Back to Journals » Infection and Drug Resistance » Volume 15
Hemorrhagic Fever with Renal Syndrome Complicated with Acute Pancreatitis and Capillary Cholangitis: A Case Report
Authors Liu T, Yang W, Li K , Guo S, Tian M, Fang X
Received 15 August 2022
Accepted for publication 14 November 2022
Published 23 November 2022 Volume 2022:15 Pages 6755—6761
DOI https://doi.org/10.2147/IDR.S386273
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Tingting Liu,* Wu Yang,* Kun Li, Siruo Guo, Manman Tian, Xueling Fang
Department of Critical Care Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xueling Fang, Department of Critical Care Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, No. 79, Qingchun Road, Shangcheng District, Hangzhou, Zhejiang, People’s Republic of China, Tel +86-13588785095, Fax +86 571-87236838, Email [email protected]
Abstract: Hemorrhagic fever with renal syndrome (HFRS), caused by hanta viruses (HTNV), can be complicated by severe complications. Seventeen percent of the HFRS patients with abdominal pain had acute pancreatitis (AP). The reported prevalence of AP among HFRS patients has a conspicuous high mortality rate. Of note, acute capillary cholangitis (ACC) among HFRS patients presenting with abdominal pain appears extremely rare, particularly independent of HFRS patients complicated with AP. The main pathophysiological mechanism of HFRS complicated with AP and ACC may be that it preferentially damages the microvascular and induces plasma leakage. To date, the management of severe HFRS cases is mainly based on supportive treatment, including extracorporeal blood purification and mechanical ventilation. Here, we describe an exceptionally rare case of a 34-year man who developed HFRS with AP and ACC while improving from HTNV infection via antiviral and supportive treatment.
Keywords: hemorrhagic fever with renal syndrome, hantaviruses, abdominal pain, acute pancreatitis, acute acalculous cholangitis
Introduction
Hemorrhagic fever with renal syndrome (HFRS) is caused by various types of hanta viruses (HTNV), which is a single-stranded RNA virus carried by specific rodent hosts, and mainly spread through their blood, saliva, urine, and feces.1–5 The primary clinical characteristics of HFRS are diverse, including high fever, headache, backache, hemorrhage, acute renal failure, or even multiple organ dysfunction.2,6 As mentioned above, these manifestations are considered clinical hallmarks of HFRS. In addition, it has been reported that a large percentage (64.4%) of HFRS patients present with a complaint of gastrointestinal symptoms, such as nausea, vomiting, diarrhea, or upper abdominal pain.7 However, several pieces of research have confirmed that gastrointestinal symptoms may be related to acute pancreatitis (AP) during the acute phase of HFRS.6,8–10
AP is a rare and life-threatening complication of HFRS, with atypical gastrointestinal symptoms and a high misdiagnosis rate.2,6,11,12 It was reported that the prevalence of AP among HFRS patients was of high variability, ranging between 2.8% and 78%.2,12,13 A previous study from South Korea confirmed that only 8 out of 73 HFRS patients manifested pancreaticobiliary syndromes, 4 with cholecystitis, 3 with AP, and 1 with acute capillary cholangitis (ACC).7 ACC is the inflammation of the bile capillary without obstruction. ACC caused by HTNV is extremely rare, too.14 Moreover, ACC remains one of the most exclusive diagnoses in various conditions, including sepsis, fasting, post-operation.14 Few cases co-existed with AP and ACC in patients with HFRS were reported. Here we reported a case of a young man with HFRS who suffered from AP and ACC caused by HTNV.
Case Report
On June 25, 2022, a 34-year man was admitted to the emergency department of the first affiliated hospital of Zhejiang University school of medicine, with complaints of fever of 5 days (the highest temperature 40°C), diarrhea, and vomiting for 2 days.
