Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Hepatectomy After Conversion Therapy for Initially Unresectable HCC: What is the Difference?
Authors Luo L , He Y , Zhu G, Xiao Y , Song S, Ge X, Wang T, Xie J, Deng W , Hu Z, Shan R
Received 13 September 2022
Accepted for publication 7 December 2022
Published 22 December 2022 Volume 2022:9 Pages 1353—1368
DOI https://doi.org/10.2147/JHC.S388965
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Laihui Luo,1,* Yongzhu He,1,* Guoqing Zhu,1,* Yongqiang Xiao,1 Shengjiang Song,1 Xian Ge,2 Tao Wang,3 Jin Xie,1 Wei Deng,1 Zhigao Hu,1 Renfeng Shan1
1Department of General Surgery, The First Affiliated Hospital of Nanchang, Nanchang, People’s Republic of China; 2Department of Pathology, The First Affiliated Hospital of Nanchang, Nanchang, People’s Republic of China; 3Department of Day Surgery Ward, The First Affiliated Hospital of Nanchang, Nanchang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Renfeng Shan, Department of General Surgery, The First Affiliated Hospital of Nanchang University, 17 Yong Wai Zheng Street, Nanchang, 330006, People’s Republic of China, Tel +86 0791-88692522, Email [email protected]
Purpose: Conversion therapy gives some patients with initially unresectable hepatocellular carcinoma (HCC) access to surgery. The purpose of this study was to evaluate the safety and efficacy of hepatectomy after conversion therapy and how it differed from those who undergoing direct hepatectomy.
Patients and Methods: From January 2018 to April 2022, 745 patients underwent hepatectomy for HCC were enrolled. Among them, 41 patients of unresectable HCC underwent hepatectomy after conversion therapy. A demographically and clinically comparable cohort was created from the remaining patients in a 1:1 ratio using propensity score matching.
Results: The median duration of conversion therapy was 108 (42– 298) days, 8 patients achieved complete response (CR) and 33 achieved partial response (PR). Conversion therapy resulted in some degree of myelosuppression, but liver function index remained good. Compared with the direct hepatectomy group, the conversion group had more blood loss (600 mL vs 400 mL, p=0.015), longer operative time (270 min vs 240 min, p=0.02), higher blood transfusion rates, and longer hospital stay (8 days vs 11 days, p< 0.001). Patients in the conversion group had significantly more complications of any grade (82.9% vs 51.2%, p=0.002) and grade 3/4 (26.8% vs 4.9%, p=0.013), and 6 patients developed post-hepatectomy liver failure (PHLF). There were no deaths in either group. All patients achieved R0 resection, 6 (6/41, 14.6%) achieved pathological complete response (pCR), 14 achieved major pathologic responses (MPR). During a median follow-up of 12.8 months, 14 patients in the conversion group experienced recurrence or metastasis, no deaths.
Conclusion: Hepatectomy after conversion therapy was more difficult than direct hepatectomy, but accurate preoperative assessment could ensure the safety of the surgery. The damage of liver function after conversion therapy was more severe than expected, PHLF should be prevented and treated. Hepatectomy was effective and necessary, postoperative pathological examination could provide guidance for adjuvant therapy.
Keywords: hepatocellular carcinoma, conversion therapy, hepatectomy, post-hepatectomy liver failure, pathological complete response
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer death worldwide in 2020.1 For patients with early-stage HCC, hepatectomy is the treatment of choice, yielding the best outcomes, with a 5 years survival of 70–80%.2,3 However, in China most patients were initially diagnosed in the intermediate or advanced stage and lost the opportunity to receive radical surgery, so-called “unresectable” HCC (u-HCC).4,5 Resection after HCC has been shrunk or downstaged is one of the important ways for patients to achieve radical cure and long-term survival. Relevant studies have shown that for these patients, if the opportunity for surgical resection is obtained through treatment, the postoperative survival rate is also better than non-surgical treatment.6,7
The aim of conversion therapy is to convert unresectable or borderline HCC into resectable tumors. In the era of lack systemic therapy, transcatheter arterial chemoembolization (TACE) is the main method of conversion therapy for u-HCC.8 In recent years, combinations of tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) for the treatment of advanced or u-HCC can achieve an ORR of about 30%.9–11 At the same time, with the advancement of drugs and technology, local regional therapy, such as TACE, hepatic artery infusion chemotherapy (HAIC), radiotherapy, etc. have shown better anti-tumor effects than before. The combination of locoregional and systemic treatments is option for u-HCC.12 In the past two years, triple therapy of TACE/HAIC, TKIs, and ICIs have been tried with encouraging results.13,14 An increasing number of patients with initially unresectable HCC were successfully undergoing radical surgery after conversion therapy.15,16
Every coin has two sides. It is undeniable that conversion therapy has certain side effects, which include not only the effects on liver function but also the effects on the whole body. Therefore, do these adverse factors affect the safety of liver resection? How does hepatectomy after conversion therapy differ from untreated direct hepatectomy? What should we focus on? In this study, we reported a group of 41 patients with initially u-HCC who underwent hepatectomy after triple combination conversion therapy (HAIC/TACE, tyrosine kinase inhibitors and programmed cell death protein-1), to explored the impact of conversion therapy on hepatectomy and how it differed from untreated direct hepatectomy.
