Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Hepatic Arterial Infusion Chemotherapy Combined with Lenvatinib Plus Humanized Programmed Death Receptor-1 in Patients with High-Risk Advanced Hepatocellular Carcinoma: A Real-World Study
Authors Chang X, Wu H, Ning S , Li X, Xie Y, Shao W, Yu J
Received 23 April 2023
Accepted for publication 20 July 2023
Published 6 September 2023 Volume 2023:10 Pages 1497—1509
DOI https://doi.org/10.2147/JHC.S418387
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Jörg Trojan
Xu Chang,1,2 Huiyong Wu,2 Shangkun Ning,3 Xinge Li,4 Yinfa Xie,2 Wenbo Shao,2 Jinming Yu1,5
1Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin Medical University, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin, 300000, People’s Republic of China; 2Department of Interventional Therapy II, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, 250117, People’s Republic of China; 3Department of Interventional Therapy I, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, 250117, People’s Republic of China; 4Department of Oncology, Central Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, 250117, People’s Republic of China; 5Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Science, Jinan, Shandong, 250117, People’s Republic of China
Correspondence: Jinming Yu, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin Medical University, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin, 300000, People’s Republic of China, Tel +86 531-67626306, Email [email protected] Wenbo Shao, Department of Interventional Therapy II, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, 250117, People’s Republic of China, Tel +86 531-67626892, Email [email protected]
Purpose: The treatment of hepatocellular carcinoma (HCC) patients with high-risk features (Vp4, and/or tumor occupancy≥ 50%) has not been standardized and has poor outcomes. The present study aimed to assess the safety, efficacy, and prognostic impact of lenvatinib, hepatic arterial infusion chemotherapy (HAIC), and humanized programmed death receptor-1 (PD-1) in treating high-risk patients and to explore the biomarkers that may predict the efficacy.
Methods: HCC patients with high-risk features treated with lenvatinib, HAIC, and PD-1 were analyzed retrospectively. Overall survival (OS), progression-free survival (PFS), duration of response (DOR), objective response rate (ORR), and disease control rate (DCR) were calculated to evaluate the antitumor efficacy. Treatment-related adverse events (TRAEs) were analyzed to assess the safety profiles.
Results: Between February 2020 and July 2022, 97 patients were enrolled in this retrospective cohort study. The median follow-up time was 447 days. During analysis, 65 patients had disease progression, and 39 patients died. The median PFS and OS were 295 and 579 days, respectively. According to RECIST 1.1 and mRECIST, the ORR was 64.9% and 78.3%, respectively, and the DCR was 92.8%. The median and intrahepatic DOR was 363 and 462 days, respectively. Treatment-related grade 3 or 4 adverse events occurred in 64 (65.9%) patients, and the most common adverse events were hypertension (9.3%), thrombocytopenia (7.2%), and elevated aspartate transaminase (7.2%). Participants with low levels of serum procalcitonin (PCT) had satisfactory prognosis.
Conclusion: Lenvatinib, HAIC, and PD-1 were safe and showed promising antitumor activity against HCC with high-risk features. The initial levels of procalcitonin might be the predictive biomarkers for the combined treatment.
Keywords: hepatocellular carcinoma, HAIC, lenvatinib, VP4, PD-1
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most common cause of cancer-related deaths worldwide.1,2 About 72% of cases of HCC occur in Asia, and >50% occur in China.3 Most patients present advanced, unresectable disease, with a poor 5-year survival probability of approximately 10–18%.4,5
Tyrosine kinase inhibitors (TKIs) such as sorafenib (SHARP and Asia-Pacific) and lenvatinib (REFLECT) have been approved as first-line systemic therapies for unresectable advanced HCC. Recently, the Food and Drug Administration (FDA) approved the combination of atezolizumab and bevacizumab for patients with unresectable or metastatic HCC who have not received prior systemic therapy, based on Phase III IMbrave150 study.6–8 Various guidelines recommend it as the preferred first-line treatment because of its outstanding results. However, the recently updated IMbrave150 showed that this treatment has limited benefit in high-risk patients [Vp4, and/or tumor occupancy≥50% (TO≥50%) of the liver] with a median overall survival (OS) of 7.6 months.9,10 Interestingly, the median survival of patients with major portal vein tumor thrombosis (Vp3 and Vp4 portal vein invasion) after sorafenib treatment is merely 3.1–6.0 months.11,12 Moreover, high-risk patients were not enrolled in the REFLECT trial. The outcome data about high-risk patients were limited because of their extremely poor prognosis and often excluded from previous trials. Consequently, the requirement of high-risk patients is not fulfilled.
