Back to Journals » Cancer Management and Research » Volume 12
Hepatic Arterial Infusion Combined with Systemic Chemotherapy for Patients with Extensive Liver Metastases from Gastric Cancer
Authors Qiang W , Shi H, Wu J, Ji M, Wu C
Received 12 January 2020
Accepted for publication 31 March 2020
Published 29 April 2020 Volume 2020:12 Pages 2911—2916
DOI https://doi.org/10.2147/CMAR.S245697
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Weiguang Qiang,1 Hongbing Shi,1 Jun Wu,1 Mei Ji,1 Changping Wu1,2
1Department of Oncology, The Third Affiliated Hospital of Soochow University, Changzhou, People’s Republic of China; 2Department of Tumor Biological Treatment, The Third Affiliated Hospital of Soochow University, Changzhou, People’s Republic of China
Correspondence: Changping Wu; Weiguang Qiang
Department of Oncology, The Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu Province, People’s Republic of China
Email [email protected]; [email protected]
Purpose: Liver metastases in patients with gastric cancer often indicate poor prognosis. Once liver metastases are extensive, it is difficult to achieve disease control by using systemic chemotherapy alone. The purpose of this study was to evaluate the effect and safety of hepatic arterial infusion (HAI) combined with systemic chemotherapy on extensive liver metastases from gastric cancer.
Patients and Methods: Between 2012 and 2019, 21 patients with extensive liver metastases from gastric cancer (LMGC) were enrolled in our study. Liver metastases were identified as unresectable and a major factor affecting prognosis mainly based on size and number of intrahepatic lesions. All patients received systemic chemotherapy with S-1 and HAI oxaliplatin plus floxuridine (FUDR).
Results: Liver metastases in 16 patients (76.2%) were evaluated as H3. The overall response rate was 76.2% (9.5% complete response). Intrahepatic and extrahepatic median progression-free survival times were 9.5 and 5.2 months, respectively. Median survival time (MST) was 12.3 months. All patients did not have the toxicity of grade 4. Grade 3 toxic effects included bone marrow suppression (14.3%) and diarrhea (9.5%). The other treatment-related toxicities were mild and reversible.
Conclusion: HAI combined with systemic chemotherapy for extensive LMGC seems to be safe and effective, which achieves a high-local response and may contribute to long survival time for patients.
Keywords: gastric cancer, liver metastases, hepatic arterial infusion, systemic chemotherapy
Introduction
Despite the decline in the incidence of gastric cancer worldwide, it is still the third most common tumor in underdeveloped countries and one of the most deadly malignancies.1 Liver metastases in advanced gastric cancer often occur, suggesting a poor prognosis. Although some progress has been made in systemic therapy, liver metastases from gastric cancer (LMGC) are still incurable and affect patients’ long-term survival.2
Theoretically, liver tumors are primarily supplied by the hepatic artery, while normal liver tissues derive blood supply from the portal vein.3 Hepatic artery infusion (HAI) chemotherapy allows most of the drugs to be delivered to the liver metastatic lesion, thus increasing antitumor activity and reducing systemic toxicity. HAI chemotherapy provides a useful treatment option for patients with liver metastases, who are initially unsuitable for surgery or ablation. In particular, the clinical effect of HAI chemotherapy in patients with liver metastases from colorectal cancer has been confirmed,4–6 which prompted us to explore the efficacy and the safety of HAI chemotherapy in patients with extensive LMGC.
Patients and Methods
Patient Selection
All patients in this retrospective study were diagnosed with gastric cancer and extensive liver metastases by histopathology and radiographic imaging between March 2012 and March 2019. Inclusion criteria included: Eastern Cooperative Oncology Group (ECOG) performance status≦2, no obvious liver and renal dysfunction, no previous history of chemotherapy. Liver metastases were identified as unresectable. The grade of liver metastases was assessed as H3, or the maximum diameter was greater than 5 cm in H1 and H2 patients. Exclusion criteria included brain metastases, other primary tumors, serious liver or renal dysfunction, obstruction or bleeding caused by primary tumor.