Methods
Patients
Between January 2018 and April 2022, 745 patients diagnosed with HCC who received hepatectomy at the Department of Hepatobiliary Surgery of The First Affiliated Hospital of Nanchang University were reviewed for eligibility. After excluding 133 cases of invalid data, a total of 612 were included in this study. Among them, 571 patients did not receive any treatment before surgery, 41 patients had initially unresectable HCC and were successfully resected after triple combination conversion therapy. These 41 patients were evaluated as unresectable HCC by the multidisciplinary team (MDT) of HCC conversion therapy in our hospital. There are two reasons why HCC is unresectable, one is unresectable in the surgical sense and the other is unresectable in the oncological or biological sense. The former includes liver function that cannot tolerate surgery or insufficient postoperative residual liver volume (<40% for patients with liver cirrhosis; <30% for patients without liver cirrhosis); the latter refers to technical resection, but after resection, it cannot obtain better curative effect than non-surgical treatment. We collected each hospitalization records of these patients during conversion therapy and surgery. The Propensity score matching (PSM) method was used to create a demographically and clinically comparable cohort of patients. Patients in the hepatectomy after conversion therapy group were matched at a 1:1 ratio with patients treated with direct hepatectomy. Demographic, clinical, and pathological data of these patients were recorded and analyzed. The flowchart of the entire process was shown in Figure 1. This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University, No. (2022) CDYFYYLK (06–009).
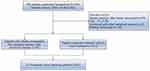 |
Figure 1 The flowchart of article. |
Conversion Therapy
The conversion therapy regimen used a triple combination conversion therapy, including regional treatment (HAIC or TACE) plus TKIs and programmed cell death protein-1 (PD-1) inhibitors. HAIC and TACE were administered every 3–6 weeks. TKIs in this study were sorafenib, apatinib and lenvatinib, and PD-1 inhibitors were camrelizumab, stintilimab and tislelizumab. The dose of these agents was administrated according to the guidelines, which would also be adjusted according to the performance status, liver function, and treatment tolerance. Hepatitis B-positive patients receive oral antiviral drugs at the same time. Before each PD-1 inhibitors treatment cycle, monitor the patient’s blood routine, liver, adrenal, thyroid, cardiac function and tumor markers. Efficacy was assessed by enhanced CT or MRI every 2 months. The results of each efficacy evaluation of the patients were discussed by the MDT and the next therapeutic regimen was formulated for the patients. After the tumor has been shrunk or downstaged and stabilized for a period of time (1–2 months), MDT would select the appropriate timing of surgery based on tumor response. Tumor response was assessed according to modified response evaluation criteria in solid tumors (mRECIST). Averse events (AE) were assessed and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0.
Hepatectomy and Complications
Preoperative evaluation included routine blood tests, blood biochemistry, thyroid function, cardiac function, pulmonary function tests, and imaging examinations. In order to ensure the safety and thoroughness of the operation, the premise of surgery was (1) achieve R0 resection; (2) preserve sufficient residual liver volume; (3) no serious AEs during conversion therapy; (4) patients had no contraindications to surgery. Patients in the conversion group needed to discontinue for a period of time before surgery according to the instructions for use of TKIs and PD-1 inhibitors.