Recently, a few immunotherapy combined therapies, such as lenvatinib plus pembrolizumab (KEYNOTE-524)13 and camrelizumab plus apatinib (RESCUE),14 have emerged as the first-line option for HCC and showed promising antitumor activity with a tolerable safety profile in treatment-naïve unresectable HCC.15 Although lenvatinib plus pembrolizumab phase III trial LEAP-002 did not meet its primary endpoint, it still had a better OS compared to lenvatinib monotherapy [21.2 vs 19.0 months, hazard ratio (HR): 0.84, 95% confidence interval (CI): 0.708–0.997, p=0.0227].16 These data suggested that further exploration of TKIs combined with immune checkpoint inhibitor (ICI) is essential.
Hepatic arterial infusion chemotherapy (HAIC) delivers a high local drug concentration into liver tumors, is associated with a substantial local antitumor effect, and has been used in primary and metastatic hepatic malignant tumors.17–20 Recent studies have explored HAIC alone or accompanied by sorafenib in advanced HCC and reported favorable results either in response rate or survival.18,19,21
Considering the different anti-malignancy mechanisms of TKIs, PD-1 inhibitors, and HAIC, combining these three modalities might exert a potential synergic effect and promising preliminary efficacy results in advanced HCC. Herein, we aimed to retrospectively explore the efficacy and safety of lenvatinib combined with PD-1 plus HAIC as the first-line treatment in advanced high-risk HCC.
Materials and Methods
Patients
A total of 97 unresectable HCC patients with high-risk treated with HAIC combined with lenvatinib plus PD-1 at the Affiliated Cancer Hospital of Shandong First Medical University between February 2020 and July 2022 were recruited in this study (Figure 1A). All patients were diagnosed with HCC based on the non-invasive criteria or biopsy. The non-invasive diagnostic criteria for HCC in patients with cirrhosis were as follows: liver cirrhosis, tumor diameter >1 cm based on four-phase multidetector computed tomography (MDCT) or dynamic magnetic resonance imaging (MRI), and arterial hypervascularization with venous or delayed phase washout.22,23 The inclusion criteria were as follows: (1) Patients aged between 18 and 80 years; (2) Liver function classification (Child–Pugh) of grade A or B; (3) Eastern Cooperative Oncology Group (ECOG) score 0–2; (4) Presence of Vp4, and/or tumor occupancy ≥50% of the liver; (5) At least one measurable intrahepatic lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; (6) No prior systemic treatments; (7) Adequate organ function (absolute neutrophil count ≥1.5×109/L, platelet count ≥60×109/L, total bilirubin <52 μmol/L, albumin ≥28 g/L, aspartate transaminase and alanine transaminase ≤5×upper limit of the normal, creatinine clearance rate of ≤1.5×upper limit of the normal, and left ventricular ejection ≥45%). The exclusion criteria were as follows: combined with other malignant tumors; incomplete medical information; loss to follow-up. This single-center retrospective study was approved by the ethics committee of the Affiliated Cancer Hospital of Shandong First Medical University. Written informed consent was acquired from all patients before the operation in accordance with the 1955 Declaration of Helsinki.
 |
Figure 1 (A) Flowchart of the patient cohort. (B) The schema of the combination therapy. |
Treatment
Patients received oral lenvatinib at 12 mg/day (for body weight >60 kg) or 8 mg/day (for body weight <60 kg), as described above 3–7 days prior to the initial HAIC to confirm tolerability. The doses were reduced due to lenvatinib-related toxicities (to 8 mg or 4 mg/day or to 4 mg every other day). Sintilimab, camrelizumab, and tislelizumab, as immune checkpoint inhibitors (ICIs), were administered intravenously at a dose of 200 mg 0–1 day after the HAIC procedure that was performed every 3 weeks (Figure 1B). A catheter/microcatheter was placed in the main feeding hepatic artery, and then the following regimen was administered via the hepatic artery: oxaliplatin 135 mg/m2 from 0–2 h on day 1; leucovorin 400 mg/m2 from 2–3 h on day 1; 5-fluorouracil 400 mg/m2 bolus at 3 h; 2400 mg/m2 over 46 h on days 1 and 2.21 After HAIC was completed, the catheter and sheath were removed. Repetitive catheterization was performed in the next HAIC cycle, which was discontinued after six cycles, and patients were treated with lenvatinib and PD-1 maintenance. In addition, when a patient becomes operable due to tumor shrinkage, the decision of surgery is made by the patient.
