was more prevalent in HBsAg- patients and chronic renal tubular/interstitial injury was severe in HBsAg+ patients. Moreover, deposition sites of immune complexes were different between the two groups. Additionally, more HBsAg+ patients were given anti-HBV drugs and less were given corticosteroid and immunosuppressants. The differences in therapeutic outcomes were not different.
HBV-GN is one of the more important extrahepatic diseases and a common secondary glomerulonephritis. The reported prevalence closely parallels geographic prevalence of HBV. Several published studies in China showed that the incidence of HBV-GN is 2.6–4.3%.13–16 Our study showed that the HBV-GN accounted 1.95% of all patents with renal biopsy. The reason of lower incidence of Guangdong Province may be associated with better economic condition and the widespread use of hepatitis B vaccination and the prevalence of HBsAg in Guangdong decreased dramatically since 2013.17 In the United States and Western Europe, the frequency of HBV-GN is low because of the lower prevalence of chronic HBV infection in general and a lower likelihood of childhood infection.18 A few studies reported the percentages of HBsAg-GN of HBV-GN were 13–35%. Our data showed 37.2%, and our higher percentage may be related to the increasing of emphasis on HBV-GN and serum HBsAg- patients were also carried on tissue immunofluorescence staining for HBV-specific antigen.
Previous studies showed the serum HBcAg level was significantly associated with higher HBV-DNA titers, high qHBsAg level and HBeAg positivity. Moreover, level of HBcAg can influence the progression of liver disease.19,20 Our data showed that the proportion of positive HBcAg was significantly higher in patients with HBsAg+ HBV-GN, which is similar to liver injury of HBV infection. Moreover, our results showed that the levels of Scr, AST, ALT were much higher in HBsAg+ group when compared with HBsAg- group, which indicated HBsAg+ HBV-GN patients had poor kidney and liver function. Previous studies have identified expression of HBV antigens in the kidneys suggesting that virus replication and direct virus-induced pathological alterations were also involved.21–23 The evidence above suggested that the activity of HBV replication in serum may directly result in renal injury except for the mechanism of immune complex deposition in renal tissue.
Patients with serum positive HBsAg had a lower level of blood complement 4 (C4) than that of patients with negative HBsAg. The relationships between pathological mechanisms and complement activation have been investigated for renal diseases such as post-infectious glomerulonephritis, lupus nephritis, and primary membranoproliferative glomerulonephritis, which are usually accompanied by hypocomplementemia.24 In pathways of C activation, C4 and C2 are cleaved, and then, C4b2a and C4b2a3b are created step by step.25 These evidences indicated that immune complexes are formed in condition of HBV replication and active hepatitis, which activates C pathway and results in inflammation reaction and severe renal injury. These immune activities may be the reason for severe renal function in HBV-GN patients with serum-positive HBsAg.
The common histologic manifestations of HBV-GN are MN, MPGN and other appearances such as MsPGN, IgAN, focal segmental (FSGS), and minimal change disease (MCD).7 In our study, we found that the histologic types were much diverse in patients with HBsAg+ HBV-GN. Our data showed that MN (60.3%), MPGN (15.9%) and MsPGN (11.1%) are common in HBV-GN patients with serum-positive HBsAg. However, MN was more prevalent (89.5%) in patients with serum HBsAg- HBV-GN. This phenomenon may be associated with the different mechanism of the two groups. Except for glomerular immune complexes depositing from circulating immune complexes and/or form in situ, HBV virus may directly infect glomerular cells contributing to the pathogenesis of HBV-GN.26 Therefore, the mechanism of HBsAg+ HBV-GN may be more complicated, resulting in diversity of histologic manifestations.
We further compared the deposition sites of immune complexes. Our results showed that the mesangial, basement membrane and subendothelial deposits were much more in HBV-GN patients with serum HBsAg+. In contrast, subepithelial deposits were significantly less in those patients. Deposition of immune complexes is the most important mechanism of HBV-GN,27 so the differences of deposition sites proved that the immune mechanism was different in HBsAg+ HBV-GN and HBsAg+ HBV-GN. The above mechanism may be the reason for diversity of pathologic preferences. MN was the main histologic manifestation of patients with HBsAg- HBV-GN, which may explain high levels of urine protein in those patients.