A total of 82 patients in both groups underwent open hepatectomy. After tracheal intubation anesthesia, a 25cm “J” shaped incision in the middle of upper abdomen was made into enterocoelia. The corresponding perihepatic ligaments were divided for liver mobilization and prepared porta hepatis tourniquet. Hepatic portal occlusion (Pringle maneuver) was performed during liver transection to control blood loss if necessary. The liver parenchyma was transected at least 1cm (ensure R0 resection) from the tumor margin with an ultrasonic scalpel, in combination with electrocoagulation to hemostasis. The ends of small vessels and bile ducts were clipped with titanium clips or Hem-o-lock. Intraoperative attention should be paid to the separation and anatomy of hepatic veins, and anatomical hepatectomy should be performed as far as possible. The principle of no tumor was ensured throughout the operation. Hilar or retroperitoneal lymph nodes were dissected as needed. Non-absorbable sutures (Prolene) were used to ligate and suture the ducts in the liver section to avoid bile leakage and bleeding. Postoperative complications were classified according to the Clavien-Dindo grading system.
Pathological Examination
The resected tumor specimens were all examined by pathology. Pathological complete response (pCR) was defined as the absence of any viable tumor cells after a complete assessment of the resected specimen, including all sampled regional lymph nodes, tumor thrombi, and distant metastases, and review of all sections. Major pathologic responses (MPR), a reduction in the proportion of viable tumors below the clinically significant threshold. (In studies of lung cancer and malignant melanoma, MPR is defined as ≤10% of surviving tumors. There is currently no definition in HCC, and we have provisionally adopted 10% as the standard.)
Propensity Score Matching
The PSM method was used to control selection bias and to create demographically and clinically comparable cohorts. Propensity score of each patient was analyzed by multivariate logistic regression including gender, age, liver cirrhosis, maximum tumor diameter, Child–Pugh grade, resection range and name of surgical operation. A 1:1 match without replacement was performed through the nearest available matching, setting the caliper as 0.05. PSM analysis was conducted with SPSS 26.0 software (SPSS Inc., Chicago, IL).
Statistical Analysis
Categorical variables in the baseline characteristics were compared using the Pearson’s χ2 test or Fisher’s exact test. Continuous variable distribution was described using mean ± standard error (SE) or standard deviation (SD) for normally distributed values, and median and range were used for non-normally distributed values. A two-tailed p value <0.05 was considered statistically significant. All data analyses were performed using SPSS 26.0 software (SPSS Inc., Chicago, IL) and GraphPad Prism (version 9.0; GraphPad, Inc.).
Results
Patients and Conversion Therapy
From January 2018 to April 2022, a total of 745 patients with HCC who underwent hepatectomy at The First Affiliated Hospital of Nanchang University. After excluding 133 invalid data, the remaining patients were divided into hepatectomy after conversion therapy group (conversion group, n = 41) and direct hepatectomy group (direct group, n = 571). Table 1 shows the characteristics of these 41 patients. These initially unresectable HCC patients had tumors staged as Barcelona Clinic Liver Cancer (BCLC) stage B or C, with a high tumor burden, half of them had vascular invasion and a few had regional lymph node metastases. All these patients achieved tumor shrinkage or downstaging after our triple combination conversion therapy, and regained the opportunity for surgery (Table 2 shows the regimen and efficacy of conversion therapy). The median time from conversion therapy to surgery for these patients was 108 days, 8 patients achieved complete response (CR), the remaining patients were partial response (PR). The median time to discontinuation in our patients was 4 days for TKIs and 33 days for PD-1 inhibitors. After PSM, excluding tumor stage, all remaining clinical features were well balanced between the conversion group (clinical data before surgery after successful conversion therapy) and direct group (Table 3). Conversion therapy was well tolerated in all patients. Most of the AEs were mild or curative after treatment, the incidence of grade 3/4 AEs were 2.4%. The top three most common treatment-related AEs were elevated ALT, elevated AST, and fatigue, respectively.