Implantation of Port System
The catheter/port system (Celsite, B. Braun, Chasseneuil, France) was implanted by fixed catheter tip method as described previously.7 Hepatic arterial angiography prior to port-catheter implantation was performed to assess arterial supply to extrahepatic adjacent organs. The gastroduodenal artery, right gastric artery and left gastric artery were embolized with metallic coils (Tornade, Cook, Bloomington, IL, USA) to prevent the gastrointestinal mucosa injury caused by chemotherapy drugs. In addition, before each hepatic arterial infusion, angiography was performed by injecting contrast agent via the port system to ensure that the port-catheter was not blocked and displaced.
Treatments
All patients received oral S-1 (80mg/m2/day, twice daily for 14 days, and rest for 7 days). After HAI with oxaliplatin (130mg/m2/30min), a 14-day continuous infusion was started by filling elastomeric infusion pump (Accufuser, Woo Young Medical Co., Ltd, Korea), connected to the port-catheter system, with floxuridine (FUDR) (0.15 mg/kg/day) and dexamethasone (DXM) (1 mg/m2/day) dissolved in 250 mL heparinized saline. Patients received the above treatment regimen repeatedly every 21 days until the disease progressed and/or intolerable side effects.
Efficacy and Toxicity Evaluation
Treatment effectiveness was assessed every 2 or 3 cycles of treatment according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.8 Complete response (CR) was defined as disappearance of all target lesions. Partial response (PR) was considered as a least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters. Drug toxicity was evaluated using the NCI Common Terminology Criteria for Adverse Events (v 5.0).
Statistical Analysis
No formal statistical comparisons between the subgroups of the patients were performed because the sample size of this study is small. The time-related parameters were obtained by the Kaplan–Meier method. All data were analyzed using SPSS version 13.0.
Results
Patient Characteristics
21 patients (16 males and 5 females) with unresectable LMGC received HAI and systemic chemotherapy. The characteristics of all patients are listed in Table 1. The median age was 68±5.9 years (range, 58 to 80). Surgical operations for primary cancer included radical operation in seven patients (33.3%) and palliative operation in 1 patient (4.8%). Baseline CT scans revealed that liver metastases in 16 patients (76.2%) were evaluated as H3. 12 patients (57.11%) were determined by CT scans to have extrahepatic metastases, five of them had lung metastases, and 10 had abdominal lymph node metastasis. The median number of treatment cycles that patients received was 5 (range 2–16).
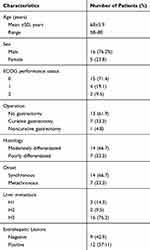 |
Table 1 Patient Characteristics |
Tumor Response and Survival
Following treatment, 14 out of 21 (66.7%) had PR. In addition, 2 patients (9.5%) achieved CR and an overall response rate (ORR) reached 76.2%. Table 2 shows the details of the tumor responses. The median survival time (MST) was 12.3 months (Figure 1). Intrahepatic and extrahepatic median progression-free times were 9.5 and 5.2 months, respectively (hazard ratio 0.85; 95% CI, 0.44–1.67; P=0.6328), which had no statistically significant difference (Figure 2). Encouragingly, 2 patients got the opportunity for surgery after treatment.
 |
Table 2 Tumor Response |
 |
Figure 1 Kaplan–Meier curves for overall survival. |
 |
Figure 2 Kaplan–Meier curves for time to intrahepatic and extrahepatic progression. |
Adverse Events
The major adverse events were bone marrow suppression, nausea/vomiting, fatigue, diarrhea, as well as hepatic dysfunction (Table 3). All patients did not have the toxicity of grade 4. Of the grade 3 adverse events, bone marrow suppression and diarrhea were observed in 3 of 21 patients (14.3%) and 2 of 21 patients (9.5%), respectively. The other treatment-related toxicities were mild and reversible.
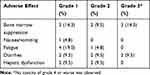 |
Table 3 Adverse Effects of the Combinational Chemotherapy |
Discussion
Liver metastases in patients with gastric cancer often indicate poor prognosis. Currently, systemic chemotherapy is the major treatment strategy. However, the efficacy of systemic chemotherapy in patients with LMGC is not satisfactory, demonstrating ORR of 50–53.3% and MST of 9.7–11.4 months.9–11 This provides the impetus for us to explore more reasonable comprehensive treatment methods. The occurrence of liver metastases is expected to determine the survival time of patients with advanced gastric cancer. Therefore, inhibiting the progression of liver metastases often determines the success or failure of treatment. Nowadays, HAI chemotherapy has been widely used in the treatment of patients with colorectal liver metastases (CRLM) and has achieved remarkable results.4–6 However, there are few clinical studies of HAI combined with systemic chemotherapy for patients with LMGC.