His manifestations began gradually and started 2 days ago (June 23, 2022) without apparent cause. At first, high fever with diarrhea and vomiting was predominant, and progression to abdominal and lumbodorsal pain. He subsequently went to the local hospital (Qian Shan County People’s Hospital) and the whole disease course of laboratory results were shown in Table 1. His blood tests at the local hospital showed that white blood cells (WBC) 4.67×109/L, the percent of neutrophile (N%) 76%, C-reactive protein (CRP) 6.81 mg/L, platelets (PLT) 131×109/L, creatinine (Cr) 91 U/L, aspartate aminotransferase (AST) 132.5 U/L, lactate dehydrogenase (LDH) 341 U/L, amylases (AMY) 50.2 U/L, total bilirubin (TBIL) 28.38 umol/L, direct bilirubin (DBIL) 10.8 μM /L, and indirect bilirubin (IBIL) 17.58 μM/L. The chest and abdomen computed tomography scan (CT) showed no abnormalities. He was treated with low-dose dexamethasone, piperacillin sodium, and tazobactam sodium for infection in the local clinic. However, he appeared to have jaundice, and his condition declined rapidly and was referred to our emergency room two days later (June 25, 2022) due to abdominal pain, oliguria, and jaundice.
 |
Table 1 Biological Parameters of the Patient |
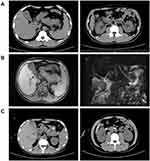
His vital signs were as follows: temperature, 36.9°C; blood pressure, 108/77 mmHg; pulse rate, 106 beats/min; and respiration rate, 19 breaths/min. The patient was in poor general condition with yellowing of the skin and sclera and oliguria (urinary output 380 mL/24 h). His abdomen had obvious tenderness with slight rebound tenderness. The bowel sounds were also reduced. He neither suffered from chronic illnesses nor significant family history. By his own account, he worked in a place overrun with rats. The routine laboratory tests showed: WBC 16.32×109/L, N% 80.3%, hemoglobin (Hb) 188 g/L, PLT 28×109/L, AST 364 U/L, Cr 147 U/L, LDH 1324 U/L, AMY 190 U/L, TBIL 68.1 μmol/L, DBIL 50.8 μmol/L, and IBIL 17.3 μmol/L, CRP 55.42 mg/L, procalcitonin (PCT) 24.58/mL, prothrombin (PT) 14.4 s, activated partial thromboplastin (APTT) 53.9s, and D-dimer 8850 μg/L, troponin I (TNI) 0.043 ng/mL, and B-type natriuretic peptide (BNP) 299 pg/mL. The abdomen CT showed acute pancreatitis, effusion around the pancreas, kidneys, paracolic sulcus, and pelvic ascites (Figure 1A). The patient was admitted to our intensive care unit (ICU) to treat AP with multiple organ dysfunction further.
Upon admission to our ICU (15:50 June 25, 2022), we received a notice from the emergency room. The patient’s IgM and IgG antibodies for HTNV (Diagnostic Kit for IgM/IgG Antibody to HTNV, DRG, German) were positive through enzyme-linked immunosorbent assays. The 2019-nCoV RNA is negative. Additional tests were negative, such as malaria parasite, leptospira, human immunodeficiency virus, hepatitis viruses, cytomegalovirus, respiratory syncytial virus, Epstein-Barr virus, and influenza virus. Based on the returned test results, we speculated that the patient with AP was caused by HFRS and mainly focused on the original disease HFRS and its related AP, taking the antiviral agent ribavirin (500mg, every 12 h), somatostatin (3 mg, every 12 h) and continuous renal replacement therapy (CRRT) to combat HTNV-induced HFRS and AP. After 3-day from admission (June 28, 2022), the patient’s condition was still aggravated, exhibiting the most obvious jaundice and irregular heartbeats. His blood tests revealed the following: a WBC of 21.11×109/L, PLT 40×109/L, CRP 31 mg/L, TBIL 159.2 μmol/L, DBIL 134.5 μmol/L, IBIL 24.7 μmol/L, ALT 170 U/L, AST 504 U/L, LDH 1767 U/L, urea nitrogen (BUN) 36.73 mmol/L, high sensitivity troponin I (hsTNI) 0.16 ng/mL, AMY 329 U/L, and Cr 536 U/L. The sputum, blood, and urine cultures were all to be normal. The patient’s abnormal liver function mainly showed a significant increase in DBIL. We examined the liver, gallbladder, spleen, and pancreas via ultrasound and merely found post-treatment alternation of AP. Next, we excluded biliary pancreatitis and used the magnetic resonance cholangiopancreatography (MRCP) test. The MRCP demonstrated no intrahepatic and extrahepatic bile duct dilation (Figure 1B). We considered that the HFRS patient co-existed with AP and ACC. As the multi-organ dysfunction aggravated dramatically, continuous renal replacement therapy (CRRT) was continually adopted to treat severe oliguria. Meanwhile, low-dose methylprednisolone (40 mg, every 12 h), ademetionine (1000 mg/d), and alprostadil injection (10 μg/d) were applied to treat AAC.