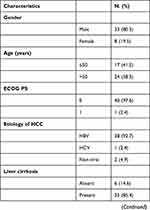 |  |  |
Table 1 Clinical Characteristics of Patients in the Conversion Group |
 |
Table 2 Conversion Therapeutic Regimen and Efficacy |
 |
Table 3 Baseline Characteristics After Propensity Score Matching Between the Two Groups |
Preoperative Examinations
Before surgery, we analyzed the results of biochemical indicators of the included patients. We also compared the relevant indicators before initial treatment and before surgery in the conversion group (Figure 2). The results showed that among the 41 patients who were successfully converted, conversion therapy had little effect on liver function, and the differences were not statistically significant (all p>0.05). However, conversion therapy had certain effects on the blood system. We observed substantial reductions in leukocytes (p<0.001) and platelets (p<0.001) in patients after conversion therapy. These effects also resulted in statistically significant differences in preoperative albumin, hemoglobin, leukocytes and platelets between the two groups of patients (All p<0.05).
Perioperative Outcomes
After PSM, the age, gender, extent of liver resection, liver function, maximum tumor diameter, presence of liver cirrhosis and American Society of Anesthesiologists (ASA) grading of the two groups of patients were basically balanced and were clinically comparable.
There were no significant differences in the incidence of intra-abdominal focal necrosis, ascites, approach of hepatectomy (anatomical or non-anatomical), hepatic hilar lymph node dissection, postoperative ICU transfer between the two groups. Compared with the direct group, the operation time (270 min vs 240 min, p=0.02) and blood loss (600 mL vs 400 mL, p=0.015) were significantly higher in the conversion group. After abdominal entry, we observed that the incidence of abdominal adhesions (85.4% vs 22.0%, p<0.001) and cholecystitis (65.9% vs 7.3%, p<0.001) was significantly higher in the conversion group (Table 4). Remarkably, both the intraoperative RBC transfusion rate (48.8% vs 19.5%, p=0.005) and the FFP transfusion rate (51.2% vs 0, p<0.001) were significantly higher in the conversion group than in the direct group. In terms of postoperative blood transfusion rate in the two groups, the conversion group was 26.8%, while the direct group was 0, with a significant difference (p<0.001). The difference was also reflected in the length of postoperative hospital stay, which was significantly shorter in the direct group compared with the conversion group (8 days vs 11 days, p<0.001). Except for 1 patient in the conversion group who underwent second operation due to postoperative bleeding, there was no death in the two groups during hospitalization and 90 days after surgery.
 |
Table 4 Comparison of Perioperative Data Between the Two Groups |
Postoperative Complications
Compared with the direct group, the conversion group had a significantly higher rate of postoperative complications (82.9% vs 51.2%, p=0.002), especially grade III–IV complications (26.8% vs 4.9%, p=0.013). The most common complications in the conversion group were pulmonary infection, fever, hyperbilirubinemia and coagulation dysfunction. Correspondingly, the direct group was pulmonary infection, fever and hyperbilirubinemia (Table 5). However, we found that post-hepatectomy liver failure (PHLF) (14.6% vs 0, p=0.026), coagulation disorders (19.5% vs 0, p=0.005), and pulmonary complications (41.5% vs 14.6%, p=0.007), especially massive pleural effusion, were more likely in the conversion group (Figure 3). Although there was no statistical difference, patients in the conversion group also had more transient liver dysfunction, abdominal infection, hyperbilirubinemia, and use of vasoactive agents after surgery. The incidence of postoperative bleeding and postoperative bile leakage was low in both groups. Patients with PHLF were graded as either grade A (n = 3) or B (n = 3).17 These patients were treated with symptomatic support, including fresh frozen plasma to improve coagulation function, albumin infusion, and diuresis. One of the patients had a continuous increase in bilirubin of up to 128.9 umol/L after surgery, which was considered to be autoimmune liver disease. Bilirubin decreased after steroid pulse therapy (it was worth noting that the use of steroid therapy in the acute phase of hepatitis may lead to fulminant hepatitis). A small number of patients in both groups had transient liver dysfunction, which may be related to the small-for-size syndrome (SFSS) after extensive liver resection, manifested as postoperative bilirubin elevation and cholestasis. Liver function returned to normal quickly in these patients after hepatic functional protection, jaundice reduction, and decreasing the pressure of portal veins. As for the patients with dyspnea caused by massive pleural effusion, the symptoms were improved after B-ultrasound-guided thoracentesis and catheter drainage, albumin supplementation and diuresis. The remaining patients recovered well after symptomatic treatment and were discharged from hospital.