Encouraged by the good results of the treatment of CRLM, researchers have turned their attention to the application of HAI chemotherapy in the treatment of LMGC. Yonemura et al12 studied the effect of systemic administration of mitomycin C (MMC), HAI of MMC and HAI of MMC plus cisplatin (CDDP) against the prognosis of patients with LMGC. The median survivals of patients were 3.1, 2.7 and 11.8 months, respectively, which indicated that HAI chemotherapy improved the survival time of LMGC compared with systemic chemotherapy. Moreover, the selection of the chemotherapeutic agents was also important. Similarly, in two retrospective studies,13,14 patients with LMGC were treated by HAI of 5-fluorouracil (5-FU) + epirubicin (EPIR) + MMC (FEM) regimen as first or second-line therapy. ORR was 42.9–73% and MST was 12.7–15 months. However, a Phase II study, including 88 patients, showed that the FEM regimen administered by HAI for unresectable LMGC induced a high-response rate, but most of them died because of the progression of extrahepatic metastases.15 In another study, Ojima et al16 retrospectively evaluated the efficacy of HAI for synchronous LMGC. Although ORR of HAI was 83%, there was no benefit in survival time. These studies suggested that liver metastases from gastric cancer were well controlled by HAI chemotherapy. However, extrahepatic growth of the tumor was not suppressed during HAI therapy alone because chemotherapy drugs were concentrated in the liver tumor. So we initially treated the patients with HAI combined with systemic chemotherapy.
In the G-SOX Phase III study,17 S-1 and oxaliplatin (SOX) regimen, as the first-line treatment for advanced gastric cancer, had similar efficacy to the S-1 and cisplatin (SP) regimen. Considering that forced hydration is not required, the SOX regimen is safer and more convenient. So it is now being often used to treat patients with advanced gastric cancer in China and Japan. In our study, the SOX regimen was chosen because the patients were in relatively poor physical condition due to extensive liver metastases. Considering that liver metastases were the primary limiting factor of survival compared with extrahepatic tumors, oxaliplatin was administered via the port-catheter system. HAI with oxaliplatin is based on some pharmacological studies, which suggest oxaliplatin is higher drug availability in the liver, and it is concentrated in the tumor.18,19 Floxuridine (fluorodeoxyuridine, FUDR), which is an antimetabolite derivative of 5-FU, has been shown to have a short half-life and high liver extraction rate (>90%).20–22 Therefore, FUDR is suggested as an ideal chemotherapy drug for HAI treatment and adopted in this study.
In our study, ORR was 76.2%, and MST of all patients was 12.3 months. Moreover, there was no statistical difference between the median times to hepatic and extrahepatic progression. The results suggested that HAI combined with systemic chemotherapy for LMGC could not only effectively control extensive liver metastases, but also prevent rapid extrahepatic progression, thus contributing to long-term survival. In the 1990s, two studies revealed HAI chemotherapy combined with hyperthermia achieved a higher response rate and longer survival time.23,24 A prospective study evaluated the efficacy of HAI chemotherapy combined with RFA for 7 patients with LMGC. The result showed ORR after HAIC was 71%. Followed by RFA, no intrahepatic recurrences occurred.25 This finding suggests that combination therapy with HAI and RFA is easier to achieve complete eradication of liver lesions and increase the survival time of patients with LMGC. In addition, the rate of recurrence after hepatectomy for LMGC is quite high, which suggests the presence of occult liver metastases after surgery.26 Therefore, two retrospectively studies27,28 evaluated the efficacy of adjuvant HAI after hepatectomy for GCLM. The findings indicated adjuvant HAI after hepatectomy for LMGC prevented remnant liver recurrence.