Finally, the therapeutic protocols and treatments were analyzed. We found that more serum HBsAg+ patients were given anti-HBV treatment. For HBV patients with MN, Kidney Disease Improving Global Outcomes (KDIGO) recommends anti-HBV therapy.28 Therefore, anti-HBV drugs were prescribed anti-HBV drugs for most of HBsAg+ patients. However, some patients with lower HBV DNA copies or HBsAg- HBV-GN were not given anti-HBV drugs. Because of more frequencies of MN in HBsAg- HBV-GN, more corticosteroid and immunosuppressants were prescribed for those patients. The ratios of clinical remission were increasing step by step from 1 month to 1 year, which indicated that continuous treatments were needed. This phenomenon is in accord with guidelines of KDIGO and another review on treatment optimization of HBV-GN.29 The tendency of remission in HBsAg+ patients is better, but the differences were not different in two group. These data showed more active treatment (corticosteroid and/or immunosuppressants) for HBsAg- GN did not bring more benefits. This result may be associated with the higher ratio of MN, higher levels of proteinuria and lower ratio of anti-HBV therapy in HBsAg- GN patients. The data above supported that anti-HBV treatment may be necessary for both serum HBsAg+ and HBsAg- HBV-GN patients, especially for those patients used corticosteroid and immunosuppressants.
In this study, we conducted research on clinical and pathologic features of HBV-GN patients with serum HBsAg+ HBV and HBsAg+. However, our study also has following limitations. Firstly, this is a retrospective study and the follow-up on proteinuria is irregular and part of data are not included. Second, therapeutic protocols are not consensus because of long research time span. Therefore, therapeutic outcome is needed with standardized protocols and prospective follow-up to establish more reliable conclusions.
Conclusion
In summary, we showed serum HBsAg+ HBV-GN patients were with poor kidney and liver function, lower levels of blood C4, more diversity in histologic types, severity of chronic renal tubular/interstitial injury, and different deposit features of immune complexes. These data indicated that the pathogenesis of serum HBsAg+ and HBsAg- HBV-GN may be different. Moreover, continuous anti-HBV treatment may be necessary for both HBsAg+ and HBsAg- HBV-GN patients, especially for patients used corticosteroid and immunosuppressants.
Ethical Approval
The study involving human participants was approved by the Ethical Committee of Guangdong Provincial People’s Hospital, written informed consent was obtained from the patients before the enrollment.
Acknowledgments
We thank all patients and support staff in the study.
Funding
This work was supported by the Natural Science Foundation of Guangdong Province, China [grant numbers 2019A1515011594, 2018A0303130251].
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Zampino R, Boemio A, Sagnelli C, et al. Hepatitis B virus burden in developing countries. World j Gastroenterol. 2015;21(42):11941–11953. doi:10.3748/wjg.v21.i42.11941
2. Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet (London, England). 2014;384(9959):2053–2063. doi:10.1016/S0140-6736(14)60220-8
3. World Health Organization. Global hepatitis report. Geneva: World Health Organization. 2017.
4. World Health Organization. Hepatitis B; 2020. Newsroom/Fact sheets/detail.
5. Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol. 2013;28(Suppl 1):7–10. doi:10.1111/jgh.12220
6. Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet (London, England). 2018;392(10161):2313–2324. doi:10.1016/S0140-6736(18)31865-8
7. Kupin WL, Viral-Associated GN. Hepatitis B and Other Viral Infections. Clin j Am Soc Nephrol. 2017;12(9):1529–1533. doi:10.2215/CJN.09180816
8. Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71(2):397–408. doi:10.1016/j.jhep.2019.03.034
9. Li D, Gao G, Jiang H, Tang Z, Yu Y, Zang G. Hepatitis B virus-associated glomerulonephritis in HBsAg serological-negative patients. Eur J Gastroenterol Hepatol. 2015;27(1):65–69. doi:10.1097/MEG.0000000000000236
10. Shen Y, Chen X. Advice of hepatitis B virus (HBV) infection related glomerulonephropathy in Beijing symposium. Zhong Hua Nei Ke Za Zhi. 1990;9:519–521.
11. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
12. Trimarchi H, Barratt J, Cattran DC, et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91(5):1014–1021. doi:10.1016/j.kint.2017.02.003
13. Hu R, Quan S, Wang Y, et al. Spectrum of biopsy proven renal diseases in Central China: a 10-year retrospective study based on 34,630 cases. Sci Rep. 2020;10(1):10994. doi:10.1038/s41598-020-67910-w
14. Zhang X, Liu S, Tang L, et al. Analysis of pathological data of renal biopsy at one single center in China from 1987 to 2012. Chin Med J. 2014;127(9):1715–1720.
15. Yang Y, Zhang Z, Zhuo L, Chen DP, Li WG. The spectrum of biopsy-proven glomerular disease in china: a systematic review. Chin Med J. 2018;131(6):731–735. doi:10.4103/0366-6999.226906
16. Nie P, Chen R, Luo M, et al. Clinical and pathological analysis of 4910 patients who received renal biopsies at a single center in Northeast China. Biomed Res Int. 2019;2019:6869179. doi:10.1155/2019/6869179
17. Zhu Q, Shao X, Chen S, et al. Epidemiological serosurvey of hepatitis B virus among children aged 1–14 years in Guangdong Province, China. Int j Infect Dis. 2018;71:25–29. doi:10.1016/j.ijid.2018.01.027
18. Shah AS, Amarapurkar DN. Spectrum of hepatitis B and renal involvement. Liver Int. 2018;38(1):23–32. doi:10.1111/liv.13498
19. Chang XJ, Sun C, Chen Y, et al. On-treatment monitoring of liver fibrosis with serum hepatitis B core-related antigen in chronic hepatitis B. World j Gastroenterol. 2019;25(32):4764–4778. doi:10.3748/wjg.v25.i32.4764
20. Lapalus M, Laouenan C, Cardoso AC, et al. Precore/Core promoter variants to predict significant fibrosis in both HBeAg positive and negative chronic hepatitis B. Liver Int. 2015;35(9):2082–2089. doi:10.1111/liv.12787
21. Lai KN, Ho RT, Tam JS, Lai FM. Detection of hepatitis B virus DNA and RNA in kidneys of HBV related glomerulonephritis. Kidney Int. 1996;50(6):1965–1977. doi:10.1038/ki.1996.519
22. Diao Z, Ding J, Yin C, Wang L, Liu W. Purified hepatitis B virus induces human mesangial cell proliferation and extracellular matrix expression in vitro. Virol J. 2013;10:300. doi:10.1186/1743-422X-10-300
23. Wang X, Wang L, Zhu N, Zhou Y, Gu LJ, Yuan WJ. Hepatitis B virus X protein modulates renal tubular epithelial cell-induced T-cell and macrophage responses. Immunol Cell Biol. 2016;94(3):266–273. doi:10.1038/icb.2015.85
24. Mizuno M, Suzuki Y, Ito Y. Complement regulation and kidney diseases: recent knowledge of the double-edged roles of complement activation in nephrology. Clin Exp Nephrol. 2018;22(1):3–14. doi:10.1007/s10157-017-1405-x
25. Nonaka M, Kimura A. Genomic view of the evolution of the complement system. Immunogenetics. 2006;58(9):701–713. doi:10.1007/s00251-006-0142-1
26. Yang Y, Wang X, Zhang Y, Yuan W. Hepatitis B virus X protein and proinflammatory cytokines synergize to enhance TRAIL-induced apoptosis of renal tubular cells by upregulation of DR4. Int J Biochem Cell Biol. 2018;97:62–72. doi:10.1016/j.biocel.2018.02.006
27. Gupta A, Quigg RJ. Glomerular diseases associated with hepatitis B and C. Adv Chronic Kidney Dis. 2015;22(5):343–351. doi:10.1053/j.ackd.2015.06.003
28. Rovin BH, Caster DJ, Cattran DC, et al. Management and treatment of glomerular diseases (part 2): conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95(2):281–295. doi:10.1016/j.kint.2018.11.008
29. Liu Y, Shi C, Fan J, Wang B, Li G. Hepatitis B-related glomerulonephritis and optimization of treatment. Expert Rev Gastroenterol Hepatol. 2020;14(2):113–125. doi:10.1080/17474124.2020.1717948
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