 |
Table 5 Comparison of Postoperative Complications Between the Two Groups |
 |
Figure 3 Comparison of postoperative complications between the two groups. |
Pathological Examination
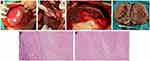
The resected tumor specimens were all examined by pathology. All patients had no residual tumor at the resection margin and achieved R0 resection. Seven patients in the conversion group achieved pCR. Microscopically, the tumor components were completely necrotic, and only the remains of the hepatocyte cords were seen, surrounded by fibrous tissue proliferation, the bile ducts were dilated, and chronic inflammatory cells (mostly lymphocytes) were infiltrated. In some necrotic tissues, deposition of yellow particles (hemosiderosis) and accumulation of foamy histiocytes were seen. The remaining patients of conversion group had a tumor necrosis rate of 60–99%,14 achieved MPR (only small clusters of cancer cells remain under the microscope). (Figure 4 shows intraoperative and pathological images of a patient who achieved pCR after conversion therapy.)
Postoperative Adjuvant Therapy and Follow Up
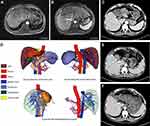
Most of the patients (28/41) in the conversion group continued to receive adjuvant HAIC/TACE plus PD-1 and TKIs according to the postoperative pathological results. Nine patients continued adjuvant therapy with PD-1 and TKIs. As of August 2022, with a median follow-up of 12.8 months (4–24 months), 14 patients in the conversion group experienced recurrence or metastasis, and no deaths occurred. (Figure 5 shows the images of the tumor changes during the conversion therapy. The surgical planning was planned with 3D reconstruction before surgery, and there was no recurrence 13 months after the surgery.)
Discussions
According to the BRIDGE study, 64% of HCC patients in China were initially diagnosed with BCLC B and C stages.18 Most of these patients had lost the chance for radical surgery, and the median survival time was only 2 years.4,19 Relevant studies have shown that for these initially unresectable HCC patients, if the tumor is shrunk or downstaged and then the opportunity for surgery is regained, it may be as effective as resection of early HCC, allowing the patients to achieve long-term survival.20 The triple-combination conversion therapy protocols show a strong anti-tumor effect, but it will bring a heavy burden to liver function and body. Whether these adverse factors affect hepatectomy after successful conversion therapy is unknown. Previous studies have shown hepatectomy after conversion therapy with PD-1 inhibitors plus tyrosine kinase inhibitors was effective and safe for advanced hepatocellular carcinoma.21,22 Our study focused on the impact of triple conversion therapy on subsequent hepatectomy and how hepatectomy after conversion therapy differs from direct hepatectomy.
Good liver function is a prerequisite for the treatment of HCC, and triple combination conversion therapy means higher requirements for liver function. We found that the Child-Pugh classification of patients remained unchanged after conversion therapy, some patients even improved their Child-Pugh scores and the corresponding biochemical indicators also showed that the effect of conversion therapy on liver function was small. Related studies have shown that maintaining the Child-Pugh score during chemotherapy was beneficial for the prognosis of patients with advanced HCC, even in patients with adequate hepatic reserve.23,24 Conversion therapy has caused certain adverse effects on the blood system, and toxic effects include neutropenia, thrombocytopenia, and anemia. This requires us to strictly monitor the toxic and side effects during chemotherapy. Surgical safety is an important content to be assessed before conversion resection.25 In addition to liver function and blood system, preoperative assessment of other organ functions is also necessary. The potential effects of systemic therapy on the body are wide-ranging, and any carelessness can have unintended consequences.
As for the timing of surgery, our center prefers to continue TKIs and PD-1 inhibitors for a period of time after the patient achieves objective response or downstaging, and performs radical surgery after keeping the lesion stable (1–2 months). The timing of discontinuation of preoperative systemic therapy is inconclusive, and continued preoperative medication does not increase the incidence of postoperative complications.26 We believe that the preoperative drug withdrawal time should be strictly in accordance with the medication instructions to minimize the impact on the surgery. If serious adverse drug reactions occur during targeted therapy or immunotherapy, surgery should be performed after the body has returned to normal.