Blood Assessment for Procalcitonin (PCT) and Determination of the Cutoff Value
PCT concentration was determined before the first cycle of the combined treatment using a chemiluminescent enzyme immunoassay. The cutoff value was defined using the software X tile.24 A blood culture was carried out for patients with PCT value >0.5 ng/mL to confirm the lack of active infectious.
Follow-Up
The primary endpoint was progression-free survival (PFS), defined as the time from the commencement of lenvatinib to progression, according to modified RECIST (mRECIST) and criteria or death from any cause, whichever occurred first. The secondary endpoints were OS, defined as the time from the commencement of lenvatinib to death from any cause, and the objective response rate (ORR), defined as the proportion of patients with complete response or partial response from the first radiological confirmation of that rate, and the disease control rate (DCR), defined as the proportion of patients with ORR plus stable disease. The duration of response (DOR) is the time from the first recorded complete or partial response to disease progression or death. The DCR and ORR were evaluated according to RECIST version 1.1 and mRECIST. The adverse events were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. Telephone follow-up and outpatient interviews were continued and ended on March 1, 2023. In case of patients out of contact during the follow-up, the OS was calculated as the time from the diagnosis to the last follow-up.
Statistical Analysis
R programming language and SPSS 26.0 were used for statistical analyses. The OS, PFS, and DOR were evaluated using the Kaplan–Meier method and Log rank test, with p<0.05 defined as a statistically significant difference. Cox proportional risk regression model was used for univariate and multivariate analyses. Post-univariate analysis, variables with p<0.2 were selected for multivariate analysis; p<0.05 indicated a statistically significant difference.
Results
Patients
Between February 2020 and July 2023, 97 patients with high-risk features were enrolled in this retrospective cohort study. The cutoff date was March 1, 2023, and the median follow-up was 447 days. The characteristics of the patients are listed in Table 1. The cohort comprised 85 females and 12 males with a mean age of 57 years (range 38–76 years). The median tumor size was 12.7cm (range 1.9–23.2cm). About 82.5% (80/97) of cases were Child-Pugh A, and the remaining were Child-Pugh B. Then, 87.6% (85/97) of patients had an underlying chronic liver disease caused by hepatitis B virus infection, and all these patients received antiviral therapy. In the cohort, 31 patients presented TO≥50%, 52 patients had Vp4, and 14 patients were accompanied with TO≥50% and Vp4. 32.0% (31/97) of patients had extrahepatic spread. All 97 patients received triple combination therapy with lenvatinib and ICIs plus HAIC. Among these patients, 57, 25, and 15 patients were treated with camrelizumab, tislelizumab, and sintilimab, respectively.
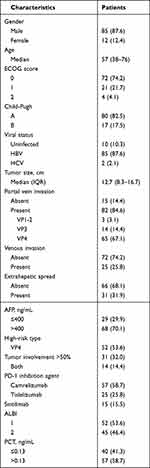 |
Table 1 Baseline Characteristics of 97 Patients |
In this study, 97 patients were treated with 375 cycles of HAIC (median four cycles). Dose adjustment of lenvatinib was observed in 17 patients, but none discontinued the target drugs. On the cutoff date, 65 patients developed disease progression, 8 did not receive second-line-treatment due to refused or impaired liver function, and 57 patients received second-line and local treatment, including transarterial chemoembolization (TACE) (number 28), radiotherapy (number 5), and systematic treatment (regorafenib monotherapy (number 24), regorafenib combined with other PD-1 antibody (number 26), other PD-1 or PD-L1 antibody monotherapy (number 5), and atezolizumab plus bevacizumab (number 2)). Notably, 8 patients underwent surgical resection after the combined treatment.