Following comprehensive management, the patient’s condition recovered. Accompanying the 24 h total urine output increased to 1350 mL on the 8-day of admission (July 2, 2022), the CRRT was discontinued. His laboratory results, meanwhile, were improved. Furthermore, his enhanced abdominal CT imaging (July 5, 2022) manifested that the pancreas was still swollen with slight effusion surrounding the pancreas (Figure 1C). On the same day (July 5, 2022), the methylprednisolone dosage was reduced to 40mg/d. As his condition became stable, the patient was transferred to the general ward for further therapy (somatostatin 3mg, QD, and methylprednisolone 20mg, QD) on July 6, 2022. The patient was discharged with the diagnosis of HFRF, AP, and ACC on July 18, 2022, and then followed up as an outpatient. The tendency changes in the laboratory results and reference range were summarized in Figure 2 and Table 1.
 |
Figure 2 (A–D) Trend charts for key liver, pancreas, and kidney function indicators since the day (June 25, 2022) transferred to the intensive care unit (ICU). |
Discussion
HFRS is a worldwide public health problem and is mainly induced by four kinds of viruses, including HTNV, Puumala virus (PUUA), Seoul virus (SEOV), and Dobrava-Belgrade virus (DOBV).15 Each virus could cause a severe outcome with high mortality. The epidemic situation in china is still grim.6,16 Generally, HFRS is primarily caused by HTNV in china.3,6
HFRS patients appeared with common clinical manifestations, such as fever, shock, hemorrhage, and acute kidney injury.15,17 Though there is no clear answer about the pathogenesis of HFRS, it may injure vascular endothelium, increase vascular permeability, emerge plasma leakage, and eventually trigger an inflammatory cascade, which influences multiple organs.5,14,18 Thus, HFRS patients suffering from HTNV could cause extrarenal organ injury. It is necessary for a comprehensive understanding of extrarenal syndromes, which would be useful in diagnosing and managing patients with HFRS presenting with various kinds of extrarenal manifestation.
Up to one-third of HFRS patients exhibited the involvement of extrarenal organs, and pancreatobiliary manifestations were the most common extrarenal manifestation, such as AP, biliary pancreatitis, and cholangitis.12 Abdominal pain is a common symptom among HFRS patients showing 49.4% of HFRS patients complained of the symptom in one study.6 Based on a retrospective review, 17% of patients with abdominal pain had pancreaticobiliary disease.6,12 The incidence rate of AP among HFRS patients is highly variable, ranging between 2.8% and 78%.2,6,7,12 It could be difficult to differentiate between abdominal pain induced by pancreatobiliary diseases and HFRS. Hence, HFRS patients with abdominal pain should test for pancreatic or liver enzyme levels.
Further imaging, including ultrasound examination or CT, should be evaluated the pancreatobiliary diseases. In this case, the rising pancreatic and liver enzyme levels could be correlated with increased vascular permeability, which showed effusion surrounding several parts according to the abdominal CT scan and ultrasonography. Viral infections are one of the causes contributing to ACC. Very few cases of ACC caused by HTNV had been reported. Although we first suspected biliary pancreatitis, ultrasonography revealed no signs of biliary blockage. Subsequently, biliary obstruction was excluded via MRCP. We still do not clarify whether the bile duct injury promoted the AP or AP-affected bile duct or whether they appeared together.