After abdominal entry, we found that most of the patients in the conversion group had abdominal adhesions of varying degrees, a few patients had necrotic foci, and the incidence of cholecystitis was significantly higher. This may be related to the chemo-inflammation caused by TACE/HAIC infusion chemotherapy drugs. As we expected, the conversion group had significantly longer operative times and more blood loss. These reasons included (1) the presence of adhesions during liver dissociation, and the inflamed gallbladder needed to be removed at the same time, which takes longer; (2) the fragility of blood vessels and liver tissue increased after conversion therapy, resulting in more bleeding and more difficult hemostasis; (3) bone marrow suppression caused by preoperative chemotherapy reduces platelet function and number; (4) after conversion therapy, it was easy to cause liver edema, resulting in unclear anatomical relationship of the pipeline structure, increasing the difficulty of surgery. This also indirectly leaded to higher intraoperative blood transfusion rates and longer postoperative hospital recovery time.
Surprisingly, 6 patients in the conversion group developed PHLF, 3 of which were grade B PHLF. Preoperative liver function tests were normal and adequate liver volume was preserved postoperatively, but the incidence of PHLF in 14.6% (6/41) did require vigilance. We think that the toxicity of conversion therapy to the liver was not as mild as we previously estimated, and a single Child-Pugh classification does not reflect the whole picture of liver reserve function. The effects of chemotherapeutic drugs on the physiological function of the liver are multi-faceted, the most common being steatosis and hepatic sinusoidal vascular changes;27–29 targeted drugs can lead to direct injury of liver cells, cholestasis or steatosis of liver cells;30 and immune checkpoint inhibitors are prone to cause hepatocellular liver injury or cholestatic liver injury.31 Published studies have shown that inflammation and immune responses after hepatectomy promote liver parenchyma cells (hepatocytes, vascular endothelial cells, hepatic sinusoidal endothelial cells, etc.) and non-parenchymal cells in the liver to secrete a variety of active substances, such as interleukin 6 (IL-6), tumor necrosis factor (TNF), hepatocyte growth factor (HGF), etc.32–34 These active substances act on the whole body through blood circulation, and together trigger the regeneration of residual liver. The effect of conversion therapy on liver regeneration and the changes in the levels of related cytokines are still unclear, which are issues worthy of further investigation.
A variety of perioperative risk factors can also contribute to the occurrence of postoperative liver failure, including (1) insufficient residual liver function; (2) intraoperative hemorrhage leading to circulatory disturbance and liver failure functional failure; (3) intraoperative hepatic blood flow obstruction leads to ischemia-reperfusion injury to the remaining liver; (4) postoperative internal environment, liver function damage caused by water, electrolyte, acid-base imbalance. Therefore, adequate preoperative surgical evaluation is required to reduce the occurrence of postoperative liver failure. For example, preoperative indocyanine green (ICG) excretion test is used to assess the reserve function of the liver. Digital 3D reconstruction technology is worth applying. The 3D visualization technology can measure the preoperative liver volume, reconstruct the intrahepatic blood vessels and bile ducts, and accurately display the spatial anatomical relationship between the intrahepatic duct system and the tumor; perform virtual surgical analysis, optimize the surgical plan, and maximize the preservation of liver parenchyma.35
All patients achieved R0 resection with a pCR rate of 17.1%, and tumor necrosis rates in the remaining patients ranged from 60% to 99%, 14 patients achieved MPR (14/41,34.1%). Tumor cells were not completely necrotic in most patients, surviving tumor cells may be the source of recurrence or metastasis, this also illustrates the necessity of radical surgical resection after conversion therapy. Studies have shown that patients with pathological response after HCC conversion and resection have longer postoperative tumor-free survival, and patients with pCR or MPR have better postoperative survival than those without pCR or MPR.20,36 It also raises the question, do we need to wait until the tumor is necrotic enough (MPR or CR) to perform a hepatectomy? In addition, pathological examination can prove whether tumor cells are sensitive to conversion therapy, and can provide guidance for postoperative adjuvant therapy.37
There were several limitations in this study. First, this was a single-center retrospective study, and even if we use PSM method, selection bias was inevitable Second, we included a small sample size, and the postoperative follow-up period was short, and the final outcome of the patients was unknown. Third, the conversion therapy regimens were not uniform, which may have different effects on the surgery. At the same time, the surgery was not completed by the same group of surgeons, which may have a certain impact on the surgical results. Fourth, the majority of participants in this study had a background of post-hepatitis B cirrhosis, and it was unknown whether the results apply to other populations. Subsequent studies are needed to reveal the potential impact of conversion therapy on the liver and the body, whether it will bring adverse factors to the safety and efficacy of liver resection, and whether initially unresectable patients can really benefit from radical surgery.