Efficacy
At the time of analysis, 65 patients had disease progression, and 39 were deceased. The median PFS was 295 days (95% CI: 248.5–341.5) (Figure 2A), and the median OS was 579 days (95% CI: 372.5–785.5) (Figure 2B). The tumor response is shown in Table 2. The median DOR was 363 days (95% CI: 243.7–482.3) (Figure 2C), and the intrahepatic DOR was 462 days (95% CI: 323.7–600.2) (Figure 2D). According to RECIST 1.1 and mRECIST, the ORR was 64.9% and 78.3%, respectively, and the DCR was 92.8%. For the intrahepatic tumors, according to RECIST 1.1 and mRECIST, the ORR was 67.0% and 81.4%, respectively, and the DCR was 96.9% (Table 2). In addition, 18 patients achieved a complete response of intrahepatic lesions based on mRECIST. The imaging scans of 48 representative participants are shown in Supplement 1: Figures 1S–48S. The median OS for patients with albumin-bilirubin (ALBI) grade 1 was not reached, and 445 days for the patients with ALBI grade 2, with no significant difference between the two groups (HR:0.56, 95% CI:0.30–1.05, p=0.075) (Figure 3A). The median OS for patients with body mass index (BMI)>25 and BMI≤25 was 675 and 480 days, respectively, with no significant difference between the two groups (HR: 0.69, 95% CI: 0.35–1.34, p=0.27) (Figure 3B). The median OS of patients with VP4, TO≥50%, and VP4+ TO≥50% was 450 days and 367 days, respectively (p=0.13) (Figure 3C). Moreover, the median OS was 651 days for patients without metastases compared to 367 days for patients with metastases, indicating a statistically significant difference between the two subgroups (HR: 0.37, 95% CI: 0.18–0.77, p=0.0084) (Figure 3D). The median OS was 579 days in patients with Child-Pugh A and 336 days in patients with Child-Pugh B, with a significant difference (HR:0.38, 95% CI: 0.16–0.89, p=0.026) (Figure 3E). In addition to various PD-1 agents, the median OS with camrelizumab, tislelizumab, and sintilimab treatment was 579 days vs 508 days vs 459 days, respectively (p=0.88) (Figure 3F). The median time to achieve response was 52 days, and the reductions in tumor size are shown Figure 4.
 |
Table 2 Summary of Best Response |
 |
Figure 2 Kaplan–Meier curves of PFS ((A) n=97), OS ((B) n=97), DOR ((C) n=76), and intrahepatic DOR ((D) n=79). |
Factors Associated with PFS and OS
Univariate analysis found that age, ALBI grade, Child-Pugh class, extrahepatic metastasis, Vp4, TO≥50%, and the PCT value were associated with PFS. In multivariate analysis, high PCT value (HR: 2.397, 95% CI: 1.325–4.334, p=0.004), ALBI Grade 2 (HR: 1.748, 95% CI: 1.028–2.972, p=0.039) were independent predictors associated with reduced PFS (Table 3). Univariate analysis found that Child-Pugh class, gender, ALBI grade, TO≥50%, extrahepatic metastasis, and PCT value were associated with OS. In multivariate analysis high PCT value (HR: 3.788, 95% CI:1.776–8.079, p=0.006) was an independent predictor associated with reduced OS (Table 4).
 |
Table 3 Univariable and Multivariable Analyses of Covariates Associated with PFS |
 |
Table 4 Univariable and Multivariable Analyses of Covariables Associated with OS |
Safety
No treatment-related deaths were recorded in this study. A total of 84 (86.6%) patients showed treatment-related adverse events (TRAEs) of any grade (Table 5). The most common TRAEs were hypertension (53.6%), thrombocytopenia (44.3%), fatigue (44.3%), elevated ALT (63.9%), elevated AST (67.0%), hypothyroidism (37.1%), and abdominal pain (43.3%). Treatment-related grade 3 or 4 adverse events occurred in 64 (65.9%) patients, and the most common events were hypertension (9.3%), thrombocytopenia (7.2%), and elevated AST (7.2%). Serious adverse events occurred in 8 patients, 6 had gastrointestinal bleeding, and 2 cases of cerebral hemorrhage caused by hypertension were observed.
 |
Table 5 Treatment-Related Adverse Events |
Immune-related adverse events of any grade were observed in 35 (36.1%) participants, and the most common was hypothyroidism (22/97, 22.7%). Immune-related hepatitis and pneumonitis occurred in 4 and 2 patients, respectively. All immune-related adverse events disappeared after participants stopped ICIs and received glucocorticoid therapy. On the cutoff date, 3 patients discontinued the ICIs due to repeat immune-related adverse events after rechallenge with the ICIs.