The diagnosis of HTNV infection, in addition to epidemiological and clinical information, can depend on a serological test, including detecting IgM/IgG antibodies of HTNV via enzyme-linked immunosorbent assays.1 The clinical course of the antibodies presented that the IgM antibody could last at least 3 months following acute HTNV infection and the IgG antibody could rise four-fold during the convalescent phase.19 However, Acute pancreaticobiliary disease among HFRS patients is easily misdiagnosed as both diseases share some common clinical manifestations, including nausea, vomiting, and abdominal pain.7 Thereinto, early recognition of HFRS patients with the acute pancreaticobiliary disease might be important to combat the original life-threatening disease. In this case, we did not perform a real-time reverse transcription-polymerase chain reaction test to confirm the serotype of the virus due to condition limitations.19 Of concern, we did not delay the clinical diagnosis and treatment of HFRS in this case.
Of note, there is no specific treatment available for HFRS, and the treatment of HFRS remains primarily supportive.20,21 Ribavirin, an antiviral drug, has been confirmed to significantly reduce the mortality rate by administration in the first 5 days after the onset of symptoms. Unfortunately, ribavirin treatment is likely ineffective during HTNV pulmonary syndrome progressing to the cardiopulmonary phase.22,23 HTNV preferentially damages the endothelial cells and triggers an inflammatory storm in the host.23–25 Despite the paradoxical results of cortisone therapy in HFRS,23,26 low-dose methylprednisolone was still used to confront the capillary endothelial injury. The patient never appeared with pulmonary syndromes since the onset of symptoms. Despite exceeding the ribavirin best therapy time, the patient improved gradually after a combination of ribavirin and low-dose methylprednisolone treatment.
Conclusion
In summary, this extremely rare HFRS case had extrarenal complications involving AP and ACC. These manifestations were associated with the pathogenic mechanism of endothelial dysfunction with plasma leakage. Early discovery, diagnosis, diagnosis, and treatment are key to improving prognosis and reducing mortality of HFRS with AP and ACC.
Consent Statement
The patient provided written informed consent to allow the case details and any accompanying images to be published. No specific ethics committee approval was required for this study.
Funding
This research received external funding from the Medical Science and Technology Project of Zhejiang Province (2019RC170) and the Department of Education of Zhejiang Province (Y201941857).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Qiu FQ, Li CC, Zhou JY. Hemorrhagic fever with renal syndrome complicated with aortic dissection: a case report. World J Clin Cases. 2020;8:5795–5801. doi:10.12998/wjcc.v8.i22.5795
2. Guo Q, Xu J, Shi Q, Du B. Acute pancreatitis associated with hemorrhagic fever with renal syndrome: a cohort study of 346 patients. BMC Infect Dis. 2021;21(1):267. doi:10.1186/s12879-021-05964-5
3. Cai Y, Wei Y, Han X, et al. Spatiotemporal patterns of hemorrhagic fever with renal syndrome in Hebei Province, China, 2001–2016. J Med Virol. 2019;91(3):337–346. doi:10.1002/jmv.25293
4. D’Souza MH, Patel TR. Biodefense implications of new-world hantaviruses. Front Bioeng Biotechnol. 2020;8. doi:10.3389/fbioe.2020.00925
5. Zhang Y, Ma R, Wang Y, et al. Viruses run: the evasion mechanisms of the antiviral innate immunity by hantavirus. Front Microbiol. 2021;12:759198. doi:10.3389/fmicb.2021.759198
6. Wang WJ, Zhao J, Yang JS, Liang MM, Ni MY, Yang JH. Clinical analysis of patients with acute pancreatitis complicated with hemorrhagic fever with renal syndrome and acute biliary pancreatitis. Medicine. 2020;99:e18916. doi:10.1097/MD.0000000000018916