Conclusion
We reported 41 patients with initially unresectable HCC who underwent successful R0 resection after conversion therapy with local regional therapy (HAIC/TACE) combined TKIs and PD-1 inhibitors. It is more and more common in clinical practice to obtain the opportunity for surgery through conversion therapy for u-HCC. However, there is a lack of evidence-based data on the timing of surgery, surgical methods, postoperative complications, postoperative recurrence time, and survival in these patients. Although hepatectomy after conversion therapy is more difficult than direct hepatectomy, we believe that accurate preoperative assessment, precise intraoperative operation, and attention to control of bleeding can ensure the safety of the operation. Attention should be paid to the prevention and treatment of PHLF, and further follow-up is required for postoperative recurrence and long-term survival of patients. Postoperative pathological analysis can provide guidance for subsequent adjuvant therapy.
Abbreviations
AE, adverse event; AFP, alpha‐fetoprotein; ALT, alanine aminotransferase; ASA, American Society of Anesthesiologists; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer stage; CNLC, Chinese Liver Cancer Stage; CR, complete response; CT, computed tomography; ECOG PS, Eastern Cooperative Oncology Group Performance Status; FFP, fresh frozen plasma; HAIC, hepatic arterial infusion chemotherapy; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; H&E, hematoxylin and eosin; HGF, hepatocyte growth factor; ICG, indocyanine green; ICI, immune checkpoint inhibitor; ICU, intensive care unit; IL-6, interleukin 6; MDT, multidisciplinary team; MPR, major pathologic responses; MRI, magnetic resonance imaging; mRECIST, modified response evaluation criteria in solid tumors; NA, not applicable; pCR, pathological complete response; PD-1, programmed cell death protein-1; PHLF, post-hepatectomy liver failure; PR, partial response; PSM, propensity score matching; PT, prothrombin time; RBC, red blood cell; SE, standard error; SFSS, small-for-size syndrome; TACE, transcatheter arterial chemoembolization; TBIL, total bilirubin; TKI, tyrosine kinase inhibitor; TNF, tumor necrosis factor; u‐HCC, unresectable HCC.
Data Sharing Statement
The study data may be provided by contacting the corresponding author.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University, No. (2022) CDYFYYLK (06-009), and in compliance with the Helsinki Declaration. Considering that patients medical data was analysed retrospectively, all informed consents were waived by the ethics committee. Of note, no patients-identifiable information was utilised.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by: the Key R&D Program of Jiangxi Provincial Department of Science and Technology, Grant Number: 20202BBGL7309.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2021;71(3):209–249. doi:10.3322/caac.21660
2. European Association For The Study Of The Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
3. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
4. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
5. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi:10.21037/hbsn-20-480
6. Zhong J, Ke Y, Gong W, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260(2):329–340. doi:10.1097/SLA.0000000000000236
7. Yin L, Li H, Li A, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan criteria: a RCT. J Hepatol. 2014;61(1):82–88. doi:10.1016/j.jhep.2014.03.012
8. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi:10.1002/hep.28453
9. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
10. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clinl Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
11. Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a Nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27(4):1003–1011. doi:10.1158/1078-0432.CCR-20-2571
12. Llovet J, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(5):293–313. doi:10.1038/s41575-020-00395-0
13. He MK, Liang RB, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:17588359211002720. doi:10.1177/17588359211002720
14. Zhang J, Zhang X, Mu H, et al. Surgical conversion for initially unresectable locally advanced hepatocellular carcinoma using a triple combination of angiogenesis inhibitors, anti-PD-1 antibodies, and hepatic arterial infusion chemotherapy: a retrospective study. Front Oncol. 2021;11:729764. doi:10.3389/fonc.2021.729764