In addition, specific abdominal pain associated with oxaliplatin infusion occurred in 18 (25.4%) patients during the HAIC procedure. This pain could be acute and severe but was quickly relieved by infusing lidocaine via microcatheter.
Furthermore, we found that the spleen volume of 21 patients increased significantly during the treatment compared to the baseline (Supplement 2: Figures 1S–12S). Among the 21 patients, 14 were treated with partial splenic embolization.
Survival Analysis According to PCT
Next, we conducted Kaplan–Meier analysis according to the baseline PCT status. Based on the X-tile software results, the optimal cutoff value of PCT was set as 0.13 ng/mL. Among the 97 patients, 14 had a PCT value >0.5ng/mL, but the blood culture was negative. Our results showed that the median OS of high PCT value (>0.13ng/mL) and low PCT value (≤0.13ng/mL) was 426 days and not reached, respectively, with a significant difference (HR:3.33, 95% CI: 1.75–6.32, p=0.0021) (Figure 5A). The median PFS of HCC with a high PCT value was significantly lower than that with a low PCT value (256 days vs 500 days, HR: 2.63, 95% CI: 1.60–4.32, p=0.0001) (Figure 5B).
 |
Figure 5 The levels of PCT before the treatment might be a predictive biomarker for the efficacy of combination therapy. Kaplan–Meier curves of PFS (A) and OS (B) between high- and low-PCT groups. |
Discussion
Hepatocellular carcinoma (HCC) is the third leading cause of cancer25 and has been estimated to be 72% of cases of HCC in Asia and >50% of cases in China.3 Most patients present advanced, unresectable disease, with a poor 5-year survival probability of approximately 10–18%.4,5,26 High-risk HCC patients with tumor thrombus in the main portal vein trunk or high tumor burden, especially those with ≥50% liver occupancy, are common in China and have a poor prognosis.27,28
Systemic therapy, such as lenvatinib or atezolizumab plus bevacizumab, is the standard treatment for advanced unresectable HCC. However, since high-risk HCC patients were excluded in the REFLECT trial and KEYNOTE-524, the safety and efficacy of lenvatinib in high-risk patients are yet to be determined.29 Furthermore, IMbrave150 showed that atezolizumab plus bevacizumab has limited benefit in high-risk patients with a median OS of 7.6 months. The therapeutic procedures for HCC patients with high risk are limited.
In 2021, the phase III trial FOHAIC-1 proved that HAIC has a higher efficacy and better survival outcome than sorafenib, and the subgroup analysis showed that HAIC achieved better OS and PFS than sorafenib in high-risk patients (10.8 vs 5.7 months, 7.7 vs 2.9 months).21 Another Phase II trial compared the efficacy and safety of sorafenib plus HAIC vs sorafenib alone for advanced HCC with major portal vein tumor thrombosis (Vp4 and Vp3).30 The median OS was 16.3 months with sorafenib plus HAIC and 6.5 months with sorafenib alone, along with a higher ORR (41% vs 3%) and a longer median PFS 9.0 vs 2.5 months. These two clinical trials demonstrated that HAIC has significant benefits for high-risk HCC patients. Recently, a Japanese study demonstrated that lenvatinib is beneficial in high-risk patients.31 The mPFS and mOS were 132 days and 229 days, 101 days and 201 days in patients with TO≥50% and Vp4, respectively. In addition, a recent study showed that lenvatinib plus PD-1 provides survival benefits in patients with Vp4 and TO≥50%.32 The median OS was 6.1 months for patients with TO≥50% and 11.39 months for patients with Vp4. On the other hand, patients with TO≥50% had significantly poor PFS, ORR, and OS. Therefore, locoregional treatments were recommended for patients with TO≥50% during systemic therapy. In the present study, we investigated the clinical benefits of lenvatinib combined with PD-1 and HAIC therapy in high-risk patients. To the best of our knowledge, this is the largest study, consisting of 97 patients with high-risk features, evaluating the applicability of the combined treatment in real-world. The median PFS was 295 days, and the median OS was 579 days. The ORR was 78.3% according to mRECIST and 64.9% according to RECIST 1.1. Notably, 16.5% showed a complete response of all lesions, and 18.5% exhibited a complete response of intrahepatic target lesions according to the mRECIST criteria. In addition, the combined treatment was effective in different high-risk types. The median OS of patients with Vp4 and TO≥50% was not reached and 450 days, respectively; also, Vp4+TO≥50% achieved an OS of 367 days. Furthermore, the median size of maximum tumor of patients enrolled in our study was 12.7cm, and 81.5% (79/97) patients had major portal vein tumor thrombosis (65 Vp4, 14 Vp3), which might have a poor prognosis. However, the median PFS, OS, and ORR in the present study were better than those in patients with early staging and low tumor burden receiving standard first-line systemic treatment.