7. Zhu Y, Chen YX, Zhu Y, Liu P, Zeng H, Lu NH. A retrospective study of acute pancreatitis in patients with hemorrhagic fever with renal syndrome. BMC Gastroenterol. 2013;13:171. doi:10.1186/1471-230X-13-171
8. Puca E, Pilaca A, Pipero P, Kraja D, Puca EY. Hemorrhagic fever with renal syndrome associated with acute pancreatitis. Virol Sin. 2012;27:214–217. doi:10.1007/s12250-012-3231-3
9. Fan H, Zhao Y, Song FC. Acute pancreatitis associated with hemorrhagic fever with renal syndrome: clinical analysis of 12 cases. Ren Fail. 2013;35(10):1330–1333. doi:10.3109/0886022X.2013.828187
10. Kang ES, Lee S, Kim W, et al. Acute pancreatitis associated with haemorrhagic fever with renal syndrome. Nephrology. 2005;10(4):421–425. doi:10.1111/j.1440-1797.2005.00428.x
11. Yotsuyanagi H, Koike K, Meng G, et al. Acute exacerbation of autoimmune liver disease associated with hantaviral infection. Scand J Infect Dis. 1998;30:81–83. doi:10.1080/003655498750002358
12. Park KH, Kang YU, Kang SJ, Jung YS, Jang HC, Jung SI. Experience with extrarenal manifestations of hemorrhagic fever with renal syndrome in a tertiary care hospital in South Korea. Am J Trop Med Hyg. 2011;84:229–233. doi:10.4269/ajtmh.2011.10-0024
13. Puca E, Harxhi A, Pipero P, et al. Pancreatitis in patients with hemorrhagic fever with renal syndrome: a five-year experience. J Infect Dev Ctries. 2017;11(11):900–903. doi:10.3855/jidc.9567
14. Kim SH, Park S, Choi J. Acalculous cholecystitis associated with hantaan virus: a case report. Ann Hepatobiliary Pancreat Surg. 2019;23:278–281. doi:10.14701/ahbps.2019.23.3.278
15. Yang X, Wang C, Wu L, Jiang X, Zhang S, Jing F. Hemorrhagic fever with renal syndrome with secondary hemophagocytic lymphohistiocytosis in West China: a case report. BMC Infect Dis. 2019;19(1):492. doi:10.1186/s12879-019-4122-0
16. Zou LX, Sun L. Analysis of hemorrhagic fever with renal syndrome using wavelet tools in Mainland China, 2004–2019. Front Public Health. 2020;8:571984. doi:10.3389/fpubh.2020.571984
17. Jiao J, Wu L, Yin J, Quan X, Chen W, Hu J. Guillain-barre syndrome associated with hemorrhagic fever with renal syndrome in China: a case report. BMC Infect Dis. 2018;18(1):143. doi:10.1186/s12879-018-3049-1
18. Pal E, Korva M, Resman Rus K, et al. Relationship between circulating vascular endothelial growth factor and its soluble receptor in patients with hemorrhagic fever with renal syndrome. Emerg Microbes Infect. 2018;7(1):89. doi:10.1038/s41426-018-0090-5
19. Avsic-Zupanc T, Saksida A, Korva M. Hantavirus infections. Clin Microbiol Infect. 2019;21:e6–e16. doi:10.1111/1469-0691.12291
20. Tariq M, Kim DM. Hemorrhagic fever with renal syndrome: literature review, epidemiology, clinical picture and pathogenesis. Infect Chemother. 2022;54(1):1–19. doi:10.3947/ic.2021.0148
21. Liu R, Ma H, Shu J, et al. Vaccines and therapeutics against hantaviruses. Front Microbiol. 2020;10:2989. doi:10.3389/fmicb.2019.02989
22. Mertz GJ, Miedzinski L, Goade D, et al. Placebo-controlled, double-blind trial of intravenous ribavirin for the treatment of hantavirus cardiopulmonary syndrome in North America. Clin Infect Dis. 2004;39(9):1307–1313. doi:10.1086/425007
23. Brocato RL, Hooper JW. Progress on the prevention and treatment of hantavirus disease. Viruses. 2019;11(7):610. doi:10.3390/v11070610
24. Jiang H, Du H, Wang LM, Wang PZ, Bai XF. Hemorrhagic fever with renal syndrome: pathogenesis and clinical picture. Front Cell Infect Microbiol. 2016;6:1. doi:10.3389/fcimb.2016.00001
25. Malinin OV, Kiryanov NA. Fatal cases of hemorrhagic fever with renal syndrome in Udmurtia, Russia, 2010 to 2019. Eur J Clin Microbiol Infect Dis. 2022;41(7):1059–1064. doi:10.1007/s10096-022-04463-y
26. Vial PA, Valdivieso F, Ferres M, et al. High-dose intravenous methylprednisolone for hantavirus cardiopulmonary syndrome in Chile: a double-blind, randomized controlled clinical trial. Clin Infect Dis. 2013;57(7):943–951. doi:10.1093/cid/cit394
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.