Complications associated with implantation of port system were infection, catheter displacement and thrombosis, while specific toxicities of HAI consist of reactive gastric or duodenal mucosal lesions, chemical hepatitis and biliary sclerosis.29–31 Fortunately, none of these adverse events occurred in this study, possibly due to the use of fixed catheter tip method, embolization of the arteries supplying adjacent organs and good postoperative nursing. Moreover, the incidence of adverse reactions related to chemotherapy was low and most observed toxicities (nausea/vomiting, fatigue and hepatic dysfunction) were tolerable. All patients did not have the toxicity of grade 4. Of the grade 3 adverse events, bone marrow suppression occurred in 14.3% of patients and diarrhea was 14.3%. The incidence of complications was lower than that of systemic chemotherapy reported to date, and this lower toxicity improved the quality of life.
We find that HAI chemotherapy is useful for inhibiting liver metastases and achieving long-term survival of patients with LMGC. Especially when liver metastases are extensive, it can effectively decrease the number and size of tumors, thus allowing patients to obtain surgical opportunities. Encouragingly, two patients in this study got the opportunity for surgery after chemotherapy and had no intrahepatic recurrence for a long time. Moreover, HAI chemotherapy has a practical value in the management of liver metastases and complete remission can be achieved in selected patients with LMGC. Finally, we acknowledge that the limitations of this study are the small sample size and retrospective study.
Conclusion
HAI combined with systemic chemotherapy for extensive LMGC seems to be effective, as reflected in the high-response rate, relatively long survival time, and mild toxicity. The results of this study provide background data for future trials. Large-scale prospective studies are needed to further clarify the clinical benefits of HAI chemotherapy.
Ethics and Consent Statement
The study was approved by the Ethics Committee of the Third Affiliated Hospital of Soochow University (Changzhou, China). The relevant informed consent forms were signed by the patients or their guardians.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Li Q, Li H, Jiang H, et al. Predictive factors of trastuzumab-based chemotherapy in HER2 positive advanced gastric cancer: a single-center prospective observational study. Clin Transl Oncol. 2018;20(6):695–702. doi:10.1007/s12094-017-1772-5
3. Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30(5):969–977.
4. Kemeny NE, Niedzwiecki D, Hollis DR, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;30(5):1395–1403. doi:10.1200/JCO.2005.03.8166
5. Kemeny NE, Melendez FD, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27(21):3465–3471. doi:10.1200/JCO.2008.20.1301
6. D’Angelica MI, Correa-Gallego C, Paty PB, et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg. 2015;261(2):353–360. doi:10.1097/SLA.0000000000000614
7. Qiang WG, Shi LR, Li XD, et al. Hepatic arterial infusion plus systemic chemotherapy as third-line or later treatment in colorectal liver metastases. Clin Transl Oncol. 2015;17(11):870–875. doi:10.1007/s12094-015-1317-8
8. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
9. Wu AW, Yuan P, Li ZY, et al. Capecitabine plus paclitaxel induction treatment in gastric cancer patients with liver metastasis: a prospective, uncontrolled, open-label Phase II clinical study. Future Oncol. 2016;12(18):2107–2116. doi:10.2217/fon-2016-0145
10. Baba H, Yamamoto M, Endo K, et al. Clinical efficacy of S-1 combined with cisplatin for advanced gastric cancer. Gastric Cancer. 2003;6(Suppl S1):45–49. doi:10.1007/s10120-003-0222-y
11. Picado O, Dygert L, Macedo FI, et al. The role of surgical resection for stage IV gastric cancer with synchronous hepatic metastasis. J Surg Res. 2018;232:422–429. doi:10.1016/j.jss.2018.06.067
12. Yonemura Y, Matuki N, Sakuma H, et al. Effect of intra-hepatoarterial infusion of MMC and CDDP for gastric cancer patients with liver metastases. Surg Today. 1992;22(3):253–259. doi:10.1007/BF00308831
13. Seki H, Ohi H, Ozaki T, Yabusaki H. Hepatic arterial infusion chemotherapy using fluorouracil, epirubicin, and mitomycin C for patients with liver metastases from gastric cancer after treatment failure of systemic S-1 plus cisplatin. Acta Radiol. 2016;57(7):781–788. doi:10.1177/0284185115603247
14. Arai Y, Endo T, Sone Y, et al. Management of patients with unresectable liver metastases from colorectal and gastric cancer employing an implantable port system. Cancer Chemother Pharmacol. 1992;31(S1):S99–S102. doi:10.1007/BF00687116
15. Kumada T, Arai Y, Itoh K, et al. Phase II study of combined administration of 5-fluorouracil, epirubicin and mitomycin-C by hepatic artery infusion in patients with liver metastases of gastric cancer. Oncology. 1999;57(3):216–223. doi:10.1159/000012034
16. Ojima H, Ootake S, Yokobori T, et al. Treatment of multiple liver metastasis from gastric carcinoma. World J Surg Oncol. 2007;5(1):70. doi:10.1186/1477-7819-5-70
17. Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26(1):141–148. doi:10.1093/annonc/mdu472
18. Guthoff I, Lotspeich E, Fester C, et al. Hepatic artery infusion using oxaliplatin in combination with 5-fluorouracil, folinic acid and mitomycin C: oxaliplatin pharmacokinetics and feasibility. Anticancer Res. 2003;23(6D):5203–5208.