A tolerable safety profile was observed with combination therapy in this study. A total of 84 (93.3%) patients showed TRAEs of any grade. Treatment-related grade 3 or 4 adverse events occurred in 64 (71.1%) patients, and the most common events were hypertension (10.0%), thrombocytopenia (7.8%), and elevated AST (8.9%). Serious adverse events occurred in 8 patients, 6 had gastrointestinal bleeding, and 2 presented cerebral hemorrhage caused by hypertension. In addition, we found that during the treatment procedure, the spleen size of some patients increased significantly compared to the baseline. Previous studies have demonstrated that oxaliplatin induces sinusoidal obstruction syndrome (SOS), leading to portal hypertension, fluid retention, and hyperbilirubinemia, aggravating the deterioration of liver function. On the other hand, increasing spleen size serves as a biomarker for the risk of oxaliplatin-induced hepatic sinusoidal injury.33 Regarding SOS, some points are crucial: (1) SOS should be considered in addition to chemotherapy drugs for patients with thrombocytopenia. In the current department, partial splenic embolization was performed for patients with refractory thrombocytopenia or significantly increased spleen size from the baseline. However, the long-term effects are yet to be determined. (2) Many patients in this study developed abnormal liver function while the tumors were well-controlled; hence, the liver damage due to lenvatinib and ICIs should be excluded, and SOS and termination of oxaliplatin should be included. In the present study, 5 patients discontinued HAIC because of severe abnormal liver function, while the intrahepatic tumors were well-controlled. Therefore, HAIC has a maximum of six cycles in the current study.
Biomarkers that predict the efficacy of systematic immunotherapy in HCC are yet to be determined.34–36 Serum C-reactive protein (CRP) is a biomarker of an acute inflammatory response and has been successfully used as a prognostic predictor for several malignancies. A recent study demonstrated the prognostic value of pretreatment serum CRP levels for advanced HCC patients and treatment with PD-1 inhibitors.37 In addition, Scheiner et al developed a novel prognostic score termed “CRAFITY” based on serum CRP and alpha-fetoprotein levels that predicts the outcomes of HCC patients receiving atezolizumab plus bevacizumab.38 PCT has been considered an excellent marker of bacterial infection and acute inflammation for over two decades. Currently, some studies reported for the first time that PCT is a prognostic factor in lung cancer patients receiving ICIs.39 Therefore, we speculated whether PCT, an indicator of inflammation, also predicts the efficacy of immunotherapy. Our results showed that the OS and PFS of HCC with low PCT value were significantly higher than those with high PCT value (not reach vs.426 days, p=0.0021, 500 days vs 256 days, p=0.0001). Additionally, the pretreatment level of serum PCT was an independent predictor associated with reduced PFS and OS. To the best of our knowledge, no study has reported an association between serum PCT levels and prognosis in HCC. Further research is needed to determine the specificity of PCT in patients with HCC, explore the potential value of PCT to monitor the response to treatment and predict prognosis.
Nevertheless, the present study has some limitations, including its retrospective nature. First, although this is the largest study evaluating high-risk HCC patients, the number of analyzed patients was not large enough. Second, this was a single-arm design, limited by the lack of a control group. Currently, we are collecting the patients who use lenvatinib monotherapy or lenvatinib combined with ICIs as the control group. Third, the follow-up was short because an insufficient number of OS events were observed. A total of 58 patients were alive at the last time point cutoff, and the long-term survival data are yet lacking. In addition, sintilimab, camrelizumab and tislelizumab are three PD-1 inhibitors that have been approved by China Food and Drug Administration (CFDA) for HCC treatment.14,40,41 In this study, patients received treatment regimens that included one of the three different PD-1 inhibitors. This is a confounding factor that may cause bias. However, in subgroup analysis, the three PD-1 drugs combined respectively with HAIC and lenvatinib have no significant difference in overall survival in high-risk patients. In the era of immuno-combined therapy, we will further explore the efficacy of lenvatinib, HAIC combined with a certain kind of ICI in HCC patients. However, our findings highlighted the short-term efficacy (ORR and PFS) of HAIC combination with lenvatinib plus PD-1 inhibitors in high-risk HCC patients. Future prospective studies are required to address our findings by evaluating many patients in a multicenter setting using the same protocols.