15. Chen S, Wu Z, Shi F, et al. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol. 2022;148(8):2115–2125. doi:10.1007/s00432-021-03767-4
16. Xiang YJ, Wang K, Yu HM, et al. Transarterial chemoembolization plus a PD-1 inhibitor with or without lenvatinib for intermediate-stage hepatocellular carcinoma. Hepatol Res. 2022;52(8):721–729. doi:10.1111/hepr.13773
17. Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group Of Liver Surgery (ISGLS). Surgery. 2011;149(5):713–724. doi:10.1016/j.surg.2010.10.001
18. Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi:10.1111/liv.12818
19. Kloeckner R, Galle PR, Bruix J. Local and regional therapies for hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):137–149. doi:10.1002/hep.31424
20. Lau WY, Ho SK, Yu SC, Lai EC, Liew CT, Leung TW. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004;240(2):299–305. doi:10.1097/01.sla.0000133123.11932.19
21. Shen Y-H, Huang C, Zhu X-D, et al. The Safety profile of hepatectomy following preoperative systemic therapy with lenvatinib plus anti-PD-1 antibodies versus hepatectomy alone in patients with hepatocellular carcinoma. Ann Surg Open. 2022;3(2):e163. doi:10.1097/AS9.0000000000000163
22. Zhang W, Hu B, Han J, et al. Surgery after conversion therapy with PD-1 Inhibitors plus tyrosine kinase inhibitors are effective and safe for advanced hepatocellular carcinoma: a pilot study of ten patients. Front Oncol. 2021;11:747950. doi:10.3389/fonc.2021.747950
23. Terashima T, Yamashita T, Arai K, et al. Beneficial effect of maintaining hepatic reserve during chemotherapy on the outcomes of patients with hepatocellular carcinoma. Liver Cancer. 2017;6(3):236–249. doi:10.1159/000472262
24. D’Avola D, Granito A, de la Torre-Alaez M, Piscaglia F. The importance of liver functional reserve in the non-surgical treatment of hepatocellular carcinoma. J Hepatol. 2021;76:1185–1198. doi:10.1016/j.jhep.2021.11.013
25. Zhu XD, Huang C, Shen YH, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer. 2021;10(4):320–329. doi:10.1159/000514313
26. Bertacco A, Vitale A, Mescoli C, Cillo U. Sorafenib treatment has the potential to downstage advanced hepatocellular carcinoma before liver resection. Per Med. 2020;17(2):83–87. doi:10.2217/pme-2018-0114
27. Aloia T, Sebagh M, Plasse M, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clinl Oncol. 2006;24(31):4983–4990. doi:10.1200/JCO.2006.05.8156
28. Nordlinger B, Benoist S. Benefits and risks of neoadjuvant therapy for liver metastases. J Clinl Oncol. 2006;24(31):4954–4955. doi:10.1200/JCO.2006.07.9244
29. Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15(3):460–466. doi:10.1093/annonc/mdh095
30. Iacovelli R, Palazzo A, Procopio G, et al. Incidence and relative risk of hepatic toxicity in patients treated with anti-angiogenic tyrosine kinase inhibitors for malignancy. Br J Clin Pharmacol. 2014;77(6):929–938. doi:10.1111/bcp.12231
31. Miller ED, Abu-Sbeih H, Styskel B, et al. Clinical characteristics and adverse impact of hepatotoxicity due to immune checkpoint inhibitors. Am J Gastroenterol. 2020;115(2):251–261. doi:10.14309/ajg.0000000000000398
32. Fathi F, Sanei B, Ganjalikhani Hakemi M, Saidi RF, Rezaei A. Liver resection promotes (regulates) proinflammatory cytokines in patients with hepatocellular carcinoma. Can J Gastroenterol Hepatol. 2021;2021:5593655. doi:10.1155/2021/5593655
33. Michalopoulos GK, Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 2021;18(1):40–55. doi:10.1038/s41575-020-0342-4
34. Campana L, Esser H, Huch M, Forbes S. Liver regeneration and inflammation: from fundamental science to clinical applications. Nat Rev Mol Cell Biol. 2021;22(9):608–624. doi:10.1038/s41580-021-00373-7
35. Fang C, An J, Bruno A, et al. Consensus recommendations of three-dimensional visualization for diagnosis and management of liver diseases. Hepatol Int. 2020;14(4):437–453. doi:10.1007/s12072-020-10052-y
36. Zhang Y, Huang G, Wang Y, et al. Is salvage liver resection necessary for initially unresectable hepatocellular carcinoma patients downstaged by transarterial chemoembolization? Ten years of experience. Oncologist. 2016;21(12):1442–1449. doi:10.1634/theoncologist.2016-0094
37. Sun HC, Zhou J, Wang Z, et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. 2022;11(2):227–252. doi:10.21037/hbsn-21-328
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.