19. Kern W, Beckert B, Lang N, et al. Phase I and pharmacokinetic study of hepatic arterial infusion with oxaliplatin in combination with folinic acid and 5-fluorouracil in patients with hepatic metastases from colorectal cancer. Ann Oncol. 2001;12(5):599–603. doi:10.1023/A:1011186708754
20. Ensminger WD, Rosowsky A, Raso V, et al. A clinical-pharmacological evaluation of hepatic arterial infusions of 5-fluoro-2ʹ-deoxyuridine and 5-fluorouracil. Cancer Res. 1978;38(11 Pt 1):3784–3792.
21. Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol. 1983;10(2):176–182.
22. Cercek A, D’Angelica M, Power D, et al. Floxuridine hepatic arterial infusion associated biliary toxicity is increased by concurrent administration of systemic bevacizumab. Ann Surg Oncol. 2014;21(2):479–486. doi:10.1245/s10434-013-3275-0
23. Hamazoe R, Murakami A, Hirooka Y, Maeta M, Kaibara N. A phase II pilot study of the combined application of hyperthermia and intra-hepato-arterial chemotherapy using cisplatinum and 5-fluorouracil. J Surg Oncol. 1991;48(2):127–132. doi:10.1002/jso.2930480211
24. Iwahashi M, Tanimura H, Nakamori M, et al. Clinical evaluation of hepatic arterial infusion of low dose-CDDP and 5-FU with hyperthermotherapy: a preliminary study for liver metastases from esophageal and gastric cancer. Hepatogastroenterology. 1999;46(28):2504–2510.
25. Yamakado K, Nakatsuka A, Takaki H, et al. Prospective study of arterial infusion chemotherapy followed by radiofrequency ablation for the treatment of liver metastasis of gastric cancer. J Vasc Interv Radiol. 2005;16(12):1747–1751. doi:10.1097/01.RVI.0000188738.84911.3B
26. Sakamoto Y, Sano T, Shimada K, et al. Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol. 2007;95(7):534–539. doi:10.1002/jso.20739
27. Kunieda K, Saji S, Sugiyama Y, et al. Evaluation of treatment for synchronous hepatic metastases from gastric cancer with special reference to long-term survivors. Surg Today. 2002;32(7):587–593. doi:10.1007/s005950200106
28. Fukami Y, Kaneoka Y, Maeda A, et al. Adjuvant hepatic artery infusion chemotherapy after hemihepatectomy for gastric cancer liver metastases. Int J Surg. 2017;46:79–84. doi:10.1016/j.ijsu.2017.08.578
29. Bouchahda M, Adam R, Giacchetti S, et al. Rescue chemotherapy using multidrug chronomodulated hepatic arterial infusion for patients with heavily pretreated metastatic colorectal cancer. Cancer. 2009;115(21):4990–4999. doi:10.1002/cncr.24549
30. Lee HJ, Lee YS, Lee KW, et al. Efficacy and safety of hepatic arterial infusion of fluorouracil with leucovorin as salvage treatment for refractory liver metastases from colorectal cancer. Korean J Intern Med. 2011;26(1):82–88. doi:10.3904/kjim.2011.26.1.82
31. Yamagami T, Kato T, Hirota T, Yoshimatsu R, Matsumoto T, Nishimura T. Embolization of accessory left gastric artery to prevent acute gastric mucosal lesions in patients undergoing repeated hepatic arterial infusion chemotherapy. Acta Radiol. 2007;48(3):280–284. doi:10.1080/02841850601182188
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