In conclusion, HAIC combined with lenvatinib plus PD-1 has a promising efficacy in high-risk HCC patients. Furthermore, this combination therapy is well-tolerated and could be a treatment option for this patient group. The levels of serum PCT might be the predictive biomarkers for triple combination therapy.
Data Sharing Statement
The dataset used for this study is available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
Informed consent was obtained from the patient to publish, and approval for this study was provided by the Research Ethics Committee of The Affiliated Cancer Hospital of Shandong First Medical University.
Funding
This research was supported by the Natural Science Foundation of Shandong Province ZR2020QH177 (the Efficacy of TACE combined with TGF-β blockade in the treatment of hepatocellular carcinoma and its impact on the immune microenvironment).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
2. Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 global cancer statistics? Cancer Commun. 2019;39:22. doi:10.1186/s40880-019-0368-6
3. Lin Q, Huang X, Zhong C, Luo T, Zeng X, Chen S. Improved survival with radiotherapy in hepatocellular carcinoma with major vascular invasion: a propensity-matched analysis of surveillance, epidemiology, and end results database. Cancer Med. 2019;8(2):515–526. doi:10.1002/cam4.1937
4. Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. Lancet Global Health. 2018;6(5):e555–e567. doi:10.1016/S2214-109X(18)30127-X
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
6. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
7. Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic therapy for advanced Hepatocellular Carcinoma: ASCO guideline. J Clin Oncol. 2020;38(36):4317–4345. doi:10.1200/JCO.20.02672
8. Di Federico A, Rizzo A, Carloni R, et al. Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expert Opin Investig Drugs. 2022;31(4):361–369. doi:10.1080/13543784.2022.2009455
9. Roy A. Updated efficacy and safety data from IMbrave150: Atezolizumab plus Bevacizumab vs. Sorafenib for Unresectable Hepatocellular Carcinoma. J Clin Exp Hepatol. 2022;12(6):1575–1576. doi:10.1016/j.jceh.2022.07.003
10. Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. doi:10.1016/j.jhep.2021.11.030
11. Katagiri S, Yamamoto M. Multidisciplinary treatments for hepatocellular carcinoma with major portal vein tumor thrombus. Surg Today. 2014;44:219–226. doi:10.1007/s00595-013-0585-6
12. Nakazawa T, Hidaka H, Shibuya A, et al. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 2014;14:84. doi:10.1186/1471-230X-14-84
13. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in patients with unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
14. Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced Hepatocellular Carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27(4):1003–1011. doi:10.1158/1078-0432.CCR-20-2571
15. Santoni M, Rizzo A, Kucharz J, et al. Complete remissions following immunotherapy or immuno-oncology combinations in cancer patients: the MOUSEION-03 meta-analysis. Cancer Immunol Immunother. 2023;72(6):1365–1379. doi:10.1007/s00262-022-03349-4
16. Finn RS, Kudo M, Merle P. Primary results from the Phase 3 LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2022;33:S1401. doi:10.1016/j.annonc.2022.08.031
17. Hu J, Bao Q, Cao G, et al. Hepatic arterial infusion chemotherapy using Oxaliplatin Plus 5-fluorouracil versus transarterial Chemoembolization/Embolization for the treatment of advanced Hepatocellular Carcinoma with major portal vein tumor thrombosis. Cardiovasc Intervent Radiol. 2020;43(7):996–1005. doi:10.1007/s00270-019-02406-3
18. Wang X, Hu J, Cao G, et al. Phase II Study of hepatic arterial infusion chemotherapy with Oxaliplatin and 5-fluorouracil for advanced perihilar cholangiocarcinoma. Radiology. 2017;283(2):580–589. doi:10.1148/radiol.2016160572
19. He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for Hepatocellular Carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi:10.1001/jamaoncol.2019.0250
20. Boehm LM, Jayakrishnan TT, Miura JT, et al. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol. 2015;111(2):213–220. doi:10.1002/jso.23781
21. Lyu N, Wang X, Li JB, et al. Arterial chemotherapy of Oxaliplatin Plus fluorouracil versus sorafenib in advanced Hepatocellular Carcinoma: a biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J Clin Oncol. 2022;40:468–480. doi:10.1200/JCO.21.01963
22. Khalili K, Kim TK, Jang HJ, et al. Optimization of imaging diagnosis of 1–2 cm hepatocellular carcinoma: an analysis of diagnostic performance and resource utilization. J Hepatol. 2011;54:723–728. doi:10.1016/j.jhep.2010.07.025
23. Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59(5):638–644. doi:10.1136/gut.2009.187286
24. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi:10.1158/1078-0432.CCR-04-0713
25. Lai Z, He M, Bu X, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: a biomolecular exploratory, phase II trial. Eur J Cancer. 2022;174:68–77. doi:10.1016/j.ejca.2022.07.005
26. Rizzo A, Ricci AD, Brandi G. Systemic adjuvant treatment in hepatocellular carcinoma: tempted to do something rather than nothing. Future Oncol. 2020;16(32):2587–2589. doi:10.2217/fon-2020-0669
27. Kuo Y-H, Wu I-P, Wang J-H, et al. The outcome of sorafenib monotherapy on hepatocellular carcinoma with portal vein tumor thrombosis. Invest New Drugs. 2018;36(2):307–314. doi:10.1007/s10637-017-0468-6
28. Arai Y, Ohtsu A, Sato Y, et al. Phase I/II study of radiologic hepatic arterial infusion of fluorouracil plus systemic irinotecan for unresectable hepatic metastases from colorectal cancer: Japan Clinical Oncology Group Trial 0208-DI. J Vasc Interv Radiol. 2012;23(10):1261–1267. doi:10.1016/j.jvir.2012.06.031
29. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
30. Zheng K, Zhu X, Fu S, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus Sorafenib for Hepatocellular Carcinoma with major portal vein tumor thrombosis: a randomized trial. Radiology. 2022;303(2):455–464. doi:10.1148/radiol.211545
31. Chuma M, Uojima H, Hiraoka A, et al. Analysis of efficacy of lenvatinib treatment in highly advanced hepatocellular carcinoma with tumor thrombus in the main trunk of the portal vein or tumor with more than 50% liver occupation: a multicenter analysis. Hepatol Res. 2021;51(2):201–215. doi:10.1111/hepr.13592
32. Sun X, Zhang Q, Mei J, Yang Z, Chen M, Liang T. Real-world efficiency of lenvatinib plus PD-1 blockades in advanced hepatocellular carcinoma: an exploration for expanded indications. BMC Cancer. 2022;22(1):293. doi:10.1186/s12885-022-09405-7
33. Overman MJ, Maru DM, Charnsangavej C, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol. 2010;28(15):2549–2555. doi:10.1200/JCO.2009.27.5701
34. Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172. doi:10.1038/s41571-021-00573-2
35. Sangro B, Sarobe P, Hervas-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(8):525–543. doi:10.1038/s41575-021-00438-0
36. Rizzo A, Cusmai A, Gadaleta-Caldarola G, et al. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expert Rev Gastroenterol Hepatol. 2022;16(4):333–339. doi:10.1080/17474124.2022.2064273
37. Zhang Y, Lu L, He Z, et al. C-reactive protein levels predict responses to PD-1 inhibitors in Hepatocellular Carcinoma patients. Front Immunol. 2022;13:808101. doi:10.3389/fimmu.2022.808101
38. Scheiner B, Pomej K, Kirstein MM, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy – development and validation of the CRAFITY score. J Hepatol. 2022;76(2):353–363. doi:10.1016/j.jhep.2021.09.035
39. Ichikawa K, Watanabe S, Miura S, et al. Prognostic significance of procalcitonin in small cell lung cancer. Transl Lung Cancer Res. 2022;11(1):43–52. doi:10.21037/tlcr-21-838
40. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, Phase 2–3 study. Lancet Oncol. 2021;22(7):977–990. doi:10.1016/S1470-2045(21)00252-7
41. Qin S, Finn RS, Kudo M, et al. RATIONALE 301 study: tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019;15(16):1811–1822. doi:10.2217/fon-2019-0097
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


